Abstract
To evaluate the persistence of PCR-detectable Bacteroides distasonis in surface water, whole human feces were dispersed into water from the Ohio River and incubated in flasks in the laboratory or in diffusion chambers in situ. Duplicate samples were taken daily, and material that pelleted at 16,000 × g was assayed by PCR. Persistence of PCR-detectable DNA from this anaerobe depended upon temperature and predation, two of the factors shown by others to influence the survival of aerobic bacteria detected by culture. B. distasonis was detected by PCR for at least 2 weeks at 4°C but for only 4 to 5 days at 14°C, 1 to 2 days at 24°C, and 1 day at 30°C. In filtered water or in the presence of cycloheximide, a eukaryotic inhibitor, persistence at 24°C was extended by at least a week.
Because fecal coliform bacteria can survive and grow in some surface waters (1, 12), their presence cannot be used to pinpoint the source of fecal pollution. It has been suggested that enteric anaerobes, such as the Bacteroides spp., might be better indicators because they survive for only a few hours in oxygenated waters (3, 13). However, the need to maintain anaerobic conditions during cultivation and identification by classical methods discourages the use of anaerobes for routine monitoring. An assay based on specific nucleic acid detection would circumvent the need to cultivate. Recently, I reported PCR-based assays to detect three of the dominant Bacteroides species from the human colon and suggested that this technique might be used to monitor fecal pollution in water (7). However, because PCR can detect DNA in dead bacteria, as well as in living and severely stressed organisms (6), the persistence of PCR-detectable bacteria in the environment must be evaluated.
To investigate the persistence of PCR-detectable Bacteroides distasonis in surface water, whole human feces were dispersed in water collected from the Ohio River and incubated in situ or in the laboratory. Feces, rather than a pure culture, were used as the source of B. distasonis because fecal organisms are the intended target. Fecal bacteria are likely to differ physiologically from cultured organisms, and other fecal components may affect persistence. Preliminary experiments showed that 10 μg of feces/ml of river water was needed for strong detection of B. distasonis with 1-ml samples, 10% of which was analyzed by PCR. This level was at least 10-fold greater than the detection limit, defined as the level at which B. distasonis was detected consistently. In previous experiments, endogenous B. distasonis was detected consistently in Ohio River water by PCR (Fig. 2 of reference 8; data not shown). However, in the present study, 200-fold less river water was analyzed, and as a result, this background level was below the detection limit.
To add feces to river water, 1 g of fresh feces was dispersed thoroughly in 100 ml of sterile deionized water in the laboratory, and 1 ml of the dispersed feces was diluted further in 1 liter of river water at the collection site. Duplicate 30-ml portions of the diluted feces in river water were incubated in the Ohio River, within 1 m of the surface, in diffusion chambers with 0.45-μm-pore-size filters (Technical Services, College of Engineering, Montana State University, Bozeman; described in reference 11). A second set of duplicate 30-ml portions were incubated in sterile 250-ml Erlenmeyer flasks in the laboratory. One-milliliter samples were taken daily. Samples from the diffusion chambers were removed with 5-cm3 syringes after water was first drawn up and down the syringe five times to mix the contents of the chamber. Samples from the Erlenmeyer flasks were removed with 5-ml serological pipettes in a similar manner. All samples were concentrated at 16,000 × g in a microcentrifuge at 4°C for 10 min, the liquid supernatant was removed with drawn Pasteur pipettes, and the pellets were stored at −70°C until analysis. For analysis, pellets were resuspended in 50 μl of 10 mM Tris-HCl–0.1 mM EDTA (pH 8.0), and 5 μl of the suspension (which contained 1 μg of feces) was added directly to 45 μl of PCR reagent. PCR and hybridization conditions were as described previously (7), except that bovine serum albumin was included in the PCR (400 ng/μl) to relieve inhibition (8). Before reuse, diffusion chambers were soaked for approximately 10 min in 10% bleach, rinsed five or six times, and autoclaved.
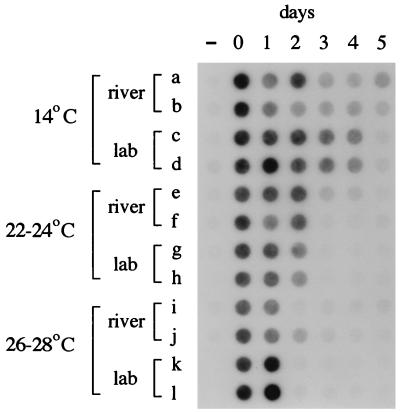
In the first experiment, conducted at the end of April 1995, B. distasonis was still detected by PCR after 5 days of incubation in the river (Fig. 1, rows a and b) but was no longer detected after 2 days in the laboratory (data not shown but similar to Fig. 1, rows g and h). It was noted, however, that the surface temperature of the Ohio River was 14°C throughout the experiment, while the room temperature in the laboratory was 24°C. Therefore, the laboratory incubations were repeated at 14°C with freshly collected feces and Ohio River water while the river was still at 14°C. As shown in Fig. 1 (rows c and d), B. distasonis was detected by PCR for 4 days in the laboratory at 14°C. Similar experiments were run in June and July, except that incubations in the river and in the laboratory were run concurrently at the same temperatures (22 to 24 and 26 to 28°C, respectively). For these, the river and laboratory results were essentially the same. Detectable B. distasonis persisted for only 2 days at 22 to 24°C (Fig. 1, rows e to h) and 1 day at 26 to 28°C (Fig. 1, rows i to l). Therefore, PCR-detectable B. distasonis persisted at least twice as long during the spring at 14°C than during the summer at 22 to 28°C (>/=4 days versus 1 to 2 days). The same results were obtained when PCR primers and a hybridization probe for B. vulgatus (7) were used to assay the samples (data not shown).
FIG. 1.
Seasonal variation in persistence in situ and in the laboratory. Feces were dispersed in raw river water, duplicate 30-ml portions were incubated in diffusion chambers in the river or in flasks in the laboratory, and daily samples were assayed for the presence of B. distasonis by PCR and dot blot hybridization as described in the text. Samples were taken before addition of feces (−), immediately after addition of feces (day 0), and after incubation for 1 to 5 days. Experiments ran from 24 to 29 April (rows a and b), from 5 to 10 May (rows c and d), from 6 to 11 June (rows e to h), and from 6 to 13 July (rows i to l), all in 1995. Surface water temperatures in the river were 14, 14, 22 to 24, and 26 to 28°C, respectively. Rows a, b, e, f, i, and j are samples taken from diffusion chambers incubated in the river. Rows c, d, g, h, k, and l are samples from flasks incubated in the laboratory at the indicated temperatures.
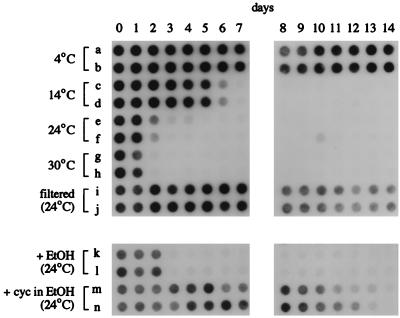
To verify that temperature influences the persistence of PCR-detectable Bacteroides, feces were dispersed in Ohio River water collected in early May 1995, when the surface temperature was 14°C, and again in mid-July 1995, when the surface temperature was 30°C. On both occasions, the water containing feces was incubated at 4, 14, 24, and 30°C in the laboratory. Results of the July experiment are shown in Fig. 2; those for May were very similar. While at 4°C, PCR-detectable B. distasonis persisted for the full 14 days of the experiment (Fig. 2, rows a and b), B. distasonis was detected for only 5 to 6 days at 14°C (rows c and d), for 1 to 2 days at 24°C (rows e and f), and for 1 day at 30°C (rows g and h). In May, B. distasonis was detected for 1 day longer at 14°C than shown for the July experiment. Therefore, persistence was inversely related to incubation temperature and varied over 10-fold between 4 and 30°C, regardless of whether the source water temperature was at 14 or 30°C.
FIG. 2.
Effect of temperature, filtration, or cycloheximide (cyc) addition on persistence of PCR-detectable bacteria. Feces were dispersed in river water, duplicate 50-ml portions were incubated in flasks in the laboratory, and samples, collected daily, were assayed for the presence of B. distasonis by PCR and dot blot hybridization as described in the text. Samples were taken immediately after addition of feces (day 0) and after incubation for 1 to 14 days at the indicated temperatures. For rows a to h and k to n, raw river water was used. For rows i and j, river water was filtered through a 0.45-μm filter with a 500-ml Millipore filtration unit before addition of feces. For rows k and l, 0.5 ml of ethanol (EtOH) and for rows m and n, 0.5 ml of 25-mg/ml cycloheximide in ethanol was added per 50 ml of river water before addition of feces.
While temperature strongly influences the rate of degradative processes, it also affects the activity of predators. To determine if predation played a role in the loss of PCR-detectable Bacteroides, Ohio River water was filtered to remove organisms larger than 0.45 μm before feces were added to one pair of flasks, and cycloheximide (250 μg/ml; reference 15) was added to raw water to inhibit eukaryotes before addition of feces to a second pair of flasks. As shown in Fig. 2 (rows i and j), B. distasonis was detected for at least 14 days in filtered river water at 24°C, at least 12 days longer than in raw water. Similarly, B. distasonis was detected for at least 10 to 12 days in raw water containing cycloheximide (which had been dissolved in absolute ethanol; Fig. 2, rows m and n), over a week longer than in raw water containing solvent only (ethanol; Fig. 2, rows k and l). Therefore, elimination of PCR-detectable B. distasonis was due, at least in part, to predation by eukaryotes. Since cycloheximide does not completely inhibit all eukaryotes (14, 16, 17), the difference between persistence of PCR-detectable Bacteroides in filtered versus cycloheximide-treated water may be due to residual grazing by protists, as well as to the activity of prokaryotes and, perhaps, to interaction with materials larger than 0.45 μm.
The results of this study show that persistence of PCR-detectable DNA from an anaerobic bacterium, B. distasonis, is influenced by temperature and predation, as has been reported for survival of aerobic bacteria detected by culture (4, 5, 10, 12, 15). Although PCR detects dead as well as living bacteria, while culture does not, this distinction is less important at temperatures at which predators are active and eliminate both targets. Other investigators have also reported rapid loss of PCR-detectable bacteria introduced into natural waters collected during summer months (2, 9). However, at low temperatures, at which predators and degradative processes are less active, the PCR target is preserved for an extended time. As a consequence, seasonal variation in persistence must be considered, as with traditional monitoring approaches.
Acknowledgments
I thank Robert Safferman of the U.S. Environmental Protection Agency for encouraging and supporting these studies.
This research was supported in part by an appointment to the Postgraduate Research Participation Program administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. DOE and the U.S. EPA.
REFERENCES
- 1.Carrillo M, Estrada E, Hazen T C. Survival and enumeration of the fecal indicators Bifidobacterium adolescentis and Escherichia coli in a tropical rain forest watershed. Appl Environ Microbiol. 1985;50:468–476. doi: 10.1128/aem.50.2.468-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupray E, Caprais M P, Derrien A, Fach P. Salmonella DNA persistence in natural seawaters using PCR analysis. J Appl Microbiol. 1997;82:507–510. doi: 10.1046/j.1365-2672.1997.00143.x. [DOI] [PubMed] [Google Scholar]
- 3.Fiksdal L, Maki J S, LaCroix S J, Staley J T. Survival and detection of Bacteroides spp., prospective indicator bacteria. Appl Environ Microbiol. 1985;49:148–150. doi: 10.1128/aem.49.1.148-150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez J M, Iriberri J, Egea L, Barcina I. Characterization of culturability, protistan grazing, and death of enteric bacteria in aquatic ecosystems. Appl Environ Microbiol. 1992;58:998–1004. doi: 10.1128/aem.58.3.998-1004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurijala K R, Alexander M. Explanation for the decline of bacteria introduced into lake water. Microb Ecol. 1990;20:231–244. doi: 10.1007/BF02543879. [DOI] [PubMed] [Google Scholar]
- 6.Josephson K L, Gerba C P, Pepper I L. Polymerase chain reaction detection of nonviable bacterial pathogens. Appl Environ Microbiol. 1993;59:3513–3515. doi: 10.1128/aem.59.10.3513-3515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreader C A. Design and evaluation of Bacteroides DNA probes for the specific detection of human fecal pollution. Appl Environ Microbiol. 1995;61:1171–1179. doi: 10.1128/aem.61.4.1171-1179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreader C A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol. 1996;62:1102–1106. doi: 10.1128/aem.62.3.1102-1106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leser T D, Boye M, Hendriksen N B. Survival and activity of Pseudomonas sp. strain B13(FR1) in a marine microcosm determined by quantitative PCR and an rRNA-targeting probe and its effect on the indigenous bacterioplankton. Appl Environ Microbiol. 1995;61:1201–1207. doi: 10.1128/aem.61.4.1201-1207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCambridge J, McMeekin T A. Protozoan predation of Escherichia coli in estuarine waters. Water Res. 1979;13:659–663. [Google Scholar]
- 11.McFeters G A, Stuart D G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972;24:805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes M W, Kator H. Survival of Escherichia coli and Salmonella spp. in estuarine environments. Appl Environ Microbiol. 1988;54:2902–2907. doi: 10.1128/aem.54.12.2902-2907.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfe R D, Hentges D J, Barrett J T, Campbell B J. Oxygen tolerance of human intestinal anaerobes. Am J Clin Nutr. 1977;30:1762–1769. doi: 10.1093/ajcn/30.11.1762. [DOI] [PubMed] [Google Scholar]
- 14.Sanders R W, Porter K G. Use of metabolic inhibitors to estimate protozooplankton grazing and bacterial production in a monomictic eutrophic lake with an anaerobic hypolimnion. Appl Environ Microbiol. 1986;52:101–107. doi: 10.1128/aem.52.1.101-107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheuerman P R, Schmidt J P, Alexander M. Factors affecting the survival and growth of bacteria introduced into lake water. Arch Microbiol. 1988;150:320–325. doi: 10.1007/BF00408301. [DOI] [PubMed] [Google Scholar]
- 16.Taylor G T, Pace M L. Validity of eucaryote inhibitors for assessing production and grazing mortality of marine bacterioplankton. Appl Environ Microbiol. 1987;53:119–128. doi: 10.1128/aem.53.1.119-128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremaine S C, Mills A L. Inadequacy of the eucaryote inhibitor cycloheximide in studies of protozoan grazing on bacteria at the freshwater-sediment interface. Appl Environ Microbiol. 1987;53:1969–1972. doi: 10.1128/aem.53.8.1969-1972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]