Abstract
Background
Hereditary angioedema (HAE) is a rare genetic disease. Patients with type II HAE have normal or elevated C1-inhibitor (C1-INH) levels but C1-INH protein is dysfunctional. C1-INH function requires careful sample handling and technical expertise and may account for the lack of diagnosed patients with type II HAE in resource-limited countries.
Objective
We sought to assess the diagnostic performance of elevated C1-INH levels in diagnosing type II HAE.
Methods
All patients with confirmed type II HAE in Hong Kong and India were analyzed. Diagnosis was confirmed by persistent low C1-INH function and/or pathogenic SERPING1 gene mutations. Their C1-INH levels were compared with those of matched controls.
Results
A total of 31 (14 Chinese, 17 Indian) patients with type II HAE and 31 matched controls were analyzed. Overall, 77.4% (24/31) of patients with type II HAE had elevated C1-INH levels compared with 38.7% (12 of 31) of controls (odds ratio, 2.00; 95% CI, 1.34-2.98; P = .017). C1-INH levels in patients with type II HAE were significantly higher than in controls (52.2 ± 20.0 mg/dL vs 29.1 ±3.6 mg/dL; P < .001). Findings were consistent when C1-INH values in the Chinese and Indian subgroups were analyzed separately. Receiver-operating characteristic curve demonstrated excellent performance for elevated C1-INH levels to diagnose patients with type II HAE with an area under the curve of 0.953 (95% CI, 0.941-0.992; P < .001). Positive and negative predictive values of both a low C4 and an elevated C1-INH level for patients with type II HAE were 100% and 82.9%, respectively.
Conclusions
Low C4 and elevated C1-INH levels may be considered as a screening tool for type II HAE, especially in countries where C1-INH function testing is not readily available.
Key words: C1 inhibitor, hereditary angioedema, function, level, screening, type 2 HAE
Introduction
Hereditary angioedema (HAE) is a rare genetic disease characterized by recurrent bradykinin-mediated angioedema. Diagnosis of HAE relies on estimation of C4, C1-inhibitor (C1-INH) antigen levels, and C1-INH function. Patients are classified as having type I HAE when C1-INH levels are low or type II HAE when C1-INH levels are normal but C1-INH protein is dysfunctional.1 A low C4 is approximately 80% to 85% sensitive in diagnosing HAE and is usually recommended as a screening test, especially with using HAE-specific cutoff values to further enhance specificity and sensitivity.2
International guidelines advocate that “all patients suspected to have HAE are assessed for blood levels of C1-INH function, C1-INH protein, and C4.”1 However, these tests are not always available, especially in resource-limited countries.3 C1-INH function, which requires careful sample handling and technical expertise, is particularly challenging and may account for the lack of diagnosed patients with type II HAE in resource-limited countries.4 During a discussion in the Asia Pacific HAE scientific meeting, it was observed that patients with type II HAE seemed to have paradoxically elevated C1-INH levels, which may be a useful adjunct to detecting abnormal C1-INH function. This international, multiethnic study was therefore performed to assess the diagnostic performance of elevated C1-INH levels in diagnosing type II HAE.
Results and discussion
All patients with confirmed type II HAE in Hong Kong (Queen Mary Hospital, University of Hong Kong) and India (Postgraduate Institute of Medical Education and Research, Chandigarh) as of March 2023 were analyzed. Diagnosis was confirmed by either persistent low C1-INH function and/or pathogenic SERPING1 gene mutations. All patients had C4 and C1-INH levels quantified by nephelometry, and the same assay (Siemens Healthineers, Marburg, Germany) was used in both centers. The suggested reference intervals for C1-INH levels by the manufacturer (21-39 mg/dL) were used. When multiple C4 or C1-INH level values were available for individual patients, blood samples with the lowest value were selected. Ethnic-matched individuals without HAE were selected as normal controls. Demographic data, such as sex and age at blood sampling, were also analyzed. C1-INH levels were compared using the chi-square test and the independent-samples t test. Pearson correlation was used to investigate the relationship between C1-INH level and C1-INH function. IBM SPSS Statistics (version 27.0; IBM, New York, NY) was used to calculate the receiver-operating characteristic curves. P values less than .05 were considered statistically significant. Informed consent was obtained from all involved patients.
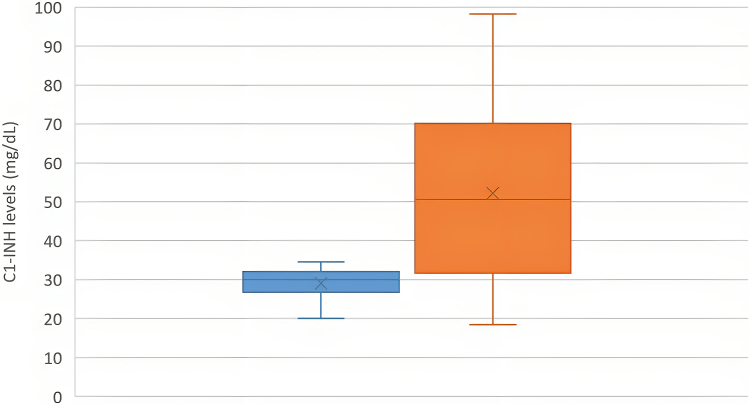
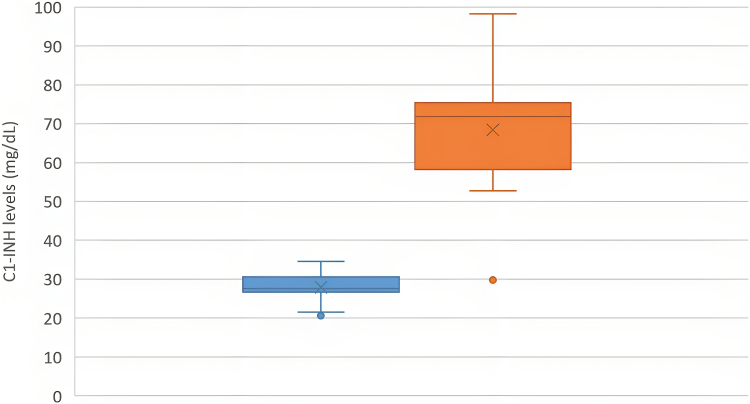
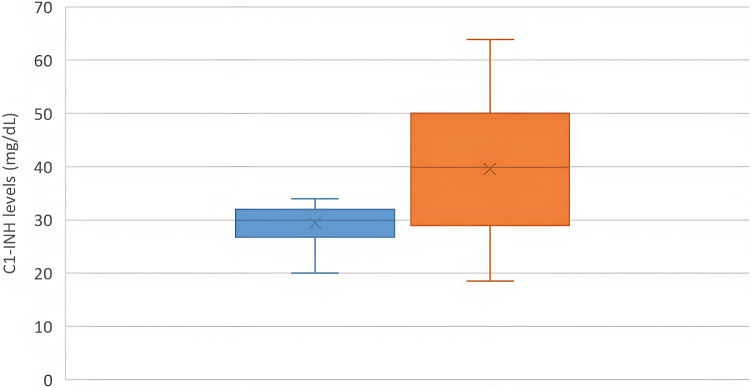
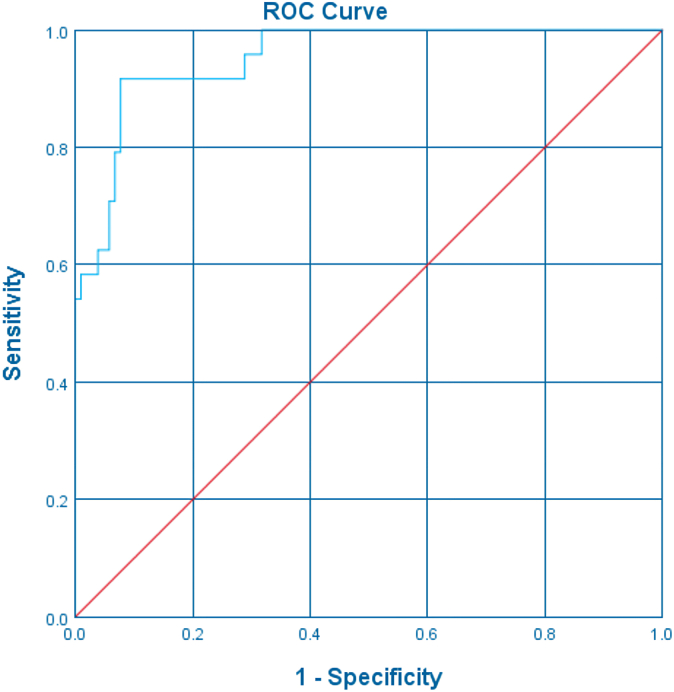
A total of 31 (14 Chinese, 17 Indian) patients with type II HAE and 31 matched controls were analyzed. Median age at the time of enrollment was 40 (range, 5-77) years, and the male-to-female ratio was 1:1.6. Sensitivity of low/absent C4 for HAE was 90.3%. Overall, 77.4% (24 of 31) of patients with type II HAE had elevated C1-INH levels compared with 38.7% (12 of 31) controls (odds ratio, 2.00; 95% CI, 1.34-2.98; P = .017). C1-INH levels in patients with type II HAE were significantly higher than in controls (52.2 ± 20.0 mg/dL vs 29.1 ± 3.6 mg/dL; P < .001) (Fig 1). Findings were consistent when C1-INH levels in the Chinese and Indian subgroups were analyzed separately (Figs 2 and 3). Receiver-operating characteristic curve demonstrated excellent performance for elevated C1-INH levels to diagnose type II HAE with an area under the curve of 0.953 (95% CI, 0.941-0.992; P < .001) (Fig 4). The positive and negative predictive values of combining a low C4 and an elevated C1-INH level for patients with type II HAE in this cohort were 100% and 82.9%, respectively.
Fig 1.
C1-INH levels between patients with type II HAE (orange) and matched controls (blue) (n = 31).
Fig 2.
C1-INH levels between Chinese patients with type II HAE (orange) and matched controls (blue) (n = 14).
Fig 3.
C1-INH levels between Indian patients with type II HAE (orange) and matched controls (blue) (n = 17).
Fig 4.
ROC-curve analysis of C1-INH levels in diagnosing type II HAE. ROC, Receiver-operating characteristic.
While evaluating patients with possible HAE, we remind physicians not to be falsely reassured by nondepressed C1-INH levels and be even more cautious of possible type II HAE when levels exceed the reference range. This may be particularly useful in screening family members with potential presymptomatic HAE. The phenomenon of elevated C1-INH levels among patients with type II HAE had been historically described in 1971 and thereafter has been mentioned in a few reports.5 However, this is the first dedicated study to assess the diagnostic performance of high C1-INH values among patients with type II HAE and demonstrate its difference compared with non-HAE controls. We postulate that this “paradoxical” increase in C1-INH levels among patients with type II HAE may be a compensatory mechanism to overcome dysfunctional or inadequate C1-INH function. Defective C1-INH may have an increased plasma half-life because of its inability to form complexes with proteases.6 However, among patients for whom both C1-INH level and C1-INH function were available, we did not observe any significant correlation between C1-INH function and C1-INH level or disease severity (r = 0.114; P = .711). A minority of patients with type II HAE had normal C1-INH levels, despite repeated sampling, even though other family members with the same pathogenic SERPING1 mutations had elevated C1-INH levels. We postulate that possible epigenetic effects or undiscovered “modifier genes” other than SERPING1 may be involved. The regulation of C1-INH homeostasis, especially among patients with type II HAE, remains to be fully elucidated, and better understanding of this phenomenon may inspire new diagnostic or therapeutic discoveries in the future.
Given its observational nature, this study has several limitations. C1-INH is also an acute-phase reactant. C1-INH levels may increase during acute-phase response, and not all patients had repeat C1-INH testing, especially when abnormal C1-INH function or pathogenic SERPING1 mutations had already been identified. Another limitation is that the matched controls were healthy volunteers. It would be valuable to compare C1-INH values between patients with type II HAE and those with other forms of bradykinin-mediated angioedema (such as angiotensin-converting enzyme–induced angioedema). Furthermore, the overall sample size was relatively small and patients other than Chinese and Indian ethnicities were not included, highlighting the need for future collaborative multiethnic studies.
Analogous to low C4 and presence of rheumatoid factor being colloquially known as the “poor man’s cryo” for screening cryoglobulinemia, we propose that low C4 and presence of elevated C1-INH levels may be considered as a screening tool for type II HAE (or the “poor man’s C1-INH function”).7 This approach would be particularly useful in resource-constrained settings in which performing C1-INH function may not be feasible for every patient. We advise that patients who are screened positive should be prioritized and referred to a facility for confirmatory testing, which may require international collaboration in terms of expertise and facilities sharing.
Significant disparities in health care impose a substantial barrier to HAE diagnosis and management in low-income countries. Because C4 and C1-INH levels are more easily available than C1-INH function or genetic testing, we believe that this approach would be particularly useful in resource-limited countries and hopefully can improve the detection rate of patients with type II HAE in these regions (eg, it is believed that >99% patients with HAE in India remain undiagnosed at present).3,4 HAE prospective studies among network Asian countries using this novel screening approach are underway, highlighting the importance of international collaborative efforts to further improve global HAE care.
Disclosure statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Clinical implications.
Low C4 and presence of elevated C1-INH antigen levels may be considered as a screening tool for type II HAE, especially in countries where C1-INH function testing is not readily available.
References
- 1.Maurer M., Magerl M., Betschel S., Aberer W., Ansotegui I.J., Aygören-Pürsün E., et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77:1961–1990. doi: 10.1111/all.15214. [DOI] [PubMed] [Google Scholar]
- 2.Wong J.C.Y., Chiang V., Lam K., Tung E., Au E.Y.L., Lau C.S., et al. Prospective study on the efficacy and impact of cascade screening and evaluation of hereditary angioedema (CaSE-HAE) J Allergy Clin Immunol Pract. 2022;10:2896–2903.e2892. doi: 10.1016/j.jaip.2022.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Li P.H., Pawankar R., Thong B.Y., Fok J.S., Chantaphakul H., Hide M., et al. Epidemiology, management, and treatment access of hereditary angioedema in the Asia Pacific region: outcomes from an international survey. J Allergy Clin Immunol Pract. 2023;11:1253–1260. doi: 10.1016/j.jaip.2022.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Jindal A.K., Reshef A., Longhurst H., Global Equity in HAE Management Workgroup Mitigating disparity in health-care resources between countries for management of hereditary angioedema. Clin Rev Allergy Immunol. 2021;61:84–97. doi: 10.1007/s12016-021-08854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen F.S., Alper C.A., Pensky J., Klemperer M.R., Donaldson V.H. Genetically determined heterogeneity of the C1 esterase inhibitor in patients with hereditary angioneurotic edema. J Clin Invest. 1971;50:2143–2149. doi: 10.1172/JCI106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prada A.E., Zahedi K., Davis A.E., III Regulation of C1 inhibitor synthesis. Immunobiology. 1998;199:377–388. doi: 10.1016/S0171-2985(98)80042-9. [DOI] [PubMed] [Google Scholar]
- 7.Damoiseaux J., Cohen Tervaert J.W. Diagnostics and treatment of cryoglobulinaemia: it takes two to tango. Clin Rev Allergy Immunol. 2014;47:299–310. doi: 10.1007/s12016-013-8390-y. [DOI] [PubMed] [Google Scholar]