Summary
Haemophagocytic lymphohistiocytosis-like toxicity following chimeric antigen receptor T cells (CAR-HLH) is being increasingly recognized, while published data are limited and criteria for recognition are elusive. We describe three patients who developed CAR-HLH after infusion of brexucabtagene autoleucel (n = 2) or axicabtagene ciloleucel (n = 1). All three patients presented following cytokine release syndrome, with fever, recurrent or worsening cytopenias, hyperferritinaemia, elevated soluble interleukin (IL)-2 receptor, hypofibrinogenaemia, hypertriglyceridaemia, elevated liver transaminases, and decreasing C-reactive protein and IL-6. Clinical improvement following treatment with anakinra (n = 2) and ruxolitinib (n = 1) was observed. Our report offers an opportunity for prompt recognition and initiation of potentially life-saving treatment for CAR-HLH.
Keywords: CAR T cells, delayed hyperinflammatory syndrome, HLH-like syndrome
SHORT REPORT
Systemic inflammatory response, in the form of cytokine release syndrome (CRS), occurs frequently and usually promptly following CD19-directed chimeric antigen receptor (CAR) T-cell infusion.1 There are rare reports of a CRS variant occurring as a severe systemic hyperinflammatory response, delayed after resolution of CRS, resembling haemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome following CAR T-cell infusion (CAR-HLH).2,3 Elucidation of mediators, patterns with diverse CAR T-cell products, evaluation, and treatment strategies are imperative for improved understanding of this underrecognized entity with high mortality. CAR-HLH has been reported following axicabtagene ciloleucel and tisagenlecleucel, as well as investigational CD22- and CD19-directed CAR T cells with 41BBζ stimulation in paediatric/young adult patients.2–5 We report three adult patients who received brexucabtagene autoleucel (two patients) or axicabtagene ciloleucel (one patient) followed by the development of CAR-HLH, and share our treatment approach. We anticipate this to aid in the recognition, evaluation and management of CAR-HLH, and contribute to better defining this entity. The relevant clinical history of the patients is elaborated in Table 1.
TABLE 1.
Patient and CAR-HLH diagnosis and treatment characteristics
| Patient | Age at CAR T/gender | Disease | Lines of therapy prior to CAR-T (response) | Date of CRS onset/ resolution and maximum grade | Treatment for CRS | Date of ICANS onset/resolution and maximum grade | Treatment for ICANS | Day CAR-HLH suspected | Reasons for CAR-HLH suspicion | Treatment of CAR-HLH | Outcome of CAR-HLH | Disease response at day +30 | Last known disease status (time from CAR T) | Last known patient status (time from CAR T) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56/M | MCL | (1) BR for 6 cycles (CR) | Day 0/Day +6 Grade 2 | Toci: 1 dose on day +6 | N/A | N/A | Day +23 | Elevated ferritin (maximum 107 748 ng/ml on day +24); hypofibrinogenaemia beginning day +20; elevated AST/ALT beginning day +22; elevated triglycerides beginning day +20; elevated soluble IL-2 receptor on day +21; persistent neutropenia, anaemia, thrombocytopenia | Steroids: MP 1 g IV on days +23 to +25 | Resolved | CR on day +48 | POD at 8 months post CAR T | Alive at 11 months post CAR T |
| (2) Maintenance rituximab for 3 cycles (POD) | Dex 10 mg IV q6h on day +26 tapered over 9 days | |||||||||||||
| (3) R-M-CHOP for 2 cycles (toxicity) | Anakinra: −200 mg SC TID starting day +24, tapered over 14 days | |||||||||||||
| (4) EAR for 1 cycle (NA) | ||||||||||||||
| (5) NMA AlloBMT (POD) | ||||||||||||||
| (6) Clinical trial of loncastuximab tesirine and ibrutinib for five cycles (fungal pneumonia) (PR) | ||||||||||||||
| (7) R2 for three cycles (POD) | ||||||||||||||
| (8) Venetoclax for four cycles (POD) | ||||||||||||||
| (9) R-DHAX for one cycle (POD) | ||||||||||||||
| 2 | 77/M | WM transformed to DLBCL | (1) BR for six cycles for WM (CR) | Day 0/Day +7 Grade 2 | Toci: 3 doses on day +1, 1 dose on day +2 | Day +5/Day +10 Grade 3 | Steroids: -MP 1 g IV on Days +6 to +11, then tapered over 7 days -Dex taper over 9 days following MP Anakinra: 200 mg SC TID on Days +5 to +12 then tapered over 3 weeks |
Day +7 | Elevated ferritin (maximum 8915 ng/ml on day +8); hypofibrinogenaemia beginning day +7; mildly elevated AST/ ALT throughout entire CAR-T course; elevated triglycerides beginning day +6; elevated soluble IL-2 receptor on day +6; Persistent anaemia and thromboc y topenia |
Steroids:a -MP 1 g IV on days +6 to +11, then tapered over 7 days -Dex taper over 9 days following MP Anakinra:b 200 mg SC TID on days +5 to +12 then tapered over 3 weeks Ruxolitinib: -10 mg PO BID on days +7 t0 + 19 -Held days +20 to +26 for suspected resolution -days +27 to +89, including taper |
Resolved | PR on day +34 | POD on day +64 | Alive at 5.5 months post CAR T |
| (2) R-CHOP for six cycles (POD) | Dex 10 mg IV Q6h on Day +1 to Day +5, when steroids escalated for ICANS | |||||||||||||
| (3) GemOx and XRT for six cycles (POD) | ||||||||||||||
| 3 | 60/M | MCL with CNS disease | (1) R-CHOP for six cycles + IT ARA-C for three doses (CR) (2) BEAM AutoBMT (POD) (3) XRT, Dex (POD) (4) Ibrutinib and venetoclax (rash) (5) Zanubrutinib and venetoclax for one cycle (NA) (6) Zanubrutinib and venetoclax + rituximab & IT MTX for one cycle (POD) (7) Rituximab, Cy, and Pred for one cycle (POD) (8) Zanubrutinib and venetoclax for 1 cycle (POD) (9) R-CVP (bridging therapy) |
Day 0/Day +8 Grade 1 | Toci: 2 doses on day +5, 1 dose on day +6 Dex: 10 mg ×1 on day +5, 10 mg BID on day +6, 10 mg q6h on day +7, then tapered over 14 days (concurrent ICANS) |
Day +6/Day +19 Grade 3 | Steroids: -Dex: 10 mg ×1 on day +5, 10 mg BID on day +6, 10 mg q6h on day +7, then tapered over 14 days -MP 1 g IV on day +15 |
Day +9 | Elevated ferritin (maximum 3872 on day +9); hypofibrinogenaemia beginning day +12; elevated soluble IL-2 on day +16; elevated triglycerides beginning day +16; persistent anaemia and thrombocytopenia | Steroids:a -Dex 10 mg daily from day +9 to +21 (started prior to day +9 for ICANS) -MP 1 g IV on day +15 Anakinra:b -200 mg SC TID on day +11, tapered over 15 days |
Resolved | CR on day +30 | CR at 2 months post CAR T | Alive at 2 months post CAR T |
Case 1
A 56-year-old man received brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma (MCL) with mediastinal and hilar lymphadenopathy. On day 0, he was admitted with grade 1 CRS and treated with antipyretics and cefepime. On day +5, he developed hypoxaemia (grade 2 CRS) and had rapid improvement following one tocilizumab dose. He was discharged on day +8, afebrile, and remained clinically stable until presenting on day +20 with neutropenic fever, hypotension, and hypoxia in the setting of new pancytopenia (neutrophils 120/mm3, haemoglobin 89 g/l, and platelets 5 × 103/μl), ferritin of 27 660 ng/ml, interleukin (IL)-6 8057 pg/ml, C-reactive protein (CRP) 12.5 mg/dl, and fibrinogen 113 mg/dl (Figure 1A). He was treated for grade 2 CRS with two additional doses of tocilizumab on day +21 and day +22 plus one dose of dexamethasone 10 mg intravenously (IV) on day +22, resulting in improvement of fevers, CRP (5.6 mg/dl), and IL-6 (2982 pg/ml). However, ferritin continued to rise with persistent hypofibrinogenaemia, pancytopenia, and elevated transaminases. Therefore, methylprednisolone 1000 mg intravenously (IV) daily was initiated on day +23 due to clinical suspicion for CAR-HLH. Anakinra was started on day +24 for recurring fever, hypoxaemia requiring supplemental oxygen, pancytopenia, rising ferritin (peak 107 748 ng/ml), persistently low fibrinogen (nadir 82 mg/dl despite cryoprecipitate transfusions), hypertriglyceridaemia 884 mg/dl, AST 783 U/l, ALT 188 U/l, and elevated soluble IL-2 receptor (sIL-2R) 15 143 pg/ml (Figure 1A). Anakinra was started at 200 mg subcutaneously (SC) three times daily (TID) resulting in improvement in fever, blood counts, and decline in ferritin level to 49 558 mg/ml on day +25 and 2788 mg/ml on day +31. Corticosteroids and anakinra were tapered over two weeks and ferritin returned to baseline by day +41. Counts improved transiently around days +33 through +35 but subsequently declined with prolonged cytopenias thereafter. A restaging positron emission topography (PET) scan at day +48 showed complete response. However, disease relapse was noted at eight months and the patient is currently undergoing treatment.
FIGURE 1.
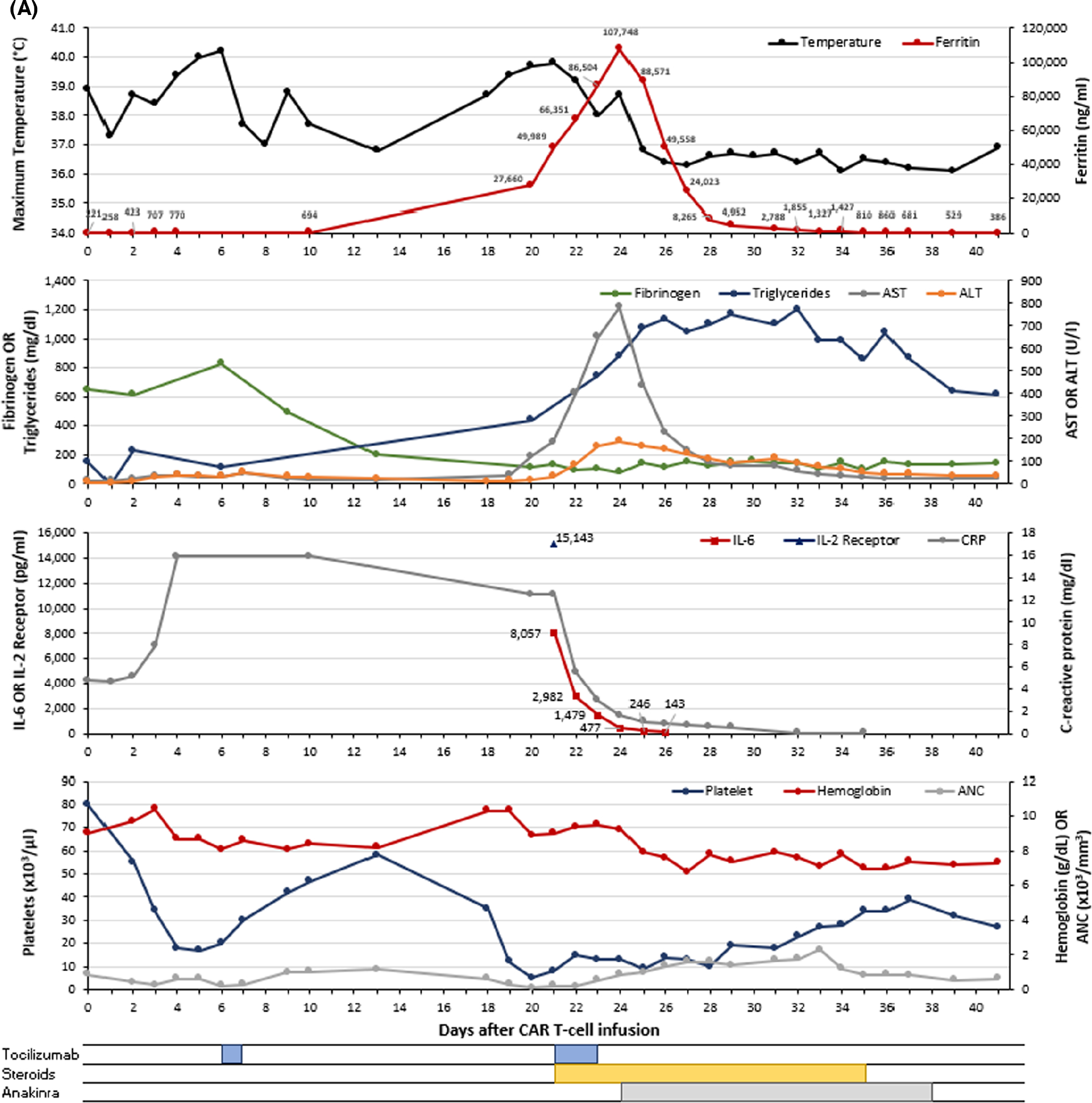
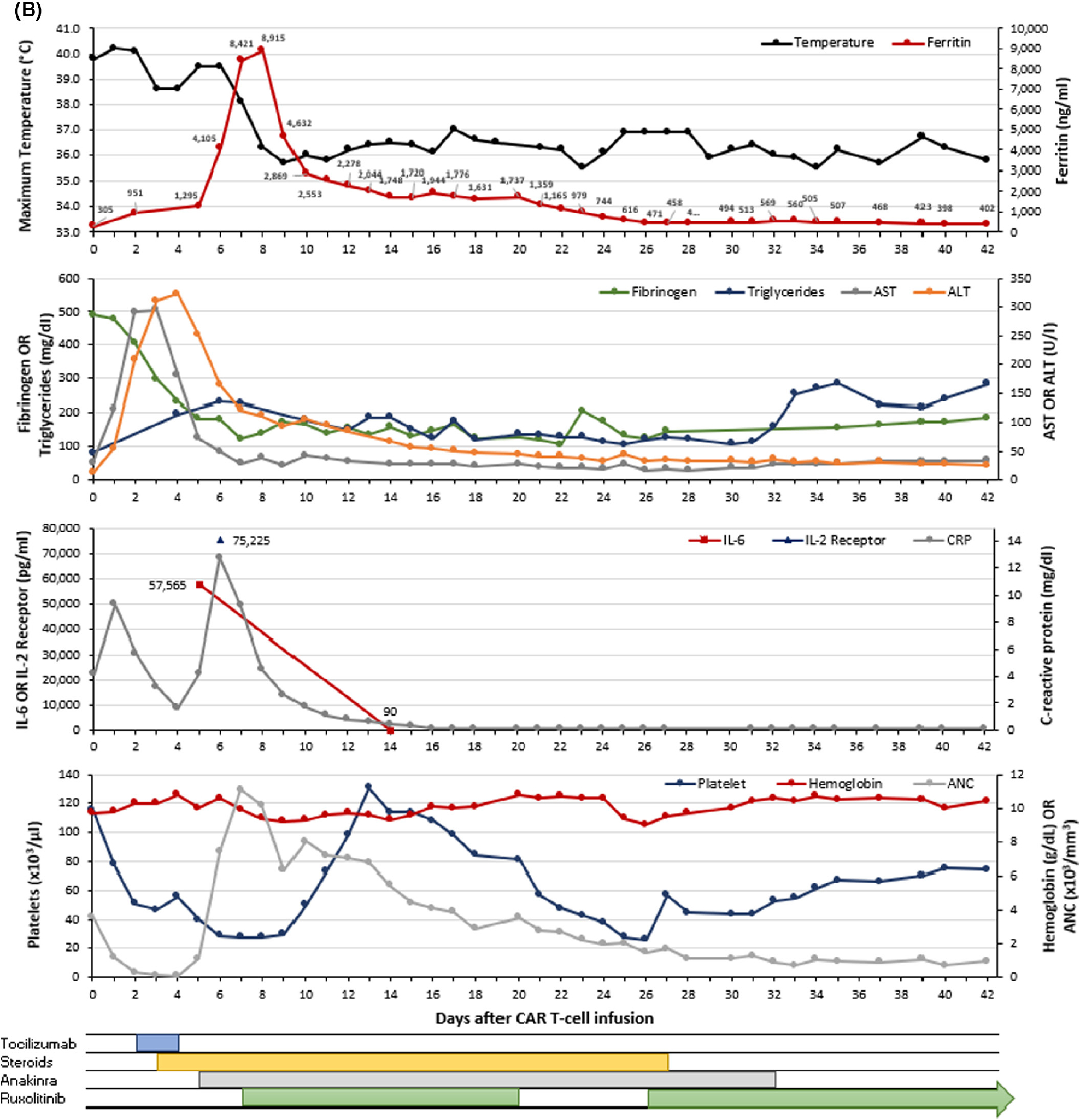
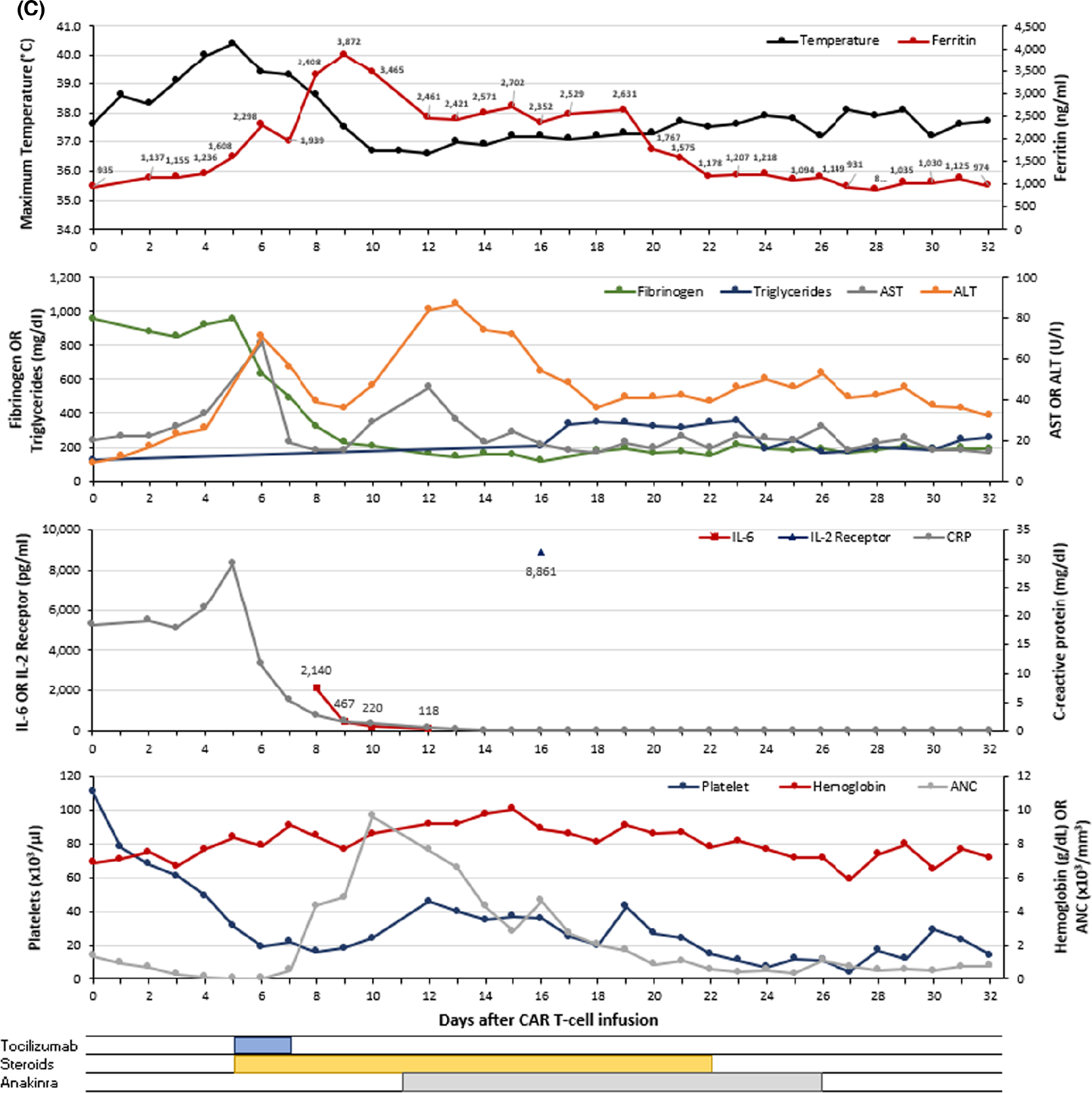
Graphical representation of the clinical course for patients with CAR-HLH after CAR T-cell therapy. Each panel (A–C) represents a unique patient (n = 3). The maximum temperature (black) and ferritin (red) are shown in the first (top) graph for each panel. The second graph presents fibrinogen (green), triglycerides (blue), AST (grey) and ALT (orange). The third graph displays IL-6 (red), soluble IL-2 receptor (blue) and C-reactive protein (grey). The fourth (bottom) graph contains platelet count (blue), haemoglobin (red), and ANC (grey). Therapeutic interventions including tocilizumab, corticosteroids, anakinra, and ruxolitinib are shown below the graphs. Values <0.3 are represented as 0.1 on the graph. ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; CAR, chimaeric antigen receptor; CRP, C-reactive protein; IL-2 receptor, soluble interleukin-2 receptor; IL-6, interleukin-6. [Colour figure can be viewed at wileyonlinelibrary.com]
Case 2
A 77-year-old man with diffuse large B-cell lymphoma of the sinuses transformed from Waldenström’s macroglobulinaemia was treated with axicabtagene ciloleucel for relapsed disease. He presented on day 0 with grade 1 CRS which worsened on day +1 with hypoxaemia, prompting tocilizumab initiation (grade 2). As CRS worsened over days +2 and +3 with new hypotension, dexamethasone 10 mg IV every six hours (q6h) was initiated following the fourth tocilizumab dose. On day +5, he had persistent fevers and a new-onset grade 1 immune effector cell-associated neurotoxicity syndrome (ICANS), which prompted initiation of anakinra 200 mg SC TID. Over the course of days +6 through +9, ICANS worsened to grade 3, fevers and hypoxaemia persisted, cytopenias ensued (platelets 28 × 103/μl, haemoglobin 92 g/l), ferritin increased (peak 8915 ng/ml), CRP (12.8–2.6 mg/dl) and fibrinogen decreased (nadir 122 mg/dl), AST and ALT increased (181 and 251 U/l respectively), and sIL-2R was 75 225 pg/ml (Figure 1B). With the working diagnosis of CAR-HLH, methylprednisolone 1000 mg IV was administered on day +6 followed by ruxolitinib 10 mg twice daily starting day +7. Given persistent hyperferritinaemia, thrombocytopenia, and low fibrinogen, anakinra was tapered over three weeks and ruxolitinib over three months. Blood counts improved by day +60. Restaging imaging demonstrated partial response at day +30, but progressive disease at day +60. Additional treatment is being pursued.
Case 3
A 59-year-old man with relapsed/refractory MCL, with previously treated central nervous system lesions, received brexucabtagene autoleucel and was admitted with grade 1 CRS on day 0. Initial treatment consisted of antipyretics and cefepime. Due to persistent fever and concurrent grade 1 ICANS, tocilizumab and dexamethasone 10 mg IV were given on day +5 followed by intermittent corticosteroids for persistent fevers. CAR-HLH was suspected on day +9 due to persistent low-grade fevers despite antipyretics and steroids, persistent anaemia (haemoglobin 76 g/l), thrombocytopenia (platelets 16 × 103/μl), elevated ferritin (peak 3872 ng/ml), decreasing fibrinogen (nadir 118), rising triglycerides, elevated sIL-2R (8861 pg/ml), lactic acidosis (range 2.5–5.5 mmol/l), in the setting of decreasing CRP and IL-6 (Figure 1C). On day +11, anakinra 200 mg SC TID was started and dexamethasone continued due to CAR-HLH concerns and ICANS worsening to grade 3. Treatment continued until day +25 upon clinical and laboratory improvement. Blood counts showed improvement by day +45. On day +30, a PET scan showed a complete response.
While CAR T cells emerge as a promising therapy for refractory haematological malignancies, inflammation-driven toxicities and cytopenias are well known.1,6,7 HLH-like phenomena have also been reported with an incidence of about 3.48%.8 Four patients who received tisagenlecleucel or SJCAR19 (CD19-directed with 4–1BBζ signalling domain) were reported to have CAR-HLH following CRS-directed therapy and were treated with anakinra before broadening to corticosteroids or ruxolitinib.3 Three patients clinically improved while the fourth died of overwhelming CAR-HLH complications.3 With CD22-directed CAR T-cells, early CRS (median onset of eight days) and a delayed CAR-HLH (median onset of 14 days) were reported in 21 of 59 patients (35.6%), a higher fraction than reported experience with commercial products.2 Sixteen patients received steroids (with/without anakinra), commonly for over two weeks. Nineteen patients had complete resolution of CAR-HLH while one died of infection, and another had CNS haemorrhage with concurrent infection.9 We report CAR-HLH with brexucabtagene ciloleucel, for the first time to our knowledge, and axicabtagene ciloleucel, which has been reported anecdotally. Overall, it appears CAR-HLH can occur with any CAR product. While previously primarily reported with severe grade CRS, our report highlights the possibility with any grade CRS.2,3 Manifestations of HLH can be difficult to distinguish from CRS due to overlapping features of fever and elevated ferritin. However, as noted in our series, rising ferritin in a setting of dropping IL-6 and CRP (suggestive of resolving CRS) should draw suspicion for CAR-HLH. Higher cytokine expression, such as interferon-gamma, IL-8, MIP-1α, and sIL-2R after CRS may substantiate the concern for CAR-HLH.2,8 Elevated IL-1β levels and marrow findings of haemophagocytosis have also been reported.2,4,5 In all patients in our series, IL-1β levels were normal (<3.9 pg/ml) although drawn after anakinra initiation, and marrows were deferred due to clinical stability. The correlation of severe inflammatory toxicity and prolonged cytopenias is well established.6 Accordingly, the mechanism of CAR-HLH is likely related to uncontrolled T-cell activation or exaggerated hyperinflammatory response following CAR T-cell infusion, similar to secondary HLH.
As efforts are underway to define diagnostic criteria for CAR-HLH, our series adds to the emerging literature guiding this recognition. Our small experience favours early initiation of treatment, making prompt recognition all the more critical. Case 1 met criteria published by Neelapu et al. and Lichenstein et al. Case 2 and 3 did not meet criteria mainly based upon the level of hyperferritinaemia, likely due to initiation of treatment which was prompted by clinical instability (Table S1).2,10 This highlights the limitations of proposed criteria for CAR-HLH which vary depending upon the timing of CRS resolution, availability of marrow biopsies, and turn-around time for IL levels. Clinical stability may vary disproportionately to hyperferritinaemia and early treatment may be life-saving without delay pending high levels of ferritin (>100 000 ng/ml). Hence, clinical characteristics and rate of ferritin increase is more informative towards initiation of treatment. Furthermore, the afore-mentioned CAR-HLH criteria do not include sIL-2R which demonstrated high specificity for levels ≥10 000 U/ml in adult HLH.11
The variability of CAR-HLH timing post CAR T-cell infusion in our series is noted. However, all cases occurred after CRS resolution, also reported by Lichenstein et al.2 Hence, while the specific days of CAR-HLH onset may vary, CAR-HLH is a ‘delayed occurrence’ after or during CRS resolution. High disease burden has been associated with increased risk for CRS, but it has not shown to increase risk for CAR-HLH.2 Lichenstein et al. reported tocilizumab use, NK-cell lymphopenia, and robust CAR T-cell and CD8 T-cell expansion as risk factors.2 While all three patients in our series received tocilizumab for CRS, standard CAR T-cell dose was used in all patients. NK-cell counts and CAR T-cell expansion were not measured.
Timely intervention is critical to improving outcomes as CAR-HLH is a serious complication potentially resulting in severe infections, bleeding and death.2,3,5 The temporal variance from CRS itself suggests distinct pathophysiology. While CRS is primarily driven by IL-6,12 these levels were downtrending at the time of CAR-HLH in our series and in the report by Lichenstein et al.2 suggesting an alternative mechanism such as IL-8, IL-18 as noted above, and underscore the unlikely benefit of IL-6-directed therapy with tocilizumab. Similarly, traditional therapies for primary HLH such as etoposide13 are likely unnecessary in CAR-HLH, both due to the underlying aetiology associated with CAR T-cell expansion and potential for CAR T-cell ablation. In our series, IL-1-directed therapy in two patients (anakinra) and JAK–STAT inhibition (ruxolitinib) in one patient appeared to demonstrate clinical improvement. Anakinra, an IL-1 receptor antagonist, in combination with intravenous immunoglobulins or corticosteroids has shown remissions in critically ill patients with HLH, including CAR-HLH.2,3,14 Based on these data, all patients initially received anakinra when CAR-HLH was suspected. The optimal anakinra dosing for CAR-HLH has yet to be elucidated. Given the short half-life, we initiated anakinra TID rather than once daily. In Case 2, anakinra did not resolve CAR-HLH symptoms and hence, ruxolitinib was started. Ruxolitinib has an emerging role in HLH treatment due to inhibition of the JAK–STAT pathway, downstream from inflammatory cytokines in HLH.15 The role of emapalumab, a human anti-interferon-gamma antibody, commercially available for primary HLH, is unclear in the setting of CAR-HLH.16
Our series is limited in sample size, but the consistent patterns substantiate the clinical trend observed with other products and will guide clinical practice for recognition as well as treatment with standard-of-care products.
Supplementary Material
Footnotes
CONFLICT OF INTERESTS
Timothy J. Porter reports consultancy with Medexus Pharmaceuticals. Jamie E. Ziggas reports consultancy with Seagen. Nina Wagner-Johnston has served on advisory boards for Epizyme and Seattle Genetics and receives research funding from ADC Therapeutics, Regeneron and Genentech. Tania Jain reports institutional research support from CTI Biopharma, Incyte and Syneos Health; advisory board participation with Care Dx, Incyte, Abbvie, Kite and Bristol Myers Squibb. All other authors report no conflicts of interest. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, or imply endorsement by the U.S. Government.
CONSENT STATEMENT
Written informed consent was obtained from all three patients for publication of this case series.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25(12):2305–21. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang HW, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138(24):2469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hines MR, Keenan C, Maron Alfaro G, Cheng C, Zhou Y, Sharma A, et al. Hemophagocytic lymphohistiocytosis-like toxicity (car-HLH) after CD19-specific CAR T-cell therapy. Br J Haematol. 2021;194(4):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Major A, Collins J, Craney C, Heitman AK, Bauer E, Zerante E, et al. Management of hemophagocytic lymphohistiocytosis (HLH) associated with chimeric antigen receptor T-cell (CAR-T) therapy using anti-cytokine therapy: an illustrative case and review of the literature. Leuk Lymphoma. 2021;62(7):1765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashmi H, Bachmeier C, Chavez JC, Song J, Hussaini M, Krivenko G, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol. 2019;187(2):e35–8. [DOI] [PubMed] [Google Scholar]
- 6.Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical utilization of chimeric antigen receptor T cells in B cell acute lymphoblastic leukemia: an expert opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(3):e76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandler RD, Tattersall RS, Schoemans H, Greco R, Badoglio M, Labopin M, et al. Diagnosis and management of secondary HLH/MAS following HSCT and CAR-T cell therapy in adults; a review of the literature and a survey of practice within EBMT centres on behalf of the autoimmune diseases working party (ADWP) and transplant complications working party (TCWP). Front Immunol. 2020;11:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masih KE, Ligon JA, Yates B, Shalabi H, Little L, Islam Z, et al. Consequences of hemophagocytic lymphohistiocytosis-like cytokine release syndrome toxicities and concurrent bacteremia. Pediatr Blood and Cancer. 2021;68(10):e29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden A, Lin M, Park S, Pudek M, Schneider M, Jordan MB, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergsten E, Horne A, Aricó M, Astigarraga I, Egeler RM, Filipovich AH, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlfarth P, Agis H, Gualdoni GA, Weber J, Staudinger T, Schellongowski P, et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–31. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A, Merrill SA, Alsawah F, Bockenstedt P, Campagnaro E, Devata S, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-Centre, pilot trial. Lancet Haematol. 2019;6(12):e630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


