Abstract
Objectives
Opioid use disorder (OUD) is a major public health concern in the USA, resulting in high rates of overdose and other negative outcomes. Methadone, an OUD treatment, has been shown to be effective in reducing the risk of overdose and improving overall health and quality of life. This study analysed the distribution of methadone for the treatment of OUD across the USA over the past decade and through the COVID-19 pandemic.
Design
Retrospective observational study using secondary data analysis of the Drug Enforcement Administration and Medicaid Databases.
Setting
USA.
Participants
Patients who were dispensed methadone at US opioid treatment programmes (OTPs).
Primary and secondary outcome measures
The primary outcomes were the overall pattern in methadone distribution and the number of OTPs in the USA per year. The secondary outcome was Medicaid prescriptions for methadone.
Results
Methadone distribution for OUD has expanded significantly over the past decade, with an average state increase of +96.96% from 2010 to 2020. There was a significant increase in overall distribution of methadone to OTP from 2010 to 2020 (+61.00%, p<0.001) and from 2015 to 2020 (+26.22%, p<0.001). However, the distribution to OTPs did not significantly change from 2019 to 2021 (−5.15%, p=0.491). There was considerable state-level variation in methadone prescribing to Medicaid patients with four states having no prescriptions.
Conclusions
There have been dynamic changes in methadone distribution for OUD. Furthermore, pronounced variation in methadone distribution among states was observed, with some states having no OTPs or Medicaid coverage. New policies are urgently needed to increase access to methadone treatment, address the opioid epidemic in the USA and reduce overdose deaths.
Keywords: COVID-19, EPIDEMIOLOGY, Health Services Accessibility, Substance misuse, REHABILITATION MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Automated Reports and Consolidated Ordering System (ARCOS) provides novel data on distribution and distributors of methadone for opioid treatment programmes over the past decade, pre-COVID-19 and post-COVID-19.
ARCOS reports methadone distribution by weight, not by patient count or prescriptions, and does not differentiate pharmacological formulations.
Incorporating Medicaid data compensates for the absence of patient-level methadone data in ARCOS.
Introduction
The US Food and Drug Administration has approved methadone, buprenorphine and naltrexone as treatments for opioid use disorder (OUD),1 but there have been several policy changes impacting OUD care during the COVID-19 pandemic. Methadone, considered a gold-standard medication for OUD (MOUD), is a long-acting synthetic opioid administered via opioid treatment programmes (OTPs).1–3 Since 2021, it has been revealed that only 27.8% of individuals with OUD have actually received MOUD treatment.4
Over recent years, methadone take-home doses have been extended to 28 days for stable patients and 14 days for less stable patients, drug screening requirements have been relaxed and telemedicine has been expanded for established patients.5 6 However, unlike for buprenorphine, starting methadone treatment requires an in-person visit. Prior to the pandemic, OUD methadone treatment also required the co-administration of counselling, and these counselling requirements have also been relaxed due to the COVID-19 pandemic.6–8
Despite its effectiveness, methadone carries the potential for serious adverse effects including respiratory depression and cardiotoxicity.3 9 10 An alternative gold-standard MOUD, buprenorphine, has also raised safety concerns, with rising respiratory depression fatalities associated with oral doses in both adult and paediatric patients, resulting in a total of 84 deaths from 2003 to 2019.11 However, it is important to note that in 2021 alone, there were 106 699 overdose deaths in the USA emphasising the urgency of providing first-line care.12
In regions where available, methadone is frequently used for severe OUD cases due to its full mu receptor agonist properties and its doses can be titrated up as needed. Buprenorphine is a partial mu receptor agonist with less higher-end dosing flexibility.13 Methadone necessitates expert handling, especially in the early stages of treatment because of its full agonism properties combined with high lipophilicity, long serum half-life and active metabolites.14
Due to its pharmacokinetic and pharmacodynamic properties,11 buprenorphine combined with naloxone may be becoming a more commonly prescribed option compared with methadone. A recent survey revealed 75% of emergency room physicians preferred buprenorphine over methadone.15 Buprenorphine was inequitably available from 2004 to 2015 driven by systemic racism and discrimination based on socioeconomic status. In particular, Black patients had a lower probability of receiving a prescription.16 Findings from a retrospective, cohort study from 1998 to 2014 suggest that patients treated with buprenorphine had a lower risk of drug-related poisoning mortality during treatment compared with those on methadone.17
Compared with buprenorphine, treatment with methadone was more effective in reducing criminal activity, HIV infection, hepatitis and overall mortality.2 18–21 Additionally, a Cochrane meta-analysis concluded that methadone was better for retaining patients in OUD treatment only if buprenorphine doses were 7 mg per day or lower, but both methadone and buprenorphine were equivalent at higher doses (online supplemental table 1).22 However, there is a nationwide accessibility problem for treatment with all potential MOUD.23 24 Although studies have shown that both treatments are effective, clinicians and patients should choose between MOUD treatments depending on their individual needs and circumstances, including the accessibility and availability of treatment programmes in their area.2 17–24
bmjopen-2023-074845supp001.pdf (333.5KB, pdf)
Methadone is a safe and effective treatment for OUD in fentanyl users and is the preferred medication over buprenorphine in this population. Methadone treatment is associated with a significant decrease in illicit drug use, including fentanyl. Patients with OUD who are using fentanyl are at increased risk of overdose and relapse, but methadone treatment can significantly reduce this risk. Additionally, patients who test positive for fentanyl use at the start of methadone treatment are just as likely to achieve remission as patients who test negative for fentanyl use. Methadone may also be protective against fentanyl overdose deaths.25 These findings suggest that methadone is a valuable tool for treating OUD in fentanyl users.
There have been over 1 million drug overdoses in the USA since the start of the opioid epidemic.26 The COVID-19 pandemic has placed tremendous stress on the healthcare system including the access to providers and availability of OUD treatments. Between 2019 and 2020, there was a +48.8% increase in overdose mortality among Black people, compared with +26.3% among white people.27 Moreover, from April 2020 to April 2021, the number of drug overdoses in the USA exceeded 100 000, a +28.5% increase over the previous year.28 With a +60% rise in overdoses compared with the previous year, May 2020 became the deadliest month on record.29
A cross-sectional study, conducted from May to June 2020 in the USA and Canada, found that new patients wishing to initiate methadone treatment were faced with a barrier in 20% of clinics.30 Similarly, prior to the pandemic, both methadone and buprenorphine-based OTPs were found to be effective in US jails and prisons. However, following the pandemic, some of these OTPs have been expanded while others were discontinued.31–33
This study obtained data from the Drug Enforcement Administration’s (DEA) Automated Reports and Consolidated Ordering System (ARCOS), a federal programme established by the 1970 Controlled Substances Act, to monitor the distribution of DEA-controlled substances from various sources including retail pharmacies, hospitals, practitioners, teaching institutions, mid-level practitioners and OTPs.34 Previous pharmacoepidemiological studies have also used the ARCOS Database.35–39 It is important to note that ARCOS does not provide information on the number of patients receiving methadone. This caveat is important because it prevents an accurate representation of the amount of methadone used for each OUD patient. However, federally funded OTPs record number of patients receiving treatment, which could be a useful resource in understanding the true scale of the OUD epidemic in the USA and the effectiveness of treatment efforts. The Substance Abuse Mental Health Services Administration’s (SAMHSA) National Survey of Substance Abuse Treatment Services’ (N-SSATS) annual report provides national data regarding alcohol and drug abuse facilities.40 The number of patients receiving methadone for OUD decreased by almost one-quarter (−23.7%) from 2019 (408 550) to 2020 (311 531)40 (online supplemental figure 1).
In addition to ARCOS, Medicaid’s State Drug Utilization Data (SDUD) Database was used in this study.41 Medicaid is a programme at the federal and state level which functions to aid in covering healthcare costs for patients with limited resources.42 Medicaid.gov publishes all prescription drugs covered by Medicaid every year for all 50 states and the District of Colombia (DC) in the SDUD Database. The State Health Official Letter, released on 30 December 2020, states that the SUPPORT Act of 2018 mandates the inclusion of Medicaid coverage for MOUD for all eligible patients with OUD. Subsequently, the Continuing Appropriations Act of 2021, which added to the SUPPORT Act, requires rebates on methadone and other MOUD starting from 1 October 2020 to 30 September 2025.43 44 However, not all states have equal coverage of medications, which can lead to discrepancies in the prescription numbers reflected by the SDUD. There is variation among states regarding methadone coverage, which in turn affects prescribing methadone patterns.45
This manuscript aims to address the paucity of research on methadone for OUD treatment over the past decade and during the COVID-19 pandemic. The impact of COVID-19-related policies on individuals with OUD is poorly understood, and this manuscript seeks to shed light on this important area of research. The use of both ARCOS and SDUD Databases provides a comprehensive picture of the distribution and utilisation of methadone for the OUD treatment over the past decade. Together, it is critical to examine the changes in methadone distribution during the COVID-19 pandemic to determine whether there are national or regional barriers to accessing this evidence-based pharmacotherapy.
Materials and methods
The quantities of methadone distributed (in grams) per state were obtained from the ARCOS yearly drug summary reports for the years 2010, 2015, 2019, 2020 and 2021. Methadone distributed to OTPs, in the ARCOS Database, was classified as an OUD treatment which excluded all methadone for pain. The number of OTPs per state was also obtained from ARCOS for 2010, 2015, 2019, 2020 and 2021. The state population estimates, including DC, were derived from the US Census Bureau’s Annual Estimates of the Resident Population for the USA.46 The US territories were examined elsewhere and were not included in figures 1–5.47 Medicaid data were collected in the year 2020 for all 50 states and DC using a filtered download from the SDUD.41 These data from Medicaid were the methadone reimbursements for use for OUD. National Drug Codes of formulations that are primarily used for OUD are provided in the online supplemental table 2. The number of methadone prescriptions per state was divided by the number of Medicaid enrollees per state in 2020. Three states—Virginia, Montana and Iowa—were excluded from the results due to being outliers (10 000–112 000 prescriptions/100 000 enrollees) which presumably reflected a Medicaid data error (mean 475.4 prescriptions/100 000 enrollees for the remaining 48 states).
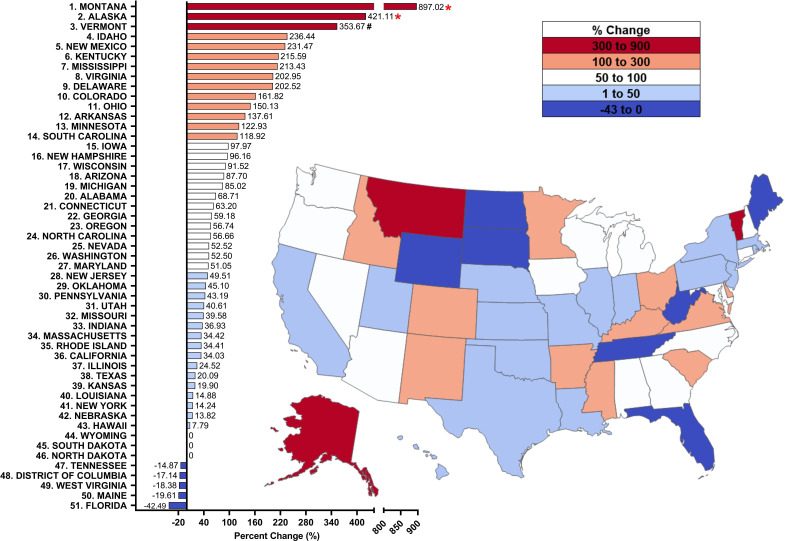
Figure 1.
Per cent change from 2010 to 2020 in methadone distribution as reported by the Drug Enforcement Administration’s Automated Reports and Consolidated Ordering System for opioid use disorder. Per cent change between 1.5 SDs and 1.959 SDs from the mean (+96.96%, SD=146.64%), indicated with a #. Per cent change >±1.96 SD from the mean was considered significant (*p<0.05).
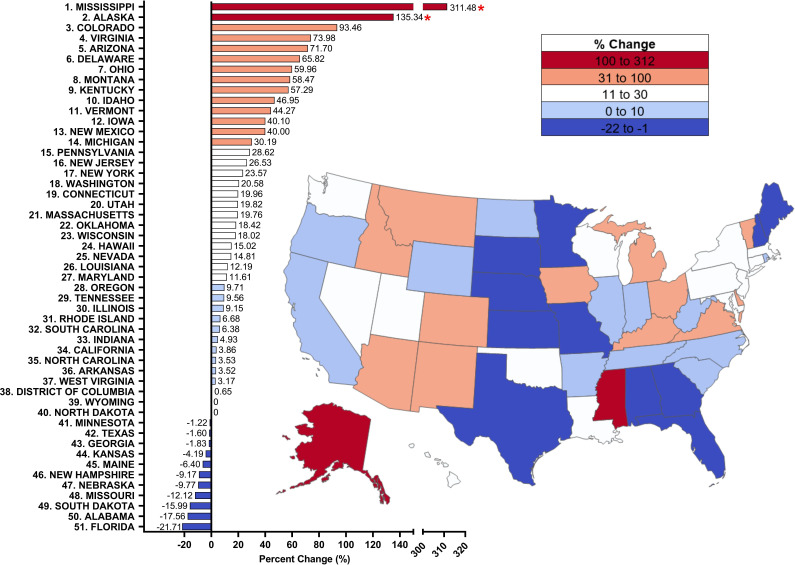
Figure 2.
Per cent change from 2015 to 2020 in methadone distribution as reported by the Drug Enforcement Administration’s Automated Reports and Consolidated Ordering System for opioid use disorder. Per cent change >±1.96 SD from the mean was considered significant (*p<0.05).
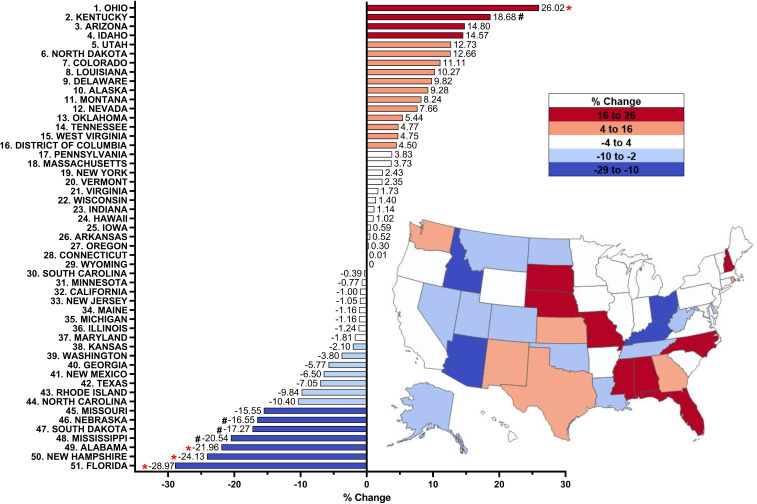
Figure 3.
Per cent change from 2019 to 2020 in methadone distribution as reported by the Drug Enforcement Administration’s Automated Reports and Consolidated Ordering System for opioid use disorder. Per cent change between 1.5 SDs and 1.959 SDs from the mean (−0.09%, SD 10.81), indicated with a #. Per cent change >±1.96 SD from the mean was considered significant (*p<0.05).
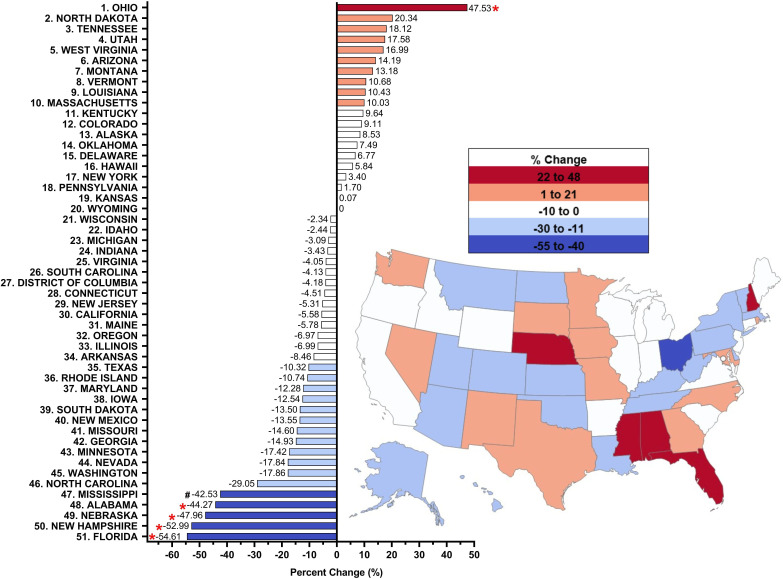
Figure 4.
Per cent change from 2019 to 2021 in methadone distribution as reported by the Drug Enforcement Administration’s Automated Reports and Consolidated Ordering System for opioid use disorder. Per cent change between 1.5 SDs and 1.959 SDs from the mean (−5.15%, SD 19.14), indicated with a #. Per cent change >±1.96 SD from the mean was considered significant (*p<0.05).
Figure 5.
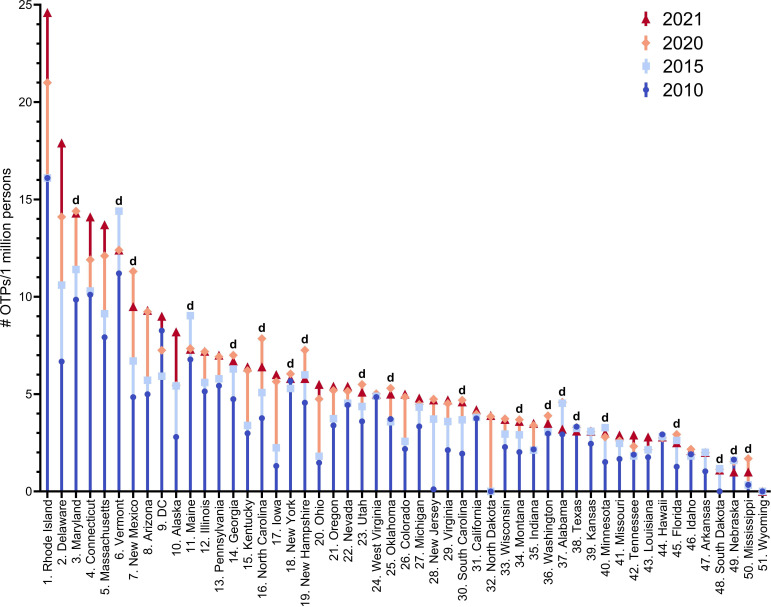
Number of opioid treatment programmes (OTPs) per 1 million persons per state all significantly (p<0.0001) different from 2010, 2015, 2020 and 2021. The 20 states that decreased in 2021 relative to 2010, 2015 or 2020 are indicated with a ‘d’. DC, District of Columbia.
The per cent change in methadone distribution for OUD was compared between states for time spans of 10 years, 5 years and 1 year, respectively. For all 50 states, the milligrams of methadone per person for the years 2010, 2015, 2020 was calculated. This calculation is the ‘amount distributed’ per year in the following equation: percentage change=(amount distributed in later year−amount distributed in earlier year)/amount distributed in earlier year×100. Data were analysed through one-way repeated-measures analysis of variance (ANOVA) with Sidak corrections to examine the effects of OTPs per 1 million persons per state in 2010, 2015, 2020, 2021. Data were similarly analysed to examine the effects of milligrams of methadone per person per state in 2010, 2015, 2019, 2020 and 2021. Heatmaps were created using JMP V.16.2.0. Figures and data analysis were completed using Microsoft Excel, GraphPad Prism V.9.4.0 and Systat V.13.1.
Patient and public involvement
None.
Results
Automated Reports and Consolidated Ordering System
Overall, the total volume of methadone distributed to OTPs in the USA increased over the last decade from 8.62 metric tons in 2010 to 10.88 tons in 2015 (+26.3%), and to 13.03 tons in 2020 (+19.7%), reflecting an increase in distribution to the majority of states. Results of the one-way repeated-measures ANOVA revealed a significant main effect of time on milligrams of methadone per person per state (F(4, 200)=24.535, p<0.001). Specifically, the average per cent change in state distribution of methadone for OUD from 2010 to 2020 significantly increased by +96.96% (SD=146.64%, Sidak post hoc p<0.001), with 43 states showing an increase, 5 states a decrease (DC, Florida, Maine, Tennessee and West Virginia) and 3 states showing no change (North Dakota, South Dakota and Wyoming). There was also a significant increase in milligrams of methadone per person per state (+61.8%) from 2010 to 2019 (p<0.001) and by +61.0% from 2010 to 2020 (p<0.001). From 2010 to 2020, there was a large (>1.5 SDs) increase in Vermont (+353.67%), and significant elevations (>1.96 SDs, p<0.05) in Alaska (+421.11%) and Montana (+897.02%) relative to the average (figure 1). These findings show that methadone distribution in the USA has increased significantly over the past decade, with most states showing increases.
Examination of 2015 relative to 2020 revealed the national average distribution of methadone for OUD increased significantly by +26.22% (SD=50.38%, p<0.001), with 38 states increasing but 11 states decreasing (Alabama, Florida, Georgia, Kansas, Maine, Minnesota, Missouri, Nebraska, New Hampshire, South Dakota and Texas). There were significant increases (p<0.05) in Alaska (+135.34%) and Mississippi (+311.48%) relative to the national mean (figure 2). In conclusion, methadone distribution increased from 2015 to 2020, with significant increases in most states.
Based on a one-way repeated-measures ANOVA with Sidak post hoc from 2019 to 2020 (ie, pre-COVID-19 to post-COVID-19 pandemic), the distribution of methadone was stable (−0.091%, SD=10.81%). Slightly over half (28) of states showed an increase and 22 states exhibited a decrease. No significant (p=1.000) differences were found between 2019 and 2020. Examination of specific states revealed an increase in Kentucky (+18.68%) and a significant increase (p<0.05) in Ohio (+26.02%) relative to the national mean. In contrast, there were appreciable decreases in Nebraska (−16.6%), South Dakota (−17.27%) and Mississippi (−20.53%), and significant decreases (p<0.05) in Alabama (−21.96%), New Hampshire (−24.13%) and Florida (−28.97%) relative to the national mean (figure 3). Overall, the distribution was stable from 2019 to 2020, with significant increases in two states and decreases in three states.
Examination of 2019–2021, a wider pre-COVID-19 to post COVID-19 pandemic timeline, the distribution of methadone to OTPs showed a decline (−5.15%, SD=19.14%), but no significant difference (p=0.491). Eighteen states showed an increase, while 31 states showed a decrease. Ohio (+47.53%) had a significant increase (p<0.05) relative to the national mean. In contrast, there was an appreciable decrease in Mississippi (−42.53%), and significant decreases (p<0.05) in Alabama (−44.27%), Nebraska (−47.96), New Hampshire (−52.99%) and Florida (−54.61%) relative to the national mean (figure 4). However, distribution in 2021 was −5.69% lower than 2019 and −5.71% lower than 2020 (online supplemental figure 2A). In summary, methadone distribution declined from 2019 to 2021, with significant decreases in four states and an increase in one state.
The average methadone distribution in the USA for OUD was 43.75 mg/person (SD=35.01) in 2021. There was significantly elevated methadone distributed in Rhode Island (155.13 mg/person), Delaware (147.27 mg/person), Connecticut (126.66 mg/person) and Vermont (125.06 mg/person) relative to the national mean (43.75, p<0.05). Therefore, the distribution was relatively uniform in 2021, with significant elevations in Rhode Island, Delaware, Connecticut and Vermont.
The total number of OTPs distributing methadone in the USA increased +18.6% from 2010 (1139) to 2015 (1351) and peaked in 2021 (1738, online supplemental figure 2B). In comparison, the number of pharmacies distributing buprenorphine (49 041) was 43.1-fold greater than OTPs distributing methadone in 2010. However, this ratio decreased to 33.3-fold in 2021. Results of the repeated-measures ANOVA revealed a significant interaction between time and OTPs per 1 million persons per state (F(3, 150)=38.067, p<0.000). The number of OTPs per 1 million persons per state significantly increased (p<0.0001) from 2010 to 2021. In addition, one-way repeated-measures ANOVA with Sidak post hoc revealed 2010–2015 (p<0.0001), 2010–2020 (p<0.0001), 2015–2020 (p<0.0001) and 2015–2021 (p<0.0001) were each significantly different. The number of OTPs per 1 million persons per state in 2020 relative to 2021 did not significantly increase (p=0.683). Twenty states had fewer OTPs in 2021 relative to 2010, 2015 or 2020 (Alabama, Florida, Georgia, Maine, Maryland, Minnesota, Mississippi, Montana, Nebraska, New Hampshire, New Mexico, New York, North Carolina, Oklahoma, South Carolina, South Dakota, Texas, Utah, Vermont and Washington). One state (Wyoming) did not have a single OTP (figure 5 and online supplemental table 3). To sum up, the number of OTPs distributing methadone increased significantly from 2010 to 2021 but plateaued in 2021. The number of OTPs per million persons per state also increased significantly, but there was no significant increase from 2020 to 2021.
Medicaid
The SDUD Database showed considerable variation in methadone prescribing between states and regions for patients covered under Medicaid (mean=475.39, SD=1097.78 with four states having values of 0). The top four states (Wisconsin, Tennessee, Oregon and Vermont) accounted for 64.03% of all methadone covered by Medicaid in 2020 (online supplemental figure 3). Four states reporting zero values suggest that some data may be missing from the SDUD Database. In conclusion, methadone prescribing for Medicaid patients varied widely across states, with the top four states disproportionally accounting for over 60% of all prescriptions.
Discussion
The key finding of this study was that the pronounced and significant increase in methadone distribution to OTPs for OUD over the past decade has reversed with non-significant decreases (−0.09%) from 2019 to 2020 and (−5.15%) from 2019 to 2021. There were significant increases in the number of OTPs per 1 million persons per state over the past decade, but no significant increases over the COVID-19 pandemic period from 2019 compared with 2021.32 These findings point to the necessity for more OTPs to combat the escalating OUD problem with this evidence-based pharmacotherapy.8 Twenty states showed a reduction in OTPs from 2010 to 2021 which could be due to funding issues or policies for OTPs in these states.48
Examination of how the COVID-19 pandemic affected OUD treatment from 2019 to 2021 revealed that methadone distribution to OTPs did not increase significantly. However, a subtle but statistically significant increase (+5.0%) was observed in the number of poison control reports of intentional methadone exposure during this period.49 In addition, the National Center for Health Statistics of the Centers for Disease Control and Prevention reported that drug overdose mortality increased by +31% between 2019 and 2020. Nonetheless, the rate of methadone overdose deaths remained low with no significant increase in this report, suggesting no change in non-prescribed methadone use during the COVID-19 pandemic.50 Conversely, others have reached the opposite conclusion.10
Since OTPs were not distributing additional methadone Morphine Mg Equivalents during the COVID-19 pandemic, and overdose rates continue to surge, this emphasises the need for expanded access to methadone treatment and reduced treatment barriers. Individuals who overdose on illicit and prescription opioids including heroin and fentanyl may be prime candidates for methadone treatment. Additional solutions can include allowing for earlier access to take-home methadone from OTPs and allowing for patients to obtain methadone prescriptions from community pharmacies after a period of OUD stability, which is currently prohibited by law outside of an OTP.51 Another uncommonly employed solution is to provide travelling methadone treatment on a daily route which can allow observed administration and decreased need for transportation to a further location.52 This would be particularly beneficial not only for rural areas but also useful for zip codes with a limited number of methadone programmes.
Despite methadone being an effective evidence-based treatment for OUD,8 only 41 states in 2018 had methadone covered under Medicaid.45 Twelve states located predominantly in the South and Midwest opted to not expand Medicaid, leaving patients in these areas vulnerable to inaccessible treatment.53 54 States where methadone was not covered included: Arkansas, Idaho, Kansas, Kentucky, Louisiana, Nebraska, North Dakota, South Carolina, Tennessee and Wyoming.45 It was also found that only four states with 5.40% of the US population accounted for the preponderance (64.03%) of prescriptions. Although this lab has prior experience with Medicaid in various capacities (clozapine, esketamine, etc), the data acquired should be viewed with substantial scepticism unless subsequently verified by others, as some states may not have uploaded all their methadone data.55 56 This pronounced disparity indicates that patients in certain states and regions have appreciably better access to this evidence-based treatment for OUD than others.2
This study suggests the importance of policy change as there is a pressing need for additional access to OUD therapy, specifically methadone treatment, in the USA. Over 400 000 people in the USA received methadone from an OTP pre-pandemic, 2019, with over 90% located in urban areas, making it challenging for rural patients to receive their medication.56–59 SAMHSA released guidelines in March 2020 allowing the regulatory authorities of all states to request blanket exceptions to allow OTP patients to take home doses of methadone and buprenorphine.60 These guidelines were extended in November 2021. However, the number of patients receiving methadone declined in the first year of the COVID-19 pandemic by one-quarter.33 Many providers and public health researchers are calling for these take-home dosage rules to be continued post-pandemic,61 62 although others that evaluated the number of overdoses involving methadone are more cautious.10 63
The number of pharmacies which dispensed buprenorphine was 34-fold greater in 2021 than the number of OTPs that dispensed methadone, indicating that buprenorphine is more easily accessible and available than methadone. On the other hand, the volume of methadone distributed by weight from OTPs was 2.4-fold higher than buprenorphine from pharmacies, hospitals and OTPs combined (online supplemental table 1). It is currently curious that there are more restrictions in the USA for prescribing methadone than for other Schedule II substances like fentanyl or oxycodone. Overcoming the ‘Not in my Backyard’ stigma surrounding OTPs is not a trivial undertaking and is exacerbated by a common lack of understanding of OUD as a chronic disease.46 It will require a paradigm shift to allow supervised administration in pharmacies, primary care offices, mobile units as are common in other western nations and even video-observed therapy.18 30 64 65
Expanding the role of specialty trained pharmacists by expanding the MOUD regulations to allow provider-delegated induction with buprenorphine in pharmacies can immediately increase access to MOUD.64–68 According to the Consolidated Appropriations Act 2023, which was passed on 29 December 2022, medical providers with a current DEA registration number are now able to prescribe buprenorphine for OUD without needing an X-DEA waiver if state law permits it.69 Overall, this is important because it aims to remove existing barriers to OUD treatment and increase access to care for individuals who need it.
During the pandemic, daily visits to a methadone clinic were a health hazard and regulations governing the clinical work of methadone clinics were changed. Nonetheless, methadone remains more challenging for patients to access on many counts. Additionally, the regulatory burden surrounding methadone maintenance means that creating clinics requires substantially more time, money and effort than even specialty addiction medicine clinics where buprenorphine can be prescribed. This means that there will always be fewer methadone clinics than buprenorphine prescribers. This difference between the two medications may account for some of the change in methadone use described in this article. With the removal of the X-DEA waiver requirement for buprenorphine prescription, and the expansion of the exception to the Ryan Haight law70 which governs prescribing scheduled drugs over telemedicine, it is reasonable to expect that an increasing proportion of people who have OUD will receive buprenorphine rather than methadone. Despite lack of robust head-to-head trials,8 methadone has long been considered to have utility when other MOUDs have been ineffective and is recommended for patients who cannot tolerate initiation or ongoing treatment with buprenorphine. Thus, there remains a strong need for wide and equitable methadone availability during this ever-worsening opioid crisis.71
The non-homogeneous distribution within states should also be a concern for policymakers. The paucity of methadone OTPs across rural states like Wyoming (253 000 km2), South Dakota (200 000 km2) and Nebraska (200 ooo km2) is an important finding. However, prior research found that another rural state, Maine, which ranked 10th in the USA, had three OTPs in a single 30 000-person city (Bangor) and none in other areas in northern Maine.58 Providing funding opportunities to train addiction medicine fellows who will focus on rural care postgraduation can result in a pipeline of addiction medicine leaders to many areas of the USA which are most in need.
The strengths of this report include novel and timely data from ARCOS which is comprehensive for both distribution and number of distributors. This investigation extends upon earlier research both pre-COVID-195 and post-COVID-19 pandemic.38 39 Potential caveats and limitations stem from the fact that methadone distribution is reported in ARCOS by weight rather than prescriptions per individual at an OTP. It is notable that ARCOS reporting does not differentiate between pharmacological formulations. Further, this pharmacoepidemiological report does not contain detailed patient-level information including medical comorbidities or social determinants of health. These contributions should be further explored in future investigations with electronic medical records. Because SAMHSA’s N-SSATS annual reports contain data on the total number of patients receiving methadone at OTPs, they could complement the ARCOS data and allow for a more detailed analysis of opioid-prescribing patterns and patient outcomes (online supplemental figure 1).40 Importantly, other pharmacoepidemiological research that compared another Schedule II substance from ARCOS with a state Prescription Drug Monitoring Program identified a high correspondence (r=+0.985).37 The inclusion of Medicaid data also, at least partially, offsets this concern. However, the substantial state-level inhomogeneity of methadone as reported by Medicaid should be viewed carefully and warrants further study. A reported value of zero for four states could possibly be explained by factors such as states not reporting data or changes in how states report these data over time. Future research should also be focused on determining which patient subgroups were most impacted by the reversal in methadone distribution. As the number of pharmacies nationally distributing buprenorphine decreased (−4.7%) from 2019 to 2021, further research should evaluate if both methadone and buprenorphine continue to be underused in the post-COVID-19 pandemic period24 (online supplemental table 1). This research is essential to guide policy and practice efforts to ensure that all individuals with OUD have access to effective MOUD treatment options.
Conclusions
In conclusion, this study highlights the trend over the past decade of methadone distribution for OUD in the USA and disparities in access to OTPs in rural and western states. The findings of this study have the potential to guide improvements in OUD treatment policies at the state level, by providing valuable information on disparities in access to OTPs that could lead to the implementation of more permanent solutions. The many policy accommodations to COVID-19 may present an important opportunity to determine which factors most impact the accessibility, adherence, safety and efficacy of methadone. Policy solutions that increase access to MOUD are urgently needed to continue to address the ongoing opioid epidemic.
Supplementary Material
Acknowledgments
Thank you to Iris Johnston for providing access to journal articles, and Olapeju Simoyan, for providing feedback. GraphPad Prism provided by grant NIH/NIEHS T32 ES007060-31A1.
Footnotes
Contributors: Conceptualisation—ALK and BJP. Data curation—ALK and JLF. Formal analysis—ALK. Investigation—ALK. Methodology—BJP. Project administration—KLM. Supervision—BJP. Visualisation—ALK. Guarantor-ALK. Writing (original draft)—ALK. Writing (review and editing)—JLF, JAF, NJM, MAJ, SDN, KLM and BJP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: BJP is supported by the Health Resources Services Administration (D34HP31025), the Pennsylvania Academic Clinical Research Center, and was (until 31 December 2021) part of an osteoarthritis research team supported by Pfizer and Eli Lilly. MAJ received consulting fees from SAMHSA, expert testimony fees, stock through US Preventative Medicine, and support for meetings through the American Board of Preventive Medicine, Addiction Medicine Examination Subcommittee, and the American Society of Addiction Medicine Board of Directors. MAJ also participates in leadership roles in the American Society of Addiction Medicine Board of Directors, Quality Improvement Council and ASAM Criteria Steering Committee. SDN is a consultant for the SAMHSA-funded Opioid Response Network providing one-on-one and didactic group provider education and is core faculty for the Maine Medical Center/VA Addiction Medicine Physician Fellowship Program. SDN also received payment for speaking about buprenorphine at the Maine Association of Psychiatric Physicians meeting and the American Association of Psychiatric Pharmacists. In addition, SDN receives support by employer at UNE to attend meetings and is supported by Lunder-Dineen Time to Ask Program alcohol advisory programme to travel to the MGH SUD conference in Florida and to the American Society of Addiction Medicine meeting to present. She has also participated in advisory boards for Maine Medical Professions Health Program, Lunder Dineen-Time to Ask and Co-Occurring Collaborative Serving Maine. Lastly, she serves as a leader on the Maine Prescription Monitoring Program Committee. The other authors have no disclosures.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Publicly available datasets were analysed in this study. These data can be found here: https://www.deadiversion.usdoj.gov/arcos/ and https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the institutional review boards of the University of New England and Geisinger.
References
- 1.Peterkin A, Laks J, Weinstein ZM. Current best practices for acute and chronic management of patients with opioid use disorder. Med Clin North Am 2022;106:61–80. 10.1016/j.mcna.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 2.Anderson IB, Kearney TE. Use of methadone. West J Med 2000;172:43–6. 10.1136/ewjm.172.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toce MS, Chai PR, Burns MM, et al. Pharmacologic treatment of opioid use disorder: A review of Pharmacotherapy, adjuncts, and toxicity. J Med Toxicol 2018;14:306–22. 10.1007/s13181-018-0685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauro PM, Gutkind S, Annunziato EM, et al. Use of medication for opioid use disorder among US adolescents and adults with need for opioid treatment, 2019. JAMA Netw Open 2022;5:e223821. 10.1001/jamanetworkopen.2022.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennalley AL, Furst JA, Mynarski NJ, et al. Methadone distribution increased from 2010 to 2019 for opioid use disorder treatment in the US. MedRxiv 2021. 10.1101/2022.03.09.22272154 [DOI] [Google Scholar]
- 6.Substance Abuse Mental Health Services Administration, Available: https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf [Accessed 15 Feb 2022].
- 7.Krawczyk N, Jent V, Hadland SE, et al. Utilization of medications for opioid use disorder across US States: relationship to treatment availability and overdose mortality. J Addict Med 2022;16:114–7. 10.1097/ADM.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 8.Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009;2009:CD002209. 10.1002/14651858.CD002209.pub2 [DOI] [PubMed] [Google Scholar]
- 9.Jones CM, Compton WM, Han B, et al. Methadone-involved overdose deaths in the US before and after federal policy changes expanding take-home methadone doses from opioid treatment programs. JAMA Psychiatry 2022;79:932–4. 10.1001/jamapsychiatry.2022.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman DE, Kennalley AL, McCall KL, et al. Examination of methadone involved overdoses during the COVID-19 pandemic. Forensic Sci Int 2023;344:111579. 10.1016/j.forsciint.2023.111579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darracq MA, Thornton SL. Respiratory depression following medications for opioid use disorder (MOUD)-Approved buprenorphine product oral exposures; National poison database system 2003-2019. Clin Toxicol (Phila) 2021;59:303–12. 10.1080/15563650.2020.1814318 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, Available: https://www.cdc.gov/nchs/nvss/index.htm [Accessed 30 Sep 2023].
- 13.Pande LJ, Arnet RE, Piper BJ. An examination of the complex pharmacological properties of the non-selective opioid modulator buprenorphine. Pharmaceuticals (Basel) 2023;16:1397. 10.3390/ph16101397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahji A, Cheng B, Gray S, et al. Reduction in mortality risk with opioid agonist therapy: a systematic review and meta-analysis. Acta Psychiatr Scand 2019;140:313–39. 10.1111/acps.13088 [DOI] [PubMed] [Google Scholar]
- 15.Heil J, Ganetsky VS, Salzman MS, et al. Attitudes on methadone utilization in the emergency department: A physician cross-sectional study. West J Emerg Med 2022;23:386–95. 10.5811/westjem.2022.2.54681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagisetty PA, Ross R, Bohnert A, et al. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry 2019;76:979–81. 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman M, Steer C, Tilling K, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction 2018;113:1461–76. 10.1111/add.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcaterra SL, Bach P, Chadi A, et al. Methadone matters: what the United States can learn from the global effort to treat opioid addiction. J Gen Intern Med 2019;34:1039–42. 10.1007/s11606-018-4801-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CM, Byrd DJ, Clarke TJ, et al. Characteristics and current clinical practices of opioid treatment programs in the United States. Drug Alcohol Depend 2019;205:107616. 10.1016/j.drugalcdep.2019.107616 [DOI] [PubMed] [Google Scholar]
- 20.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction 1998;93:515–32. 10.1046/j.1360-0443.1998.9345157.x [DOI] [PubMed] [Google Scholar]
- 21.Otiashvili D, Piralishvili G, Sikharulidze Z, et al. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior--outcomes of a randomized trial. Drug Alcohol Depend 2013;133:376–82. 10.1016/j.drugalcdep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;2:CD002207. 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute on Drug Abuse, Available: https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/overview [Accessed 23 Dec 2022].
- 24.Presnall NJ, Butler GC, Grucza RA. Consumer access to buprenorphine and methadone in certified community behavioral health centers: A secret shopper study. J Subst Abuse Treat 2022;139:108788. 10.1016/j.jsat.2022.108788 [DOI] [PubMed] [Google Scholar]
- 25.Stone AC, Carroll JJ, Rich JD, et al. One year of methadone maintenance treatment in a fentanyl endemic area: safety, repeated exposure, retention, and remission. J Subst Abuse Treat 2020;115:108031. 10.1016/j.jsat.2020.108031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention, Available: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm [Accessed 22 Feb 2022].
- 27.Friedman JR, Hansen H. Evaluation of increases in drug overdose mortality rates in the US by race and Ethnicity before and during the COVID-19 pandemic. JAMA Psychiatry 2022;79:379–81. 10.1001/jamapsychiatry.2022.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention, Available: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm [Accessed 31 Mar 2022].
- 29.Friedman J, Akre S. COVID-19 and the drug overdose crisis: Uncovering the deadliest months in the United States, January‒July 2020. Am J Public Health 2021;111:1284–91. 10.2105/AJPH.2021.306256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joudrey PJ, Adams ZM, Bach P, et al. Methadone access for opioid use disorder during the COVID-19 pandemic within the United States and Canada. JAMA Netw Open 2021;4:e2118223. 10.1001/jamanetworkopen.2021.18223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandara S, Kennedy-Hendricks A, Merritt S, et al. Early effects of COVID-19 on programs providing medications for opioid use disorder in jails and prisons. J Addict Med 2020;14:e257–60. 10.1097/ADM.0000000000000718 [DOI] [PubMed] [Google Scholar]
- 32.Bandara S, Kennedy-Hendricks A, Merritt S, et al. Methadone and buprenorphine treatment in United States jails and prisons: lessons from early Adopters. Addiction 2021;116:3473–81. 10.1111/add.15565 [DOI] [PubMed] [Google Scholar]
- 33.Maine Department of Corrections, Available: https://www.maine.gov/corrections/sites/maine.gov.corrections/files/inline-files/MAT_Year_One_ReviewFinal.pdf [Accessed 19 Oct 2022].
- 34.U.S. Department of Justice Drug Enforcement Administration Diversion Control Division, Available: https://www.deadiversion.usdoj.gov/arcos/ [Accessed 4 Oct 2023].
- 35.Furst JA, Mynarski NJ, McCall KL, et al. Pronounced regional disparities in United States methadone distribution. Ann Pharmacother 2022;56:271–9. 10.1177/10600280211028262 [DOI] [PubMed] [Google Scholar]
- 36.Kennalley AL, Boureghda YA, Ganesh JG, et al. Declining national codeine distribution in united states hospitals and pharmacies from 2011 to 2019. Pain Medicine [Preprint] 2022. 10.1101/2022.04.12.22273805 [DOI]
- 37.Piper BJ, Shah DT, Simoyan OM, et al. Trends in medical use of opioids in the U.S., 2006–2016. Am J Prev Med 2018;54:652–60. 10.1016/j.amepre.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 38.Chen AY, Powell D, Stein BD. Changes in buprenorphine and methadone supplies in the US during the COVID-19 pandemic. JAMA Netw Open 2022;5:e2223708. 10.1001/jamanetworkopen.2022.23708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterman NJ, Palsgaard P, Vashi A, et al. Demographic and geospatial analysis of buprenorphine and methadone prescription rates. Cureus 2022;14:e25477. 10.7759/cureus.25477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Substance Abuse and Mental Health Services Administration, Available: https://www.samhsa.gov/data/report/national-survey-substance-abuse-treatment-services-n-ssats-2020-data-substance-abuse [Accessed 11 Feb 2023].
- 41.Medicaid.Gov. Available: https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html [Accessed 26 Jul 2022].
- 42.Chorniy A, Currie J, Sonchak L. Exploding asthma and ADHD Caseloads: the role of Medicaid managed care. J Health Econ 2018;60:1–15. 10.1016/j.jhealeco.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medicaid.gov . Mandatory Medicaid state plan coverage of Medication-Assisted Treatment, . 2020Available: https://www.medicaid.gov/federal-policy-guidance/downloads/sho20005.pdf [Accessed 26 Jul 2022].
- 44.Saloner B, Stoller KB, Barry CL. Medicaid coverage for methadone maintenance and use of opioid agonist therapy in specialty addiction treatment. Psychiatr Serv 2016;67:676–9. 10.1176/appi.ps.201500228 [DOI] [PubMed] [Google Scholar]
- 45.Kaiser Family Foundation, Available: https://www.kff.org/infographic/medicaids-role-in-addressing-opioid-epidemic/ [Accessed 22 Sep 2022].
- 46.40 United States Census Bureau, Available: https://www.census.gov/topics/population.html [Accessed 23 Jan 2022].
- 47.Cabrera FF, Gamarra ER, Garcia TE, et al. Opioid distribution trends (2006-2017) in the US territories. PeerJ 2019;7:e6272. 10.7717/peerj.6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CBR. Socio-spatial stigmatization and the contested space of addiction treatment: Remapping strategies of opposition to the disorder of drugs. Soc Sci Med 2010;70:859–66. 10.1016/j.socscimed.2009.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh C, Doyon S, Hart K. Methadone exposures reported to poison control centers in the United States following the COVID-19-related loosening of Federal methadone regulations. Int J Drug Policy 2022;102:103591. 10.1016/j.drugpo.2022.103591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention, Available: https://www.cdc.gov/nchs/products/databriefs/db428.htm [Accessed 23 Jan 2022].
- 51.Bart G, Wastvedt S, Hodges JS, et al. Did drug use increase following COVID-19 relaxation of methadone take-out regulations? 2020 was a complicated year. J Subst Abuse Treat 2022;133:108590. 10.1016/j.jsat.2021.108590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breve F, Batastini L, LeQuang JAK, et al. Mobile narcotic treatment programs: on the road again Cureus 2022;14:e23221. 10.7759/cureus.23221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grissinger M. Keeping patients safe from methadone overdoses. P T 2011;36:462–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser Family Foundation . Available: https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D [Accessed 22 Sep 2022].
- 55.Benito RA, Gatusky MH, Panoussi MW, et al. Thirteen-fold variation between states in clozapine prescriptions to United States Medicaid patients. Schizophr Res 2023:S0920-9964(23)00103-2. 10.1016/j.schres.2023.03.010 [DOI] [PubMed] [Google Scholar]
- 56.Aguilar AG, Beauregard BA, Conroy CP, et al. Pronounced regional variation in esketamine and ketamine prescribing to US Medicaid patients. J Psychoactive Drugs 2023:1–7. 10.1080/02791072.2023.2178558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Pew Charitable Trusts . Available: https://www.pewtrusts.org/en/research-and-analysis/articles/2021/04/22/more-flexible-methadone-access-should-continue-post-pandemic [Accessed 31 Mar 2022].
- 58.Hyde TF, Bekoe-Tabiri AD, Kropp Lopez AK, et al. County and demographic differences in drug arrests and controlled substance use in Maine. Journal of Maine Medical Center 2021;3. 10.46804/2641-2225.1074 [DOI] [Google Scholar]
- 59.Jehan S, Zahnd WE, Wooten NR, et al. Geographic variation in availability of opioid treatment programs across U.S. communities. J Addict Dis 2023:1–11. 10.1080/10550887.2023.2165869 [DOI] [PubMed] [Google Scholar]
- 60.Substance Abuse Mental Health Services Administration . Available: https://www.samhsa.gov/medication-assisted-treatment/statutes-regulations-guidelines/methadone-guidance [Accessed 15 Feb 2022].
- 61.Amram O, Amiri S, Panwala V, et al. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. Am J Drug Alcohol Abuse 2021;47:722–9. 10.1080/00952990.2021.1979991 [DOI] [PubMed] [Google Scholar]
- 62.Regulatory Studies Center . Available: https://regulatorystudies.columbian.gwu.edu/extending-pandemic-flexibilities-opioid-use-disorder-treatment [Accessed 2 Mar 2022].
- 63.Kleinman RA, Sanches M. Methadone-involved overdose deaths in the United States before and during the COVID-19 pandemic. Drug Alcohol Depend 2023;242:109703. 10.1016/j.drugalcdep.2022.109703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darnton JB, Bhatraju EP, Beima-Sofie K, et al. Sign me up”: a qualitative study of video observed therapy (VOT) for patients receiving expedited methadone take-homes during the COVID-19 pandemic. Addict Sci Clin Pract 2023;18:21. 10.1186/s13722-023-00372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibbons JB, Stuart EA, Saloner B. Methadone on wheels-A new option to expand access to care through mobile units. JAMA Psychiatry 2022;79:187–8. 10.1001/jamapsychiatry.2021.3716 [DOI] [PubMed] [Google Scholar]
- 66.Peckham AM, Ball J, Colvard MD, et al. Leveraging pharmacists to maintain and extend buprenorphine supply for opioid use disorder amid COVID-19 pandemic. Am J Health Syst Pharm 2021;78:613–8. 10.1093/ajhp/zxab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Substance Abuse and Mental Health Services Administration . Available: https://www.samhsa.gov/medications-substance-use-disorders/removal-data-waiver-requirement [Accessed 1 Feb 2023].
- 68.Green TC, Serafinski R, Clark SA, et al. Physician-delegated unobserved induction with buprenorphine in pharmacies. N Engl J Med 2023;388:185–6. 10.1056/NEJMc2208055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Congress.gov . Available: https://www.congress.gov/bill/117th-congress/house-bill/2617 [Accessed 11 Feb 2023].
- 70.American Psychiatric Association , Available: https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act [Accessed 10 Mar 2023].
- 71.The Washington Post . Available: https://www.washingtonpost.com/health/2023/03/05/methadone-addiction-treatment-prescrption/ [Accessed 10 Mar 2023].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074845supp001.pdf (333.5KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Publicly available datasets were analysed in this study. These data can be found here: https://www.deadiversion.usdoj.gov/arcos/ and https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html.