Abstract
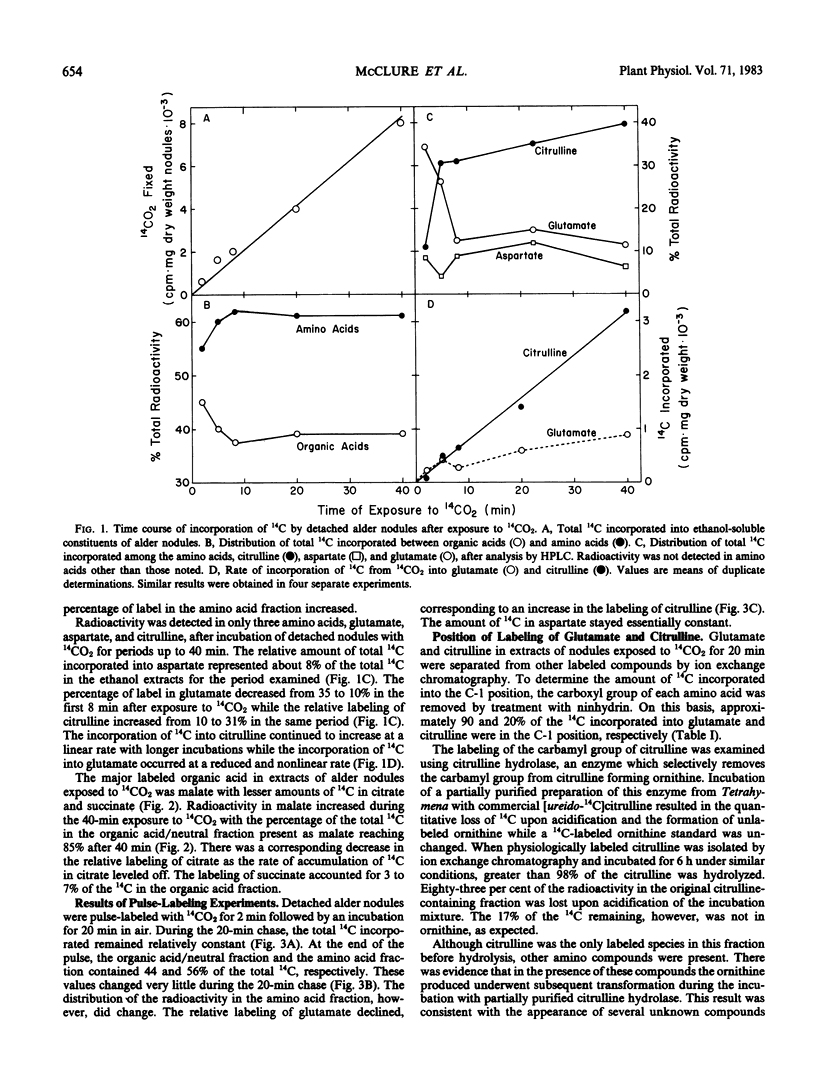
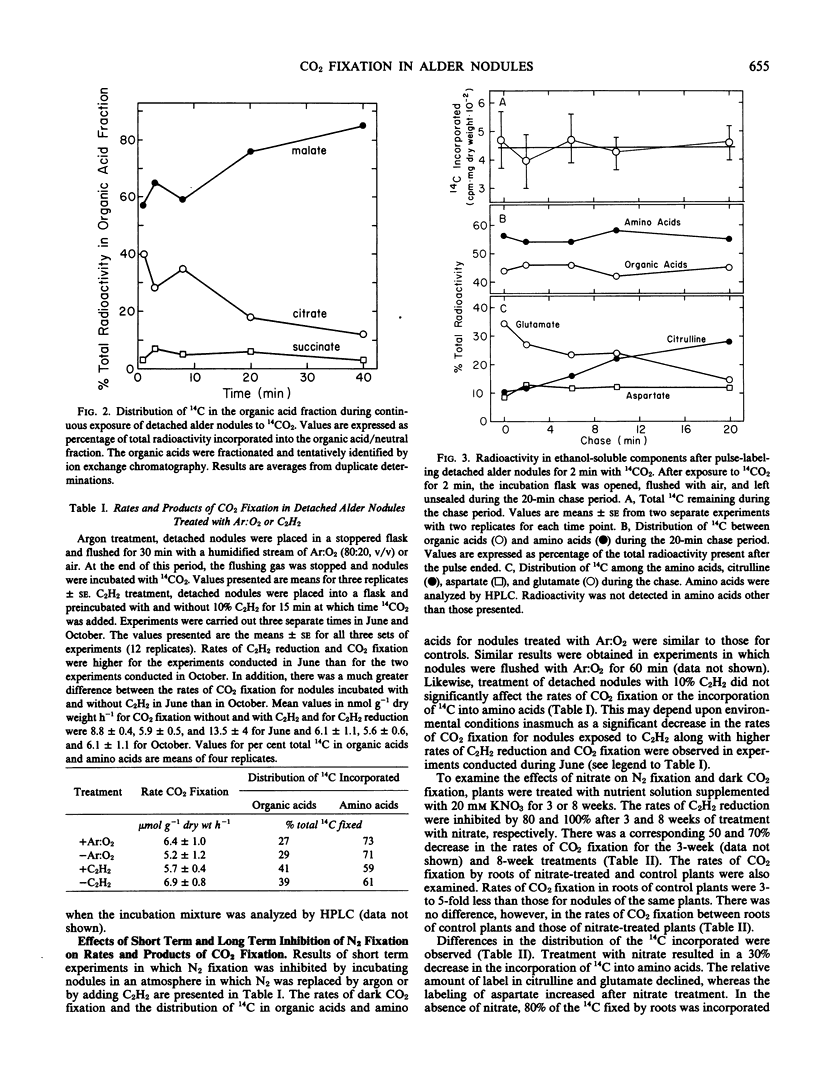
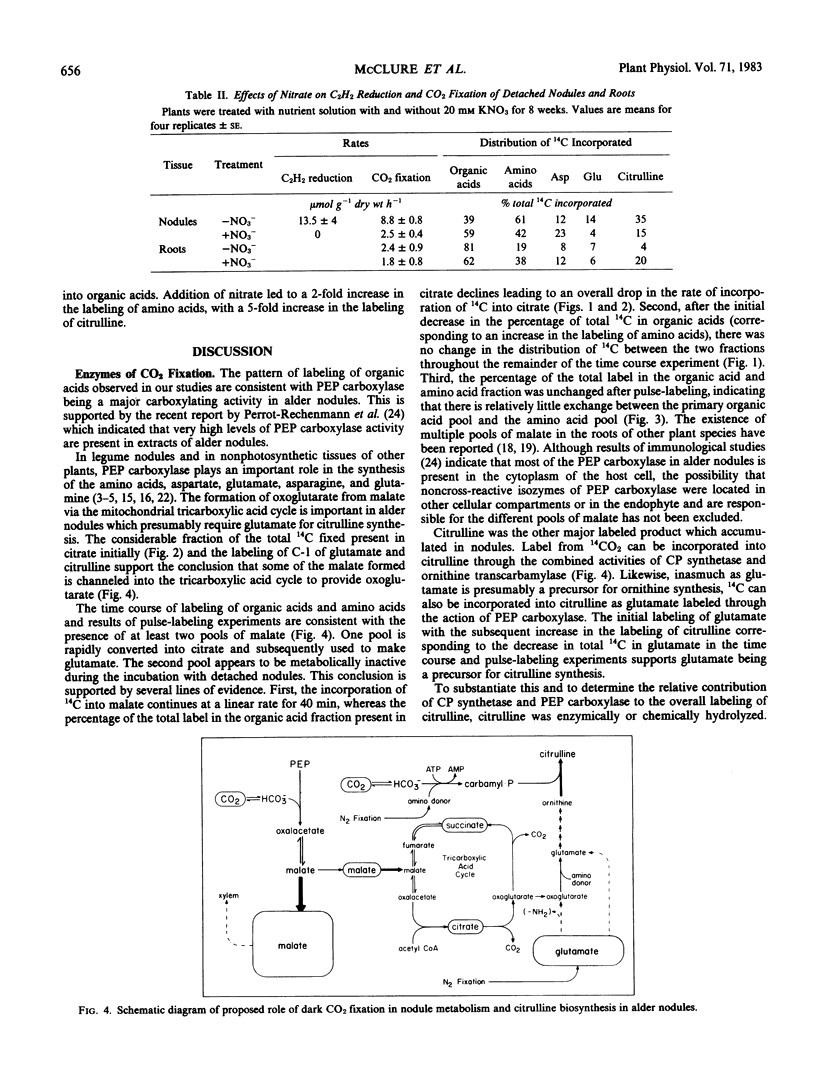
Detached roots and nodules of the N2-fixing species, Albus glutinosa (European black alder), actively assimilate CO2. The maximum rates of dark CO2 fixation observed for detached nodules and roots were 15 and 3 micromoles CO2 fixed per gram dry weight per hour, respectively. The net incorporation of CO2 in these tissues was catalyzed by phosphoenolpyruvate carboxylase which produces organic acids, some of which are used in the synthesis of the amino acids, aspartate, glutamate, and citrulline and by carbamyl phosphate synthetase. The latter accounts for approximately 30 to 40% of the CO2 fixed and provides carbamyl phosphate for the synthesis of citrulline. Results of labeling studies suggest that there are multiple pools of malate present in nodules. The major pool is apparently metabolically inactive and of unknown function while the smaller pool is rapidly utilized in the synthesis of amino acids. Dark CO2 fixation and N2 fixation in nodules decreased after treatment of nodulated plants with nitrate while the percentage of the total 14C incorporated into organic acids increased. Phosphoenolpyruvate carboxylase and carbamyl phosphate synthetase play key roles in the synthesis of amino acids including citrulline and in the metabolism of N2-fixing nodules and roots of alder.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERL S., TAKAGAKI G., CLARKE D. D., WAELSCH H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962 Aug;237:2562–2569. [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHERTOGH A. A., MAYEUX P. A., EVANS H. J. THE RELATIONSHIP OF COBALT REQUIREMENT TO PROPIONATE METABOLISM IN RHIZOBIUM. J Biol Chem. 1964 Aug;239:2446–2453. [PubMed] [Google Scholar]
- Gardner I. C., Leaf G. Translocation of Citrulline in Alnus Glutinosa. Plant Physiol. 1960 Nov;35(6):948–950. doi: 10.1104/pp.35.6.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Nagarajan B. Separation and estimation of arginine-related metabolites in tissues. Anal Biochem. 1980 Sep 15;107(2):318–323. doi: 10.1016/0003-2697(80)90390-5. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Chambers P. The biosynthesis of proline by Tetrahymena pyriformis. Biochim Biophys Acta. 1967 Nov 28;148(2):435–447. doi: 10.1016/0304-4165(67)90140-7. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of organic acids in corn roots I. Differential labeling of 2 malate pools. Plant Physiol. 1966 Apr;41(4):709–712. doi: 10.1104/pp.41.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Coker G. T. Ammonia Assimilation in Alnus glutinosa and Glycine max: SHORT-TERM STUDIES USING [N]AMMONIUM. Plant Physiol. 1981 Apr;67(4):662–665. doi: 10.1104/pp.67.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNOCK L. G., VAN EYS J. Normal carbohydrate metabolism in Tetrahymean pyriformis. J Cell Comp Physiol. 1962 Aug;60:53–60. doi: 10.1002/jcp.1030600108. [DOI] [PubMed] [Google Scholar]


