Abstract
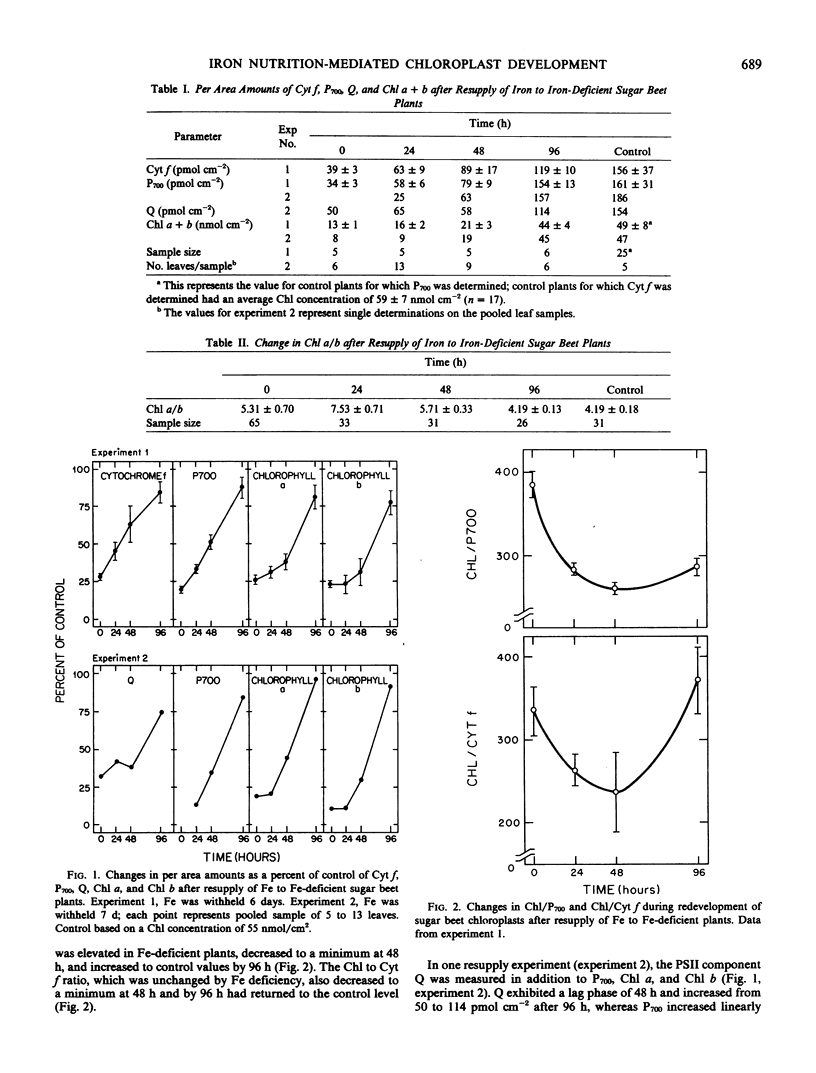
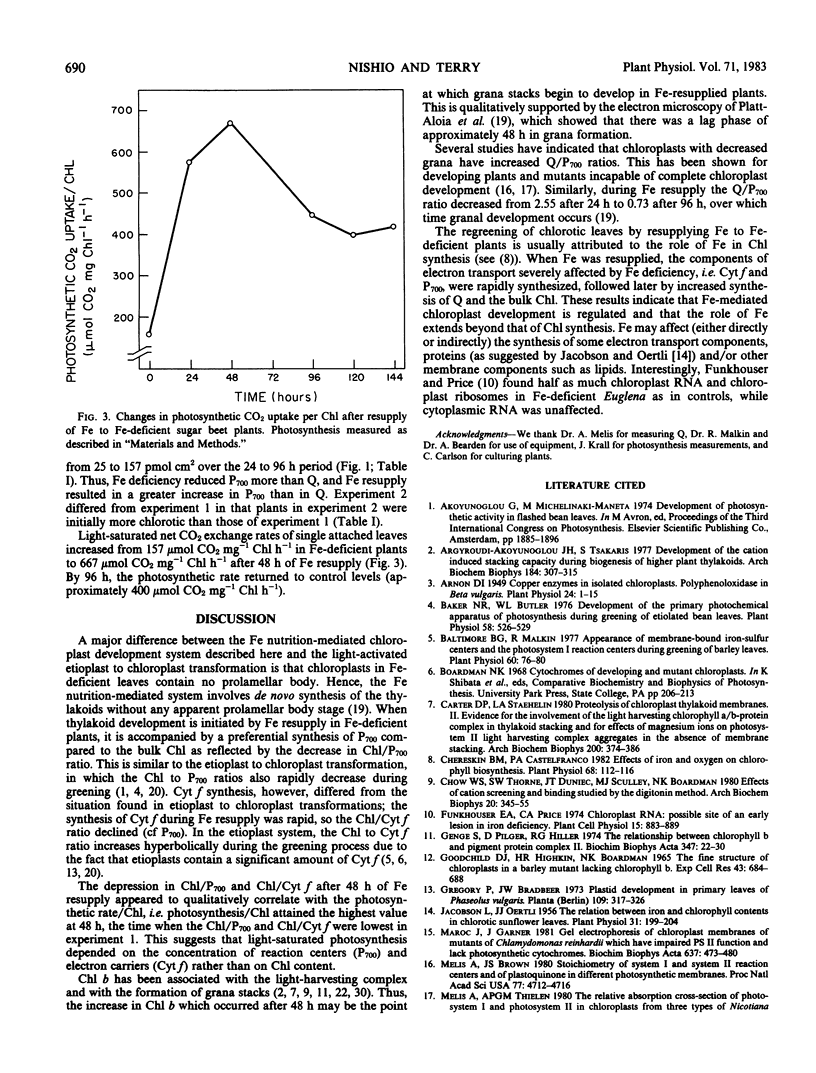
Membrane development in chloroplasts was explored by resupplying iron to iron-deficient sugar beet (Beta vulgaris L. cv F58-554H1) and monitoring changes in lamellar components during regreening. The synthesis of chlorophyll a, chlorophyll b, and Q, the first stable electron acceptor of photosystem II, exhibited a lag phase during the first 24 to 48 hours of resupply. In contrast, the per area amounts of P700 and cytochrome f increased linearly over the first 48 hours. During the early regreening period, the Q to P700 ratio was 2.6 and decreased to 0.7 after 96 hours of regreening. The rate of photosynthesis (net CO2 uptake) per chlorophyll increased during the first 48 hours of resupply, then by 96 hours decreased to values typical of control plants. The results suggest that there was preferential synthesis of the measured photosystem I components during the first 24 to 48 hours, while from 48 to 96 hours there was rapid synthesis of all components. The iron nutrition-mediated chloroplast development system provides a useful experimental approach for studying biomembrane synthesis and structural-functional relations of the photosynthetic apparatus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyroudi-Akoyunoglou J. H., Tsakiris S. Development of the cation-induced stacking capacity during the biogenesis of higher plant thylakoids. Arch Biochem Biophys. 1977 Nov;184(1):307–315. doi: 10.1016/0003-9861(77)90355-1. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Butler W. L. Development of the Primary Photochemical Apparatus of Photosynthesis during Greening of Etiolated Bean Leaves. Plant Physiol. 1976 Oct;58(4):526–529. doi: 10.1104/pp.58.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore B. G., Malkin R. Appearance of Membrane-bound Iron-Sulfur Centers and the Photosystem I Reaction Center during Greening of Barley Leaves. Plant Physiol. 1977 Jul;60(1):76–80. doi: 10.1104/pp.60.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. P., Staehelin L. A. Proteolysis of chloroplast thylakoid membranes. II. Evidence for the involvement of the light-harvesting chlorophyll a/b-protein complex in thylakoid stacking and for effects of magnesium ions on photosystem II-light-harvesting complex aggregates in the absence of membrane stacking. Arch Biochem Biophys. 1980 Apr 1;200(2):374–386. doi: 10.1016/0003-9861(80)90367-7. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genge S., Pilger D., Hiller R. G. The relationship between chlorophyll b and pigment-protein complex II. Biochim Biophys Acta. 1974 Apr 23;347(1):22–30. doi: 10.1016/0005-2728(74)90196-0. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Oertli J. J. The Relation between Iron and Chlorophyll Contents in Chlorotic Sunflower Leaves. Plant Physiol. 1956 May;31(3):199–204. doi: 10.1104/pp.31.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J., Anderson J. M., Goodchild D. J. The role of the light-harvesting chlorophyll a/b protein complex in chloroplast membrane stacking. Cation-induced aggregation of reconstituted proteoliposomes. Eur J Biochem. 1980 Jun;107(2):345–354. doi: 10.1111/j.1432-1033.1980.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Spiller S., Terry N. Limiting Factors in Photosynthesis: II. IRON STRESS DIMINISHES PHOTOCHEMICAL CAPACITY BY REDUCING THE NUMBER OF PHOTOSYNTHETIC UNITS. Plant Physiol. 1980 Jan;65(1):121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E., Terry N., Huston R. P. Limiting Factors in Photosynthesis: III. Effects of Iron Nutrition on the Activities of Three Regulatory Enzymes of Photosynthetic Carbon Metabolism. Plant Physiol. 1982 Nov;70(5):1541–1543. doi: 10.1104/pp.70.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Photosynthesis, growth, and the role of chloride. Plant Physiol. 1977 Jul;60(1):69–75. doi: 10.1104/pp.60.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Waldron J. C., Anderson J. M. Chlorophyll-protein complexes from thylakoids of a mutant barley lacking chlorophyll b. Eur J Biochem. 1979 Dec 17;102(2):357–362. doi: 10.1111/j.1432-1033.1979.tb04250.x. [DOI] [PubMed] [Google Scholar]


