Abstract
4-Hydroxy-2-keto-pentanoic acid aldolase from Escherichia coli was identified as a class I aldolase. The enzyme was found to be highly selective for the acetaldehyde acceptor but would accept α-ketobutyric acid or phenylpyruvic acid in place of the pyruvic acid carbonyl donor.
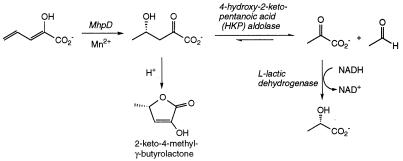
Aldolase-catalyzed reactions are involved in the latter stages of many bacterial catabolic pathways responsible for the degradation of aromatic compounds (3). In particular, many of the bacterial meta cleavage pathways for aromatic degradation proceed via the common intermediate 4-hydroxy-2-keto-pentanoic acid (HKP) (3), whose cleavage to acetaldehyde and pyruvic acid (Fig. 1) is catalyzed by an aldolase enzyme. Previous biochemical work on HKP aldolase is limited to the enzyme activity from Pseudomonas strains. HKP aldolase from Pseudomonas sp. strain CF600 was shown to be activated by Mn2+ ions (9), whereas 4-hydroxy-4-methyl-2-oxoglutarate aldolase from Pseudomonas putida (11) requires Mg2+ ions for catalytic activity, and these enzymes appear to fall into the class II family of aldolases.
FIG. 1.
Reaction catalyzed by HKP aldolase. Also illustrated are the previous reaction on the phenylpropionate catabolic pathway, the lactone derivative of HKP, and the method used for the coupled enzyme assay.
It has been reported previously that the HKP aldolase activity in extracts of Escherichia coli would process both enantiomers of the substrate, unlike the corresponding activities from Pseudomonas and Acinetobacter spp., which were enantioselective (2). We have previously established that the preceding enzyme on the phenylpropionic acid catabolic pathway, namely, 2-hydroxypentadienoic acid hydratase (MhpD), catalyzes a stereospecific hydration reaction (8). We therefore wished to examine the stereoselectivity and substrate selectivity of HKP aldolase from E. coli.
HKP aldolase activity was detectable in extracts of E. coli W3110 in a stopped assay involving treatment of HKP with extract for 30 min, followed by heat treatment and then addition of lactate dehydrogenase and NADH. Levels of enzyme activity were low (7.0 mU/mg of protein) and were not enhanced by inclusion of phenylpropionic acid in the growth media (1). The enzyme was purified by precipitation with 50 to 80% ammonium sulfate, phenyl-agarose hydrophobic-interaction chromatography, Q Sepharose anion-exchange fast protein liquid chromatography (FPLC), and Mono Q anion-exchange FPLC. The purified enzyme had a specific activity of 184 mU/mg of protein, a 26-fold purification overall. Although not purified to homogeneity, the purified enzyme was entirely free of background NADH oxidase activity and could be examined in a continuous assay by incubation with lactate dehydrogenase and NADH. Maximum activity was obtained in the pH range 6.25 to 6.75, with sharp inflections of activity at pH 6.0 and 8.0.
The purified HKP aldolase showed no observable dependence on divalent metal ions, unlike the purified Pseudomonas aldolases (9, 11). Furthermore, treatment of the enzyme with the metal chelator EDTA at 10 mM resulted in no loss of enzyme activity. Moreover, treatment of enzyme with sodium borohydride in the presence of substrate HKP resulted in 100% loss of activity, indicative of an imine linkage. Treatment with sodium borohydride in the absence of substrate resulted in only a slight (<10%) loss of activity; thus, the imine linkage is formed only upon addition of the substrate. These data imply that the E. coli enzyme is a class I aldolase utilizing an imine linkage between the C-2 carbonyl of HKP and the ɛ-amino group of a lysine residue at the active site. Since 80% amino acid sequence identity has been determined between the Pseudomonas strain CF600 DmpG and E. coli MhpE gene products corresponding to the respective HKP aldolases (4), the difference in behavior between the E. coli and Pseudomonas enzymes is most surprising. We note that although the Pseudomonas HKP aldolase was reported to show a six- to eightfold activation by Mn2+, the enzyme retained residual activity after EDTA treatment and the presence of an imine linkage was not investigated (9).
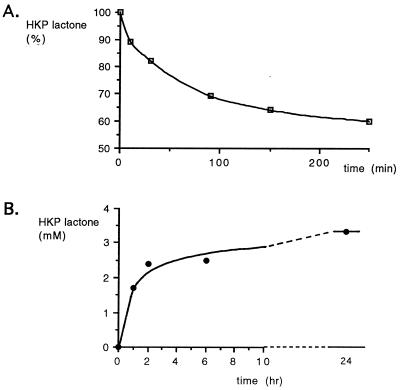
The stereoselectivity of the enzymatic reaction was examined by treatment of racemic HKP with the enzyme for various reaction times, followed by acid-catalyzed lactonization of the remaining HKP substrate to give 2-keto-4-methyl-γ-butyrolactone, followed by organic acids HPLC analysis. Time-dependent consumption of HKP was observed, but after long reaction times approximately 60% of the substrate remained (Fig. 2A). Kinetic studies subsequently showed that the equilibrium position lies strongly in favor of the forward reaction; therefore, these data imply that the enzyme utilizes only one enantiomer of the substrate. Since the earlier study of the E. coli enzyme stereospecificity was carried out with crude extract (2), it is possible that there is a second aldolase with the opposite stereospecificity in E. coli, although we observed only one peak of activity upon enzyme purification.
FIG. 2.
Analysis of HKP aldolase-catalyzed reaction via HPLC analysis of the lactone derivative α-methyl-γ-methyl-γ-butyrolactone. (A) Forward reaction using racemic HKP as the substrate (percentage of initial peak area). (B) Reverse reaction using pyruvic acid and acetaldehyde as substrates (concentrations determined by peak area, versus authentic standards).
The reverse reaction was assayed by incubation of enzyme with acetaldehyde and pyruvate, followed by lactonization of the HKP product under acidic conditions, and HPLC analysis. Time-dependent formation of product was observed (Fig. 2B), indicating that HKP aldolase also catalyzes the reverse reaction. Concentrations of product were deduced by calibration with known amounts of synthetic 2-keto-4-methyl-γ-butyrolactone (10); thus, after 24 h the reaction mixture contained 3.7 mM HKP. Initial concentrations of acetaldehyde and pyruvic acid were 360 mM and 180 mM, respectively; thus, a Keq of 17 M in favor of the forward reaction can be deduced. From the rate of reaction over the first hour, it was calculated that the reverse reaction proceeds at 13% of the rate of the forward reaction.
The substrate selectivity for the reverse reaction catalyzed by HKP aldolase was examined with respect to the carbonyl donor pyruvic acid and the carbonyl acceptor acetaldehyde. No product formation was observed when propionaldehyde was used in place of acetaldehyde; thus, the enzyme is highly selective for the carbonyl acceptor. However, lactone products with similar retention times and λmaxs were observed by HPLC using either α-ketobutyric acid or phenylpyruvic acid as the substrate for the reverse reaction. The apparent rates of formation of the new lactone derivatives are similar to those observed with pyruvic acid as the substrate (Table 1); thus, α-ketobutyric acid and phenylpyruvic acid appear to be converted efficiently by the enzyme.
TABLE 1.
Substrate selectivity for the MhpE-catalyzed forward and reverse reactionsa
| Substrate(s) (concn) | Assay method (retention time)b | vrelc |
|---|---|---|
| 4-Hydroxy-2-keto-pentanoic acid (200 μM) | LDH/NADH | 1.00 |
| Acetaldehyde + pyruvic acid (both 100 mg/ml) | HPLC (34 min) | 0.13 |
| Propionaldehyde + pyruvic acid (both 100 mg/ml) | HPLC | — |
| Acetaldehyde + 2-keto-butyric acid (both 100 mg/ml) | HPLC (37 min) | 0.12 |
| Acetaldehyde + phenylpyruvic acid (both 100 mg/ml) | HPLC (33 min) | 0.13 |
Assay methods for forward and reverse reactions are described in the text and are illustrated in Fig. 1. Assays were conducted at 20°C in 50 mM potassium phosphate buffer, pH 7.0.
Retention time for α-keto-γ-butyrolactone derivatives observed on organic acids HPLC (Bio-Rad HPX-87H column, eluent 0.005 mM H2SO4, flow rate 0.6 ml/min). LDH, lactate dehydrogenase.
Reaction rate relative to HKP forward reaction. —, no reaction observed.
In summary, HKP aldolase from E. coli is shown to be a class I aldolase enzyme, which proceeds via an imine linkage between the substrate and the enzyme active site. Only a small number of class I aldolases have been found in prokaryotes (5–7), including an aldolase enzyme involved in bacterial naphthalene sulfonate degradation (6). The partially purified enzyme is shown to be selective for one enantiomer of the substrate, presumably the 4S enantiomer produced by the preceding enzyme on the pathway (8). The enzyme shows a high selectivity for the acetaldehyde acceptor, which would constrain the degradation of ortho-substituted phenylpropionic acids via this pathway. The relaxed specificity for the α-keto acid carbonyl donor offers the possibility of using HKP aldolase for stereospecific carbon-carbon bond formation reactions with nonnatural substrates.
Acknowledgments
We thank BBSRC for the award of an earmarked studentship (J.R.P.).
REFERENCES
- 1.Burlingame R, Chapman P J. Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J Bacteriol. 1983;155:113–121. doi: 10.1128/jb.155.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burlingame R, Chapman P J. Stereospecificity in meta-fission catabolic pathways. J Bacteriol. 1983;155:424–426. doi: 10.1128/jb.155.1.424-426.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- 4.Ferrandez A, Garcia J L, Diaz E. Genetic characterization and expression in heterologous hosts of the 3-(3-hydroxyphenyl)propionate catabolic pathway of Escherichia coli K-12. J Bacteriol. 1997;179:2573–2581. doi: 10.1128/jb.179.8.2573-2581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Götz F, Fischer S, Schleifer K H. Purification and characterisation of an unusually heat-stable and acid/base-stable class I fructose-1,6-bisphosphate aldolase from Staphylococcus aureus. Eur J Biochem. 1980;108:295–301. doi: 10.1111/j.1432-1033.1980.tb04723.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuhm A E, Knackmuss H J, Stolz A. Purification and properties of 2′-hydroxybenzalpyruvate aldolase from a bacterium that degrades naphthalenesulfonates. J Biol Chem. 1993;268:9484–9489. [PubMed] [Google Scholar]
- 7.Meloche H P, Wood W A. The mechanism of 2-keto-3-deoxy-6-phospho-gluconic aldolase. J Biol Chem. 1964;239:3511–3514. [PubMed] [Google Scholar]
- 8.Pollard J R, Bugg T D H. Purification, characterisation and reaction mechanism of monofunctional 2-hydroxypentadienoic acid hydratase from Escherichia coli. Eur J Biochem. 1997;251:98–106. doi: 10.1046/j.1432-1327.1998.2510098.x. [DOI] [PubMed] [Google Scholar]
- 9.Powlowski J, Sahlman L, Shingler V. Purification and properties of the physically associated meta-cleavage pathway enzymes 4-hydroxy-2-ketovalerate aldolase and aldehyde dehydrogenase from Pseudomonas sp. strain CF600. J Bacteriol. 1993;175:377–385. doi: 10.1128/jb.175.2.377-385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi A, Schinz H. Alcuni α-cheto-γ-lattoni con sostituenti alchilici in posizione γ. Helv Chim Acta. 1948;31:473–488. doi: 10.1002/hlca.19480310226. [DOI] [PubMed] [Google Scholar]
- 11.Tack B F, Chapman P J, Dagley S. Purification and properties of 4-hydroxy-4-methyl-2-oxoglutarate aldolase. J Biol Chem. 1972;247:6444–6449. [PubMed] [Google Scholar]