Abstract
Background
Metastasizing Ameloblastoma (MA) is an aggressive variant of ameloblastoma (AM) with the ability to metastasize without cytological malignant changes. Thus it aims to comprehensively review the clinico-pathological and prognostic aspects of MA through integration of current literature.
Methods
Electronic searches were conducted in PubMed-MEDLINE, Scopus, Web of Science and Google Scholar. Two independent reviewers screened abstracts and evaluated paper eligibility. AMSTAR2 checklist was used to assessed methodological quality of included systematic reviews (SRs).
Results
From 390 initial papers, 279 underwent eligibility screening, with five systematic reviews (SRs) meeting inclusion criteria. Six hundred sixty-one MA cases were found in five SRs that were included. MA predominantly affects men, exhibits mandible preference, and occurs in individuals in their fourth or fifth decade. Benign metastatic deposits commonly manifest in lungs and lymph nodes. Distant metastasis probability rises with multiple recurrences and incomplete surgical removal. Tumor recurrence and metastasis unfavorably impact clinical outcomes. Quality of evidence assessment was absent across SRs; four SRs were critically low in methodological quality.
Conclusions
AM's metastatic potential lacks predictability. Early/multiple recurrences post-treatment may signal poor prognosis, warranting vigilant follow-up. Methodical analysis of each AM case is imperative to comprehend the metastatic-benign histology relationship.
Keywords: Ameloblastoma, Odontogenic tumor, Meta-analysis, Prognosis
Graphical abstract
Highlights
-
•
Metastasizing ameloblastoma is a rare clinical entity.
-
•
This paper summarizes the comprehensive clinic-pathological analysis of MA.
-
•
It is challenging to forecast the likelihood of developing metastasis in ameloblastoma.
-
•
Tumour metastasis and recurrence were linked to a poor clinical outcome in MA.
1. Introduction
Ameloblastoma (AM) is a benign, locally invasive, intraosseous epithelial odontogenic tumour that exhibits slow, gradual growth.1 Folkson originally characterised this tumour in 1879, while Churchil coined the word "ameloblastoma" in 1933.2 Remains of odontogenic epithelium, odontogenic cyst lining, and overlaying mucosa are thought to constitute the origin of the tumour.3,4 Ameloblastic carcinoma (AC) is the malignant counterpart of AM, and metastasizing ameloblastoma (MA) is the term used to describe an AM that has spread to regional and distant sites. When viewed from a histological perspective, the MA does not exhibit cytological atypia, whereas the AC does, even at the location of distant metastatic spread.5
A systematic review (SR) of systematic reviews (also known as overview of reviews, umbrella review) is an increasingly popular form of evidence synthesis as they are considered to provide tertiary level of evidence as per recent scales of evidence.6 These overviews of SRs7 highlight the evidence from several SRs on a crucial broad topic at a variety of different levels, providing clinicians and decision-makers with a high-quality evidence foundation. The ability to do these overviews more quickly to address a research topic with a requirement for constrained resources is their main benefit.8
Metastasis is one of the distinctive hallmark that separates a malignant tumour from a benign one. The main factor in both morbidity and mortality associated with tumours is metastasis, which frequently results in significant clinical problems. However, while having benign histological characteristics, several benign entities, including benign metastasizing leiomyoma,9 metastasizing pleomorphic adenoma,10 and giant cell tumour of the bone,11 exhibit metastasis. The current investigation focused on MA, which also exhibits benign metastatic deposits. Though not universally, WHO (2017) classed MA as a benign tumour in the new classification of odontogenic tumours.12 With MA being classified as a benign entity, it could be inferred that pathologists have been baffled by MA, and the argument over its precise biological nature is far from settled.
Therefore, it is crucial to examine the peculiar conduct in AM presenting as MA as well as its prognostic ramifications. Research on this uncommon pathology is scarce. The SRs that have been released thus far are inconclusive. In order to better understand the clinicopathological and molecular mechanisms that influence the development of this clinicopathological entity of complex biology, more study with a high level of evidence is required. Therefore, employing appropriate and widely recognized diagnostic criteria, the present overview of systematic reviews aimed to review demographic data, recurrence and metastasis rates, as well as survival prognosis in individuals with MA.
2. Materials and methods
2.1. Study design
The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) [registration number:CRD42023378222]. This registration ensured transparency and minimized the risk of bias in the review process. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to report overview of SRs6 (Table S1) which was not previously published or registered and adhered to the Cochrane Collaboration's guidelines for conducting an overview of reviews.13 The permission of an institutional review board was not deemed necessary given the nature of the current investigation.
An overview of the reviews was carried out to summarize the findings of systematic reviews on the bizarre behaviour in AM presenting as MA along with its prognostic implications using the following PICO elements:
P (Population): AM presenting as MA along with its prognostic implications.
I (Intervention): Not applicable.
C (Comparator): Not applicable.
O (Outcome): Clinico-pathological features and prognosis of metastasizing ameloblastoma.
2.2. Data sources
A detailed automated electronic literature search was conducted to identify the relevant papers on PubMed-MEDLINE, Scopus, Web of Science and Google Scholar without language limitations on 25th July 2023.
2.3. Search strategy
According to the PRESS initiative,13 search words were created and searches were carried out by fusing the free terms and thesaurus terms utilised by the databases (Table S2). We developed a specific keyword search string for each database such as PubMed ((((((Metastatic ameloblastoma) OR (Metastasizing ameloblastoma)) OR (malignant ameloblastoma)) OR (Malignant odontogenic tumours)) AND (systematic review)), Scopus (ALL (metastatic AND ameloblastoma OR metastasizing AND ameloblastoma OR malignant AND ameloblastoma OR malignant AND odontogenic AND tumours OR ameloblastoma) AND (systematic AND review)) and Web of Science ((((((ALL= (Metastasizing ameloblastoma)) OR ALL= (Metastatic ameloblastoma)) OR ALL= (malignant ameloblastoma)) OR ALL= (Malignant odontogenic tumours)) OR ALL= (Ameloblastoma)) AND ALL= (systematic review)). As “Google Scholar” search engine retrieved many results, we only examined the first 100 results based on relevance. Only “Metastasizing ameloblastoma” and “systematic review” keywords were used to search Google Scholar. All of the included papers' references were meticulously hand-searched to find any overlooked pertinent reviews that might have affected their eligibility.
2.4. Eligibility criteria
According to Condition, Context, and Population CoCoPop framework,14 eligible papers were full text articles on SRs with or without meta-analysis (study design), evaluating patients followed up with a diagnosis of AM (population) which later resulted into MA (condition). All relevant papers without restriction (context) on language, date, follow-up periods and country of origin of the study were included. The studies needed to contain enough clinico-histopathological information to confirm the diagnosis. The definitions and criteria of the World Health Classification of Tumours—Head and Neck Tumours book (last updated in 2022), were used to diagnose the tumours. Studies with (i) no defined research question, search strategy or defined process of paper selection, and (ii) topics other than metastasizing ameloblastoma were excluded.
2.5. Selection of studies
Two reviewers (GS and SG) independently evaluated the titles and abstracts of the applicable publications to ascertain their eligibility. Subsequently, the whole texts of all potentially acceptable papers were acquired, and the same reviewers carried out a second independent assessment (GS and SG). After any disagreements were discussed with the third reviewer (SS), a list of the articles to be included in this overview was agreed.
2.6. Data extraction
Full-text papers were screened by two reviewers (GS and SG) independently. From each article, the following data were extracted: journal, publication year, first author, study design, study characteristics such as patient sex and age, diagnostic criteria, primary lesion location (maxilla/mandible), location of metastatic lesions, treatment method (surgery, chemotherapy, radiotherapy, neck dissection), follow-up time, recurrences, and prognosis (death). Any discrepancies were cross-checked by both reviewers during a second review, after which a consensus was reached.
2.7. Risk of bias assessment
Quality assessment of the included SRs was undertaken with AMSTAR2 checklist15 by one reviewer (SG) and cross checked by second (GS) and discrepancies were resolved by discussion.
3. Results
3.1. Results of database search
A total of 390 papers (PubMed-MEDLINE: 65, Scopus: 240, Web of Science: 85) were retrieved from the electronic databases, and six results were found through manual searching. 279 potentially eligible papers were discovered after duplicates were eliminated. Nine papers were deemed to be eligible for full text review after carefully examining their titles and abstracts for eligibility. Of those nine papers, four articles were eliminated for failing to meet the minimal eligibility requirements (Table S3). Finally, the present review contained five SRs16, 17, 18, 19, 20 that satisfied the selection criteria (Fig. 1). There were no further, helpful papers found when the references to these five articles were examined.
Fig. 1.
Prisma flow diagram.
3.2. Review characteristics
Each of the five included SRs was published in different journals namely, Stomatology Oral & maxillofacial Surgery,16 Dental Oral Biology and Craniofacial Research,17 Frontiers in Oral Health,18 International Journal of Oral & maxillofacial Surgery19 and Journal of Oral Pathology & Medicine.20 Table 2 shows the characteristics of the methodological design and clinical criteria used by all five SRs who reported information about MA. The SRs presented in this overview had sample sizes ranged between 14 studies (18 cases of MA) and 312 studies (507 cases of MA) with a total of 477 studies (661 cases of MA). Among five, 2 SRs a priori designed a protocol on the SR methodology and registered in PROSPERO [Yang, Marin] and all five SRs followed PRISMA SRs reporting guidelines.16, 17, 18, 19, 20 In compliance to Cochrane Collaboration criteria, MEDLINE was searched by all five included reviews,16, 17, 18, 19, 20 however Embase was searched in SRs of Yang et al.19 and Marin et al.18 Four SRs applied English language restriction,16,17,19,20 while one SR impose English and Spanish language restriction.18 None of the SR conducted meta-analysis16, 17, 18, 19, 20 and risk of bias analysis was performed by only one SR.19
Table 2.
Characteristics of the studies included in the systematic review.
| Author (Year/Journal) | No. of included studies | Patient (no.) | Study design | Follow-up Period |
Protocol registered (PROSPERO no.) | Systematic review guidelines | Databases searched [Range (years)] | Language accessed | Meta-analysis performed | Risk of bias analysis (Tools) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hosalkar et al. (2020/Stomatology Oral & maxillofacial Surgery)16 | 50 | 65 | Case reports and Case Series | 72–344.3 months | No | PRISMA | PubMed, Science Direct, Cochrane database [Jan 2000–March 2019] |
English | No | No |
| Zambrano & Coyago (2021/Dental Oral Biology and Craniofacial Research)17 | 14 | 18 | Case reports and Case Series | Not available | No | PRISMA | PubMed, Science Direct, Cochrane [2011–2020] |
English | No | No |
| Marin et al. (2021/Frontiers in Oral Health)18 | 312 | 507 | Case reports and Case Series | Not available | PROSPERO CRD42021248757 |
PRISMA | PubMed, Web of Science, Embase, [1946–1974] |
English and Spanish | No | No |
| Yang et al. (2022/International Journal of Oral & maxillofacial Surgery)19 | 24 | 28 | Case reports and Case Series | 5–444 months | PROSPERO CRD42020209981 |
PRISMA | PubMed, Web of Science, Embase, Cochrane [Jan2000-August2020] |
English | No | Yes, (JBI) |
| Chrcanovic et al. (2022/Journal of Oral Pathology & Medicine)20 | 77 | 43 | Case reports and Case Series | 24-554 (224.2 ± 150.8) Months |
No | PRISMA | PubMed, Web of Science, Science Direct, Google Scholar [no time restrictions] |
English | No | No |
JBI: Joanna Briggs Institute; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO: International Prospective Register of Systematic Reviews CRD: Centre for Reviews and Dissemination.
3.3. Demographic and clinical characteristics associated with metastasizing ameloblastoma
Six hundred sixty-one MA cases were found in five SRs that were included. According to our data, MA is more prevalent in men,16,18,20 and the mean age at diagnosis ranges from 31.7 to 46 years. Although just one SR17 offered details, it was obvious that radiolucent/hypodense multiloculated radiographic presentation and/or irregular borders dominated the initial lesion's radiographic appearance. Only 2 SRs17,19 provided information on the histological sub-variant of the initial tumour, with a higher representation of the follicular followed by plexiform subtype. All five SRs reported that the initial tumour originated in the mandible. The lung followed by cervical lymph nodes were the preferred locations for metastasis, and two SRs indicated that there were more distant metastases than local ones.16,17 Furthermore, Yang et al.'s SR reported bilateral lung metastatic involvement. Additionally, it was discovered that mandibular AM metastasized more frequently. In the SRs covered by this analysis, surgical management accounted for the majority of AM treatments, with adjuvant therapies such radiation, chemotherapy, combination therapy, and neck dissection being used. Regarding recurrence of the tumour, a bit variable results were observed with the highest reported in the SR of Chrcanovic et al.20 at 71.1 % and the lowest in the SR of Hosalkar et al. at 24.6 %.16 In their prognosis reports, three SRs noted that 18.4 %–25 % of MA cases were deceased16,19,20 (Table 3).
Table 3.
Descriptive clinical analysis of the included papers.
| Author (Year/Journal) | No. of included studies | Patient (n) | Mean +SD Age (Years) | Sex (n) | Common Site | Histology pattern of the primary tumour | Metastasis (n) | Recurrence | Prognosis | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| Hosalkar et al. (2020/Stomatology Oral & maxillofacial Surgery)16 | 50 | 65 | 45 SD Not available |
M − 42, F-23 | Mandible-61.5 % | Not reported | Distant- 67.7 %, local-32.3 % | 24.6 % | Dead- 18.4 % |
|
| Zambrano & Coyago (2021/Dental Oral Biology and Craniofacial Research)17 | 14 | 18 | 46 SD not available |
M − 9, F-9 |
Mandible-77.8 % | Follicular (46.2 %), Plexiform (30.8 %) |
Distant-14(77.8 %) ocoregional-4(22.2 %) | 44.8 % | Not reported |
|
| Marin et al. (2021/Frontiers in Oral Health)18 | 312 | 507 | 45.8 (4 months–90 years) | M-63 %, F-37 % |
Mandible-74.3 % | Not reported | distant- 40 %, locoregional-16.2 % | 26.8 % | Not reported |
|
| Yang et al. (2022/International Journal of Oral & maxillofacial Surgery)19 | 24 | 28 | 45.3 ± 15.0 | M − 14, F-14 |
Mandible-78.6 % | Follicular (64.3 %) | distatnt-22 cases, Bilateral involvement-78.6 % | 60.7 % | Dead- 21.4 % |
|
| Chrcanovic et al. (2022/Journal of Oral Pathology & Medicine)20 | 77 | 43 | 31.7 ± 14.6 | M − 23, F-18; | Mandible-76.7 %; | Not reported | Distant- 29 cases, locoregional- 11 | 71.1 % | Dead- 25 % |
|
M: Male; F: Female; RoB: Risk of Bias; SD: Standard Deviation.
3.4. Risk of bias of individual studies
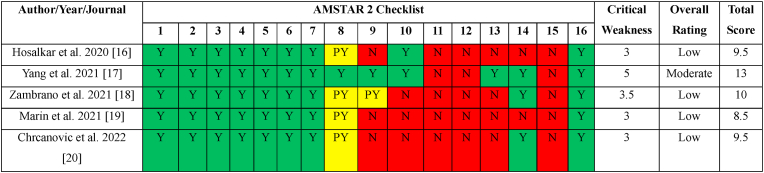
Not all SRs were conducted with the same level of rigour, according to our qualitative analysis utilising the AMSTAR (A Measurement Tool to Assess Systematic Reviews) 2 tool (Table 1). Using the latest AMSTAR2 scoring methodology, four SRs16,17,19,20 received a critically low-quality overall rating, while SR by Yang et al. was the sole SR to receive a moderate overall grade. One SR (19) had a high total score of 13 (out of 16) based on the evaluation of the various items utilising the AMSTAR2 instrument, while the other scores ranged from 8.5 to 10 out of 16 for each of the other SRs respectively.16,17,19,20
Table 1.
Risk of Bias Assessment using AMSTAR2 tool.
Y: Yes; N:No; PY: Partial Yes; AMSTAR: A MeaSurement Tool to Assess systematic Reviews.
3.5. Quality of evidence assessment
Overall, no SR included in this overview of SRs evaluated the strength of the body of evidence except SR by Yang et al.19 Therefore, the reliability of the conclusions drawn from primary-level investigations in these SRs is weak. As a result, there can be a discrepancy between the summary estimations provided by these SRs.
4. Discussion
The WHO acknowledged the significant histological difference between "ameloblastic carcinoma" and "metastasizing (malignant) ameloblastoma" in 2005. The WHO (2005) uses the term "metastasizing ameloblastoma" to define this entity as a histologically benign ameloblastoma that has metastasized,21 despite the nomenclature of "metastasizing (malignant) ameloblastoma" in the International Classification of Diseases for Oncology (ICD-O) code (9310/3). The term "metastasizing ameloblastoma" was used in the present overview of SRs in accordance with the WHO (2005) criteria. According to the current comprehensive analysis, MA are more prevalent in men, have a strong preference for the mandible, and occur in individuals who are typically in their fourth or fifth decade of life. Follicular and plexiform subtypes of primary tumours that metastasized were found to be the most prevalent histological subtypes; the lung was the preferred site of metastasis, followed by cervical lymph nodes. Despite the study's limitations, this information supports the notion that MA are unique clinicopathologic entities.
SRs and meta-analysis offer high-quality evidence but demand significant time and effort, making them suitable for specific cases.22 Out of five SRs, only two were PROSPERO registered and all reviewed SRs were published within a concise two-year span, advocating for transparency to reduce research waste and benefit other researchers' understanding of SR methodology.23 Enhancing the likelihood of obtaining pertinent data and minimizing reporting biases entails searching various electronic databases. Additional papers may surface through perusing initial search article references and exploring gray literature, especially when unindexed in bibliographic databases. Zambrano et al.'s SR17 limited database scanning, likely explaining their limited findings. Meanwhile, Yang et al.19 and Marin et al.18 searched gray literature but only in English or English & Spanish, respectively.
All five of the SRs covered in this overview included mainly case reports and case series and Chrcanovic et al. additionally included clinical trials, cohort studies, case-control and cross-sectional studies.20 Since there is a dearth of large-scale studies that provide comprehensive information on MA, we believe that an SR of case reports and case series with well-defined specific features and potential prognostic factors would provide appropriate support for the subjects mentioned in this article.
As it possesses traits of both the benign odontogenic tumour AM and its malignant counterpart AC, MA is an ambiguous odontogenic tumour. Uncertainty exists regarding the underlying process that causes a benign tumour like AM to metastasis. Its formation is attributed by many authors to tumour spillage or inadequate removal during main or recurrent therapy. Based on research on benign tumours with comparable behaviour, such as benign pleomorphic adenoma or benign fibrous cutaneous metastasizing histiocytoma, it is hypothesised that incomplete removal of the primary tumour or a history of multiple surgical interventions for recurrent tumour favour haematogenous spread of tumour cells with subsequent metastasis.9,11
Similar results imply that subjects with numerous recurrences are more likely to develop metastasis in benign fibrous cutaneous metastasizing histiocytoma and leiomyoma.9,11,24 Others disagreed with this theory, however, as distant foci of benign cancer cells are typically eliminated by the natural immune mechanisms.25 In his review on malignant odontogenic tumours, Praetorius makes the following suggestions as potential clinical predictors of metastasis: the size and evolution of the primary tumour, mandibular location, repeated and incomplete surgical interventions, or a primary tumour treated with chemotherapy and radiotherapy.26 The fundamental mechanisms by which each of these factors affects the pathogenesis of MA have not yet been fully understood.
The findings of the current comprehensive analysis revealed that MA are more prevalent in men and are present in patients who are typically in their fourth or fifth decade of life. Though, according to Zambrano et al. in their SR, there are no variations based on sex in the likelihood of identifying an MA when the main tumour was found before the age of 30.17 According to the clinical characteristics of the initial tumour, the mandible was more frequently involved in the current umbrella review. In fact, earlier research points to the mandible as a potential indicator of MA.26 The plexiform or mixed (follicular/plexiform) variety typically demonstrates a higher chance of metastasis with regard to histological subtype, according to prior research.27 The most common histological subtype in the current overview was follicular, which was followed by plexiform.17,19 The follicular variety, according to Pandiar et al., histologically exhibits an abundance of bud-like structures and a development pattern resembling odontogenesis just before morphodifferentiation. They also proposed the idea that these entities might separate and implant in different places during surgery.28
Distant metastases were more frequently seen than locoregional metastases across the SRs that make up the current review. About two thirds of patients who experienced distant metastasis had their most common organ affected being the lungs. The lungs are the most typical site of metastasis, accounting for 72.7 % of patients, according to earlier MA literature.29 There have also been reports of other regions, including the kidneys, pelvis, and brain. The majority of the cases included in the SRs got a conservative surgical approach in the management of their initial AM with regard to the treatment plan with which the main tumour was handled. Additionally, a lot of the recurring cases had a history of surgical therapy of AM and presented with metastases. This shows that it is impossible to rule out the possibility of partial removal or tumour leakage during the treatment of AM that causes MA. Given the recorded cases of MA at many sites, including the bone, liver, brain, skin, lung, and lymph nodes, the route of cancer cells' dissemination in an AM is not totally known, but, according to Laughlin, the predominant route of dissemination in an AM would be the haematogenous route.30 Additionally, Hosalkar et al. discovered in their SR that recurrence in MA was significantly linked with a poor clinical outcome (P 0.05).16 Unfortunately, there are currently no reliable methods for determining how, when, and which cases of AM may metastasize. A few clinical markers, such as a large primary tumour, fast local invasion, a protracted clinical course, inadequate primary surgical excision, and multiple recurrences, however, indicate a higher likelihood of metastasis in AM. Therefore, we firmly feel that periodic chest imaging screening for AM should be a component of routine follow-up, particularly in cases where there has been a history of local recurrences. The information shows that making aggressive decisions and managing AM meticulously with enough margins may be crucial in preventing MA.
Zambrano et al.17 showed primary tumour positivity for non-MAPK pathway genes such p63 and high expression for Ki67 with regard to the molecular characteristics linked with MA. This is imilar to a review by Ganjre al.31 which reported that these genes were found to be present in few MA cases. Additionally, Zambrano et al. hypothesised that a primary tumor's association with positive for non-MAPK pathway genes increases the likelihood of identifying an AM with distant metastases.17 However, there is yet no solid proof that immunostaining is more effective than histology alone. Instead, for diagnosis, morphology, immunostaining, and cytogenetic methods should work in concert, particularly when the pace of cell proliferation and the presence of cell variations are unclear.
Improving the quality of individual studies on MA is essential to contribute to a stronger evidence base that will improve the quality of future SRs. The quality of such studies can be improved with prospective study designs, defining clear study objectives, large sample size, establishing clear and comprehensive inclusion criteria, adequate follow-up durations and standardized data collection. Nonetheless it is difficulty to achieve these parameters due to rarity of the MA.
Due to its limitations, the conclusions of this overview should be interpreted with care. First, because all of the included studies were retrospective reports with insufficient records, they were inevitably flawed. The statistical analyses could have been of higher quality if it had been possible to retrieve data for all variables from all cases. Second, the follow-up period was frequently brief in the published cases, which may have caused the real survival rate to be underestimated. So, in order to better predict the biological behaviour and prognosis of an AM, we think that a thorough anamnesis and a thorough description of the primary tumour in each case are necessary. The presence of primary studies that may have been duplicated throughout included reviews is another key limitation of this review. The current overview of SRs demonstrates robustness despite its limitations because of the comprehensive study design, exhaustive search strategy, strict eligibility criteria, and in-depth analysis used to assist remove selection bias. The biological behaviour and prognosis of instances presenting with this puzzling odontogenic tumour were also the subject of critically crucial interpretations.
5. Conclusion
The scarcity of large-scale studies on MA due to its rarity poses a challenge in obtaining comprehensive information. Many published cases featured short follow-up periods, potentially leading to an underestimation of the true survival rate. Recent research highlights the difficulty in predicting MA development based solely on clinical presentation or histological criteria. Instead, early or multiple recurrences following AM treatment could serve as prognostic indicators, warranting vigilant follow-up.
Given the high recurrence rate associated with AM, conservative surgical treatments should be favoured over this approach. Despite benign cytological characteristics, tumor metastasis and recurrence correlate with poor clinical outcomes in MA. Therefore, a systematic analysis of each AM case is essential to explore the relationship between metastasis and benign histology. The included studies exhibited significant methodological limitations, underscoring the need for more robust systematic reviews (SRs) in the future to gain a deeper understanding of MA and inform improved therapeutic management.
Funding source
None declared.
Declaration of competing interest
All the author associated with present manuscript declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2023.10.006.
Contributor Information
Gargi Sarode, Email: gargi14@gmail.com.
Shailesh M. Gondivkar, Email: shailesh_gondivkar@yahoo.com.
Akanksha Gore, Email: akankshagore08@gmail.com.
Rahul Anand, Email: rahul.anand303@gmail.com.
Namrata Sengupta, Email: dr.namrata.sengupta@gmail.com.
Vini Mehta, Email: vini.mehta@dpu.edu.in.
Sachin C. Sarode, Email: drsachinsarode@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.WHO Classification of Tumors Editorial Board . fifth ed. Vol. 9. International Agency for Research on Cancer; 2022. Head and Neck Tumours. [Google Scholar]
- 2.Churchill H. Histological differentiation between certain dentigerous cysts and ameloblastoma. Dent Cosmet. 1934;76:1173e1178. [Google Scholar]
- 3.Zwahlen R.A., Gratz K.W. Maxillary ameloblastomas: a review of the literature and of a 15-year database. J Cranio-Maxillo-Fac Surg. 2002;30(5):273–279. doi: 10.1016/s1010-5182(02)90317-3. PMID 12377199. [DOI] [PubMed] [Google Scholar]
- 4.Masthan K.M., Anitha N., Krupaa J., Manikkam S. Ameloblastoma. J Pharm Bioallied Sci. 2015;7(Suppl 1):S167–S170. doi: 10.4103/0975-7406.155891. S167eS170. PMID 26015700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dam S.D., Unni K.K., Keller E.E. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg. 2010;68(12):2962–2974. doi: 10.1016/j.joms.2010.05.084. PMID 20970910. [DOI] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul 21;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker L., Oxman A.D. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P., Oxford G.S., editors. The Cochrane Collaboration; 2009. Overviews of reviews; p. 2009. [Google Scholar]
- 8.Caird J., Sutcliffe K., Kwan I., Dickson K., Thomas J. Mediating policy-relevant evidence at speed: are systematic reviews of systematic reviews a useful approach? Evid Policy. 2015;11(1):81–97. doi: 10.1332/174426514X13988609036850. [DOI] [Google Scholar]
- 9.Barnas E., Ksiazek M., Ras R., Skret A., Magierlo J.S., Gajzlerska E.D. Benign metastisizing leiomyoma: a review of current literature in respect to the time and type of previous gyenecological surgery. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yayan J. Increased risk of Lng metastases in patients with giant cell bone tumors: a systematic review. Adv Exp Med Biol. 2019;1176:1–17. doi: 10.1007/5584_2019_372. PMID 30989587. [DOI] [PubMed] [Google Scholar]
- 11.Knight J., Ratnasingham K. Metastasising pleomorphic adenoma: systematic review. Int J Surg. 2015;19:137–145. doi: 10.1016/j.ijsu.2015.04.084. PMID 25958295. [DOI] [PubMed] [Google Scholar]
- 12.Speight P.M., Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472(3):331–339. doi: 10.1007/s00428-017-2182-3. PMID 28674741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. PMID 27005575. [DOI] [PubMed] [Google Scholar]
- 14.Aromataris E., Munn Z. JBI; 2020. JBI Manual for Evidence Synthesis. [Google Scholar]
- 15.Shea B.J., Reeves B.C., Wells G., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. PMID 28935701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosalkar R., Saluja T.S., Swain N., Singh S.K. Prognostic evaluation of metastasizing ameloblastoma: a systematic review of reported cases in literature. J Stomatol Oral Maxillofac Surg. 2021;122(2):192–198. doi: 10.1016/j.jormas.2020.07.001. PMID 32659412. [DOI] [PubMed] [Google Scholar]
- 17.Zambrano J.F.B., Coyago M.L.D. Metastasizing ameloblastoma: a systematic review in search of clinicopathological predictors. Dent Oral Biol Craniofac Res. 2021;4:3–10. [Google Scholar]
- 18.Marin C., Dave M., Hunter K.D. Malignant odontogenic tumours: a systematic review of cases reported in literature. Front Oral Health. 2021;2 doi: 10.3389/froh.2021.775707. PMID 35048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Zhou K., Tao Y., Ge S., Shang W., Song K. Treatment efficacy and prognosis of pulmonary metastasizing ameloblastoma: a systematic review. Int J Oral Maxillofac Surg. 2022;51(5):579–590. doi: 10.1016/j.ijom.2021.07.016. PMID 34462177. [DOI] [PubMed] [Google Scholar]
- 20.Chrcanovic B.R., Martins-Chaves R.R., Pontes F.S.C., Fonseca F.P., Gomez R.S., Pontes H.A.R. Comparison of survival outcomes between ameloblastic carcinoma and metastasizing ameloblastoma: a systematic review. J Oral Pathol Med. 2022;51(7):603–610. doi: 10.1111/jop.13334. PMID 35822408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biernat W. World Health Organization Classification of Tumours; Lyon: 2005. Pathology and Genetics: Head and Neck Tumours; p. 284. [Google Scholar]
- 22.Garner P., Hopewell S., Chandler J., et al. When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354:i3507. doi: 10.1136/bmj.i3507. PMID 27443385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Booth A., Stewart L. How to reduce unnecessary duplication: use PROSPERO. BJOG. 2014;121(7):784–786. doi: 10.1111/1471-0528.12657. PMID 24629162. [DOI] [PubMed] [Google Scholar]
- 24.Doyle L.A., Fletcher C.D.M. Metastasizing ”benign” cutaneous fibrous histiocytoma: a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2013;37(4):484–495. doi: 10.1097/PAS.0b013e31827070d4. PMID 23426120. [DOI] [PubMed] [Google Scholar]
- 25.Houston G., Davenport W., Keaton W., Harris S. Malignant (metastatic) ameloblastoma: report of a case. J Oral Maxillofac Surg. 1993;51(10):1152–1155. doi: 10.1016/s0278-2391(10)80458-6. discussion 1156. PMID 8410456. [DOI] [PubMed] [Google Scholar]
- 26.Praetorius F. 2017. Malignant Odontogenic Tumours. Surg Pathol Head Neck Odontogenic Tumours. New York. [Google Scholar]
- 27.Kunze E., Donath K., Luhr H.G., Engelhardt W., De Vivie R. Biology of metastasizing ameloblastoma. Pathol Res Pract. 1985;180(5):526–535. doi: 10.1016/S0344-0338(85)80017-0. PMID 4080638. [DOI] [PubMed] [Google Scholar]
- 28.Pandiar D., Anand R., Kamboj M., Narwal A., Shameena P.M., Devi A. Metastasizing ameloblastoma: a 10 year clinicopathological review with an insight into pathogenesis. Head Neck Pathol. 2021;15(3):967–974. doi: 10.1007/s12105-020-01258-5. PMID 33394372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y., He J.F., Li Z.Y., Liu J.H. Ameloblastoma with varied sites of metastasis: report of two cases and literature review. J Cranio-Maxillo-Fac Surg. 2014;42(5):e301–e304. doi: 10.1016/j.jcms.2013.10.010. PMID 24280106. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin E.H. Metastasizing ameloblastoma. Cancer. 1989;64(3):776–780. doi: 10.1002/1097-0142(19890801)64:3<776::aid-cncr2820640335>3.0.co;2-8. PMID 2663133. [DOI] [PubMed] [Google Scholar]
- 31.Ganjre A.P., Sarode G., Sarode S. Molecular characterization of metastasizing ameloblastoma: a comprehensive review. J Cancer Res Therapeut. 2019;15(3):455–462. doi: 10.4103/jcrt.JCRT_268_17. PMID 31169204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.