Abstract
Background
Emergency percutaneous coronary intervention (PCI) can quickly restore myocardial perfusion after acute coronary syndrome. Whether and which lipid-lowering regimens are effective in reducing major adverse cardiovascular events (MACEs) and mortality risk after PCI remain unclear.
Objective
This study assessed the benefits of different lipid-lowering regimens on the risk of MACEs and mortality in the post-PCI population by network meta-analysis.
Methods
Public databases, including PubMed, Embase and the Cochrane Library, were searched from inception to August 2022. Randomised controlled trials (RCTs) on lipid-lowering regimens in post-PCI populations were included and analysed. The outcomes were the incidence of all-cause mortality and MACEs, whether reported as dichotomous variables or as HRs.
Results
Thirty-nine RCTs were included. For MACEs, alirocumab plus rosuvastatin (OR: 0.18; 95% CI: 0.07 to 0.44), evolocumab plus ezetimibe and statins (OR: 0.19; 95% CI: 0.06 to 0.59), eicosapentaenoic acid (EPA) plus pitavastatin (HR: 0.67; 95% CI: 0.49 to 0.96) and icosapent ethyl plus statins (HR: 0.73; 95% CI: 0.62 to 0.86) had significant advantages and relatively high rankings. For mortality, rosuvastatin (OR: 0.30; 95% CI: 0.11 to 0.84), ezetimibe plus statins (OR: 0.55; 95% CI: 0.43 to 0.89) and icosapent ethyl plus statins (OR: 0.66; 95% CI: 0.45 to 0.96) had significant advantages compared with the control.
Conclusion
EPA, especially icosapent ethyl, plus statins had a beneficial effect on reducing the risk of MACEs and mortality in post-PCI patients. Proprotein convertase subtilisin/kexin type-9 inhibitors plus statins were able to reduce the risk of MACEs, but the risk of mortality remained unclear.
PROSPERO registration number
CRD42018099600.
Keywords: Coronary heart disease, Coronary intervention, Lipid disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Only randomised controlled trials with high overall design quality were considered for inclusion.
Major adverse cardiovascular event (MACE) and mortality were adopted as outcomes with little influence from subjective factors. This meta-analysis was based on the study level instead of the individual level.
The criteria for defining MACEs varied among studies.
Many included studies only reported dichotomous outcomes but did not report the HR results.
Introduction
Acute coronary syndrome (ACS) is a term used to refer to a range of conditions associated with acute myocardial ischaemia and/or infarction, which are usually due to coronary artery occlusion and acute ischaemic necrosis of the myocardium due to the progression of coronary atherosclerotic lesions.1 2 Emergency percutaneous coronary intervention (PCI) can quickly restore myocardial perfusion.3 Although the development of technological and procedural PCI has resulted in substantial improvements in clinical outcomes, recurrent coronary events may still occur after PCI.4
The view of ‘residual cardiovascular risk’ was introduced because major adverse cardiovascular events (MACEs) still occur in some patients who underwent PCI during follow-up. PCI can treat focal manifestations of systemic progressive disease, but the residual risk of ACS is largely related to the systemic proatherosclerotic effect of poorly controlled cardiovascular risk factors.4 Lowering lipid levels, especially Low density lipoprotein - cholesterol (LDL-C), can halt the progression of coronary atherosclerosis and improve cardiovascular outcomes. Based on this view, it is believed that long-term optimal lipid-lowering therapy is effective in reducing long-term cardiovascular events after PCI. However, this view was still subject to challenges.
Based on data from the ‘Korea Acute Myocardial Infarction Registry’, the proponents concluded that patients treated with statins had significantly lower rates of MACEs, all-cause death and cardiac death during the 2-year follow-up period after PCI application.5 However, a study of postoperative follow-up of patients with PCI enrolled in the Melbourne Interventional Group registry concluded that statins have no significant beneficial effect on MACEs after PCI.6 The controversy may be explained by two concepts: on the one hand, the optimal lipid reduction target may not be achieved by using single statins.7 8 On the other hand, long-term high-dose application of statins increases the risk of intracerebral haemorrhage and other side effects.9 10
There is a consensus on preloading high-dose statins to reduce MACEs in the perioperative period with PCI.11 12 However, there is still insufficient evidence for the continued application of lipid-lowering drugs to reduce the risk of long-term MACEs and mortality. This study assessed the benefits of different lipid-lowering regimens on the risk of MACEs and mortality in the post-PCI population by network meta-analysis (NMA).
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The study was registered with PROSPERO.
Patient and public involvement
None.
Search strategy
Public literature databases, including PubMed, Embase and the Cochrane Library, were searched from inception to August 2022 without language restrictions using the following search terms: (lipid-lowering or statin or simvastatin or rosuvastatin or atorvastatin or fluvastatin or lovastatin or pravastatin or pitavastatin or mevastatin or ezetimibe or “eicosapentaenoic acid” or “icosapent ethyl” or “bempedoic acid” or fibrate or bezafibrate or gemfibrozil or fenofibrate or ciprofibrate or evolocumab or alirocumab or evinacumab or volanesorsen or vupanorsen or pelacarsen or olezarsen or inclisiran or olpasiran) and (“percutaneous coronary intervention” or “coronary angioplasty”) and (random* or randomized or randomized). The details of the full search strategy are listed in the online supplemental file 1. The references of relevant systematic reviews and meta-analyses were also searched to avoid omissions. The two authors conducted literature retrieval independently, and any conflicts were resolved through discussion with the third author.
bmjopen-2022-070827supp001.pdf (33KB, pdf)
Inclusion and exclusion criteria
The literature was included if it met the following criteria: (1) the study adopted a randomised controlled study design; (2) the study included patients who underwent PCI surgery or reported the subgroup of the population that underwent PCI; (3) the lipid-lowering regimen was applied to the population of the intervention group; (4) the control group used a different lipid-lowering agent or regimen and (5) the study reported the outcome of mortality and/or MACEs. The exclusion criteria were as follows: (1) as preloading of statins before PCI was shown to have clear benefits, to determine whether application of lipid-lowering drugs after PCI also had beneficial effects, this work excluded studies on the preloading application of lipid-lowering drugs before PCI; and (2) although high-dose lipid-lowering agents, such as statins, have a better lipid-lowering effect, long-term application may bring potential side effects.9 13 Therefore, only studies in which all agents were considered to be applied at reasonable doses were included, and dose–response studies were excluded. In addition, repeatedly published studies, protocols, conference abstracts, reviews, comments and editorials were also excluded.
Data extraction and quality assessment
Two authors independently extracted the information from the included studies. The contents include the name of the first author, publication year, study location, sample size (population that underwent PCI), study abbreviation and registration number, lipid-lowering intervention and control and follow-up time.
The outcomes analysed were the incidence of all-cause mortality and MACEs, whether reported as dichotomous or HR statistics based on Cox regression. The MACE outcome was selected to most closely approximate the composite endpoint, including mortality, Myocardial infarction (MI), stroke, coronary revascularisation and restenosis. Study quality was assessed by two investigators using the Cochrane risk of bias assessment tool, which included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential biases.
Statistical analysis
We conducted a frequentist NMA using random-effects models weighted by the inverse variance method. ORs and 95% CIs were used for dichotomous outcomes. The HRs and 95% CIs based on Cox regression results were also pooled for reporting. If the HR value was not reported but there was a Kaplan-Meier survival curve, the HR value was extracted from the curve by GetData Graph Digitizer software V.2.24.
In network plots, the direct comparisons among treatment arms are shown, the end of each line indicates a treatment arm, and the thickness of the lines indicates the number of studies comparing the two treatments. Forest plots were used to describe the network comparison results between each treatment and the control.
The restricted maximum likelihood estimation was used to quantify network heterogeneity. The Q statistic was used to assess the sum of statistics for heterogeneity (within designs) and for overall inconsistency (between designs).14
The ranking probabilities of each regimen were estimated using the surface under the cumulative ranking curve (SUCRA), which was the ratio of the area under the curve to the entire area. A comparison-adjusted funnel plot was used to examine potential publication biases in the NMA. P values of <0.05 were considered to indicate statistical significance. The NMA was performed using R language with the ‘netmeta’ package.
Results
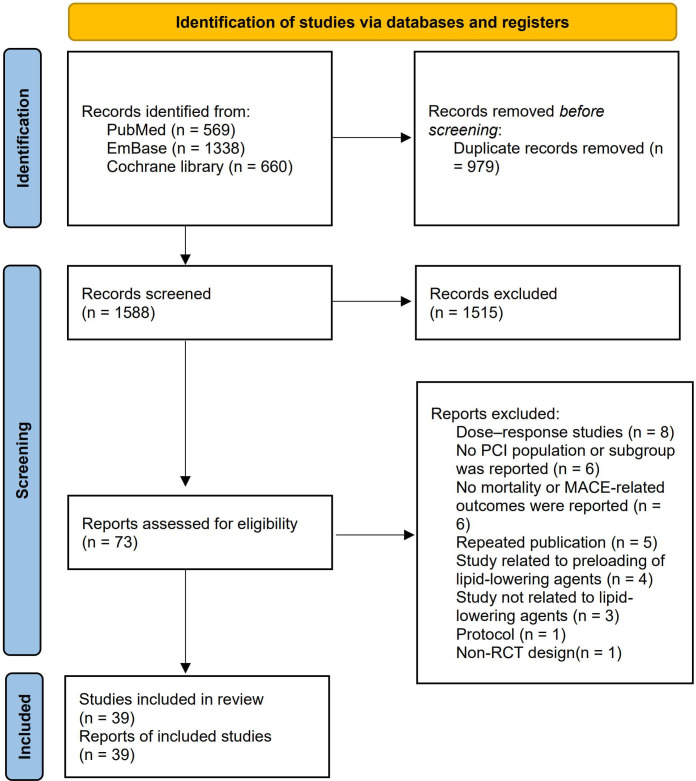
After removing duplicates, we obtained 1588 literature items. After screening the titles and abstracts, 1515 irrelevant studies were excluded. Seventy-three articles were screened for full text. The following articles were excluded: dose–response studies (8); those where no PCI population or subgroup was reported (6); those where no mortality or MACE-related outcomes were reported (6); repeated publications (5); studies related to preloading of lipid-lowering agents (4); studies unrelated to lipid-lowering agents (3); a protocol study (1) and a study with a non-randomised controlled trial (RCT) design (1). Finally, 39 articles were included, containing 54 478 post-PCI patients15–53 (figure 1).
Figure 1.
Flowchart of the study selection process for eligible studies. *Consider, if feasible to do so, reporting the numbers of records identified from each database or register searched (rather than the total number across all database/registers). **If automation tools were used, indicate how many records were exculded by a human and how many were exculded by automation tools. MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention; RCT, randomised controlled trial.
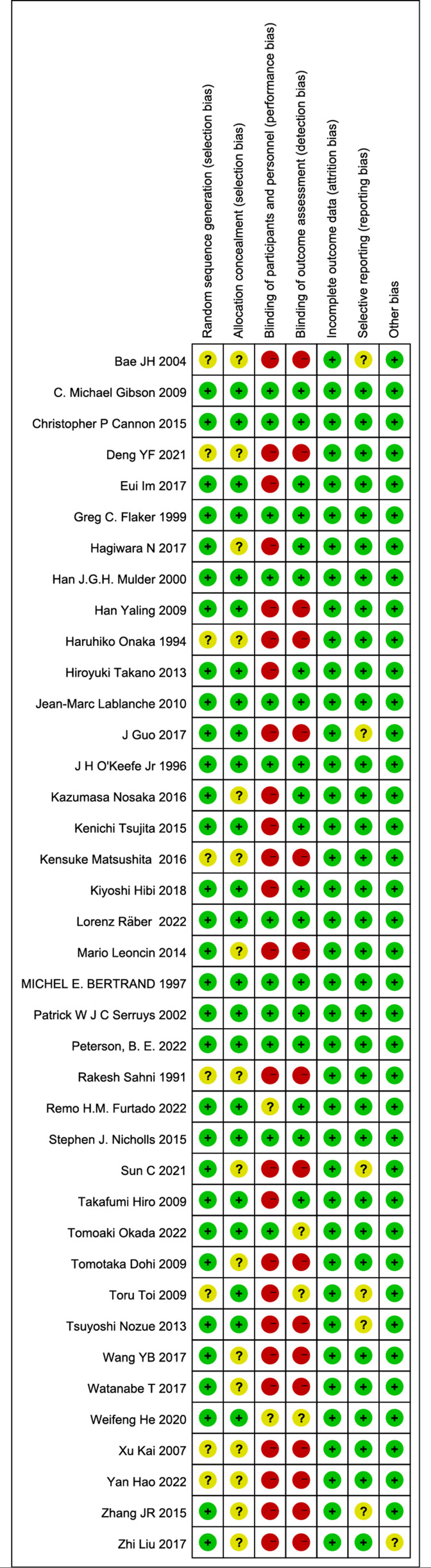
Among the included studies, the publication period ranged from 1991 to 2022. The research locations were mainly in Asia (China, Japan and South Korea), Europe (Netherlands, France and Italy), America and multiple centres. There were 10 studies with sample sizes greater than 1000 patients. There were also 22 studies with publicly available clinical study registration numbers (table 1). In terms of design quality, all included studies were RCTs. Therefore, the design quality was generally high. The main factors potentially affecting design quality were the blinding of participants and personnel and blinding of outcome assessment (figure 2). However, as the desired outcomes were mortality and MACEs, the subjective factors of the investigator had little influence on the outcomes.
Table 1.
The characteristics of included studies
| Study | Location | Sample size | Abbreviation | Register ID | Intervention | Control | Follow-up* |
| Räber et al15 2022 | European | 300 | PACMAN-AMI | NCT03067844 | Alirocumab; rosuvastatin | Placebo; rosuvastatin | 52W |
| Peterson et al16 2022 | Multicentre | 3408 | REDUCE-IT PCI | NCT01492361 | Icosapent ethyl; statins | Placebo; statins | 4.8Y |
| Furtado et al17 2022 | Multicentre | 17 073 | FOURIER | NCT01764633 | Evolocumab; statins | Placebo; statins | 2.2Y |
| Okada et al18 2022 | Japan | 102 | – | UMIN000028729 | Evolocumab; pitavastatin | Pitavastatin | 4W |
| Hao et al19 2022 | China | 136 | – | – | Evolocumab; atorvastatin; ezetimibe | Ezetimibe; atorvastatin | 3M |
| Deng et al20 2021 | China | 90 | – | – | Ezetimibe; atorvastatin | Atorvastatin | 1Y |
| Sun et al21 2021 | China | 171 | – | ChiCTR-IPR-17012219 | Ezetimibe; rosuvastatin | Rosuvastatin | 3M |
| He et al22 2020 | China | 192 | – | – | Atorvastatin vs Rosuvastatin vs Simvastatin | – | 6M |
| Hibi et al23 2018 | Japan | 128 | Ezetimibe-ACS | NCT00549926 | Ezetimibe; pitavastatin | Pitavastatin | 1Y |
| Im et al24 2017 | Korea | 2000 | NCT01557075 | Atorvastatin | Pravastatin | 1Y | |
| Hagiwara et al25 2017 | Japan | 1734 | HIJ-PROPER | UMIN000002742 | Ezetimibe; pitavastatin | Pitavastatin | 36M |
| Guo et al26 2017 | China | 137 | – | – | Rosuvastatin | Control | 1Y |
| Wang et al27 2017 | China | 132 | – | ChiCTR-IPR-15007035 | Pitavastatin | Atorvastatin | 6M |
| Watanabe et al28 2017 | Japan | 193 | CHERRY | UMIN000002815 | EPA; pitavastatin | Pitavastatin | 6–8M |
| Liu et al29 2017 | China | 102 | – | – | Ezetimibe; atorvastatin | Atorvastatin 20 mg/day | 1Y |
| Nosaka et al30 2016 | Japan | 241 | – | UMIN000016723 | EPA; pitavastatin | Pitavastatin | 1Y |
| Matsushita et al31 2016 | Japan | 118 | Yokohama-ACS | NCT00549926 | Atorvastatin vs Pitavastatin vs Pravastatin vs Fluvastatin | – | 10.3M |
| Cannon et al32 2015 | Multicentre | 12 941 | IMPROVE-IT | NCT00202878 | Ezetimibe; simvastatin | Simvastatin | 6M |
| Tsujita et al33 2015 | Multicentre | 246 | PRECISE-IVUS | NCT01043380 | Ezetimibe; atorvastatin | Atorvastatin | 1Y |
| Nicholls et al34 2015 | Multicentre | 3295 | VISTA-16 | NCT01130246 | Varespladib; atorvastatin | Placebo; atorvastatin | 6M |
| Zhang et al35 2015 | China | 104 | – | – | Atorvastatin | Rosuvastatin | 6M |
| Leoncini et al36 2014 | Italy | 333 | PRATO-ACS | NCT01185938 | Rosuvastatin | Control | 6M |
| Takano et al37 2013 | Japan | 458 | PEARL | UMINC000000428 | Pitavastatin | Control | 35.5M |
| Nozue et al38 2015 | Japan | 164 | TRUTH | UMIN000004627 | Pitavastatin | Pravastatin | 2Y |
| Lablanche et al39 2010 | Multicentre | 887 | CENTAURUS | NCT00296387 | Rosuvastatin | Atorvastatin | 3M |
| Gibson et al40 2009 | USA | 2868 | PROVE IT-TIMI 22 | NCT00382460 | Atorvastatin | Provastatin | 2Y |
| Han et al41 2009 | China | 1275 | – | NCT00405717 | Atorvastatin | Provastatin | 1Y |
| Hiro et al42 2009 | Japan | 307 | JAPAN-ACS | NCT00242944 | Pitavastatin | Atorvastatin | 1Y |
| Dohi et al43 2009 | Japan | 180 | Extended-ESTABLISH trial | – | Atorvastatin | Control | 4Y |
| Toi et al44 2009 | Japan | 160 | – | – | Pitavastatin | Atorvastatin | 17D |
| Xu et al45 2007 | China | 648 | – | – | Atorvastatin | Control | 2Y |
| Bae et al46 2004 | Korea | 205 | – | – | Atorvastatin | Control | 6M |
| Serruys et al47 2002 | Multicentre | 1677 | LIPS | – | Fluvastatin | Placebo | 3.9Y |
| Mulder et al48 2000 | Netherland | 201 | REGRESS | – | Pravastatin | Placebo | 2Y |
| Flaker et al49 1999 | Multicentre | 1154 | CARE trial | – | Pravastatin | Placebo | 6Y |
| Bertrand et al50 1997 | France | 695 | PREDICT | – | Pravastatin | Placebo | 6M |
| O'Keefe Jr et al51 1996 | USA | 200 | APPLE | – | Probucol; lovastatin | Placebo | 6M |
| Onaka et al52 1994 | Japan | 66 | – | – | Pravastatin | Control | 5M |
| Sahni et al53 1991 | USA | 157 | – | – | Lovastatin | Control | 6M |
*Follow-up period.
D, days; EPA, eicosapentaenoic acid; M, months; W, weeks; Y, years.
Figure 2.
Methodological quality assessment of included studies.
As the two studies did not specify the types of statins, the NMA was divided into two parts. One part was analysed based on specific types of statins, and the other was based on taking statins as a whole. For the dichotomous results of MACEs, the NMA based on specific types of statins included 18 lipid-lowering regimens. The Q test for heterogeneity (p=0.07) and inconsistency (p=0.16) was non-significant, indicating no evidence of heterogeneity or inconsistency in the NMA.
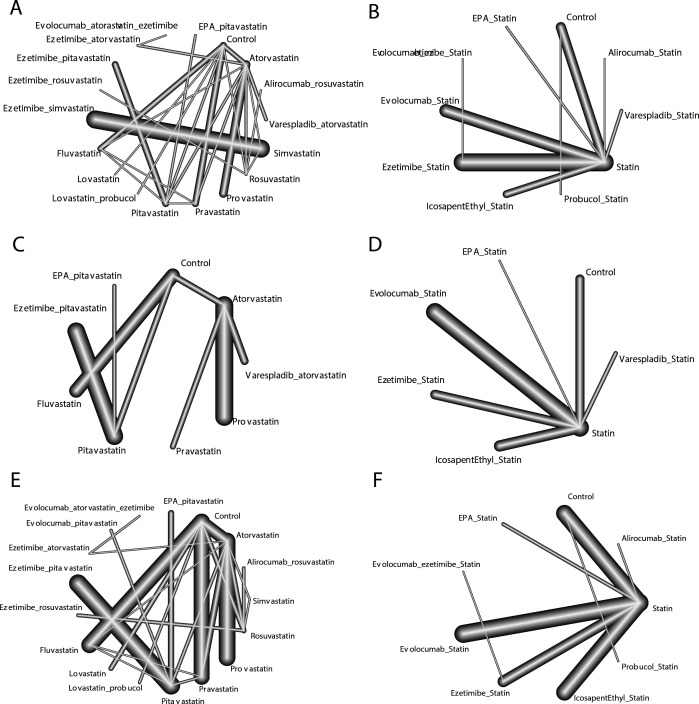
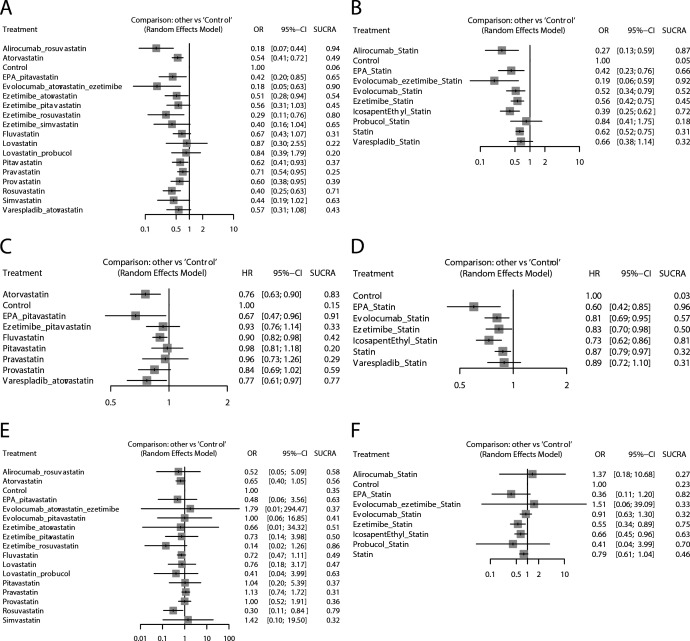
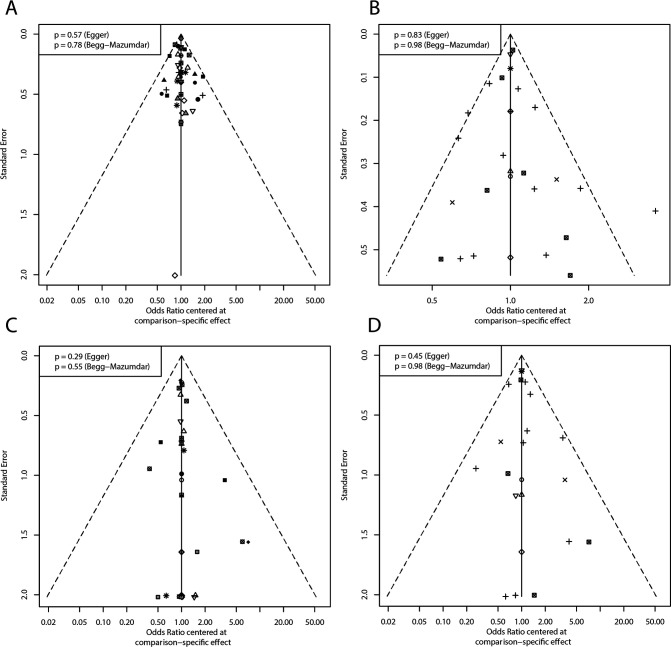
In pairwise comparisons with the control, alirocumab plus rosuvastatin (OR: 0.18; 95% CI: 0.07 to 0.44; SUCRA: 0.94), evolocumab plus atorvastatin and ezetimibe (OR: 0.18; 95% CI: 0.05 to 0.63; SUCRA: 0.90) and ezetimibe plus rosuvastatin (OR: 0.29; 95% CI: 0.11 to 0.76; SUCRA: 0.80) had significant advantages and relatively high SUCRA rankings. No potential publication bias was found according to the comparison-adjusted funnel plot (figure 3).
Figure 3.
Network plots of comparisons for major outcomes included in the analyses. (A) Dichotomous results of MACE based on specific types of statins. (B) Dichotomous results of MACE based on taking statins as a whole. (C) HR results of MACE based on specific types of statins. (D) HR results of MACE based on taking statins as a whole. (E) Dichotomous results of mortality based on specific types of statins. (F) Dichotomous results of mortality based on taking statins as a whole. MACE, major adverse cardiovascular event.
In the NMA based on taking statins as a whole, 10 regimens were analysed. Evolocumab plus ezetimibe and statins (OR: 0.19; 95% CI: 0.06 to 0.59; SUCRA: 0.92), alirocumab plus statins (OR: 0.27; 95% CI: 0.13 to 0.59; SUCRA: 0.87) and icosapent ethyl plus statins (OR: 0.39; 95% CI: 0.25 to 0.62; SUCRA: 0.72) had significant advantages and relatively high SUCRA rankings. No potential publication bias was found.
For the HR results of MACEs, the NMA based on specific types of statins included nine regimens. The Q test for heterogeneity was non-significant (p=0.964) because the network comparisons lacked loops. Therefore, the results were considered consistent. Compared with the control, eicosapentaenoic acid (EPA) plus pitavastatin (HR: 0.67; 95% CI: 0.49 to 0.96; SUCRA: 0.91), atorvastatin (HR: 0.76; 95% CI: 0.63 to 0.90; SUCRA: 0.83) and varespladib plus atorvastatin (HR: 0.77; 95% CI: 0.61 to 0.97; SUCRA: 0.77) had significant advantages and relatively high SUCRA rankings. Potential publication bias was not analysed due to the small number of included studies.
In the NMA based on taking statins as a whole, seven regimens were analysed. EPA plus statins (HR: 0.60; 95% CI: 0.42 to 0.85; SUCRA: 0.96) and icosapent ethyl plus statins (HR: 0.73; 95% CI: 0.62 to 0.86; SUCRA: 0.81) had significant advantages over the control.
For the dichotomous mortality results, the NMA based on specific types of statins included 17 lipid-lowering regimens. The Q test for heterogeneity (p=0.78) and inconsistency (p=0.99) was non-significant. Due to the rare occurrence of events, the results of the comparison had low precision with a large SE. Compared with the control, only rosuvastatin (OR: 0.30; 95% CI: 0.11 to 0.84; SUCRA: 0.79) showed a significantly better effect. Ezetimibe plus rosuvastatin had a relatively high SUCRA ranking, but there was no significant difference compared with the control (OR: 0.14; 95% CI: 0.02 to 1.26; SUCRA: 0.86). No potential publication bias was found (figure 4).
Figure 4.
Forest plots of lipid-lowering therapy compare to control for outcomes in network meta-analysis with SUCRA ranking results. (A) Dichotomous results of MACE based on specific types of statins. (B) Dichotomous results of MACE based on taking statins as a whole. (C) HR results of MACE based on specific types of statins. (D) HR results of MACE based on taking statins as a whole. (E) Dichotomous results of mortality based on specific types of statins. (F) Dichotomous results of mortality based on taking statins as a whole. MACE, major adverse cardiovascular event; SUCRA, surface under the cumulative ranking curve.
In the NMA based on taking statins as a whole, nine regimens were analysed. Ezetimibe plus statins (OR: 0.55; 95% CI: 0.43 to 0.89; SUCRA: 0.75) and icosapent ethyl plus statins (OR: 0.66; 95% CI: 0.45 to 0.96; SUCRA: 0.63) had significant advantages compared with the blank control group. No potential publication bias existed. NMA was not performed due to the small number of studies reporting HRs for mortality (figure 5).
Figure 5.
The comparison-adjusted funnel plot for assessing all main outcomes. (A) Dichotomous results of MACE based on specific types of statins. (B) Dichotomous results of MACE based on taking statins as a whole. (C) Dichotomous results of mortality based on specific types of statins. (D) Dichotomous results of mortality based on taking statins as a whole. MACE, major adverse cardiovascular event.
Discussion
This study analysed the benefits of lipid-lowering therapy on mortality and MACE outcomes in patients who underwent PCI by NMA. The results showed that several lipid-lowering regimens could reduce the risk of MACEs compared with the blank control. Icosapent ethyl plus statins had the benefit of reducing both the risk of MACEs and mortality. However, EPA plus statins had more advantages in reducing the risk of MACEs. Of note, based on the current evidence, alirocumab and evolocumab plus statins had obvious advantages in reducing the risk of MACEs but had no obvious benefit in reducing the risk of mortality.
EPA is a long-chain omega-3 polyunsaturated fatty acid. Long-term intake of EPA can reduce the residual cardiovascular risk to reduce the risk of MACEs.54 In terms of pathological mechanisms, EPA combined with pitavastatin was shown to reduce the lipid volume of coronary artery plaques and total atherosclerotic plaque volume in patients who underwent PCI, which may be the reason for the reduced risk of MACEs.55
Icosapent ethyl is a highly purified and stable EPA ethyl ester that has potential higher anti-inflammatory, antioxidant, plaque stability and cell membrane stability effects.56 In the NMA results, icosapent ethyl plus statins had significant benefits for reducing the risk of either mortality or MACEs in patients who underwent PCI, which was an ideal regimen for the population.
Ezetimibe inhibits the absorption of cholesterol and has a synergistic lipid-lowering pharmacological effect with statins to further reduce the risk of death and MACEs. In particular, when combined with rosuvastatin, ezetimibe has a stronger lipid-lowering effect with a high safety profile without the risk of drug interactions.57 Our NMA results also showed that ezetimibe can reduce the risk of MACEs and mortality. According to the guidelines for the management of dyslipidaemia from the European Society of Cardiology and the European Atherosclerosis Society, ezetimibe was recommended if the LDL-C target was not reached.58 59 The American College of Cardiology guidelines also recommend adding ezetimibe when using maximally tolerated statin therapy and if LDL-C levels remained ≥70 mg/dL.60 These benefits have also been demonstrated in the secondary prevention of PCI.
Alirocumab and evolocumab are both proprotein convertase subtilisin/kexin type-9 inhibitors (PCSK9is), which can increase the level of LDL receptor in the liver, thus improving the ability of the liver to bind LDL-C and reducing the level of peripheral LDL-C.61 There was also a synergistic lipid-lowering pharmacological effect when PCSK9is were combined with statins that resulted in a significantly reduced LDL-C concentration and atherosclerosis event risk; however, there was still controversy regarding the mortality risk reduction.62 It has been suggested that the powerful effect of PCSK9is on reducing LDL-C predisposes patients to hypocholesterolaemia, which will not increase the risk of cerebral haemorrhage. PCSK9is may be the preferred lipid-lowering agents in patients with elevated Intra-Cerebral Hemorrhage (ICH) risk.63–65 On the other hand, PCSK9is did not reduce serum inflammatory factors in one study, suggesting that they may not reduce the risk of residual inflammation in the post-PCI population.66
In the results of this study, lipid-lowering therapy strategies had general advantages in reducing MACE risk. However, for all-cause mortality, the advantage of lipid-lowering therapy was not obvious. Based on dichotomous outcomes of mortality, some strategies may even have a tendency to increase the mortality risk. This challenges the opinion that lipid-lowering therapy is recommended after PCI.67 A large sample size retrospective study suggests that statins can reduce the risk of all-cause death in patients with coronary artery disease undergoing PCI, regardless of individual cholesterol levels.68 Alternatively, the ‘lipid paradox’ view has been proposed and indicates that higher levels of LDL-C and triglycerides on admission are associated with better clinical outcomes. Especially in patients with ST-elevation myocardial infarction, lower LDL-C levels were associated with worse mortality outcomes.69 However, this view is also controversial.70
On the other hand, it is possible that the contribution of LDL-C reduction to the risk of mortality outcomes is obscured by other confounding factors. For example, inflammatory status may also have had an important impact on patient mortality risk. In a cohort of post-PCI patients with low LDL-C levels, residual inflammatory risk also had a significant effect on overall mortality.71 C reactive protein can also predict long-term mortality in post-PCI patients independent of LDL-C levels.72 In addition, cardiac remodelling also has an important impact on the survival outcome of post-PCI patients.73
There are several limitations in this study. First, this analysis was based on the study level instead of the individual level, making it difficult to consider the individual confounding factors in the analysis. Second, the two included studies did not specify the type of statins, so our study had to be analysed separately according to whether all statins were considered as a whole. Third, the criteria for defining MACEs varied among studies, which contributed to heterogeneity among the study results. Fourth, many included studies only reported dichotomous outcomes but did not report the HR results, resulting in missing relevant data for the analysis.
In conclusion, the results of this study suggested that EPA, especially icosapent ethyl, plus statins had a beneficial effect on reducing the risk of MACEs and mortality in post-PCI patients. PCSK9is plus statins were able to reduce the risk of MACEs, but the effects on the risk of mortality remained unclear.
Supplementary Material
Acknowledgments
We thank Professor Xiang Xie for his support.
Footnotes
Contributors: C-JD completed the manuscript. JY, T-TW, YP and Y-YZ guided the data analysis and the production of the figures. X-GH, S-FW, SS, MA and XX read and approved the final manuscript. XX was responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Yoshihara S. Acute coronary syndrome on non-electrocardiogram-gated contrast-enhanced computed tomography. World J Radiol 2022;14:30–46. 10.4329/wjr.v14.i2.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferri N, Ruscica M, Lupo MG, et al. Pharmacological rationale for the very early treatment of acute coronary syndrome with monoclonal antibodies anti-PCSK9. Pharmacol Res 2022;184:106439. 10.1016/j.phrs.2022.106439 [DOI] [PubMed] [Google Scholar]
- 3.Feng K-F, Wu M, Ma L-K. Factors associated with the prognosis of patients with acute myocardial infarction and cardiogenic shock. Med Sci Monit 2021;27:e929996. 10.12659/MSM.929996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koskinas KC, Mach F, Räber L. Lipid-lowering therapy and percutaneous coronary interventions. EuroIntervention 2021;16:1389–403. 10.4244/EIJ-D-20-00999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YH, Her A-Y, Jeong MH, et al. Two-year outcomes of statin therapy in patients with acute myocardial infarction with or without dyslipidemia after percutaneous coronary intervention in the era of new-generation drug-eluting stents within Korean population: data from the Korea acute myocardial infarction registry. Catheter Cardiovasc Interv 2019;93:1264–75. 10.1002/ccd.27985 [DOI] [PubMed] [Google Scholar]
- 6.Chin KL, Wolfe R, Reid CM, et al. Does statin benefits patients with heart failure undergoing percutaneous coronary intervention? findings from the Melbourne interventional group registry. Cardiovasc Drugs Ther 2018;32:57–64. 10.1007/s10557-018-6769-y [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–89. 10.1093/eurjpc/zwaa047 [DOI] [PubMed] [Google Scholar]
- 8.Rea F, Savaré L, Corrao G, et al. Adherence to lipid-lowering treatment by single-pill combination of statin and ezetimibe. Adv Ther 2021;38:5270–85. 10.1007/s12325-021-01892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandit AK, Kumar P, Kumar A, et al. High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand 2016;134:22–8. 10.1111/ane.12540 [DOI] [PubMed] [Google Scholar]
- 10.Bellosta S, Corsini A. Statin drug interactions and related adverse reactions: an update. Expert Opin Drug Saf 2018;17:25–37. 10.1080/14740338.2018.1394455 [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Lan X, Zhang X-L, et al. Intensive vs non-intensive statin pretreatment before percutaneous coronary intervention in Chinese patients: a meta-analysis of randomized controlled trials. World J Clin Cases 2022;10:1557–71. 10.12998/wjcc.v10.i5.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borovac JA, Leth-Olsen M, Kumric M, et al. Efficacy of high-dose atorvastatin or Rosuvastatin loading in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a meta-analysis of randomized controlled trials with GRADE qualification of available evidence. Eur J Clin Pharmacol 2022;78:111–26. 10.1007/s00228-021-03196-9 [DOI] [PubMed] [Google Scholar]
- 13.Vinci P, Panizon E, Tosoni LM, et al. Statin-associated myopathy: emphasis on mechanisms and targeted therapy. Int J Mol Sci 2021;22:11687. 10.3390/ijms222111687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Chaimani A, Furukawa TA, et al. Visualizing the evolution of evidence: cumulative network meta-analyses of new generation antidepressants in the last 40 years. Res Synth Methods 2021;12:74–85. 10.1002/jrsm.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Räber L, Koskinas KC. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022;328:891–2. 10.1001/jama.2022.11836 [DOI] [PubMed] [Google Scholar]
- 16.Peterson BE, Bhatt DL, Steg PG, et al. Treatment with Icosapent ethyl to reduce ischemic events in patients with prior percutaneous coronary intervention: insights from REDUCE-IT PCI. J Am Heart Assoc 2022;11:e022937. 10.1161/JAHA.121.022937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furtado RHM, Fagundes AA, Oyama K, et al. Effect of evolocumab in patients with prior percutaneous coronary intervention. Circ Cardiovasc Interv 2022;15:e011382. 10.1161/CIRCINTERVENTIONS.121.011382 [DOI] [PubMed] [Google Scholar]
- 18.Okada T, Miyoshi T, Doi M, et al. Effect of early initiation of evolocumab on lipoprotein(A) in patients with acute myocardial infarction. J Cardiovasc Dev Dis 2022;9:153. 10.3390/jcdd9050153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao Y, Yang Y-L, Wang Y-C, et al. Effect of the early application of evolocumab on blood lipid profile and cardiovascular prognosis in patients with extremely high-risk acute coronary syndrome. Int Heart J 2022;63:669–77. 10.1536/ihj.22-052 [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, He S, Wang D, et al. Clinical study of effect of ezetimibe combined with statins on residual lipoprotein cholesterol and MACE events in patients undergoing emergency intervention with acute coronary syndrome. Chinese J Pharmacol Ther 2021;26:1048–52. [Google Scholar]
- 21.Sun C, Zheng W, Liang L, et al. Ezetimibe improves rosuvastatin effects on inflammation and vascular endothelial function in acute coronary syndrome patients undergoing PCI. J Interv Cardiol 2021;2021:2995602. 10.1155/2021/2995602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He W, Cao M, Li Z. Effects of different doses of atorvastatin, rosuvastatin, and simvastatin on elderly patients with ST-elevation acute myocardial infarction (AMI) after percutaneous coronary intervention (PCI). Drug Dev Res 2020;81:551–6. 10.1002/ddr.21651 [DOI] [PubMed] [Google Scholar]
- 23.Hibi K, Sonoda S, Kawasaki M, et al. Effects of ezetimibe-statin combination therapy on coronary ain acute coronary syndrome. Circ J 2018;82:757–66. 10.1253/circj.CJ-17-0598 [DOI] [PubMed] [Google Scholar]
- 24.Im E, Cho Y-H, Suh Y, et al. High-intensity statin treatments in clinically stable patients on aspirin monotherapy 12 months after drug-eluting stent implantation: a randomized study. Rev Esp Cardiol (Engl Ed) 2018;71:423–31. 10.1016/j.rec.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara N, Kawada-Watanabe E, Koyanagi R, et al. Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J 2017;38:2264–76. 10.1093/eurheartj/ehx162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Zhang W-Z, Zhao Q, et al. Study on the effect of different doses of rosuvastatin on ventricular remodeling in patients with acute coronary syndrome after emergency percutaneous coronary intervention. Eur Rev Med Pharmacol Sci 2017;21:4457–63. [PubMed] [Google Scholar]
- 27.Wang Y-B, Fu X-H, Gu X-S, et al. Effects of intensive pitavastatin therapy on glucose control in patients with non-ST elevation acute coronary syndrome. Am J Cardiovasc Dis 2017;7:89–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017;70:537–44. 10.1016/j.jjcc.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Hao H, Yin C, et al. Therapeutic effects of atorvastatin and ezetimibe compared with double-dose atorvastatin in very elderly patients with acute coronary syndrome. Oncotarget 2017;8:41582–9. 10.18632/oncotarget.15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosaka K, Miyoshi T, Iwamoto M, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int J Cardiol 2017;228:173–9. 10.1016/j.ijcard.2016.11.105 [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Hibi K, Komura N, et al. Effects of 4 statins on regression of coronary plaque in acute coronary syndrome. Circ J 2016;80:1634–43. 10.1253/circj.CJ-15-1379 [DOI] [PubMed] [Google Scholar]
- 32.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 33.Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention. J Am Coll Cardiol 2015;66:495–507. 10.1016/j.jacc.2015.05.065 [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Kastelein JJP, Schwartz GG, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 2014;311:252–62. 10.1001/jama.2013.282836 [DOI] [PubMed] [Google Scholar]
- 35.Zhang J-R, Wang D-Q, Du J, et al. Efficacy of clopidogrel and clinical outcome when clopidogrel is coadministered with atorvastatin and lansoprazole: a prospective. Medicine (Baltimore) 2015;94:e2262. 10.1097/MD.0000000000002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leoncini M, Toso A, Maioli M, et al. Early high-dose rosuvastatin and cardioprotection in the protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome (PRATO-ACS) study. Am Heart J 2014;168:792–7. 10.1016/j.ahj.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 37.Takano H, Mizuma H, Kuwabara Y, et al. Effects of pitavastatin in Japanese patients with chronic heart failure: the pitavastatin heart failure study (PEARL study). Circ J 2013;77:917–25. 10.1253/circj.cj-12-1062 [DOI] [PubMed] [Google Scholar]
- 38.Nozue T, Fukui K, Yamamoto S, et al. C-reactive protein and future cardiovascular events in statin-treated patients with angina pectoris: the extended TRUTH study. J Atheroscler Thromb 2013;20:717–25. 10.5551/jat.18705 [DOI] [PubMed] [Google Scholar]
- 39.Lablanche J-M, Leone A, Merkely B, et al. Comparison of the efficacy of rosuvastatin versus atorvastatin in reducing apolipoprotein B/apolipoprotein A-1 ratio in patients with acute coronary syndrome: results of the CENTAURUS study. Arch Cardiovasc Dis 2010;103:160–9. 10.1016/j.acvd.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 40.Gibson CM, Pride YB, Hochberg CP, et al. Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. J Am Coll Cardiol 2009;54:2290–5. 10.1016/j.jacc.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 41.Han Y-L, Zhang Z-L, Li Y, et al. Comparison on long-term effects of atorvastatin or pravastatin combined with clopidogrel for patients undergoing coronary stenting: a randomized controlled trial. Zhonghua Yi Xue Za Zhi 2009;89:2240–4. [PubMed] [Google Scholar]
- 42.Hiro T, Kimura T, Morimoto T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric Intravascular ultrasound using pitavastatin versus atorvastatin. JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome]. J Am Coll Cardiol 2009;54:293–302. 10.1016/j.jacc.2009.04.033 [DOI] [PubMed] [Google Scholar]
- 43.Dohi T, Miyauchi K, Okazaki S, et al. Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (extended-ESTABLISH trial): a follow-up study. Atherosclerosis 2010;210:497–502. 10.1016/j.atherosclerosis.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Toi T, Taguchi I, Yoneda S, et al. Early effect of lipid-lowering therapy with pitavastatin on regression of coronary atherosclerotic plaque. Comparison with atorvastatin. Circ J 2009;73:1466–72. 10.1253/circj.cj-08-1051 [DOI] [PubMed] [Google Scholar]
- 45.Xu K, Han Y-L, Jing Q-M, et al. Lipid-modifying therapy in diabetic patients with high plasma non-high-density lipoprotein cholesterol after percutaneous coronary intervention. Exp Clin Cardiol 2007;12:48–50. [PMC free article] [PubMed] [Google Scholar]
- 46.Bae J-H, Bassenge E, Kim K-Y, et al. Effects of low-dose atorvastatin on vascular responses in patients undergoing percutaneous coronary intervention with stenting. J Cardiovasc Pharmacol Ther 2004;9:185–92. 10.1177/107424840400900306 [DOI] [PubMed] [Google Scholar]
- 47.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287:3215–22. 10.1001/jama.287.24.3215 [DOI] [PubMed] [Google Scholar]
- 48.Mulder HJ, Bal ET, Jukema JW, et al. Pravastatin reduces restenosis two years after percutaneous transluminal coronary angioplasty (REGRESS trial). Am J Cardiol 2000;86:742–6. 10.1016/s0002-9149(00)01073-0 [DOI] [PubMed] [Google Scholar]
- 49.Flaker GC, Warnica JW, Sacks FM, et al. Pravastatin prevents clinical events in revascularized patients with average cholesterol concentrations. cholesterol and recurrent events CARE investigators. J Am Coll Cardiol 1999;34:106–12. 10.1016/s0735-1097(99)00145-x [DOI] [PubMed] [Google Scholar]
- 50.Bertrand ME, McFadden EP, Fruchart JC, et al. Effect of pravastatin on angiographic restenosis after coronary balloon angioplasty. The PREDICT trial investigators prevention of restenosis by elisor after transluminal coronary angioplasty. J Am Coll Cardiol 1997;30:863–9. 10.1016/s0735-1097(97)00259-3 [DOI] [PubMed] [Google Scholar]
- 51.O’Keefe JH, Stone GW, McCallister BD, et al. Lovastatin plus probucol for prevention of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol 1996;77:649–52. 10.1016/s0002-9149(97)89325-3 [DOI] [PubMed] [Google Scholar]
- 52.Onaka H, Hirota Y, Kita Y, et al. The effect of pravastatin on prevention of restenosis after successful percutaneous transluminal coronary angioplasty. Jpn Circ J 1994;58:100–6. 10.1253/jcj.58.100 [DOI] [PubMed] [Google Scholar]
- 53.Sahni R, Maniet AR, Voci G, et al. Prevention of restenosis by lovastatin after successful coronary angioplasty. Am Heart J 1991;121(6 Pt 1):1600–8. 10.1016/0002-8703(91)90002-y [DOI] [PubMed] [Google Scholar]
- 54.Kita Y, Watanabe M, Kamon D, et al. Effects of fatty acid therapy in addition to strong statin on coronary plaques in acute coronary syndrome: an optical coherence tomography study. J Am Heart Assoc 2020;9:e015593. 10.1161/JAHA.119.015593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B-H, Yin F, Qiao Y-N, et al. Triglyceride and triglyceride-rich lipoproteins in atherosclerosis. Front Mol Biosci 2022;9:909151. 10.3389/fmolb.2022.909151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 57.Strilchuk L, Tocci G, Fogacci F, et al. An overview of rosuvastatin/ezetimibe association for the treatment of hypercholesterolemia and mixed dyslipidemia. Expert Opin Pharmacother 2020;21:531–9. 10.1080/14656566.2020.1714028 [DOI] [PubMed] [Google Scholar]
- 58.Baigent C, F, Catapano AL, Koskinas KC, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019;41:111–88. [DOI] [PubMed] [Google Scholar]
- 59.Ray KK, Del Prato S, Müller-Wieland D, et al. Alirocumab therapy in individuals with type 2 diabetes mellitus and atherosclerotic cardiovascular disease: analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN studies. Cardiovasc Diabetol 2019;18:149. 10.1186/s12933-019-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Y, Parco C, Karathanos A, et al. Clinical efficacy and safety outcomes of bempedoic acid for LDL-C lowering therapy in patients at high cardiovascular risk: a systematic review and meta-analysis. BMJ Open 2022;12:e048893. 10.1136/bmjopen-2021-048893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Wen D, Chen Y, et al. Pcsk9 inhibitors for secondary prevention in patients with cardiovascular diseases: a Bayesian network meta-analysis. Cardiovasc Diabetol 2022;21:107. 10.1186/s12933-022-01542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt AF, Carter J-PL, Pearce LS, et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2020;10:CD011748. 10.1002/14651858.CD011748.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An SJ, Kim TJ, Yoon B-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 2017;19:3–10. 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasta A, Cremonini AL, Pisciotta L, et al. PCSK9 inhibitors for treating hypercholesterolemia. Expert Opin Pharmacother 2020;21:353–63. 10.1080/14656566.2019.1702970 [DOI] [PubMed] [Google Scholar]
- 65.Sanz-Cuesta BE, Saver JL. Lipid-lowering therapy and hemorrhagic stroke risk: comparative meta-analysis of statins and Pcsk9 inhibitors. Stroke 2021;52:3142–50. 10.1161/STROKEAHA.121.034576 [DOI] [PubMed] [Google Scholar]
- 66.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–43. 10.1161/CIRCRESAHA.116.308537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang T, Fu X, Fu P, et al. The value of fragmented QRS in predicting the prognosis of chronic total occlusion patients with myocardial infarction history undergoing percutaneous coronary intervention: A 24-months follow-up study. Clin Cardiol 2021;44:537–46. 10.1002/clc.23573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ndrepepa G, Holdenrieder S, Cassese S, et al. Hypocholesterolaemia and mortality in patients with coronary artery disease. Eur J Clin Invest 2020;50:e13194. 10.1111/eci.13194 [DOI] [PubMed] [Google Scholar]
- 69.Sia C-H, Zheng H, Ho AF-W, et al. The lipid paradox is present in ST-elevation but not in non-ST-elevation myocardial infarction patients: insights from the Singapore myocardial infarction registry. Sci Rep 2020;10:6799. 10.1038/s41598-020-63825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun H, Li Z, Song X, et al. Revisiting the lipid paradox in ST-elevation myocardial infarction in the Chinese population: findings from the CCC-ACS project. Eur Heart J Acute Cardiovasc Care 2021;10:978–87. 10.1093/ehjacc/zuab053 [DOI] [PubMed] [Google Scholar]
- 71.Guedeney P, Claessen BE, Kalkman DN, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol 2019;73:2401–9. 10.1016/j.jacc.2019.01.077 [DOI] [PubMed] [Google Scholar]
- 72.Razzouk L, Muntner P, Bansilal S, et al. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J 2009;158:277–83. 10.1016/j.ahj.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 73.Abbate A, Biondi-Zoccai GGL, Appleton DL, et al. Survival and cardiac remodeling benefits in patients undergoing late percutaneous coronary intervention of the infarct-related artery: evidence from a meta-analysis of randomized controlled trials. J Am Coll Cardiol 2008;51:956–64. 10.1016/j.jacc.2007.11.062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070827supp001.pdf (33KB, pdf)
Data Availability Statement
Data are available upon reasonable request.