Abstract
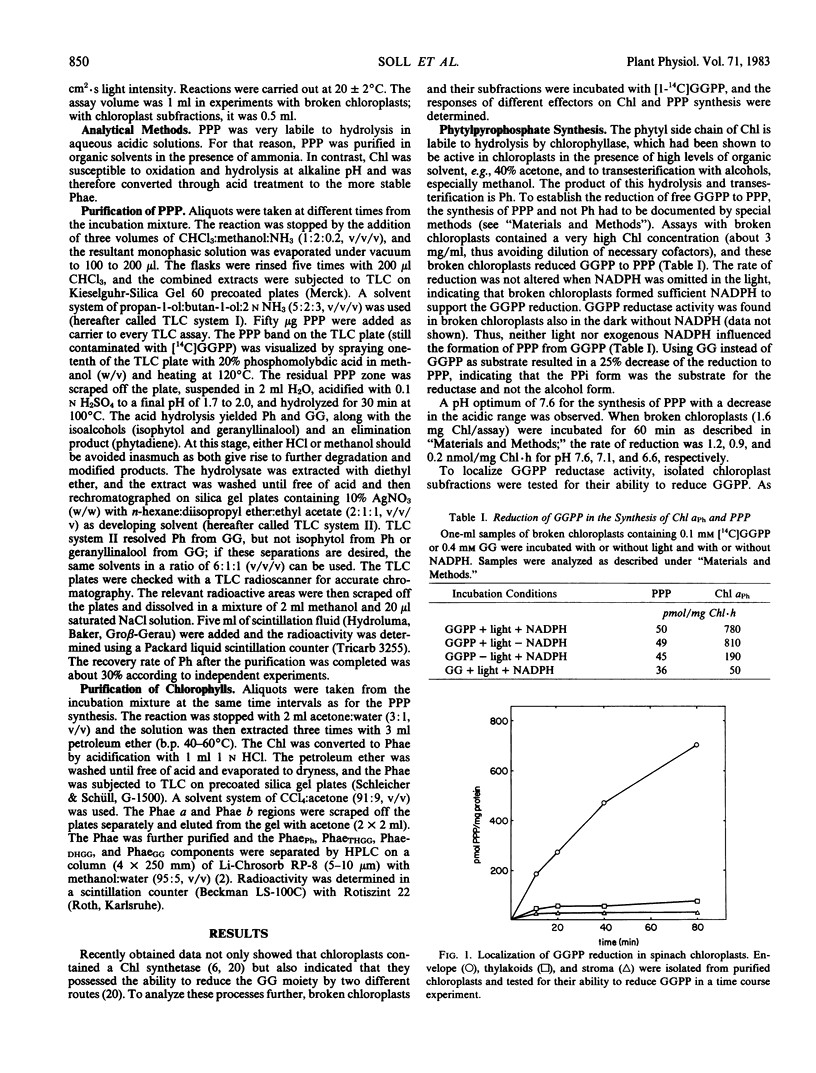
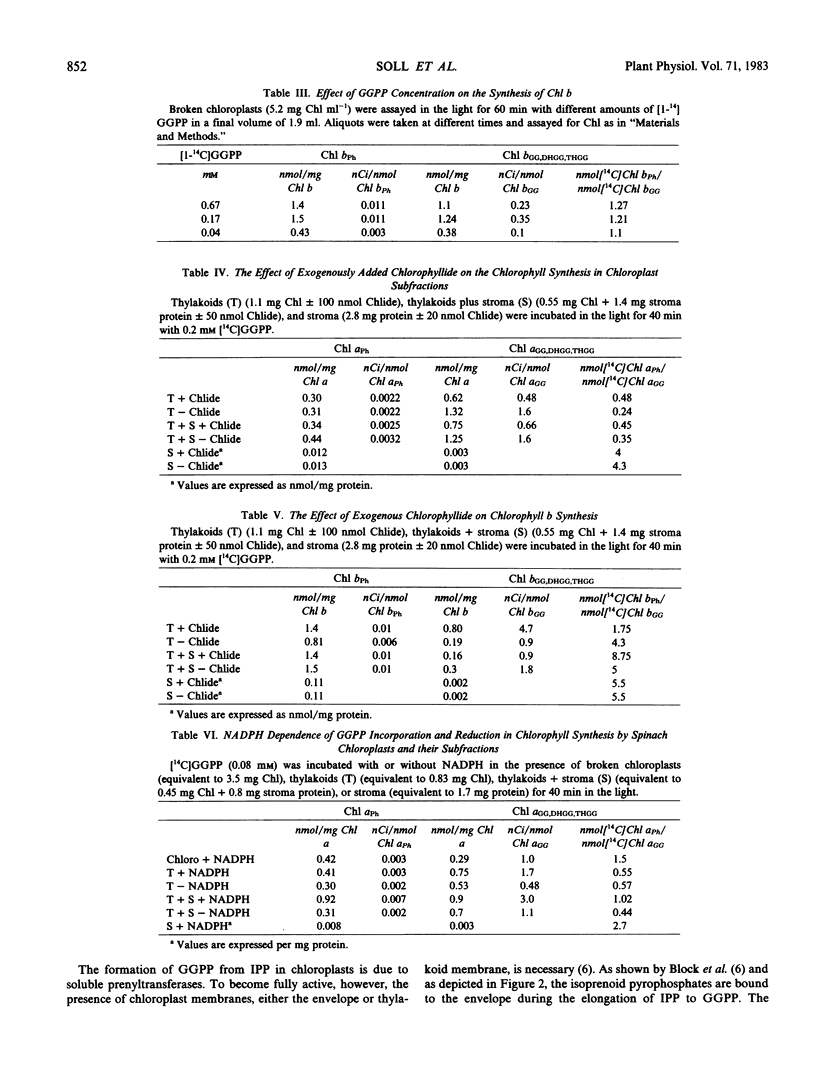
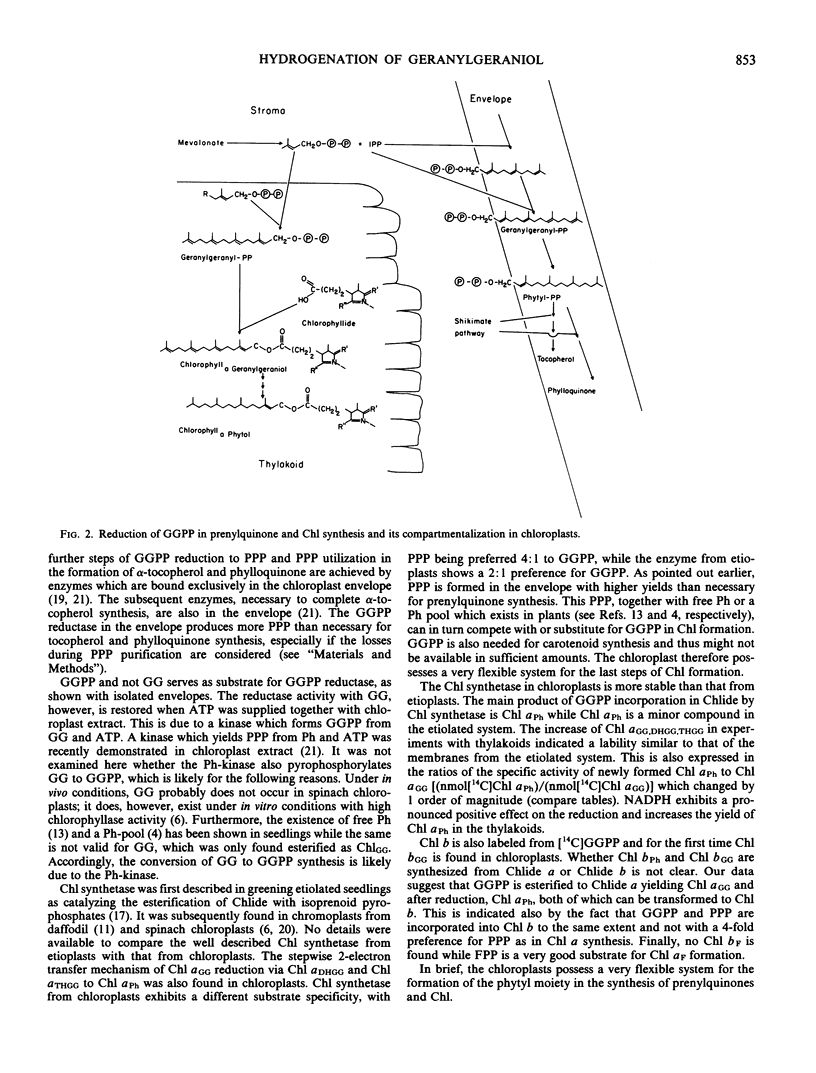
The reduction of geranylgeranylpyrophosphate to phytylpyrophosphate in spinach chloroplasts is described for the first time. The reductase is localized in the chloroplast envelope. By contrast, the reduction of the geranylgeranyl moiety in Chl synthesis is catalyzed in the thylakoids (via Chl synthetase). NADPH functions as electron donor in both reactions. Chl synthetase is firmly bound to the thylakoid membranes, and very little activity is found in the stroma fraction. Chl synthetase in chloroplasts can use the pyrophosphate ester of either phytol, geranylgeraniol, or farnesol, phytylpyrophosphate being the preferred substrate. Exogenous Chlide exhibits no influence on Chl synthesis by chloroplast subfractions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Joyard J., Douce R. Site of synthesis of geranylgeraniol derivatives in intact spinach chloroplasts. Biochim Biophys Acta. 1980 Aug 1;631(1):210–219. doi: 10.1016/0304-4165(80)90069-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Rüdiger W., Benz J., Guthoff C. Detection and partial characterization of activity of chlorophyll synthetase in etioplast membranes. Eur J Biochem. 1980 Aug;109(1):193–200. doi: 10.1111/j.1432-1033.1980.tb04784.x. [DOI] [PubMed] [Google Scholar]
- Schultz G., Ellerbrock B. H., Soll J. Site of prenylation reaction in synthesis of phylloquinone (vitamin K1) by spinach chloroplasts. Eur J Biochem. 1981 Jul;117(2):329–332. doi: 10.1111/j.1432-1033.1981.tb06341.x. [DOI] [PubMed] [Google Scholar]
- Soll J., Kemmerling M., Schultz G. Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys. 1980 Oct 15;204(2):544–550. doi: 10.1016/0003-9861(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Soll J., Schultz G. Phytol synthesis from geranylgeraniol in spinach chloroplasts. Biochem Biophys Res Commun. 1981 Apr 15;99(3):907–912. doi: 10.1016/0006-291x(81)91249-3. [DOI] [PubMed] [Google Scholar]
- Watts R. B., Kekwick R. G. Factors affecting the formation of phytol and its incorporation into chlorophyll by homogenates of the leaves of the french bean Phaseolus vulgaris. Arch Biochem Biophys. 1974 Feb;160(2):469–475. doi: 10.1016/0003-9861(74)90423-8. [DOI] [PubMed] [Google Scholar]
- Widmaier R., Howe J., Heinstein P. Prenyltransferase from Gossypium hirsutum. Arch Biochem Biophys. 1980 Apr 1;200(2):609–616. doi: 10.1016/0003-9861(80)90394-x. [DOI] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]


