Abstract
Like other chenopods, sugarbeets (Beta vulgaris L. cv Great Western D-2) accumulate glycine betaine when salinized; this may be an adaptive response to stress. The pathway of betaine synthesis in leaves of salinized (150-200 millimolar NaCl) sugarbeet plants was investigated by supplying [14C]formate, phosphoryl[14C]monomethylethanolamine ([14C][unk] MME) or phosphoryl[14C]choline ([14C][unk] choline) to leaf discs and following 14C incorporation into prospective intermediates. The 14C kinetic data were used to develop a computer model of the betaine pathway.
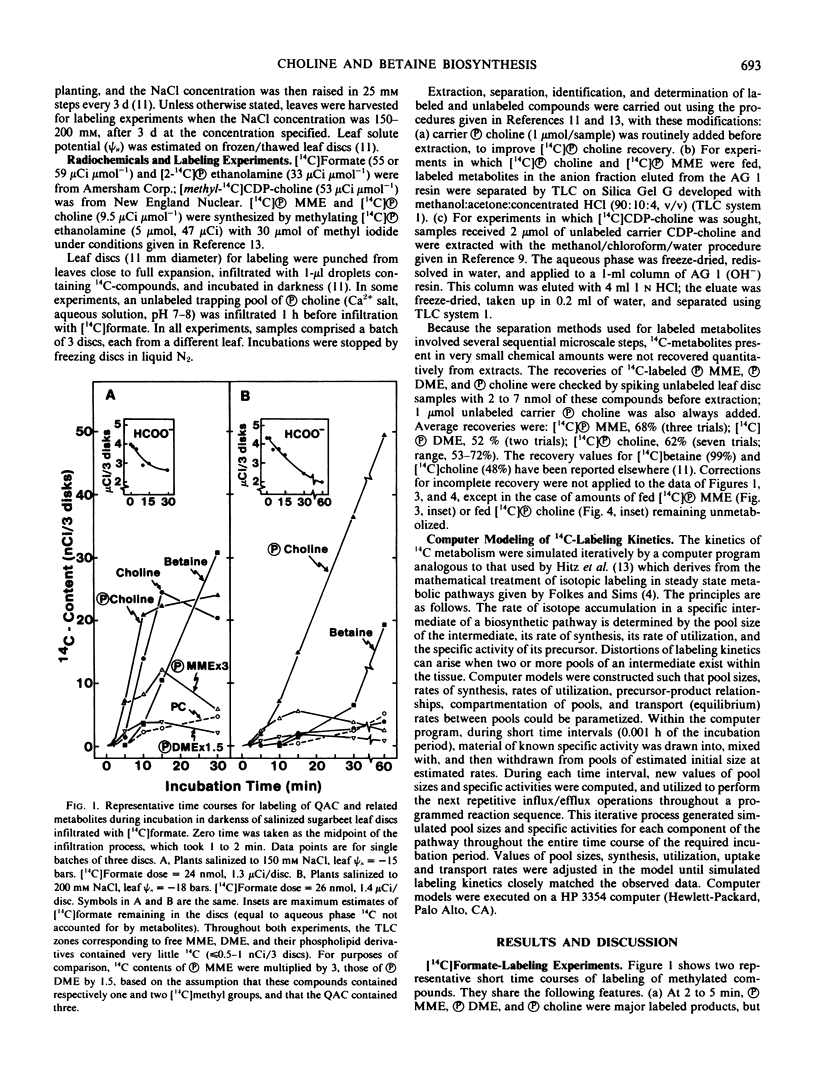
When [14C]formate was fed, [unk] MME, phosphoryldimethylethanolamine ([unk] DME) and [unk] choline were the most prominent methylated products at short labeling times, after which 14C appeared in free choline and in betaine. Phosphatidylcholine labeled more slowly than [unk] choline, choline, and betaine, and behaved as a minor end product. Very little 14C entered the free methylethanolamines. When [14C][unk] MME was supplied, a small amount was hydrolyzed to the free base but the major fate was conversion to [unk] DME, [unk] choline, free choline, and betaine; label also accumulated slowly in phosphatidylcholine. Label from supplied [14C][unk] choline entered choline and betaine rapidly, while phosphatidylcholine labeled only slowly and to a small extent.
These results are consistent with the pathway [unk] MME →[unk] DME → [unk] choline → choline → → betaine, with a minor side branch leading from [unk] choline into phosphatidylcholine. This contrasts markedly (a) with the pathway of stress-induced choline and betaine synthesis in barley, in which phosphatidylcholine apparently acts as an intermediate (Hitz, Rhodes, Hanson 1981, Plant Physiol 68: 814-822); (b) with choline biogenesis in mammalian liver and microorganisms. Computer modeling of the experimental data pointed strongly to regulation at the [unk] choline → choline step, and also indicated that the rate of [unk] choline synthesis is subject to feedback inhibition by [unk] choline.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DELWICHE C. C., BREGOFF H. M. Pathway of betaine and choline synthesis in Beta vulgaris. J Biol Chem. 1958 Aug;233(2):430–433. [PubMed] [Google Scholar]
- Folkes B. F., Sims A. P. The significance of amino acid inhibition of NADP-linked glutamate dehydrogenase in the physiological control of glutamate synthesis in Candida utilis. J Gen Microbiol. 1974 May;82(1):77–95. doi: 10.1099/00221287-82-1-77. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Nelsen C. E. Betaine Accumulation and [C]Formate Metabolism in Water-stressed Barley Leaves. Plant Physiol. 1978 Aug;62(2):305–312. doi: 10.1104/pp.62.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Scott N. A. Betaine Synthesis from Radioactive Precursors in Attached, Water-stressed Barley Leaves. Plant Physiol. 1980 Aug;66(2):342–348. doi: 10.1104/pp.66.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Wyse R. Biosynthesis, translocation, and accumulation of betaine in sugar beet and its progenitors in relation to salinity. Plant Physiol. 1982 Oct;70(4):1191–1198. doi: 10.1104/pp.70.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz W. D., Rhodes D., Hanson A. D. Radiotracer evidence implicating phosphoryl and phosphatidyl bases as intermediates in betaine synthesis by water-stressed barley leaves. Plant Physiol. 1981 Oct;68(4):814–822. doi: 10.1104/pp.68.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. G. An assessment of quaternary ammonium and related compounds as osmotic effectors in crop plants. Basic Life Sci. 1979;14:155–170. doi: 10.1007/978-1-4684-3725-6_12. [DOI] [PubMed] [Google Scholar]
- Pan S. M., Moreau R. A., Yu C., Huang A. H. Betaine accumulation and betaine-aldehyde dehydrogenase in spinach leaves. Plant Physiol. 1981 Jun;67(6):1105–1108. doi: 10.1104/pp.67.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno D. M., Beeler D. A. The biosynthesis of phospholipids and their precursors in rat liver involving de novo methylation, and base-exchange pathways, in vivo. Biochim Biophys Acta. 1973 Dec 20;326(3):325–338. doi: 10.1016/0005-2760(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Storey R., Jones R. G. Responses of Atriplex spongiosa and Suaeda monoica to Salinity. Plant Physiol. 1979 Jan;63(1):156–162. doi: 10.1104/pp.63.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Akesson B. Biosynthesis of phosphatidylethanolamines and phosphatidylcholines from ethanolamine and choline in rat liver. Biochem J. 1975 Feb;146(2):309–315. doi: 10.1042/bj1460309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Tolbert N. E., Gohlke A. F. Choline kinase and phosphorylcholine phosphatase in plants. Plant Physiol. 1966 Feb;41(2):307–312. doi: 10.1104/pp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Inhibition of the Development of Induced Respiration and Cyanide-insensitive Respiration in Potato Tuber Slices by Cerulenin and Dimethylaminoethanol. Plant Physiol. 1977 Jul;60(1):11–16. doi: 10.1104/pp.60.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]


