Abstract
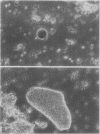
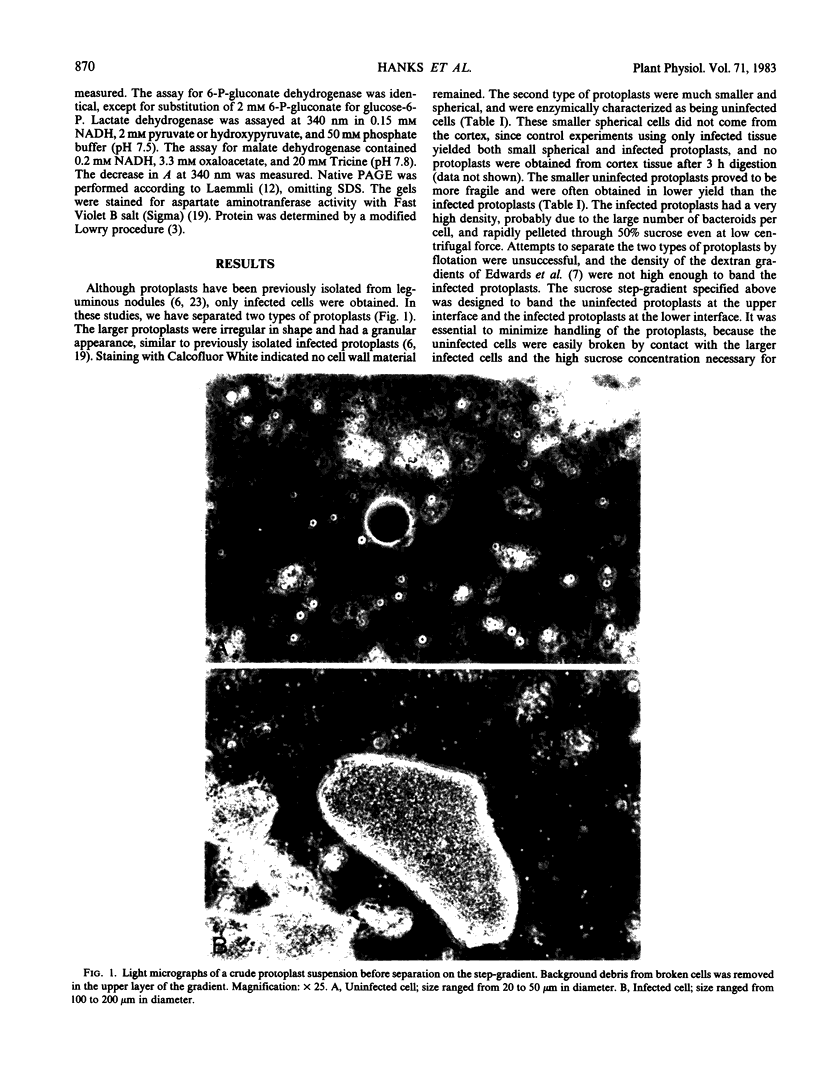
The distribution of organelles and associated enzymes between cells containing bacteroids and uninfected cells from nodules of Glycine max L. Merr. cv Amsoy 71 was investigated by separation of protoplasts on a sucrose step-gradient. Infected protoplasts were much larger, irregular in shape, and more dense than uninfected protoplasts. The peroxisomal enzymes, uricase and catalase, were present at much higher specific activity in the uninfected cell fraction. Allantoinase, an enzyme of the endoplasmic reticulum, had a greater specific activity in the uninfected cell fraction. Several enzymes whose products are required for purine biosynthesis, including phosphoglycerate dehydrogenase, aspartate aminotransferase, 6-phosphogluconate dehydrogenase, and glucose-6-phosphate dehydrogenase, exhibited a higher specific activity in the uninfected cell fraction. Isozymes of aspartate aminotransferase were separated on native gels and located by an activity stain. The soluble isozyme was predominantly found in the uninfected cell fraction. These data suggest that peroxisomes, containing uricase and catalase for conversion of uric acid to allantoin, are present only in the uninfected cells of soybean nodules. The uninfected cells also appear to be the site of the allantoinase reaction.
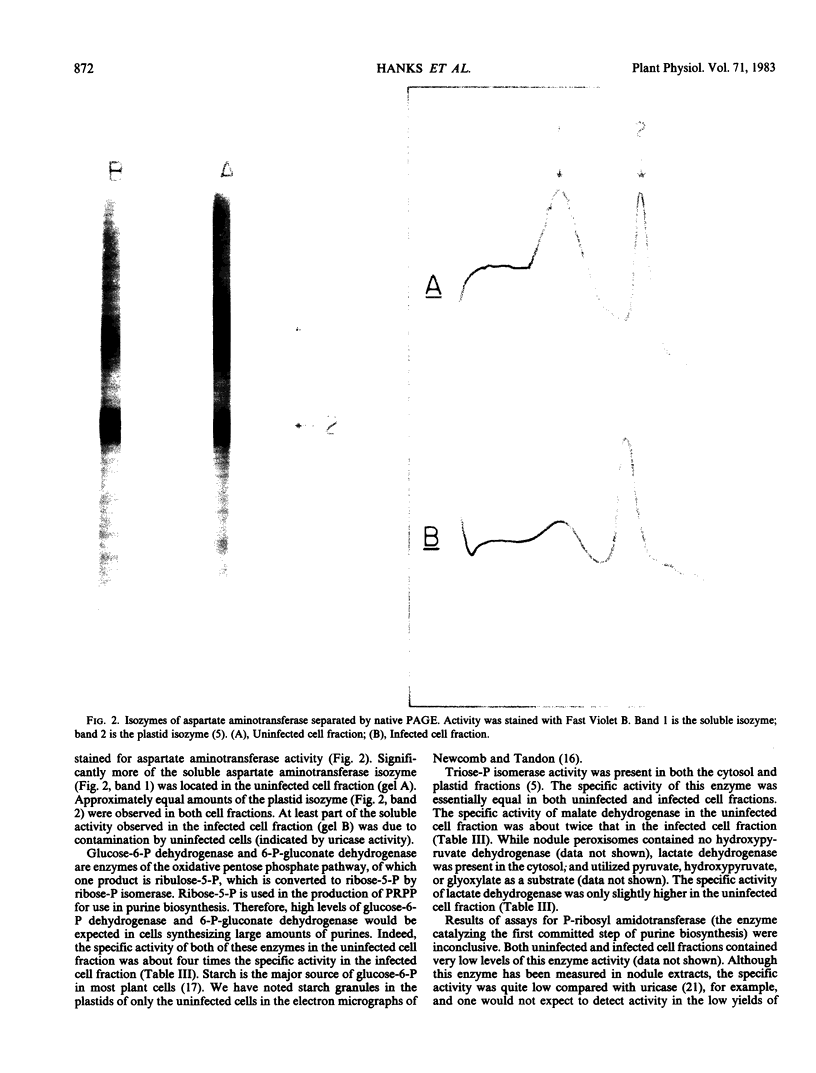
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Edwards G. E. Isolation of Intact and Functional Chloroplasts from Mesophyll and Bundle Sheath Protoplasts of the C(4) Plant Panicum miliaceum. Plant Physiol. 1979 May;63(5):821–827. doi: 10.1104/pp.63.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. W., McDonald J. A., McCord J. M., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Kinetic and regulatory properties. J Biol Chem. 1973 Jan 10;248(1):144–150. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb E. H., Tandon S. R. Uninfected cells of soybean root nodules: ultrastructure suggests key role in ureide production. Science. 1981 Jun 19;212(4501):1394–1396. doi: 10.1126/science.212.4501.1394. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Reynolds P. H., Boland M. J., Blevins D. G., Schubert K. R., Randall D. D. Enzymes of amide and ureide biogenesis in developing soybean nodules. Plant Physiol. 1982 Jun;69(6):1334–1338. doi: 10.1104/pp.69.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]