Abstract
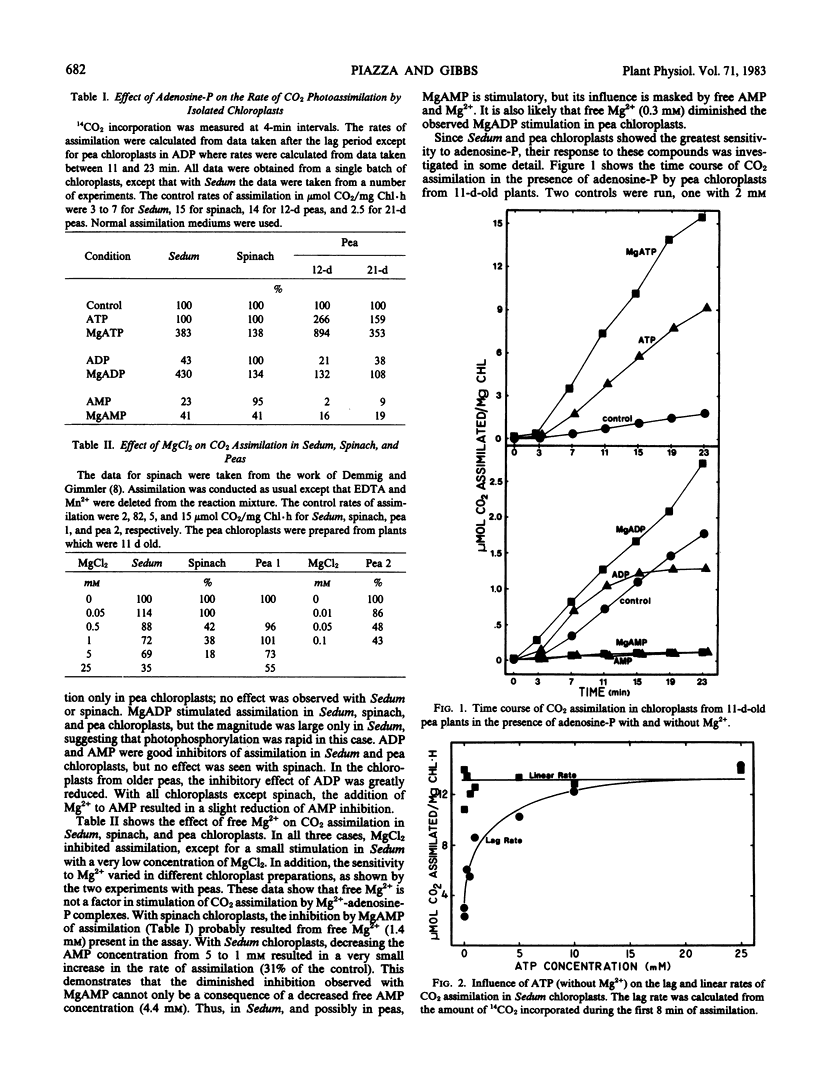
14CO2 photoassimilation in the presence of MgATP, MgADP, and MgAMP was investigated using intact chloroplasts from Sedum praealtum, a Crassulacean acid metabolism plant, and two C3 plants: spinach and peas. Inasmuch as free ATP, ADP, AMP, and uncomplexed Mg2+ were present in the assays, their influence upon CO2 assimilation was also examined. Free Mg2+ was inhibitory with all chloroplasts, as were ADP and AMP in chloroplasts from Sedum and peas. With Sedum chloroplasts in the presence of ADP, the time course of assimilation was linear. However, with pea chloroplasts, ADP inhibition became progressively more severe, resulting in a curved time course. ATP stimulated assimilation only in pea chloroplasts. MgATP and MgADP stimulated assimilation in all chloroplasts. ADP inhibition of CO2 assimilation was maximal at optimum orthophosphate concentrations in Sedum chloroplasts, while MgATP stimulation was maximal at optimum or below optimum concentrations of orthophosphate. MgATP stimulation in peas and Sedum and ADP inhibition in Sedum were not sensitive to the addition of glycerate 3-phosphate (PGA).
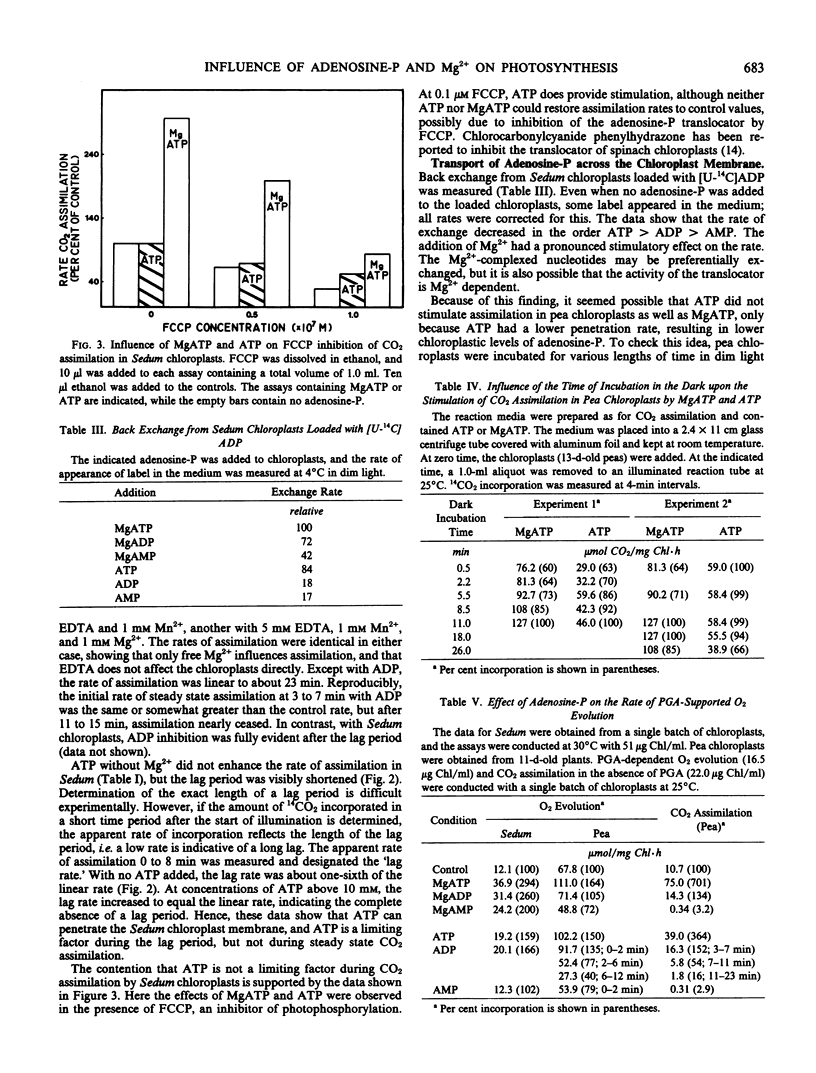
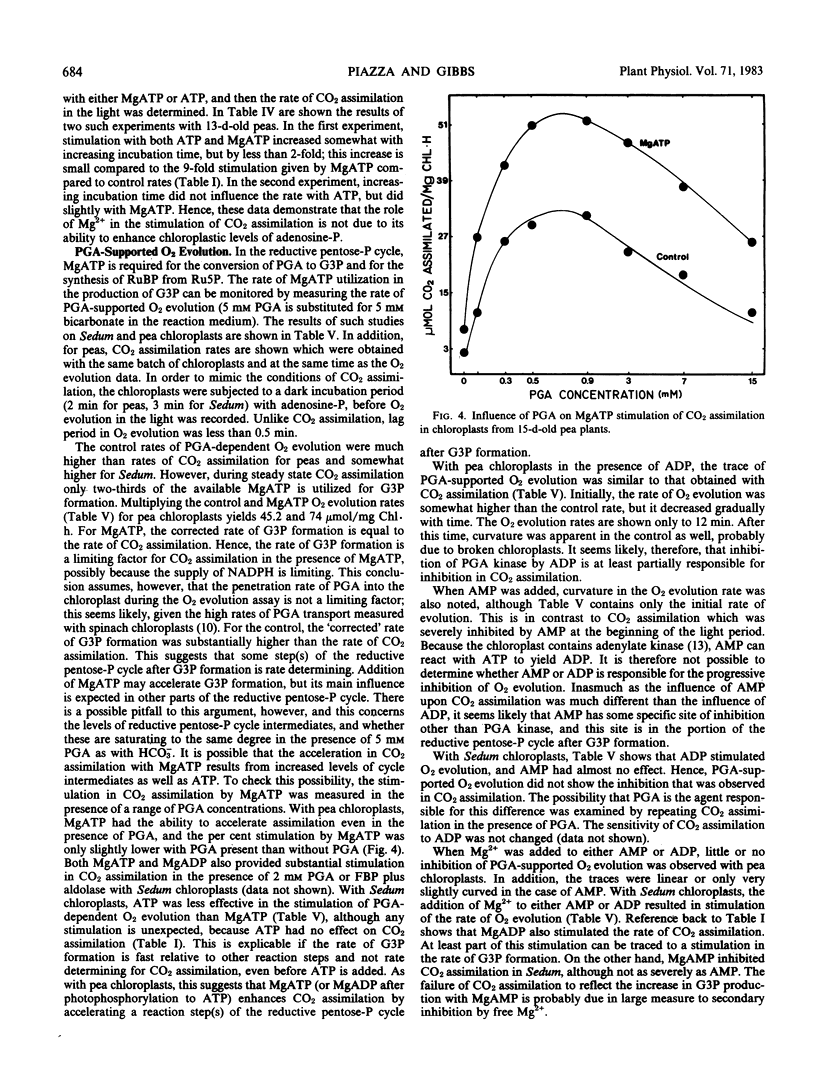
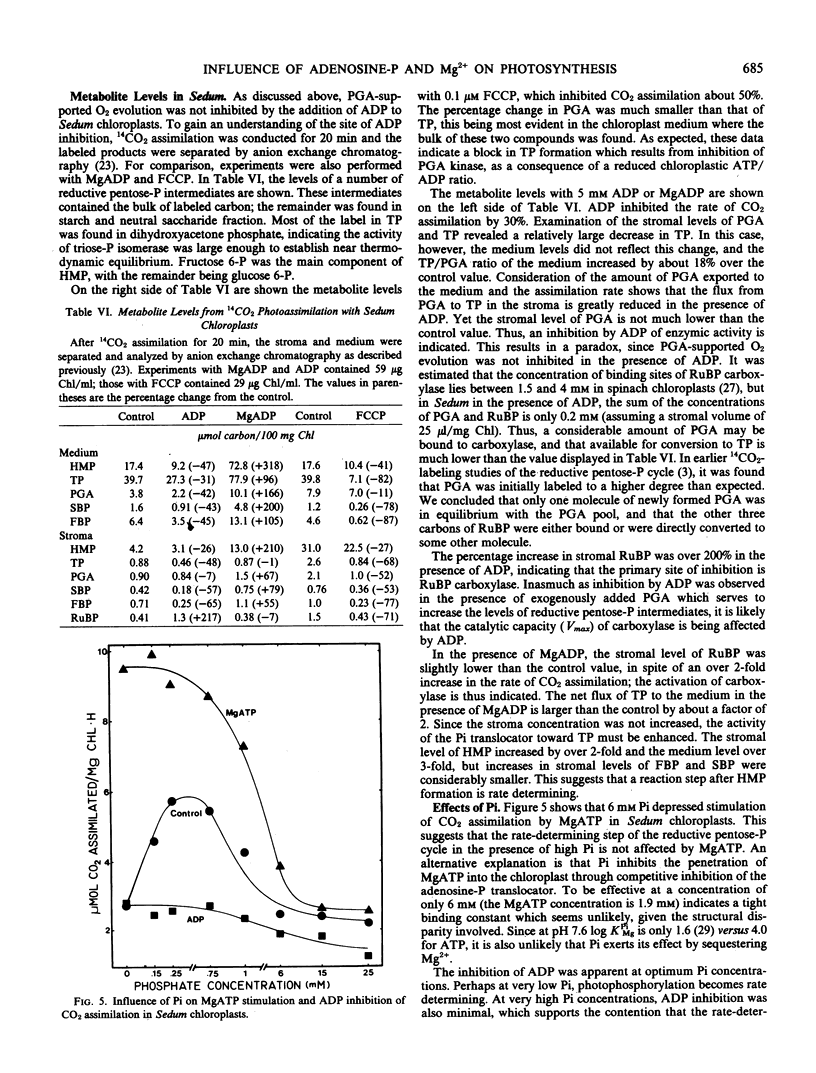
PGA-supported O2 evolution by pea chloroplasts was not inhibited immediately by ADP; the rate of O2 evolution slowed as time passed, corresponding to the effect of ADP on CO2 assimilation, and indicating that glycerate 3-phosphate kinase was a site of inhibition. Likewise, upon the addition of AMP, inhibition of PGA-dependent O2 evolution became more severe with time. This did not mirror CO2 assimilation, which was inhibited immediately by AMP. In Sedum chloroplasts, PGA-dependent O2 evolution was not inhibited by ADP and AMP. In chloroplasts from peas and Sedum, the magnitude of MgADP and MgATP stimulation of PGA-dependent O2 evolution was not much larger than that given by ATP, and it was much smaller than MgATP stimulation of CO2 assimilation. Analysis of stromal metabolite levels by anion exchange chromatography indicated that ribulose 1,5-bisphosphate carboxylase was inhibited by ADP and stimulated by MgADP in Sedum chloroplasts.
The appearance of label in the medium was measured when [U-14C] ADP-loaded Sedum chloroplasts were challenged with ATP, ADP, or AMP and their Mg2+ complexes. The rate of back exchange was stimulated by the presence of Mg2+. This suggests that ATP, ADP, and AMP penetrate the chloroplast slower than their Mg2+ complexes. A portion of the CO2 assimilation and O2 evolution data could be explained by differential penetration rates, and other proposals were made to explain the remainder of the observations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Regulation of pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta. 1973 Oct 10;321(2):484–488. doi: 10.1016/0005-2744(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruist M. F., Hammes G. G. Further characterization of nucleotide binding sites on chloroplast coupling factor one. Biochemistry. 1981 Oct 27;20(22):6298–6305. doi: 10.1021/bi00525a003. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P. Regulation of ribulose 1,5-diphosphate carboxylase in the photosynthetic assimilation of carbon dioxide. J Biol Chem. 1973 Jul 25;248(14):4956–4964. [PubMed] [Google Scholar]
- Davis L. C., Kotake S. Regulation of nitrogenase activity in aerobes by Mg2+ availability: an hypothesis. Biochem Biophys Res Commun. 1980 Apr 14;93(3):934–940. doi: 10.1016/0006-291x(80)91165-1. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Yushok W. D. Noninvasive 31P NMR probes of free Mg2+, MgATP, and MgADP in intact Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2487–2491. doi: 10.1073/pnas.77.5.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Gibbs M. Characterization of a Photosynthesizing Reconstituted Spinach Chloroplast Preparation : REGULATION BY PRIMER, ADENYLATES, FERREDOXIN, AND PYRIDINE NUCLEOTIDES. Plant Physiol. 1982 Jan;69(1):179–186. doi: 10.1104/pp.69.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C., Srivastava P. C. Binding of 2-azidoadenosine [beta-32P]diphosphate to the receptor on intact human blood platelets which inhibits adenylate cyclase. Biochemistry. 1982 Feb 2;21(3):544–549. doi: 10.1021/bi00532a020. [DOI] [PubMed] [Google Scholar]
- O'Sullivan W. J., Smithers G. W. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- Peavey D. G., Gibbs M. Photosynthetic enhancement studied in intact spinach chloroplasts. Plant Physiol. 1975 May;55(5):799–802. doi: 10.1104/pp.55.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza G. J., Smith M. G., Gibbs M. Characterization of the Formation and Distribution of Photosynthetic Products by Sedum praealtum Chloroplasts. Plant Physiol. 1982 Dec;70(6):1748–1758. doi: 10.1104/pp.70.6.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Chon C. J., Mosbach A., Heldt H. W. Fructose-and sedoheptulosebisphosphatase. The sites of a possible control of CO2 fixation by lightdependent changes of the stromal Mg2+ concentration. Biochim Biophys Acta. 1977 Aug 10;461(2):313–325. doi: 10.1016/0005-2728(77)90181-5. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Bahr J. T., Jensen R. G. Measurement of ribulose 1,5-bisphosphate from spinach chloroplasts. Plant Physiol. 1979 Nov;64(5):876–879. doi: 10.1104/pp.64.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic Z. S., Walker D. A. Photosynthesis by isolated pea chloroplasts: some effects of adenylates and inorganic pyrophosphate. Plant Physiol. 1977 Mar;59(3):428–432. doi: 10.1104/pp.59.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Jensen R. G. Photosynthetic CO(2) Fixation at Air Levels of CO(2) by Isolated Spinach Chloroplasts. Plant Physiol. 1982 Jun;69(6):1263–1267. doi: 10.1104/pp.69.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]


