Abstract
The role of choline in osmoprotection in the moderate halophile Halomonas elongata has been examined. Transport and conversion of choline to betaine began immediately after addition of choline to the growth medium. Intracellular accumulation of betaine synthesized from choline was salt dependent up to 2.5 M NaCl. Oxidation of choline was enhanced at 2.0 M NaCl in the presence or absence of externally provided betaine. This indicates that the NaCl concentration in the growth medium has major effects on the choline-betaine pathway of H. elongata.
Microorganisms must be able to adapt to changes in the osmolarity of their environment. To adapt to these changes, bacteria accumulate some compounds, named compatible solutes, that confer protection against the deleterious effect of the low water activity (8). Moderately halophilic bacteria are defined as those which can grow optimally between 0.5 and 2.5 M salt (9). Among this heterogeneous group of microorganisms, Halomonas elongata has a very promising potential for use in biotechnology (13), and since it can grow at a very wide range of salinities (from 0.5 to 3 M NaCl in a minimal medium) (4), it is also a good model organism to study the molecular basis of prokaryotic osmoadaptation (14). To cope with changes in the salt concentration of the environment, H. elongata is able to synthesize ectoine and hydroxyectoine (5, 8) and to take up a variety of organic compounds which can serve as osmoprotectants when present externally (4). Accordingly, exogenous glycine betaine (called betaine hereafter), choline, and choline-O-sulfate have been demonstrated to play an osmoprotective role in H. elongata (4). Many organisms, including gram-positive (1, 15) and gram-negative (11) bacteria, as well as higher plants (12, 18), can generate betaine for osmoprotection by oxidation of choline. We have investigated the transport of choline and its conversion to the osmoprotectant compound betaine in the moderately halophilic bacterium H. elongata. The role of salinity and betaine in the regulation of this osmoprotective mechanism has also been investigated.
Effect of choline on the salinity growth range of H. elongata.
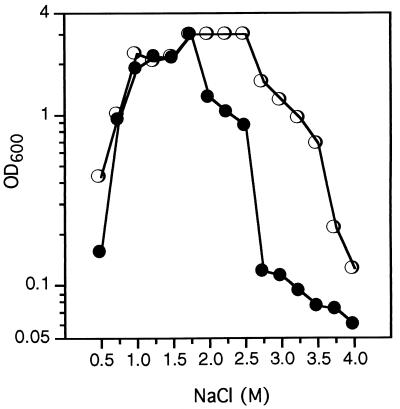
H. elongata DSM 3043 (16) has been shown to be able to grow in minimal medium M63 in a wide range of salt concentrations in the absence of any added osmoprotectant (4). To test the effect of choline on the growth of H. elongata at different salinities, cells were inoculated in M63 (6) (with 20 mM glucose as sole carbon source) plus 0.5 to 4.0 M NaCl in the presence or absence of 1 mM choline, and the optical density at 600 nm was measured after 24 h of incubation at 37°C (Fig. 1). Choline stimulated the growth of H. elongata in minimal medium at salinities above the optimal concentration of 2 M NaCl, so that it extended the salinity range of growth to at least 3.5 M NaCl. It is noteworthy that the growth of H. elongata was also stimulated by choline at 0.5 M NaCl (Fig. 1). This result is consistent with our earlier observation that betaine was likewise stimulatory for this bacterium at all salinities, including the suboptimal concentration of 0.5 M NaCl (4). Thus, compared to a culture grown at 0.5 M NaCl, the growth rate of H. elongata can be increased by two seemingly contradictory additives: increased NaCl concentration up to 2 M NaCl or an osmoprotectant such as betaine or its precursor, choline. These observations present somewhat of a paradox, because these two additives have contradictory effects: increasing the NaCl concentration presumably increases osmotic stress, while the osmoprotectants are believed to alleviate the inhibitory effects of high osmolarity. Resolution of this apparent paradox may lie in the hypothesis that while H. elongata requires high concentrations of NaCl (up to 2 M) for some unknown biochemical reason(s), it is nevertheless under osmotic stress, even at 0.5 M NaCl, and addition of osmoprotectants relieves the inhibitory effects of high osmolarity.
FIG. 1.
Effect of choline on growth of H. elongata in M63-glucose-minimal medium with increasing salinity. Cultures were grown at 37°C in M63 containing the indicated concentrations of NaCl, and the optical density was determined after 24 h. Symbols: •, M63 alone; ○, M63 with 1 mM choline. For every growth experiment, data are the averages of three different repeats which on no occasion varied by more than 5%. OD600, optical density at 600 nm.
Transport of choline and its intracellular conversion to betaine.
To study the effect of salinity on the transport of choline, cells were incubated in M63 with 0.5 or 2.0 M NaCl and 10 μM [methyl-14C]choline. Samples (0.5 ml) were taken at different time intervals, and cells were collected after 2 min of centrifugation in a microcentrifuge. Cell pellets were frozen in liquid nitrogen. For each sample, 0.3 ml of supernatant was used for scintillation counting to measure the choline remaining in the culture medium. At 2.0 M NaCl, almost the entire exogenous choline was taken up within 5 min, while at 0.5 M NaCl, the cells required 10 min to transport 85% of the external choline, and transport was completed only after 20 min of incubation (data not shown).
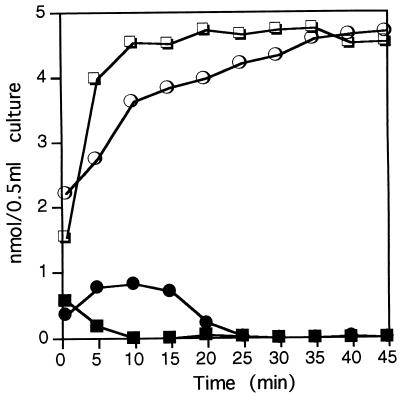
To determine the rate of intracellular oxidation of choline to betaine in response to salt stress, cell pellets of each sample were extracted with 80% methanol and betaine and choline were separated by thin-layer chromatography with 90:10:4 (vol/vol/vol) methanol-acetone-hydrochloric acid as running solvent (17). The radioactive metabolites were visualized by autoradiography and identified by comparison with [14C]betaine and [14C]choline standards. The distribution of choline and betaine in the thin-layer chromatogram was analyzed in the exposed films by computing densitometry (ImageQuant; Molecular Dynamics). Figure 2 shows that the transformation of choline was much faster at 2.0 M than at 0.5 M NaCl. Although almost all choline added was taken up by the cells after 10 min at 0.5 M NaCl, it took about 35 min to complete its oxidation to betaine. However, at 2.0 M NaCl oxidation was completed in 10 min. These data indicate that at high salinity there was a stimulation of the intracellular transformation of choline to betaine. Both at low (0.5 M NaCl) and at high (2.0 M NaCl) salinity, transport and intracellular conversion of choline to betaine by H. elongata began immediately after addition of choline to the minimal medium (Fig. 2). However, we cannot infer from these data that the genes encoding the choline-betaine pathway in this moderate halophile are constitutively expressed in the absence of osmotic stress. The stimulation of transport and oxidation of choline by high osmolarity also occurs in Escherichia coli, where the betT and betIAB operons are induced by osmotic stress. Expression of the E. coli bet genes is further enhanced by the addition of choline and is repressed by betaine, anaerobiosis, or low temperature (7, 10). In contrast, high osmolarity alone did not stimulate gbsAB expression in Bacillus subtilis, where these genes were found to be induced by choline (2). Uptake of choline in this bacterium is mediated by an efficient transport system, which is osmotically regulated at the level of transport activity and expression of its structural gene(s) (1). H. elongata also possesses a high-affinity transport system for choline, whose Km is about 10 μM (3).
FIG. 2.
Analysis of the conversion of choline to betaine in response to salinity. Choline and betaine in cell extracts were separated by thin-layer chromatography and visualized by autoradiography. Signals corresponding to choline (closed symbols) and betaine (open symbols) in the autoradiogram were analyzed by computing densitometry. Cells were grown at 0.5 (circles) or 2.0 (squares) M NaCl. Experiments (at both salt concentrations) are means of duplicates which on no occasion varied by more than 5%.
Effect of betaine in the intracellular oxidation of choline.
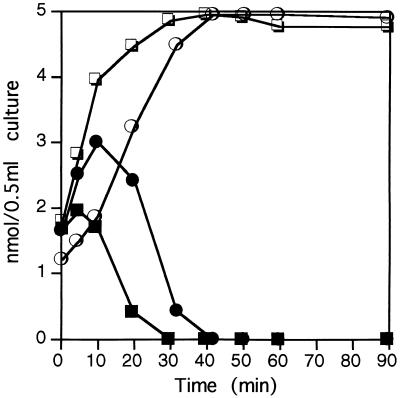
The uptake and conversion of choline were assayed in the presence of betaine. Cells were incubated in M63 plus 0.5 or 2.0 M NaCl with 10 μM [methyl-14C]choline and 10 μM betaine, and the disappearance of choline from the medium and the rate of intracellular oxidation of choline to betaine were determined as described above. Although the initial uptake of choline was slightly delayed at both salt concentrations, choline was completely taken up by 10 min at 2.0 M NaCl or 20 min at 0.5 M NaCl (data not shown). After 10 min of incubation at 2.0 M NaCl, 34% of choline remained in the cytoplasm in the presence of betaine (Fig. 3), whereas no choline could be detected at the same time in the absence of betaine (Fig. 2). After 10 min of incubation at 0.5 M NaCl, 60% of nonoxidized choline remained in the cytoplasm in cells grown with betaine (Fig. 3), whereas at the same time less than 20% of the choline remained in the cytoplasm of cells grown without betaine (Fig. 2). Thus, whereas betaine exerted a slight inhibition of the uptake of choline in H. elongata (maybe due to a competition for the choline transporter), its major negative regulatory effect occurred at the oxidation of choline to betaine. This inhibition seems to be stronger at high salinity, where the almost instantaneous conversion of choline to betaine was delayed by betaine. However, the fact that both the transport and the oxidation of choline were faster at 2 M NaCl than at 0.5 M NaCl, regardless of the presence or absence of betaine, indicates that the salinity of the medium is the main factor that regulates the choline-betaine pathway of H. elongata under the experimental conditions used.
FIG. 3.
Effect of betaine on the intracellular oxidation of choline. H. elongata was grown in M63 containing 0.5 (circles) or 2.0 (squares) M NaCl and [methyl-14C]choline (closed symbols) and betaine (open symbols). Oxidation of choline was determined as described in the legend to Fig. 2. Experiments (at both salt concentrations) were repeated twice, and the data correspond to means which on no occasion varied by more than 5%.
Accumulation of betaine from choline in response to salt stress.
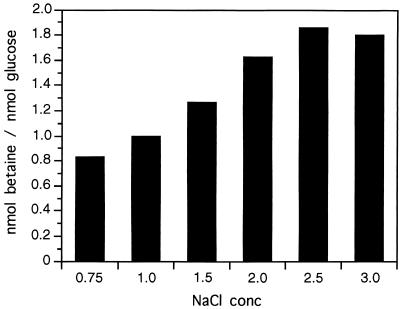
The intracellular betaine level derived from choline was determined from cells grown in M63 containing 0.75 to 3 M NaCl, 10 mM [methyl-14C]choline, and 20 mM [3H]glucose as described previously (4) (Fig. 4). Because choline was converted rapidly to betaine (see above), the radioactivity contained in the cells was due to betaine. The accumulation of betaine by synthesis from choline was salt dependent and increased as the NaCl concentration was raised up to 2.5 M NaCl. At 3 M NaCl, the level of betaine accumulated was slightly lower than at 2.5 M NaCl, suggesting that the transport of choline, its transformation to betaine, or its retention at this high salinity was affected by an uncharacterized mechanism. Studies of the accumulation of betaine by a high-affinity transport system (4) are in good agreement with the data reported here. In both cases (with choline or betaine added externally), the levels of betaine accumulated in the cytoplasm at different salt concentrations were nearly identical, suggesting a common regulatory mechanism for the two systems.
FIG. 4.
Accumulation of betaine from choline by H. elongata in M63 at increasing osmolarities. Cells were grown overnight in the presence of [methyl-14C]choline and [3H]glucose. Samples were centrifuged and washed, and the accumulated radioactivity was measured by scintillation counting. Experiments (at both salt concentrations) were repeated twice, and the data correspond to means which on no occasion varied by more than 5%. conc, concentration.
Data presented in this work on the role of choline in osmoprotection and regulation of betaine synthesis will be helpful for further studies on the regulation of the genes involved in this pathway.
Acknowledgments
This research was financially supported by grants from the BIOTECH Program of the European Commission (Generic Project Extremophiles as Cell Factories, grant BIO4-CT96-0488), from the Ministerio de Educación y Ciencia, Spain (grant PB97-0722), and from the Junta de Andalucía. D. Cánovas is supported by a fellowship from the Spanish Ministerio de Educación y Cultura.
REFERENCES
- 1.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boch J, Kempf B, Schmid R, Bremer E. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of gbsAB genes. J Bacteriol. 1996;178:5121–5129. doi: 10.1128/jb.178.17.5121-5129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cánovas, D., C. Vargas, E. Bremer, A. Ventosa, and J. J. Nieto. Unpublished data.
- 4.Cánovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J Bacteriol. 1996;178:7221–7226. doi: 10.1128/jb.178.24.7221-7226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cánovas D, Vargas C, Iglesias-Guerra F, Csonka L N, Rhodes D, Ventosa A, Nieto J J. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Biol Chem. 1997;272:25794–25801. doi: 10.1074/jbc.272.41.25794. [DOI] [PubMed] [Google Scholar]
- 6.Cohen G N, Rickenberg R H. Concentration specifique reversible des aminoi acides chez E. coli. Ann Inst Pasteur Paris. 1956;91:693–720. [PubMed] [Google Scholar]
- 7.Eshoo M W. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J Bacteriol. 1988;170:5208–5215. doi: 10.1128/jb.170.11.5208-5215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;1995:273–328. [PubMed] [Google Scholar]
- 9.Kushner D J. Life in high salt and solute concentrations: halophilic bacteria. In: Kushner D J, editor. Microbial life in extreme environments. London, United Kingdom: Academic Press; 1978. pp. 317–368. [Google Scholar]
- 10.Lamark T, Røkenes T P, McDougall J, Strøm A R. The complex bet promoters of Escherichia coli: regulation by oxygen (ArcA), choline (BetI), and osmotic stress. J Bacteriol. 1996;178:1655–1662. doi: 10.1128/jb.178.6.1655-1662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landfald B, Strøm A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCue K F, Hanson A D. Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol Biol. 1992;18:1–11. doi: 10.1007/BF00018451. [DOI] [PubMed] [Google Scholar]
- 13.Ventosa A, Nieto J J. Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol. 1995;11:85–94. doi: 10.1007/BF00339138. [DOI] [PubMed] [Google Scholar]
- 14.Ventosa A, Nieto J J, Oren A. The biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijaranakul U, Nadakavukaren M J, Bayles D O, Wilkinson B J, Jayaswal R K. Characterization of a NaCl-sensitive Staphylococcus aureus mutant and rescue of the NaCl-sensitive phenotype by glycine betaine but not by other compatible solutes. Appl Environ Microbiol. 1997;63:1889–1897. doi: 10.1128/aem.63.5.1889-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vreeland R H, Litchfield C D, Martin E L, Elliot E. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol. 1980;30:485–495. [Google Scholar]
- 17.Weigel P, Lerma C, Hanson A D. Choline oxidation by intact spinach chloroplasts. Plant Physiol. 1988;86:54–60. doi: 10.1104/pp.86.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weretilnyk E A, Hanson A D. Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci USA. 1990;87:2745–2749. doi: 10.1073/pnas.87.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]