Abstract
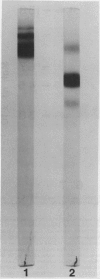
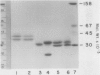
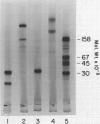
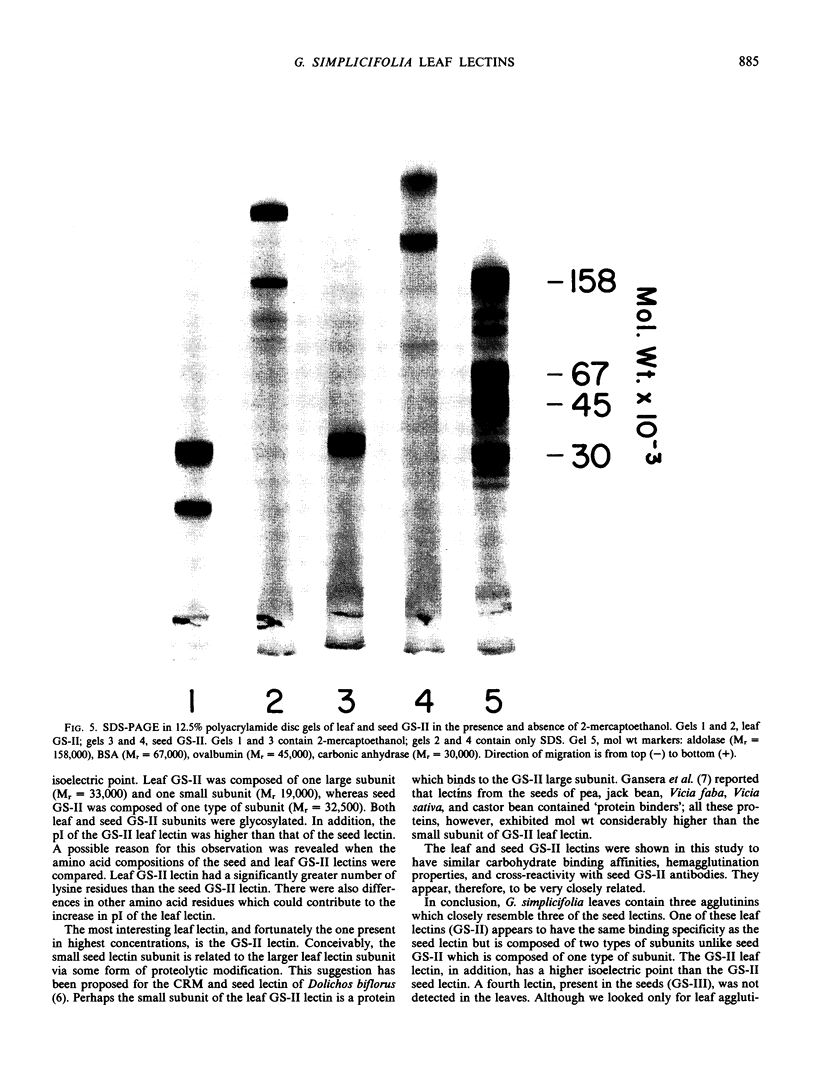
Leaves from mature Griffonia simplicifolia plants were examined for the presence of leaf lectins possessing sugar binding specificities similar to the four known seed lectins (GS-I, GS-II, GS-III, GS-IV). Three (GS-I, -II, -IV) of the four known G. simplicifolia seed lectins were present in the leaves. Leaf G. simplicifolia lectins I and IV were similar to the respective seed lectins. Leaf GS-II, however, was composed of two types of subunits (Mr = 33,000 and 19,000), whereas the seed lectin consists of only one type of subunit (Mr 32,500). Seed and leaf GS-II lectins also had different isoelectric points. All leaf and seed lectins were similar with respect to their hemagglutination and glycoconjugate precipitation properties and all subunits contained covalently bound carbohydrate. Leaf GS-IV appeared slightly under-glycosylated compared to seed GS-IV.
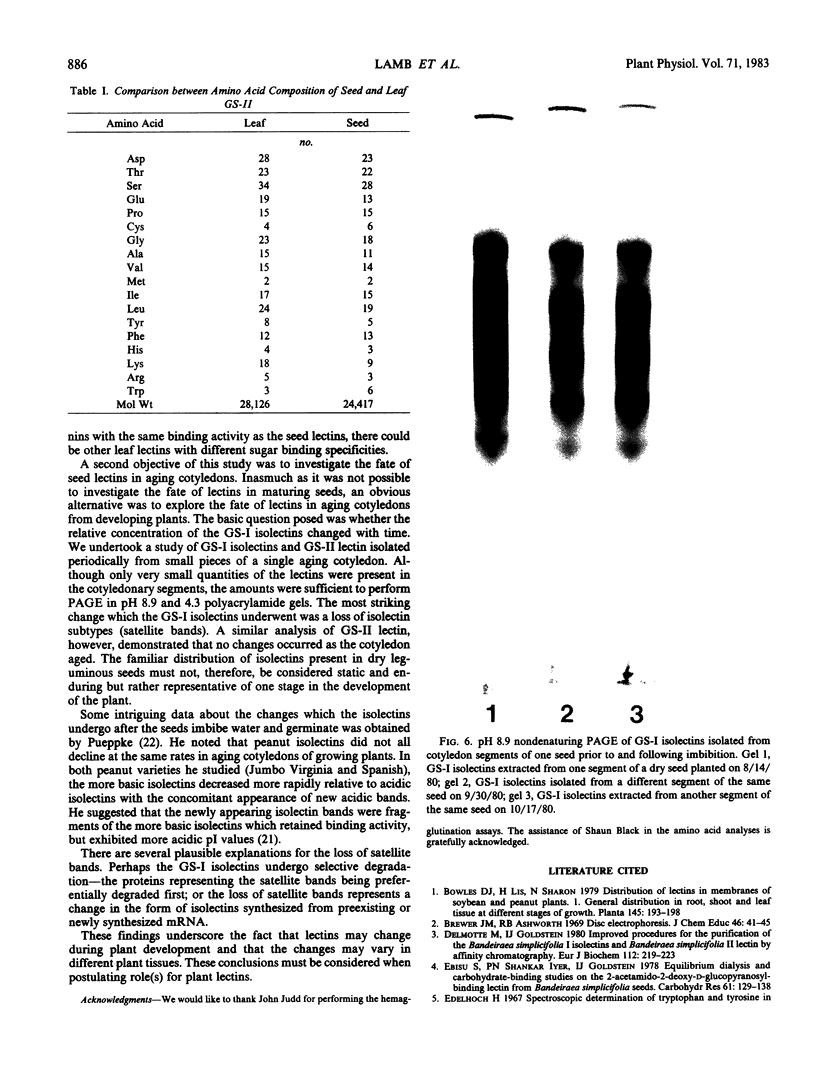
The fate of GS-I and GS-II seed lectins in aging cotyledons was investigated. GS-I isolectins usually contain isolectin subtypes associated with each main isolectin. Upon inbibition and germination, these GS-I isolectin subtypes disappeared. Over time, GS-II lectin did not change its disc gel electrophoretic properties.
Full text
PDF

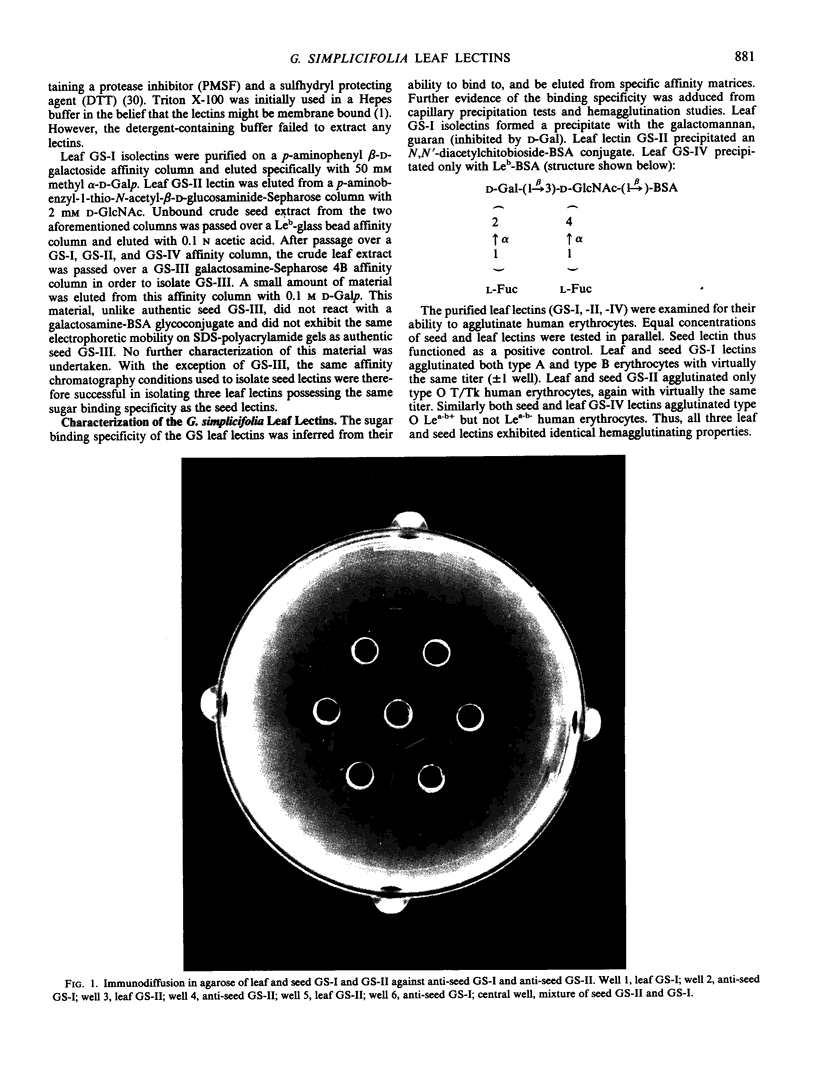

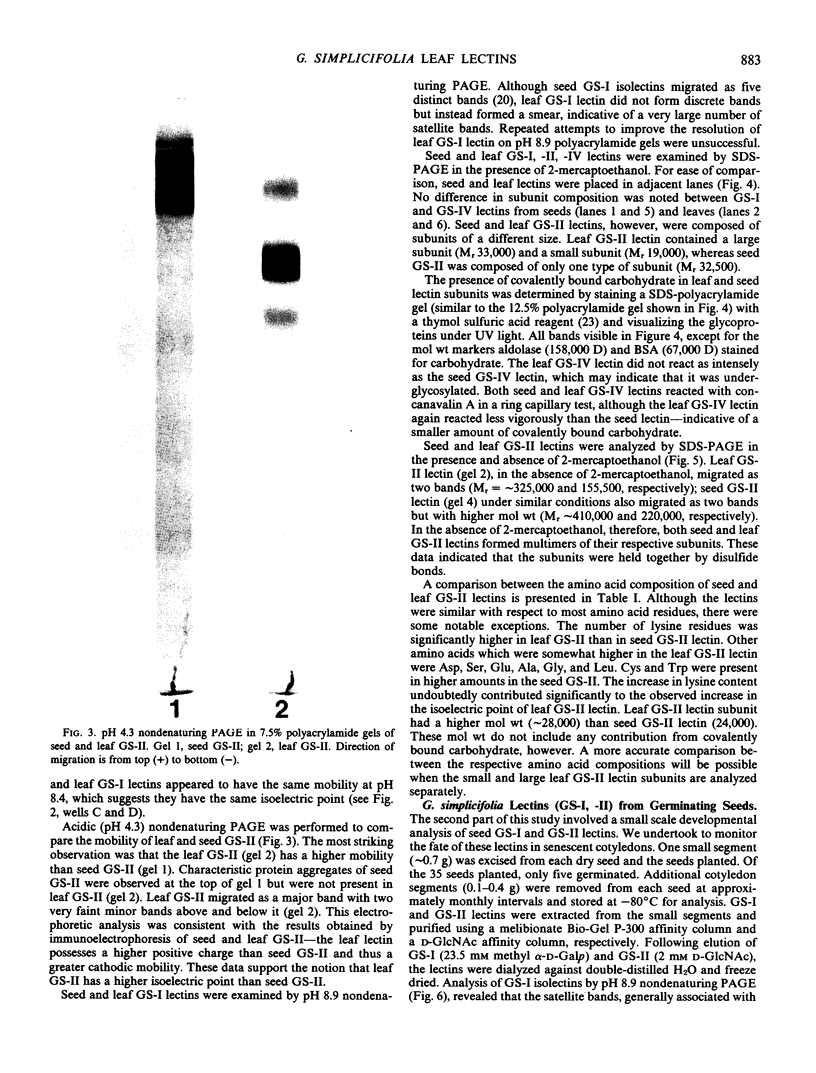
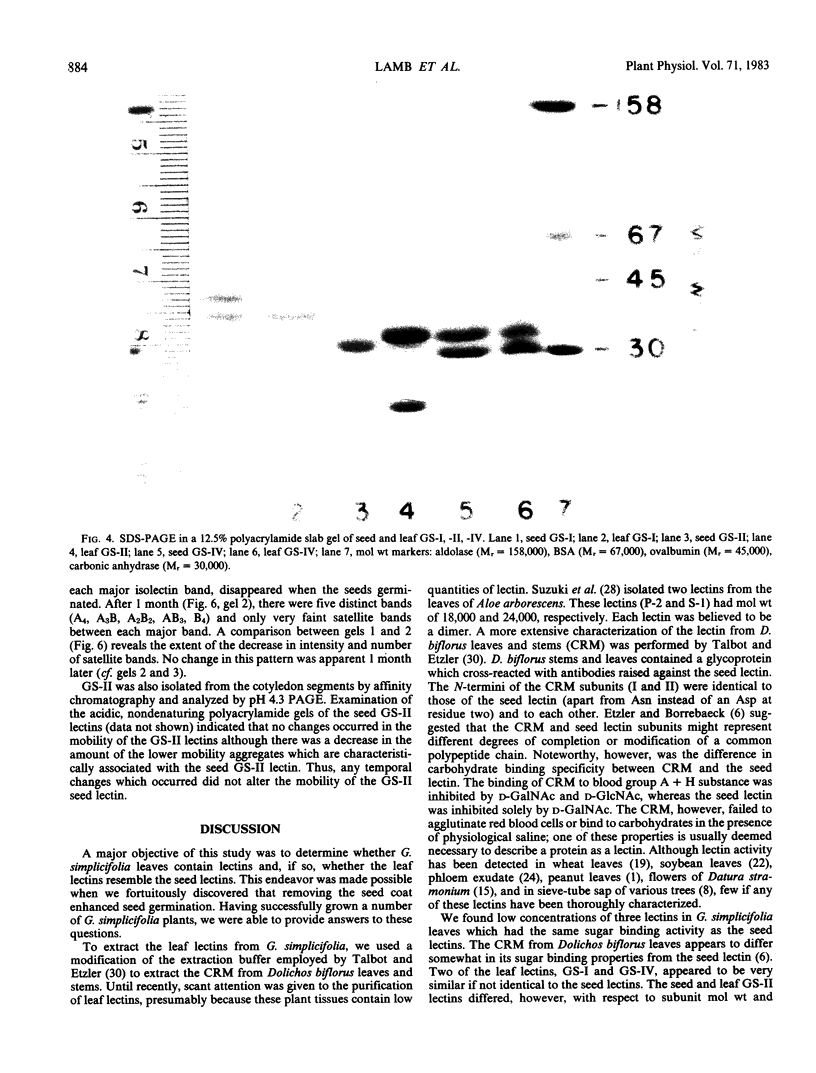

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Delmotte F. M., Goldstein I. J. Improved procedures for purification of the Bandeiraea simplicifolia I isolectins and Bandeiraea simplicifolia II lectin by affinity chromatography. Eur J Biochem. 1980 Nov;112(2):219–223. doi: 10.1111/j.1432-1033.1980.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Borrebaeck C. Carbohydrate binding activity of a lectin-like glycoprotein from stems and leaves of Dolichos biflorus. Biochem Biophys Res Commun. 1980 Sep 16;96(1):92–97. doi: 10.1016/0006-291x(80)91185-7. [DOI] [PubMed] [Google Scholar]
- Gansera R., Schurz H., Rüdiger H. Lectin-associated proteins from the seeds of Leguminosae. Hoppe Seylers Z Physiol Chem. 1979 Nov;360(11):1579–1585. doi: 10.1515/bchm2.1979.360.2.1579. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Hoy T. G., Ferdinand W., Harrison P. M. A computer-assisted method for determining the nearest integer ratios of amino acid residues in purified proteins. Int J Pept Protein Res. 1974;6(3):121–140. doi: 10.1111/j.1399-3011.1974.tb02369.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. C., Yeoman M. M., Gould A. R. Tissue and subcellular distribution of the lectin from Datura stramonium (thorn apple). Biochem J. 1979 Nov 15;184(2):215–219. doi: 10.1042/bj1840215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. E., Bookstein F. L., Goldstein I. J., Newton L. E. Bandeiraea simplicifolia I isolectins reveal a development sequential relationship. J Biol Chem. 1981 Jun 10;256(11):5874–5878. [PubMed] [Google Scholar]
- Lyer P. N., Wilkinson K. D., Goldstein L. J. An -N-acetyl-D-glycosamine binding lectin from Bandeiraea simplicifolia seeds. Arch Biochem Biophys. 1976 Nov;177(1):330–333. doi: 10.1016/0003-9861(76)90444-6. [DOI] [PubMed] [Google Scholar]
- Mishkind M., Keegstra K., Palevitz B. A. Distribution of wheat germ agglutinin in young wheat plants. Plant Physiol. 1980 Nov;66(5):950–955. doi: 10.1104/pp.66.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. A., Goldstein I. J. Five alpha-D-galactopyranosyl-binding isolectins from Bandeiraea simplicifolia seeds. J Biol Chem. 1977 Jul 10;252(13):4739–4742. [PubMed] [Google Scholar]
- Pueppke S. G., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: II. Distribution of Soybean Lectin in Tissues of Glycine max (L.) Merr. Plant Physiol. 1978 May;61(5):779–784. doi: 10.1104/pp.61.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Distribution of Lectins in the Jumbo Virginia and Spanish Varieties of the Peanut, Arachis hypogaea L. Plant Physiol. 1979 Oct;64(4):575–580. doi: 10.1104/pp.64.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen D. Glycoprotein detection in polyacrylamide gel with thymol and sulfuric acid. Anal Biochem. 1979 Nov 1;99(2):474–476. doi: 10.1016/s0003-2697(79)80035-4. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IV. Application of the quantitative precipitin method to polysaccharide-concanavalin A interaction. J Biol Chem. 1967 Apr 10;242(7):1617–1622. [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Saito H., Inoue S., Migita S., Takahashi T. Purification and characterization of two lectins from Aloe arborescens Mill. J Biochem. 1979 Jan;85(1):163–171. doi: 10.1093/oxfordjournals.jbchem.a132306. [DOI] [PubMed] [Google Scholar]
- Talbot C. F., Etzler M. E. Isolation and characterization of a protein from leaves and stems of Dolichos biflorus that cross reacts with antibodies to the seed lectin. Biochemistry. 1978 Apr 18;17(8):1474–1479. doi: 10.1021/bi00601a018. [DOI] [PubMed] [Google Scholar]