Abstract
Cardiovascular disease is one of the leading causes of mortality and morbidity worldwide. Atherosclerosis imaging has traditionally focused on detection of obstructive luminal stenoses or measurements of plaque burden. However, with advances in imaging technology it has now become possible to noninvasively interrogate plaque composition and disease activity, thereby differentiating stable from unstable patterns of disease and potentially improving risk stratification. This manuscript reviews multimodality imaging in this field, focusing on carotid and coronary atherosclerosis and how these novel techniques have the potential to complement current imaging assessments and improve clinical decision making.
Keywords: atherosclerosis, plaque burden, positron emission tomography, vulnerable plaque
Cardiovascular disease remains the leading cause of death globally, placing a major burden on health care services worldwide (1). Atherosclerosis is the main pathophysiological process responsible for ischemic heart disease and cerebrovascular disease. It is a systemic, multifocal process that starts early in life with a long quiescent phase before the manifestation of overt disease (2) and symptoms that vary depending on the morphology and characteristics of the underlying plaque. Obstructive atherosclerotic disease in the coronary arteries leads to the development of myocardial ischemia and angina and is usually characterized by stable plaque. Alternatively, unstable atherosclerotic plaque is prone to abrupt rupture or erosion, which is often clinically silent but can result in thrombotic vessel occlusion and acute stroke or myocardial infarction (MI).
Modern imaging techniques now allow for the direct and noninvasive imaging of atherosclerotic plaque in the carotid and coronary arteries, for the first time facilitating the assessment of plaque composition and disease activity (Central Illustration). In this review we discuss how these novel approaches might differentiate stable from unstable patterns of atherosclerosis, and complement existing imaging techniques. Ultimately these advances hold potential in improving the care of patients with carotid and coronary atherosclerosis, although it should be acknowledged that there are few randomized data, beyond the recent SCOT-HEART (Scottish Computed Tomography of the Heart) trial, demonstrating an improvement in outcome with use of even well-established imaging approaches, resulting in weak guideline recommendations (3).
CENTRAL ILLUSTRATION.
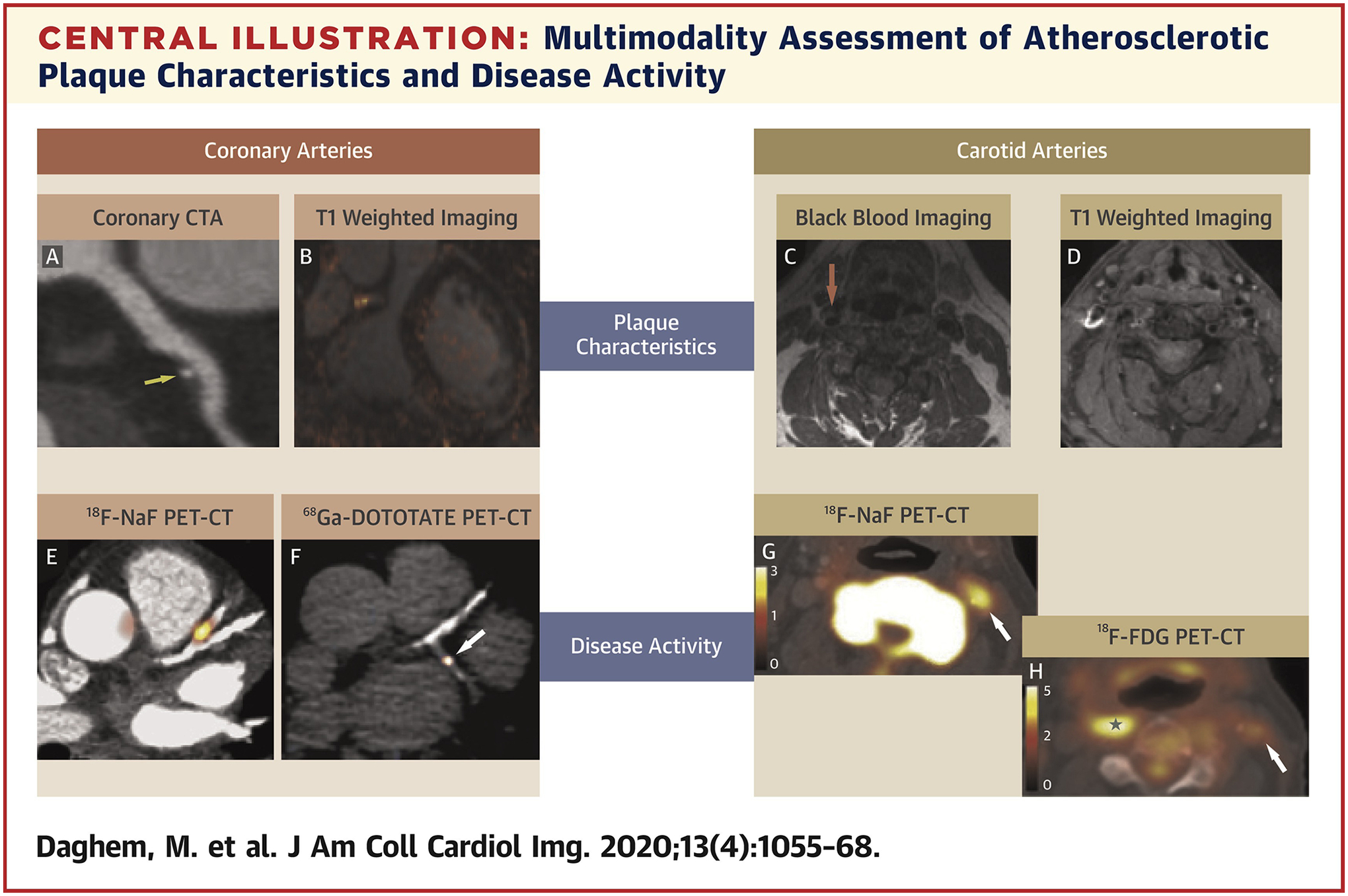
Multimodality Assessment of Atherosclerotic Plaque Characteristics and Disease Activity
Multimodality noninvasive imaging allows comprehensive analysis of atherosclerotic plaque in the coronary and vascular bed. Beyond the standard assessment of luminal stenosis and disease burden, emerging techniques enable clinical assessment of plaque characteristics and disease activity. Computed tomography (A) and magnetic resonance (B to D) provide the registered anatomic background information relating to plaque morphology, which can be combined with positron emission tomography data 18F-sodium fluoride (E, G), 68Ga-DOTATATE (F), and 18F-fluorodeoxyglucose (H), which has potential to examine multiple markers of plaque biology. F reproduced with permission from Tarkin et al. (53).
CURRENT IMAGING STRATEGIES
The clinical approach to atherosclerosis imaging has for many decades been based around the detection of obstructive luminal stenoses and the clinical sequelae that can ensue. In the carotid arteries the degree of luminal stenosis, assessed by ultrasound, coronary computed tomography angiography (CTA), or magnetic resonance (MR) angiography, is routinely used to inform decisions regarding surgical revascularization (4). However, a significant number of strokes occur in patients with nonobstructive carotid plaque. Indeed, the presence of a severe stenosis provides only modest risk prediction of future events, particularly in asymptomatic individuals (5). In the coronary arteries, CTA can directly assess luminal stenosis severity aided by the functional assessment provided by computed tomography (CT) fractional flow reserve, whereas myocardial stress perfusion imaging and stress echocardiography detect the myocardial consequences of obstructive plaques. However, although stable angina and symptoms of cardiac ischemia are associated with severe coronary artery stenoses, most MIs occur at sites of non-obstructive plaque on antecedent angiography (6). These findings, coupled with the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) and BARI-2D (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trials (7,8) in which percutaneous coronary intervention failed to reduce the risk of MI despite effective relief of ischemia, suggest that the relationship between obstructive stenoses, myocardial ischemia, and adverse outcomes may not be causal. Consequently, there has been significant interest in alternative imaging strategies targeting different aspects of the atherosclerotic disease process.
One such strategy has been to quantify the total atherosclerotic plaque burden, the rationale being that the more plaques a patient has, the more likely it is that a plaque rupture will occur and cause a clinical event. Indeed, regardless of the specific modality used, all imaging measures of atherosclerotic plaque burden seem to provide powerful prognostic information (9,10). In the carotid arteries, ultrasound-derived plaque burden assessments improve risk prediction beyond that provided by cardiovascular risk scores (11,12). In the coronary arteries, CT calcium scoring quantifies macroscopic deposits of calcification and provides a surrogate of total coronary atherosclerotic burden. This technique offers powerful prognostication of incremental value to cardiovascular risk scores. The relationship between calcium score and major adverse cardiovascular events including all-cause mortality, cardiovascular events, and non-fatal MI has been established in several studies (13,14). A large observational study involving 25,253 patients in the United States with a mean follow-up of 6.8 years showed that survival varied significantly by the extent of coronary artery calcification (CAC) (p < 0.0001) with survival rates of 99.4%, 94.7%, and 87.8%, respectively, for CAC scores of 0, 400 to 699, and ≥1,000 (p < 0.0001) (13). There have been multiple studies examining the very low event rates in patients with CAC of zero (15,16). In their meta-analysis of 13 studies assessing the relationship of CAC with adverse cardiovascular outcomes in 71,595 asymptomatic patients, Sarwar et al. (15) demonstrated that 0.47% without CAC had a cardiovascular event during follow-up, as compared with 4.14% with CAC. There is also increasing interest in using incidental CAC observed on chest CTs performed for noncardiac indications to improve patient risk prediction and to guide preventative therapy (16).
However, although CAC scoring provides a surrogate of the atherosclerotic plaque burden at relatively low radiation doses (~1 mSv) it actually quantifies calcified plaques that are themselves relatively unlikely to cause clinical events. It has therefore been suggested that atherosclerosis imaging and risk prediction may be improved by considering not only plaque burden, but also plaque type. Broadly, imaging assessments of plaque type can be categorized into anatomic assessments of plaque composition and molecular information about disease activity, both of which seek to differentiate stable from unstable atherosclerosis.
STABLE VERSUS UNSTABLE ATHEROSCLEROTIC PLAQUE
To understand how best to image plaque composition and disease activity it is first important to highlight some important pathophysiological aspects of atherosclerotic disease. Atherosclerosis is an inflammatory process that affects the arterial intima with extensive lipid deposition, foam cell formation, and vascular smooth muscle cells migration (17). The resulting plaque can cause progressive luminal stenosis, ischemia, and symptoms of stable angina (stable disease) or alternatively rupture abruptly, resulting in thrombus formation, vessel occlusion, and myocardial or cerebral infarction (unstable disease). Indeed, plaque rupture is the main method by which atherosclerosis causes clinical events, accounting for 60% to 70% of acute MIs (18) and approximately 90% of ischemic strokes (19).
Unstable plaques that are at high risk of rupture, so-called “vulnerable plaques,” have certain pathological characteristics that are distinct from those seen in stable lesions (Figure 1). Although stable lesions are associated with a thick fibrous cap, macrocalcification and extensive fibrous tissue, vulnerable plaques are characterized by a large necrotic core, thin fibrous cap (<65 μm), inflammation (predominantly in the form of macrophage infiltration), angiogenesis, plaque hemorrhage, and microcalcification (20). Interestingly, although culprit lesions are often associated with a large area of plaque they frequently have a luminal stenosis of <75% because of positive remodeling (20). These adverse plaque characteristics are frequently found together within the well-described thin cap fibroatheroma (21), and each represents a potential imaging target.
FIGURE 1.
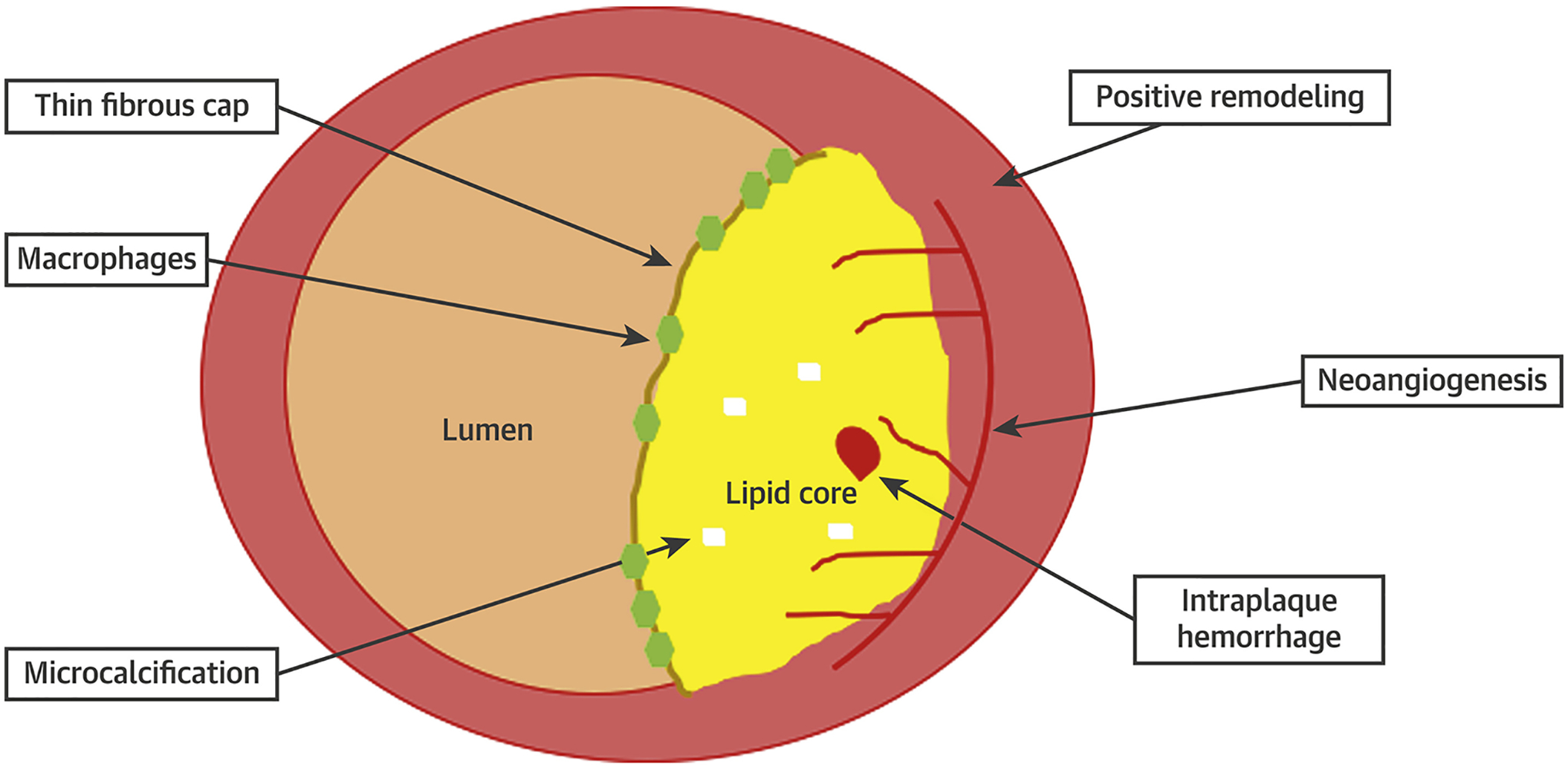
Pathological Features Associated With Culprit Atherosclerotic Plaque
Typical adverse plaque characteristics include macrophage accumulation, a large lipid core, positive remodeling, a thin fibrous cap, and microcalcification. Intraplaque microvessels result from angiogenesis driven by hypoxia and inflammatory stimuli within the necrotic core. These vessels can result in intraplaque hemorrhage, which increases the risk of plaque destabilization. Each of these represents a potential noninvasive imaging target.
RATIONALE FOR IMAGING STABLE VERSUS UNSTABLE PLAQUE
Initial attempts to identify vulnerable, unstable plaques were based around invasive imaging strategies, predominantly intravascular ultrasound (IVUS) (22), optical coherence tomography (23), and more recently near-infrared spectroscopy (24). These approaches provide excellent spatial resolution and detailed assessments of plaque morphology that are yet to be exceeded by noninvasive techniques. The ability of virtual histology IVUS (VH-IVUS) to detect adverse plaques and then to predict outcomes was investigated in the landmark PROSPECT (A Prospective Natural-History Study of Coronary Atherosclerosis) study (25). Stone et al. (25) prospectively enrolled 697 patients with acute coronary syndromes undergoing percutaneous revascularization and performed 3-vessel VH-IVUS, identifying 596 high-risk plaques (VH-IVUS-defined thin cap fibroatheromas). However, after 3 years’ follow-up only 21 MIs were observed, indicating that most vulnerable plaques either heal or rupture subclinically without causing hard clinical events. What then is the point of identifying vulnerable plaques? The rationale makes more sense at the level of the patient by applying noninvasive techniques to image the entire coronary or carotid vasculature (26). Although individual plaques are themselves relatively unlikely to cause an event, patients who develop unstable patterns of disease seem at higher risk of adverse outcomes (27). One potential explanation is that patients with a propensity to develop adverse plaque characteristics develop a greater number of such lesions at multiple sites and in different vascular beds over time. Although most heal, the risk of 1 of these many unstable plaques rupturing and causing a clinical event is increased, even if it is unlikely to be the lesion first identified on imaging.
THE CAROTID ARTERIES
Cerebrovascular disease is 1 of the main causes of death and the most important cause of disability globally, placing a significant economic burden on health care services worldwide (28). The underlying pathology and risk factors are similar to those of coronary artery disease, even though a significant proportion of events are attributable to other causes, such as cardioembolic disease and small-vessel ischemia. However, because of their location, large size, and stationary nature, imaging of the carotid arteries is considerably less challenging than imaging the coronary vasculature. As a consequence most novel plaque imaging techniques are first applied to the carotid arteries, with the added advantage that histo-logical validation is also feasible if imaging is performed before carotid endarterectomy. To date, ultrasound, CT, MR, and positron emission tomography (PET) have all been used to investigate carotid plaque composition and disease activity, with MR and PET techniques holding the greatest promise (Figure 2).
FIGURE 2.
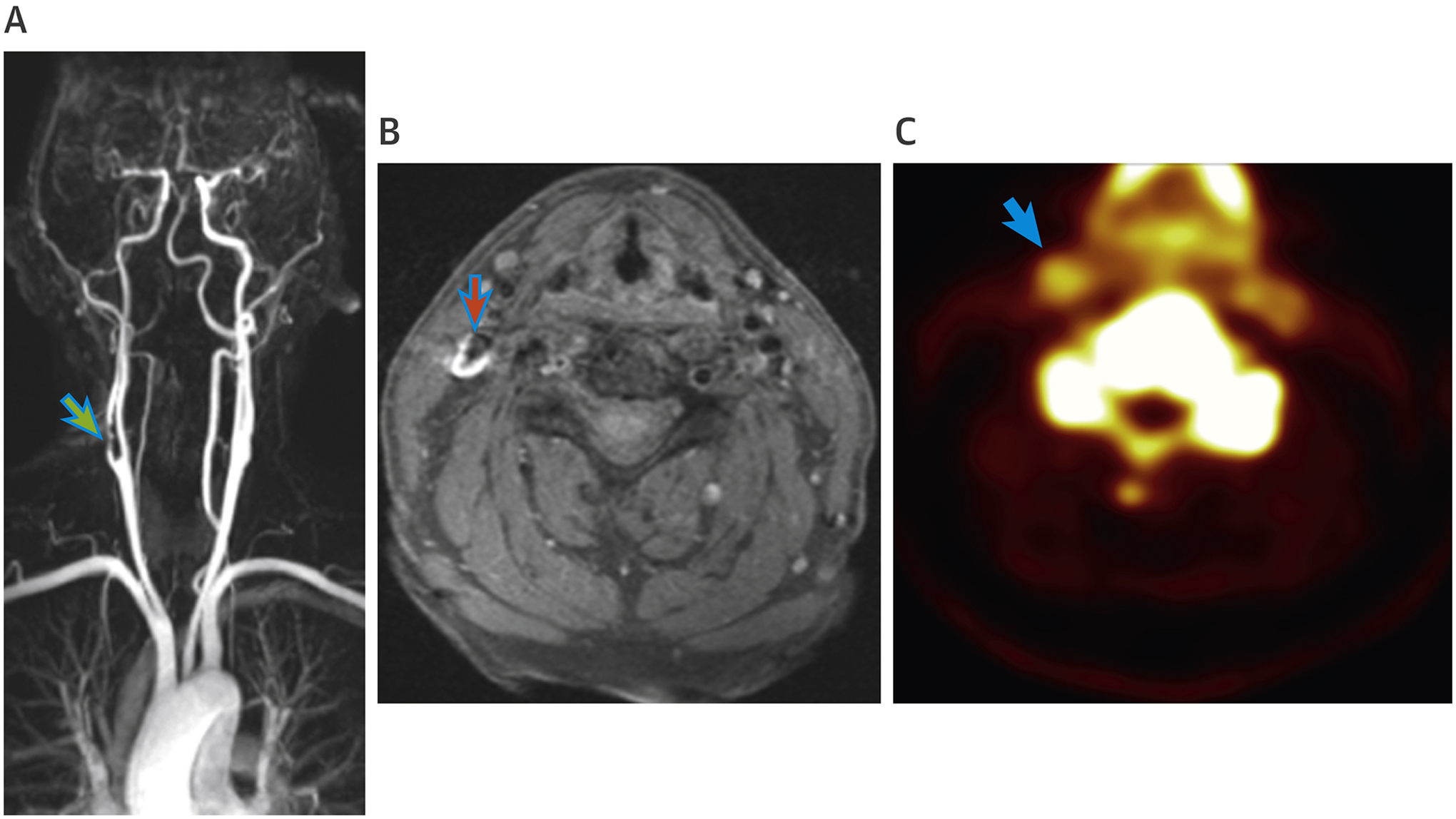
Multiparametric Assessments of the Carotid Arteries in a Patient Post–Transient Ischemic Attack
(A) Magnetic resonance angiogram of a patient with transient ischemic attack with time of flight image demonstrating a complex culprit lesion in the right internal carotid artery (green arrow). (B) T1-weighted magnetic resonance images of the same patient with a high T1 signal (red arrow) associated with methemoglobin within areas of fresh thrombus. (C) Positron emission tomography/magnetic resonance imaging with 18F-sodium fluoride showing increased signal intensity (blue arrow) in the right internal carotid artery corresponding with stenosis and symptoms.
PLAQUE COMPOSITION.
Although ultrasound is widely used to assess carotid luminal stenosis clinically it has a limited ability to assess plaque composition. Ultrasound can make some distinctions between different plaque types with echolucent heterogeneous plaques representative of more unstable disease (29) and echogenic plaques reflective of calcific stable lesions (30), but this distinction is yet to find a clinical role. CTA of the carotid arteries provides high-resolution anatomic imaging that may be useful in determining stenosis severity where ultrasound is difficult to perform (31). Many of the adverse plaque characteristics that can be identified in the coronaries (discussed next) can also be assessed in the carotids. However, this approach requires radiation and iodinated contrast and is less sensitive and specific than MR angiography, which is therefore usually preferred (32).
MR is well suited to atherosclerotic plaque characterization given the soft tissue contrast it provides. Carotid plaque imaging can be performed with fast high-resolution MR black-blood techniques that provide good blood suppression and high spatial resolution, providing detailed visualization of the adjacent vessel and plaque composition (33). In addition to measurement of carotid plaque thickness, volume, and area (34), multispectral MR imaging can categorize carotid plaque into different tissue types, quantify lipid content, and identify the presence of a ruptured fibrous cap (35). Imaging after administration of gadolinium contrast can further improve the ability of MR to discriminate lipid core from the overlying fibrotic cap (36). T1-weighted techniques hold particular promise in the identification of intra-luminal thrombosis and intraplaque hemorrhage (37). Methemoglobin, a break down product of hemoglobin, causes T1 shortening, resulting in signal amplification on T1-weighted images in areas of unstable plaque containing recent thrombus or hemorrhage (38). Finally, MR has the ability to target atherosclerotic plaque inflammation, using ultrasmall superparamagnetic particles of iron oxide (USPIO). When USPIO particles are administered intravenously, they are taken up by macrophages via surface scavenger receptors. The powerful T2* effects of USPIOs subsequently result in signal hypointensity in areas with active macrophage infiltration. Using this approach, increased USPIO uptake has been demonstrated in culprit carotid plaques post-stroke and asymptomatic carotid stenoses when compared with nonculprit or healthy control vessels (39,40). Larger clinical trials are required to establish a clear role for USPIOs beyond the research setting.
Increasing data suggest that the presence of adverse carotid plaque features on MR identifies patients at increased risk of future cardiovascular events (41–43). Takaya et al. (41) showed that in asymptomatic patients with 50% to 70% stenoses, arteries with thinned or ruptured fibrous caps, intraplaque hemorrhage, and a large lipid core on MR were associated with subsequent cerebrovascular events, a finding that was confirmed in a systematic review of 9 studies (44). Studies have also demonstrated an association between adverse plaque characteristics in the carotids and clinical events arising from the coronary arteries (42,43), highlighting the systemic nature of atherosclerosis, and the potential for unstable plaque detection in one territory to inform about disease status in other vascular beds.
DISEASE ACTIVITY.
Advances in hybrid scanners now allow combined noninvasive assessment of disease activity using hybrid PET imaging alongside the anatomic information provided by CT or MR. Targeted PET radiotracers are injected intravenously and accumulate in areas where the disease process of interest is active. The photons ejected following collision of the emitted positron with a free electron can be detected and localized by the PET scanner. This information is then fused with the anatomic datasets. In principle this approach can be used to investigate the activity of any pathological process, subject to the availability of a relevant radiotracer. In practice the availability of tracers approved for human use has previously been limited, although this is rapidly changing with the advent of multiple novel tracers specifically targeting pathological processes relevant to cardiovascular disease.
18F-fluorodeoxyglucose (18F-FDG) is a commonly used tracer that labels cells with high glycolytic metabolic requirements. These include activated inflammatory cells that express high levels of glucose transporters and rapidly accumulate 18F-FDG. Increased 18F-FDG uptake in carotid plaque demonstrates a close association with plaque macrophage infiltration leading to its use as a surrogate of vascular inflammation (45,46). Using 18F-fluoromisonidazole PET imaging to quantify hypoxia in atherosclerotic plaques, Joshi et al. (47) showed that symptomatic carotid plaques were more hypoxic than asymptomatic lesions and that hypoxia contributes to increased FDG uptake. Chowdhury et al. (46) showed that culprit carotid plaque following a cerebrovascular event demonstrated increased 18F-FDG activity compared with the contralateral carotid artery. More recent studies have indicated that 18F-FDG uptake in the carotids correlates with predicted cardiovascular risk, although it sometimes fails to discriminate culprit and nonculprit lesions (48,49). Although prospective outcome data regarding carotid 18F-FDG uptake are lacking, a recent retrospective study of 309 patients who underwent 18F-FDG PET-CT, mostly because of the clinical suspicion of cancer, demonstrated that uptake in the ascending aorta was a predictor of future coronary heart disease events independent of Framingham risk scores, and was associated (50). 18F-FDG uptake has also been used to monitor vascular wall inflammation in response to antiatherogenic therapies. In a recent meta-analysis of 7 studies with a combined total of 287 participants, statin treatment was associated with a significant reduction in arterial wall inflammation, based on tissue-to-background ratio (TBR) measurement by 18F-FDG PET-CT imaging (51). Multicenter prospective studies are now required to confirm the clinical role of 18F-FDG in assessment of atherosclerotic plaque inflammation, discriminating stable from unstable plaques, predicting cardiovascular prognosis, and monitoring response to therapies.
Although 18F-FDG is convenient and cheap, it is a glucose analogue an(53)d therefore not specific for inflammation. There has therefore been growing interest in the use of more inflammation-specific tracers, such as DOTATATE and 18-kDa translocator protein (TSPO) tracers. DOTATATE targets the somatostatin receptor subtype-2 (SSTR2), which is abundant on the surface of proinflammatory M1 macrophages. Pedersen et al. (52) demonstrated increased in vivo uptake of 64Cu-DOTATATE in carotid plaque, with correlation of this activity with macrophage burden on histology. Tarkin et al. (53) demonstrated increased 68Ga-DOTATATE PET uptake in culprit carotid plaque, with this tracer seeming to outperform 18F-FDG.
The 18-kDa translocator protein (TSPO), formally known as the peripheral benzodiazepine receptor, is found within the outer membrane of mitochondria and is highly expressed within macrophages and microglia in the brain (54). Several PET tracers targeting this protein have been explored as inflammation-specific tracers, including the prototypical 11C-PK11195, which has demonstrated increased uptake in culprit carotid plaques following stroke, independent of vessel stenosis, and correlated with inflammatory cell burden on histology (55). In patients with carotid disease, 11C-PK11195 uptake can distinguish between recently symptomatic and asymptomatic plaque and improves risk stratification (55). However, this tracer is limited by high nonspecific binding and a short half-life (20 min) that necessitates onsite cyclotron facilities (56). Second-generation TSPO tracers have also been disappointing, primarily because of their variable binding in patients with different forms of the rs6971 genetic polymorphism (57). Third-generation TSPO radiotracers are under evaluation that seem to be less sensitive to TSPO binding affinity and the rs6971 polymorphism.
Finally, tracers investigating other pathological processes are also being explored. 18F- sodium fluoride (18F-NaF) is a cheap and widely available PET tracer that preferentially binds to areas of newly developing microcalcification (58). 18F-NaF therefore targets the unstable early phase of the calcification process and provides information about calcification activity that is different and complementary to the stable macrocalcific plaques identified on CT. 18F-NaF PET has been used to study vascular calcification activity in a range of conditions including aortic stenosis (59), abdominal aortic aneurysm disease (60), and both carotid and coronary atherosclerosis (61). 18F-NaF uptake in the carotid arteries has been validated against histology, with increased uptake observed in plaques with multiple different adverse characteristics, and 18F-NaF offering improved discrimination of culprit plaque post-stroke compared with 18F-FDG (48). Larger studies investigating the role of 18F-NaF in the assessment of carotid atherosclerosis are currently underway.
THE CORONARY ARTERIES
Ischemic heart disease is the most common global cause of death (28). There is consequently a major focus on primary and secondary prevention, with an attendant need to accurately identify patients who might benefit from preventative therapies. As in the carotids, there is interest in using advanced imaging to differentiate stable coronary atheroma from the unstable patterns of disease associated with plaque rupture and clinical events. However, these approaches are considerably more challenging in the small-caliber, highly mobile coronary arteries (62). Over recent years advances in contrast CTA technology have overcome many of these hurdles. Indeed, contrast CT is being used increasingly in the clinical assessment of patients with suspected coronary artery disease, supported by data from 2 large randomized controlled trials: PROMISE (Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease) (63) and SCOT-HEART (64). In SCOT-HEART the use of CTA increased diagnostic certainty, changed clinical management, and reduced fatal or nonfatal MI at 5 years compared with standard care (2.3% vs. 3.9%; hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.41 to 0.84) (65). PROMISE recruited a lower risk population with a shorter duration of follow-up. After 25 months, there was no difference in the composite primary outcome (death, MI, hospitalization for unstable angina, or major procedural complication), although at 12 months the risk of death or nonfatal MI was lower in the CTA group compared with the control arm (mandated stress imaging) (HR: 0.66; 95% CI: 0.44 to 1.00) (63). When these trials were considered as part of a meta-analysis again CT was associated with a reduction in MI, although there was no difference in mortality (66). It is important to note that in both trials roughly one-half of the observed MIs occurred in patients with nonobstructive disease on their baseline scan (63). Indeed, the value of CT imaging probably lies in its ability to identify obstructive and nonobstructive plaque and to provide an overall assessment of plaque burden. On the back of this growing clinical adoption the next step is to assess whether CT or hybrid assessments of plaque type can further refine patient assessment (67,68).
PLAQUE COMPOSITION.
Most simply, CT can be used to categories plaques as noncalcified, partially calcified, and calcified based on their attenuation values (Hounsfield units) (69). One study of patients with suspected coronary artery disease demonstrated that at the patient level, higher rates of 3-year major adverse cardiovascular events were observed in patients with noncalcified versus calcified plaque 22.7% versus 5.5% (70). Data from the ICONIC (Incident COroNary Syndromes Identified by Computed Tomography) register found that culprit lesions had a 92% higher volume of noncalcific plaque than control subjects (71). However, CT can also offer more detailed information and identification of specific adverse plaque characteristics (Figure 3). These include positive remodeling, spotty calcification (a marker of early macrocalcification rather than true microcalcification), the napkin ring sign (a signature CT lesion appearance of low-attenuation surround by a rim of high-attenuation), and low attenuation plaque (<30 HU) as a marker of a large necrotic core. These adverse plaque features have been well validated against intravascular imaging, are more frequently observed in patients with MI compared with patients with stable angina (72,73), and are associated with an increased incidence of future cardiovascular events (74,75). Motoyama et al. (76) investigated 3,158 patients who underwent CTA for suspected or known coronary artery disease. They identified adverse plaque features of positive remodeling or low attenuation (<30 HU) in 294 patients, 16% of whom went on to have acute coronary syndrome compared with only 1.4% of patients without adverse plaques (Figure 3). Similarly, a recent prospective study of 245 patients with nonobstructive coronary artery disease on CTA reported that when adjusted for clinical variables the presence of at least 2 adverse plaque features was associated with a statistically higher rate of cardiac death and/or acute coronary syndrome (HR: 7.54; 95% CI: 2.43 to 23.34; p = 0.0005) (77).
FIGURE 3.
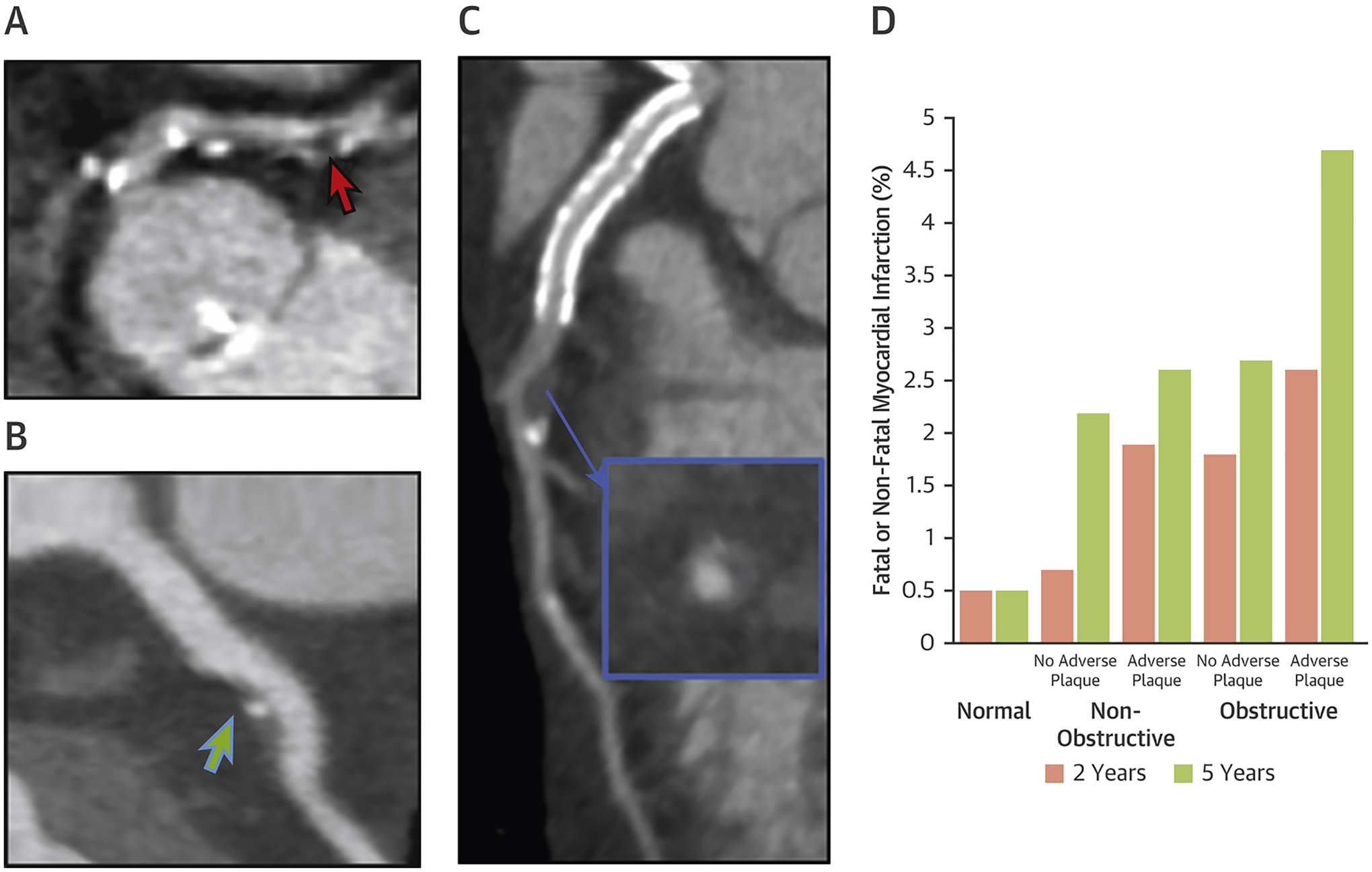
Assessment of Coronary Plaque Characteristics on Coronary Computed Tomography Angiography
(A) Low-attenuation plaque (red arrow) in the proximal left anterior descending artery with spotty calcification and associated vessel stenosis. (B) Nonobstructive plaque in proximal left anterior descending artery with spotty calcification (green arrow). (C) An atherosclerotic plaque with positive remodeling (blue arrow) and low-attenuation plaque in the left anterior descending artery. (D) Williams et al. (68) demonstrated that patients with obstructive disease and 1 or more high-risk plaque had a 10-fold increased risk of subsequent myocardial infarctions than patients with normal coronaries. Figure 3D reproduced with permission from Williams et al. (68).
Secondary analyses exploring the associations between adverse plaque characteristics and outcomes have been performed in both the PROMISE and SCOT-HEART cohorts. The PROMISE data showed that even after adjusting for risk factors and stenosis severity, the presence of adverse plaque features is associated with an increased risk of major adverse cardiac events (HR: 1.72; 95% CI: 1.89 to 3.93), with stronger associations observed in younger patients, those with nonobstructive disease, and women (67). This is consistent with the SCOT-HEART data that demonstrated a 3-fold increase in events in patients with adverse plaque (positive remodeling or low-attenuation plaque) (68). The presence of both obstructive disease and adverse plaque had a synergistic effect with a resultant 10-fold increase in these patients compared with those with normal coronary arteries (68). Importantly, however, the predictive effect of adverse plaque features and obstructive disease was not significant when adjusted for calcium score, which emerged as the only independent predictor of fatal or nonfatal MI. Although these data support the hypothesis that patients with adverse plaque features and an unstable pattern of disease are at increased risk of future events, they highlight an important caveat: that no study of high-risk plaque identification has yet demonstrated incremental prognostic benefit over and above more simple markers of overall plaque burden, namely the calcium score.
A recent and novel CT method for identifying unstable coronary atherosclerosis is the assessment of epicardial adipose tissue. This is the visceral fat surrounding the heart that is thought to interact with adjacent coronary atherosclerotic plaque in a bidirectional manner. For many years paracrine effects of pericardial fat on coronary plaque have been proposed, modulating underlying atheroma via activation of local inflammatory pathways and increased neovascularization (78). Recent studies have shown that higher epicardial fat volumes are observed in patients with noncalcified compared with calcified plaques (79) and associated with an increased rate of future cardiovascular events (80). However, the improved spatial and temporal resolution of contemporary CTA now allows study of this interaction in the other direction. The rationale is that coronary plaque inflammation alters the composition of the adjacent epicardial fat, an effect that can be detected by changes in CT attenuation. This technique was validated by Antonopoulos et al. (81), and has been shown to identify patients at increased risk of death in a recent retrospective multicenter outcome study (82). With further validation this approach could become a useful technique to measure inflammation retrospectively on routinely acquired CT scans, although further work is required to understand where in the coronary vasculature it is best measured, the effects of coronary stents on attenuation measurements, and whether this approach predicts MI as well as death (83).
MR angiography techniques are less developed than CT and not in widespread clinical use. MR is able to visualize coronary plaque and detect adverse features (62), such as positive remodeling (84), but such approaches have largely been limited to individual coronary territories, restricting potential utility. However, T1-weighted approaches looking for plaque hemorrhage and luminal thrombus can be applied across the entire coronary vasculature, with recent studies confirming increased signal in culprit plaques post-MI (85) (Figure 4), and that the presence of such high-intensity coronary plaque identifies patients at increased future risk of cardiovascular events (86). This approach is deserving of further study.
FIGURE 4.
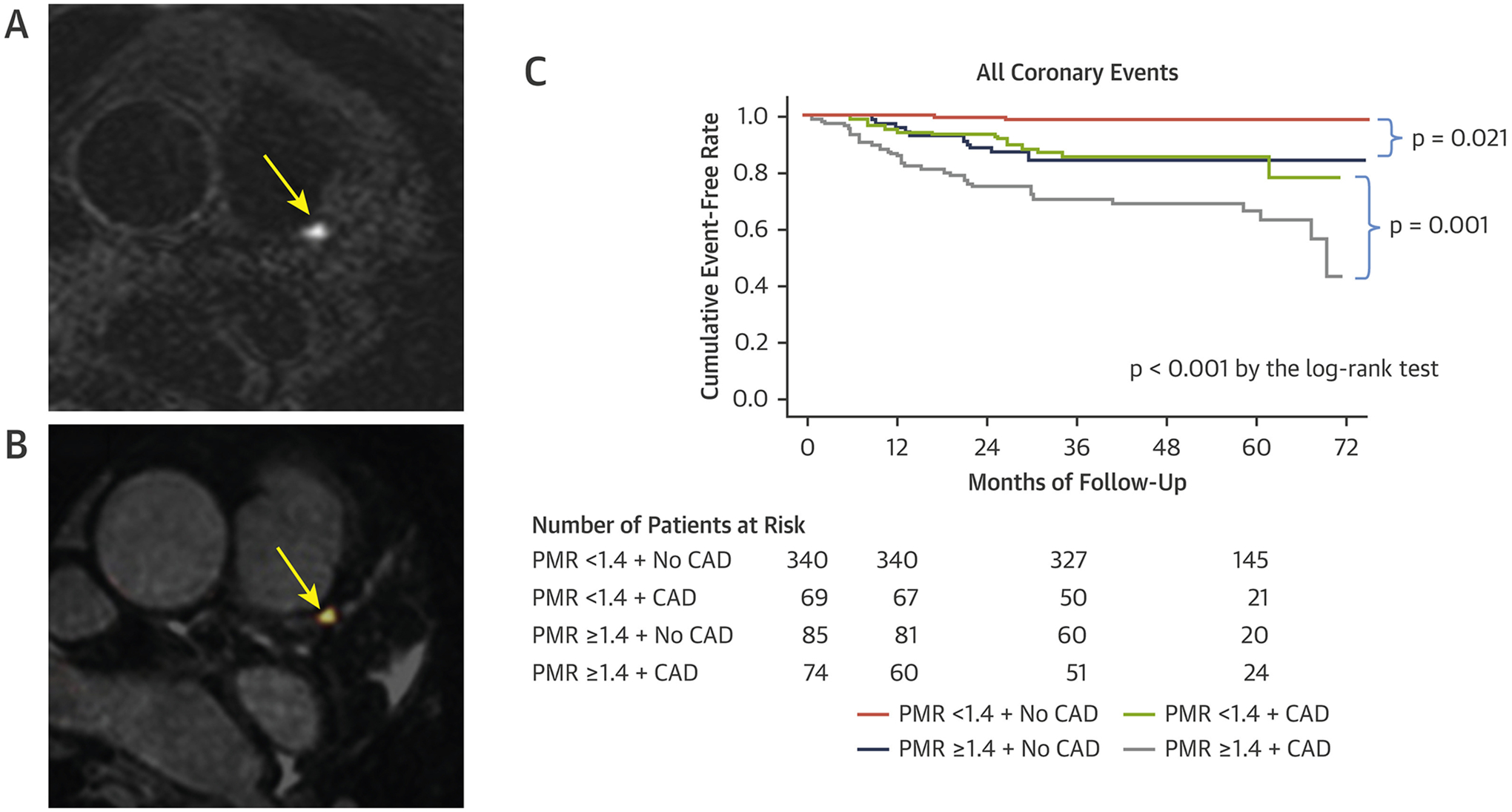
Assessment of Coronary Plaque Characteristics on Magnetic Resonance
(A) High-intensity plaque in the proximal left anterior descending artery (yellow arrow) on noncontrast T1-weighted magnetic resonance. (B) T1-weighted images coregistered with magnetic resonance coronary angiogram, increased signal at site of high-intensity plaque (yellow arrow). (C) Kaplan-Meier curves comparing the probability of coronary events in patients with and without high-intensity plaque on noncontrast T1-weighted imaging. Adverse plaque characteristic conferred a higher incidence of adverse clinical events. Reproduced with permission from Noguchi et al. (86). CAD = coronary artery disease; PMR = plaque-to-myocardium signal intensity ratio.
DISEASE ACTIVITY.
With modern PET-CT scanners, accurate coregistration, improved blood-pool correction, and state-of-the-art motion correction have facilitated the measurement of disease activity in the coronary arteries (87,88). Unfortunately, 18F-FDG imaging in the coronary arteries is challenging because of the high metabolic activity of the myocardium, which obscures signal in adjacent coronary plaque in a third of patients, even despite dietary restrictions (89). This has led to interest in more specific tracers, including 68Ga-DOTATATE, which similar to the carotids localizes to culprit coronary plaques post-MI (53).
18F-NaF is characterized by very low uptake in the myocardium (roughly half to two-thirds lower than in the blood-pool) (90), which makes this tracer well suited to the detection of signal in the coronary arteries. Increased 18F-NaF uptake is observed in coronary plaques (Figure 5) with multiple adverse features on CT, VH-IVUS, and optical coherence tomography (OCT), and in the culprit plaques of patients post-MI (61). At the patient level increased 18F-NaF activity is observed in patients with higher Framingham risk scores (91). To further investigate the clinical utility of 18F-NaF in the coronary arteries and its role in risk prediction, the prospective multicenter PREFFIR (Prediction of Recurrent Events With 18F-Fluoride) trial is currently underway (NCT02278211).
FIGURE 5.
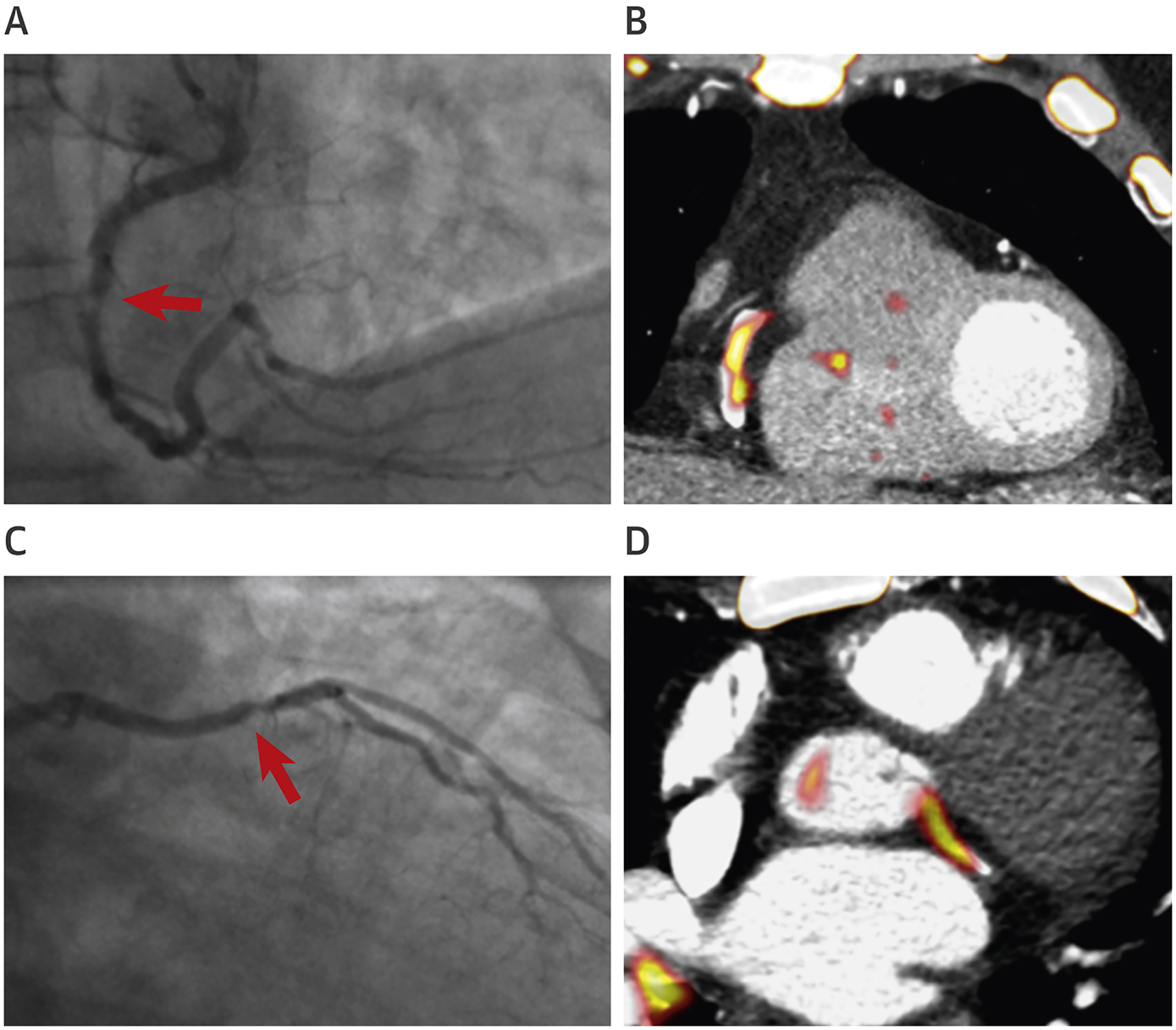
Assessment of Disease Activity in the Coronary Arteries
Positron emission tomography/computed tomography imaging of the coronaries with 18F-sodium fluoride. (A) Invasive coronary angiography showing a culprit lesion in right coronary artery (red arrow). (B) Intense focal 18F-sodium fluoride uptake is observed at the site of the culprit plaque on positron emission tomography/computed tomography. (C) Invasive coronary angiography showing culprit plaque in the left circumflex artery (red arrow). (D) Intense focal 18F-sodium fluoride uptake is observed at the site of the culprit plaque on positron emission tomography/computed tomography.
FUTURE DIRECTIONS
With advances in scanner technology it is now possible to image atherosclerotic plaque composition and disease activity and to differentiate stable from unstable patterns of disease in the carotid and coronary vessels. Although several of these techniques have now become well established, their incremental clinical value over and above more standard imaging approaches has yet to be established. Large-scale prospective studies are required for this purpose and in particular need to demonstrate added value to simpler plaque burden measurements before the added time and resources in assessing plaque type can be justified.
Of the available plaque composition assessments, CTA seems to offer the greatest potential given the advanced plaque characterization that it provides and the growing clinical role that this technique is assuming. More advanced molecular imaging techniques, such as PET, hold great promise in improving pathological understanding of atherosclerosis, particularly with the development of novel tracers targeting macrophages, microcalcification, angiogenesis, and thrombus formation (Table 1). With ongoing technological advances these PET techniques may also find a clinical role, further improving risk stratification and potentially guiding the use of invasive or expensive treatments in patients with advanced disease. However, any such benefits need to be substantial to justify the additional radiation exposure and expense associated with molecular imaging.
TABLE 1.
Positron Emission Tomography–Based Molecular Imaging in Carotid and Coronary Disease
| Tracer | Pathological Process | Biological Target | Research Application and Limitations |
|---|---|---|---|
| 18 F-FDG | Inflammation | Glucose analogue | Increased uptake seen in carotid disease (46), but myocardial uptake limits its use in coronaries. |
| 18 F-NaF | Microcalcification | Hydroxyapatite | Increased uptake demonstrated in both culprit coronary and carotid plaque (61). Limited myocardial uptake, but overspill from bones can affect interpretation. |
| Ga68- or Cu64- DOTATATE | Inflammation | Somatostatin receptor subtype-2 (SSTR2) | Localizes to culprit coronary and carotid plaque (53). No physiological uptake in the myocardium. In some cases lack of availability limits its use even in research. Further validation studies are needed. |
| 11 C-PK11195 | Inflammation | Translocator protein (TSPO) | Increased uptake in culprit carotid plaques following stroke, independent of vessel stenosis (55). Limited by high nonspecific binding and short half-life. Further validation studies are needed. |
| USPIO | Inflammation | Surface scavenger receptors | Has been used in cardiac magnetic resonance, with uptake demonstrated in culprit carotid plaques and asymptomatic carotid stenoses 39,40. High blood pool signal may affect signal interpretation. |
18F-FDG = 18F-fluorodeoxyglucose; 18F-NaF = 18F-sodium fluoride; USPIO = ultrasmall superparamagnetic iron oxide.
CONCLUSIONS
Rapid advances in noninvasive cardiovascular imaging now allow assessment of plaque composition and atherosclerotic disease activity in the aorta, carotid, and coronary arteries and differentiation of stable versus unstable disease states. Although this has provided important pathophysiological insights, further research is now required to investigate whether such approaches provide any incremental clinical information beyond standard patient assessments.
HIGHLIGHTS.
Assessment of coronary and carotid atherosclerotic plaque composition is possible using computed tomography and magnetic resonance imaging.
Disease activity in coronary and carotid atherosclerotic plaque can be assessed using molecular imaging techniques, such as positron emission tomography.
These imaging assessments of plaque type can be used to differentiate stable from unstable patterns of atherosclerosis and potentially to improve patient risk stratification.
ACKNOWLEDGEMENTS
The authors thank Mr. Jakub Kaczynski and Dr. Michelle Williams (British Heart Foundation Centre for Cardiovascular Science, University of Edinburgh) for their contributions to the development of Figures 2 and 3.
Dr. Fayad is supported by National Institutes of Health/National Heart, Lung, and Blood Institute P01 HL131478, R01 HL071021, R01 HL128056, R01HL135878, R01HL144072, and R01HL143814; National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering R01 EB009638; and American Heart Association (14SFRN20780005). Dr. Dweck is supported by the Sir Jules Thorn Biomedical Research Award 2015 (15/JTA) and by the British Heart Foundation (FS/14/78/31020). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAC
coronary artery calcification
- CI
confidence interval
- CT
computed tomography
- CTA
coronary computed tomography angiography
- 18F-FDG
18F-fluorodeoxyglucose
- 18F-NaF
18F-sodium fluoride
- HR
hazard ratio
- IVUS
intravascular ultrasound
- MI
myocardial infarction
- MR
magnetic resonance
- PET
positron emission tomography
- USPIO
ultrasmall superparamagnetic particles of iron oxide
- VH
virtual histology
REFERENCES
- 1.Jankovic N, Geelen A, Streppel MT, et al. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr 2015;102:745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Patho-biological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA 1990;264: 3018–24. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64:1929–49. [DOI] [PubMed] [Google Scholar]
- 4.Jonas DE, Feltner C, Amick HR, et al. Screening for asymptomatic carotid artery stenosis: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2014;161:336–46. [DOI] [PubMed] [Google Scholar]
- 5.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491–502. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005;111:3481–8. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, O’Rourke RA, Teo KK. Optimal medical therapy with or without PCI for stable coronary disease. J Vasc Surg 2007;45:1286. [DOI] [PubMed] [Google Scholar]
- 8.BARI 2D Study Group, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. J Am Coll Cardiol Img 2012;5: 681–9. [DOI] [PubMed] [Google Scholar]
- 10.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128–33. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015;131:2104–13. [DOI] [PubMed] [Google Scholar]
- 12.Baber U, Mehran R, Sartori S, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol 2015;65:1065–74. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–70. [DOI] [PubMed] [Google Scholar]
- 14.Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015;163:14–21. [DOI] [PubMed] [Google Scholar]
- 15.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. J Am Coll Cardiol Img 2009;2: 675–88. [DOI] [PubMed] [Google Scholar]
- 16.Jairam PM, Gondrie MJA, Grobbee DE, et al. Incidental imaging findings from routine chest CT used to identify subjects at high risk of future cardiovascular events. Radiology 2014;272:700–8. [DOI] [PubMed] [Google Scholar]
- 17.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb 1994;14:840–56. [DOI] [PubMed] [Google Scholar]
- 18.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664–72. [DOI] [PubMed] [Google Scholar]
- 19.Spagnoli LG, Mauriello A, Sangiorgi G, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004; 292:1845–52. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47 Suppl. 8:C13–8. [DOI] [PubMed] [Google Scholar]
- 21.Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 2001;16:285–92. [DOI] [PubMed] [Google Scholar]
- 22.Sathyanarayana S, Carlier S, Li W, Thomas L. Characterisation of atherosclerotic plaque by spectral similarity of radiofrequency intravascular ultrasound signals. EuroIntervention 2009;5: 133–9. [DOI] [PubMed] [Google Scholar]
- 23.Tearney GJ, Yabushita H, Houser SL, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003;107:113–9. [DOI] [PubMed] [Google Scholar]
- 24.Schuurman A-S, Vroegindewey M, Kardys I, et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur Heart J 2018;39: 295–302. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–35. [DOI] [PubMed] [Google Scholar]
- 26.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol 2015;65:846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanadis C, Antoniou C-K, Tsiachris D, Pietri P. Coronary atherosclerotic vulnerable plaque: current perspectives. J Am Heart Assoc 2017;6:e005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohsfeldt RL, Gandhi SK, Fox KM, Bullano MF, Davidson M. Medical and cost burden of atherosclerosis among patients treated in routine clinical practice. J Med Econ 2010;13:500–7. [DOI] [PubMed] [Google Scholar]
- 29.Polak JF, Shemanski L, O’Leary DH, et al. Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology 1998;208:649–54. [DOI] [PubMed] [Google Scholar]
- 30.Joakimsen O, Bønaa KH, Mathiesen EB, Stensland-Bugge E, Arnesen E. Prediction of mortality by ultrasound screening of a general population for carotid stenosis: the Tromsø Study. Stroke 2000;31:1871–6. [DOI] [PubMed] [Google Scholar]
- 31.Koelemay MJW, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke 2004;35:2306–12. [DOI] [PubMed] [Google Scholar]
- 32.Wardlaw JM, Chappell FM, Best JJK, Wartolowska K, Berry E, NHS Research and Development Health Technology Assessment Carotid Stenosis Imaging Group. Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367:1503–12. [DOI] [PubMed] [Google Scholar]
- 33.Hartog den AG, Bovens SM, Koning W, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013;45:7–21. [DOI] [PubMed] [Google Scholar]
- 34.Makris GC, Teng Z, Patterson AJ, et al. Advances in MRI for the evaluation of carotid atherosclerosis. Br J Radiol 2015;88:20140282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996;94:932–8. [DOI] [PubMed] [Google Scholar]
- 36.Wasserman BA, Smith WI, Trout HH II., Cannon RO II., Balaban RS, Arai AE. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging—initial results. Radiology 2002;223: 566–73. [DOI] [PubMed] [Google Scholar]
- 37.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081–91. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen SF, Thrysøe SA, Robich MP, et al. Assessment of intramyocardial hemorrhage by T1-weighted cardiovascular magnetic resonance in reperfused acute myocardial infarction. J Cardiovasc Magn Reson 2012;14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang T, Howarth SPS, Miller SR, et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 2006;37:2266–70. [DOI] [PubMed] [Google Scholar]
- 40.Smits LP, Tiessens F, Zheng KH, Stroes ES, Nederveen AJ, Coolen BF. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017;263:211–8. [DOI] [PubMed] [Google Scholar]
- 41.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke 2006;37:818–23. [DOI] [PubMed] [Google Scholar]
- 42.Zavodni AEH, Wasserman BA, McClelland RL, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014;271:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Zhao X-Q, Balu N, et al. Carotid plaque lipid content and fibrous cap status predict systemic cv outcomes: the MRI Substudy in AIM-HIGH. J Am Coll Cardiol Img 2017;10:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk. Stroke 2013; 44:3071–7. [DOI] [PubMed] [Google Scholar]
- 45.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24. [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury MM, Tarkin JM, Evans NR, et al. 18F-FDG uptake on PET/CT in symptomatic versus asymptomatic carotid disease: a meta-analysis. Eur J Vasc Endovasc Surg 2018;56:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi FR, Manavaki R, Fryer TD, et al. Vascular imaging with 18F-fluorodeoxyglucose positron emission tomography is influenced by hypoxia. J Am Coll Cardiol 2017;69:1873–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vesey AT, Jenkins WSA, Agnese Irkle, et al. 18F-Fluoride and 18F-fluorodeoxyglucose positron emission tomography after transient ischemic attack or minor ischemic stroke. Clinical perspective: case–control study. Circ Cardiovasc Imaging 2017;10:e004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J-M, Lee ES, Park K-Y, Seok JW, Kwon O-S. Comparison of [18F]-FDG and [18F]-NaF positron emission tomography on culprit carotid atherosclerosis: a prospective study. J Am Coll Cardiol Img 2018;12. 2740–372. [DOI] [PubMed] [Google Scholar]
- 50.Iwatsuka R, Matsue Y, Yonetsu T, et al. Arterial inflammation measured by 18 F-FDG-PET-CT to predict coronary events in older subjects. Atherosclerosis 2018;268:49–54. [DOI] [PubMed] [Google Scholar]
- 51.Pirro M, Simental-Mendía LE, Bianconi V, Watts GF, Banach M, Sahebkar A. Effect of statin therapy on arterial wall inflammation based on 18F-FDG PET/CT: a systematic review and meta-analysis of interventional studies. J Clin Med 2019;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen SF, Sandholt BV, Keller SH, et al. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol 2015;35:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by 68Ga-DOTA-TATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006;27:402–9. [DOI] [PubMed] [Google Scholar]
- 55.Gaemperli O, Shalhoub J, Owen DRJ, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 2012;33:1902–10. [DOI] [PubMed] [Google Scholar]
- 56.Pugliese F, Gaemperli O, Kinderlerer AR, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol 2010;56:653–61. [DOI] [PubMed] [Google Scholar]
- 57.Kreisl WC, Jenko KJ, Hines CS, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013;33:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dweck MR, Jenkins WSA, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014; 7:371–8. [DOI] [PubMed] [Google Scholar]
- 60.Forsythe RO, Dweck MR, McBride OMB, et al. 18F–sodium fluoride uptake in abdominal aortic aneurysms: the SoFIA3 Study. J Am Coll Cardiol 2018;71:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. [DOI] [PubMed] [Google Scholar]
- 62.Dweck MR, Puntman V, Vesey AT, Fayad ZA, Nagel E. MR imaging of coronary arteries and plaques. J Am Coll Cardiol Img 2016;9:306–16. [DOI] [PubMed] [Google Scholar]
- 63.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015; 372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 65.SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. [DOI] [PubMed] [Google Scholar]
- 66.Bittencourt MS, Hulten EA, Murthy VL, et al. Clinical outcomes after evaluation of stable chest pain by coronary computed tomographic angiography versus usual care. Circ Cardiovasc Imaging 2016;9:e004419. [DOI] [PubMed] [Google Scholar]
- 67.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART Study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu F-Z, Wu M-T. 2014 SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2015;9:e3. [DOI] [PubMed] [Google Scholar]
- 70.Hou Z-H, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. J Am Coll Cardiol Img 2012;5:990–9. [DOI] [PubMed] [Google Scholar]
- 71.Chang H-J, Lin FY, Lee S-E, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 2018;71:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–26. [DOI] [PubMed] [Google Scholar]
- 73.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014; 64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 75.Nakanishi K, Fukuda S, Shimada K, et al. Nonobstructive low attenuation coronary plaque predicts three-year acute coronary syndrome events in patients with hypertension: multidetector computed tomographic study. J Cardiol 2012;59: 167–75. [DOI] [PubMed] [Google Scholar]
- 76.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 77.Conte E, Annoni A, Pontone G, et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: a long-term follow-up study. Eur Heart J Cardiovasc Imaging 2016;170:200. [DOI] [PubMed] [Google Scholar]
- 78.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010;210:150–4. [DOI] [PubMed] [Google Scholar]
- 79.Dong D-D, Wang K, Wang D, Zhang T, Tu Y-F, Shen B-Z. Relationship between epicardial adipose tissue volume measured using coronary computed tomography angiography and atherosclerotic plaque characteristics in patients with severe coronary artery stenosis. J Int Med Res 2013;41: 1520–31. [DOI] [PubMed] [Google Scholar]
- 80.Hajsadeghi F, Nabavi V, Bhandari A, et al. Increased epicardial adipose tissue is associated with coronary artery disease and major adverse cardiovascular events. Atherosclerosis 2014;237: 486–9. [DOI] [PubMed] [Google Scholar]
- 81.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9:398. [DOI] [PubMed] [Google Scholar]
- 82.Mahabadi AA, Rassaf T. Imaging of coronary inflammation for cardiovascular risk prediction. Lancet 2018;392:894–6. [DOI] [PubMed] [Google Scholar]
- 83.Dweck MR, Fayad ZA. Imaging: perivascular fat. An unheralded informant of coronary inflammation. Nat Rev Cardiol 2017;14:573–4. [DOI] [PubMed] [Google Scholar]
- 84.Miao C, Chen S, Macedo R, et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2009;53:1708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jansen CHP, Perera D, Makowski MR, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 2011;124: 416–24. [DOI] [PubMed] [Google Scholar]
- 86.Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol 2014;63:989–99. [DOI] [PubMed] [Google Scholar]
- 87.Kwiecinski J, Adamson PD, Lassen ML, et al. Feasibility of coronary 18F-sodium fluoride positron-emission tomography assessment with the utilization of previously acquired computed tomography angiography. Circ Cardiovasc Imaging 2018;11:e008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lassen ML, Kwiecinski J, Cadet S, et al. Data-driven gross patient motion detection and compensation: implications for coronary 18F-NaF PET imaging. J Nucl Med 2019;60: 830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 2009;50:563–8. [DOI] [PubMed] [Google Scholar]
- 90.Trivieri MG, Dweck MR, Abgral R, et al. 18F-Sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 2016;68: 2712–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dweck MR, Chow MWL, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012; 59:1539–48. [DOI] [PubMed] [Google Scholar]


