Abstract
Background
Intrahepatic cholestasis of pregnancy (ICP) is likely to lead to unfavorable consequences. Total bile acid (TBA) is thought to be the sole ICP indicator available as of now, but it comes with some kind of restrictions in terms of sensitivity and specificity. We were endeavoring to find potential diagnostic biomarkers for ICP in this investigation.
Methods
This case-control study with a prospective nature included 40 females in the stage of pregnancy who were diagnosed with ICP. It also included another 20 females who were also pregnant but with sound physical condition(control). Placental and plasma samples were collected from all females that were in the stage of pregnancy, except for 20 ICP patients, in which only plasma was collected. We used four-dimensional data-independent acquisition followed by enzyme-linked immunosorbent assay and immunohistochemistry to identify and validate plasma and placental profiles in ICP patients and controls. Bioinformatics was adopted in an effort to demonstrate the relevant biological processes and signalling pathways. Correlation analysis was used to analyse the consistency of tissue and plasma protein expression and the correlation between sequencing and experimental results.
Results
The expression levels of nectin-1 (NECTIN1), Kunitz-type protease inhibitor 1 (SPINT1), and inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3) were remarkably higher in ICP patients than in controls. However, heparin cofactor 2 (SERPIND1) expression levels in female participants in the stage of pregnancy who were diagnosed with ICP were remarkably lower than those pregnant females with good physical fitness. In addition to the negative correlation between SERPIND1 and TBA, NECTIN1, SPINT1, and ITIH3 expression positively correlated with TBA. Area under the receiver operating characteristic curve (AUC) values of 0.7925, 0.8313, 0.8163, and 0.9025, respectively, were used to assess the diagnostic accuracies of NECTIN1, SPINT1, ITIH3, and SERPIND1. AUC (0.9438) was considerably greater when NECTIN1, SPINT1, and SERPIND1 were integrated, according to binary logistic regression. The AUC of the ROC curve for various combinations of SERPIND1 and other indicators was higher than itself, thus providing a more reliable ICP diagnosis. Furthermore, according to the bioinformatics analysis, the NECTIN1, SPINT1, ITIH3, and SERPIND1 were identified as secreted proteins because they were localized in the extracellular region.
Conclusions
This research discovered new non-invasive ICP indicators. On top of this, it sheds new light on the crucial diagnostic function of secreted proteins in ICP.
Keywords: Intrahepatic cholestasis of pregnancy (ICP), Secreted protein, Biomarker
1. Introduction
The most prevalent liver condition specific to pregnancy is known as intrahepatic cholestasis of pregnancy (ICP). It primarily affects women in their second and third trimesters. The incidence of ICP ranges from 0.3 to 15 %; it varies according to different regions and races [1]. Its incidence is higher in Chile, Sweden, and the Yangtze River Basin of China. The main clinical manifestations are pruritus and upregulated serum total bile acid (TBA) levels. ICP is a benign condition in pregnant women that spontaneously resolves after delivery. However, it may cause serious adverse effects in the perinatal foetus, including preterm birth, neonatal asphyxia, and even stillbirth [2]. Uncertainty exists regarding the mechanisms behind the onset and progression of ICP. As cholesterol metabolism progresses, it produces TBA in the final stage. It is essential to absorb cholesterol and dietary esters owing to its amphiphilicity [3]. Currently, the clinical diagnostic criteria for ICP include elevated TBA levels and typical pruritus. However, the sensitivity and specificity of the TBA diagnosis are limited. According to Sentillhes et al. [4], a case of abrupt foetal arrest at 39.4 weeks occurred in an ICP patient with low bile acid readings (13 UI/L). As a result, it is likely that TBA's diagnostic accuracy was overstated. Taking this into consideration, novel biomarkers appear exceedingly crucial in enhancing ICP diagnosis and prediction.
Fortunately, state-of-the-art technologies such as next-generation sequencing (NGS)-based transcriptomics or data-independent acquisition (DIA)-based proteomics permit large-scale and high-throughput analysis of RNAs or proteins. Diverse omics research techniques have been used to identify a number of new biomarkers for the diagnosis of ICP. For example, our earlier research discovered that the novel biomarkers miR-4271, miR-1275, and miR-6891-5p and their mutual combinations are useful for enhancing ICP diagnosis and prognostic prediction using NGS [5]. Zou et al. [6]used DIA proteomics to screen a combination of four proteins as promising diagnostic markers for ICP. These new sequencing techniques have driven early prediction, diagnosis, and prognosis of ICP. However, no relevant studies have used four-dimensional DIA (4D-DIA) to explore ICP biomarkers. Traditional DIA can render spectrograms highly complex and pose significant challenges for data analysis. Thus, controversy remains regarding its quantitative reliability. To address this problem, we first applied the most advanced 4D-DIA method to reveal the proteomic patterns of the placenta and plasma in a clinical cohort [7].
Using 4D-DIA, we examined the protein expression patterns of the placenta and plasma in this study and discovered proteins that were differentially expressed between the two tissues. Next, enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry (IHC) experiments with an expanded sample size were performed to validate the candidate proteins. The simultaneous validation of plasma and placental samples from pregnant women improved the justification and relevance of our study. To shed new light on probable molecular mechanisms underlying ICP, Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes (KEGG) analyses were carried out. The ultimate goal of this work was to identify the secreted proteins that are specific to ICP and may be used as biomarkers to diagnose ICP.
2. Materials and methods
2.1. Patients and samples
In this study, 20 physically sound female participants at the pregnancy stage served as controls. This study also included 40 pregnant females with ICP. In addition to 20 ICP pregnant women whose placental samples were not collected, the placenta and plasma samples of the other 40 pregnant women were sampled simultaneously. The participants were in the third trimester of pregnancy, which was over 28 weeks of time. From September 2021 to June 2022, all patients who underwent treatment at the Nanjing Medical University-affiliated Wuxi Maternity and Child Health Care Hospital were free of severe acute respiratory syndrome coronavirus 2. Placental and plasma samples from eight patients with ICP and eight physically sound females at the stage of pregnancy were collected for quantitative proteomic analysis. Subsequently, 20 ICP patients and 20 healthy controls were selected to verify the variation in terms of the expression of the target proteins, both in placenta and plasma, between the two groups mentioned above. We also examined the correlation between the protein expression levels and the clinical indicators, as well as the gestational week of the pregnant women. Simultaneously, plasma samples from 40 ICP patients and 20 controls were used to draw receiver operator characteristic (ROC) curves to evaluate the clinical merits of the target proteins in the ICP diagnosis.
All participants were Chinese primiparas with singleton births. ICP was identified in pregnant women who had elevated bile acid levels linked to classic pruritus. After delivery, every symptom was resolved. Both the inclusion and exclusion criteria as well as the diagnostic criteria for ICP have been illustrated previously [6]. Briefly, patients with liver dysfunction attributed to other causes that could potentially elevate bile acids were excluded from the analysis. Preeclampsia, viral hepatitis, hemolysis, increased liver enzymes, low platelets syndrome, acute fatty liver of pregnancy, primary biliary cirrhosis, and any abnormalities in ultrasonography that could result in biliary obstruction are some of the aforementioned causes.
Following a caesarean delivery, 40 pregnant females had their placentas harvested in accordance with a methodology that had previously been published. It comprised 20 patients with ICP and 20 physically sound females as controls [8]. After the caesarean section, fine needle biopsy samples from various sites on each placenta were pooled at random and rinsed with cold saline to remove any blood that was contaminated. Subsequently, placenta tissues were fixed in 4 % paraformaldehyde fixative for 24 h at 4 °C, dehydrated in gradient ethanol concentrations, and embedded in paraffin. A 2 mm pinhole was used to extract samples from the paraffin block. These samples were subsequently embedded and sectioned into a series of immunohistochemical chips, resulting in 40 samples available for staining. Venous plasma samples were collected from all participants and separated at 4 °C through centrifugation at 3000 rpm for 10 min. All plasma sample centrifugation supernatants were preserved in a −80 °C freezer before it was used for subsequent tests. Before blood samples were taken, none of the patients had received ursodeoxycholic acid treatment. The Institutional Review Board of the Wuxi Maternal and Child Health Hospital approved the study's ethical affairs, and each subject granted their written consent (Wuxi Maternal and Child Health Hospital Ethical Review [2020-01-0310-08]).
2.2. Proteomics
The placenta was mixed with SDT buffer and put into 2 mL tubes with quartz sand. Using an MP Fastprep-24 automatic homogenizer (6.0 M/S, 30s, twice), the lysate was homogenized. After being sonicated, the homogenates were kept in boiling water for 15 min. The supernatant was filtered through 0.22 m filters after being centrifuged at 14,000 g for 40 min. Using the BCA Protein Assay Kit (P0012, Beyotime), the filtrate was quantified. The samples were stored at −20 °C. Contrastingly, plasma samples did not require homogenization or ultrasound steps.
SDT buffer (30 μL) was used to incorporate 200 μg of proteins for each sample; this buffer containing 4 % SDS, 100 mM DTT, and 150 mM Tris-HCl pH 8.0. UA buffer (8 M Urea, 150 mM Tris-HCl, pH 8.5) was employed to eliminate the detergent, DTT, and other low-molecular-weight components using repeated ultrafiltration. After that, 100 μL of iodoacetamide was added to block reduced cysteine residues, and the samples were incubated for 30 min in the dark. The filters were initially rinsed three times with 100 μl of UA buffer and then twice with 100 μl of 0.1 m TEAB buffer. The protein suspensions were then digested with 4 μg of trypsin (Promega) in 40 μL of 0.1 M TEAB buffer over the course of an entire night at 37 °C. As a filtrate, the peptides that were produced were collected. On the basis of the frequency of tryptophan and tyrosine in vertebrate proteins, the peptide content was estimated using UV light spectral density at 280 nm and an extinction coefficient of 1.1 of a 0.1 % solution [9].
Peptides from each sample were pooled and then fractionated using Agilent 1260 Infinity II HPLC equipment with RP chromatography. The peptide mixture was loaded onto an XBridge Peptide BEH C18 Column, 130 Å, 5 μm, 4.6 mm 100 mm column after being diluted with buffer A(10 mM HCOONH4, 5 % can, pH 10.0). At a flow rate of 1 mL/min, the peptides were eluted using a gradient of buffer B (10 mM HCOONH4, 5 % can, pH 10.0), ranging from 0 % to 7 % for 5 min, 7–40 % for 5–40 min, 40%–100 % for 45–50 min, and 100 % for 50–65 min. Elution was monitored at 214 nm based on the UV light trace, and the fractions were collected every 1 min for 5–50 min. Vacuum centrifugation was employed to dry the obtained fractions down at 45 °C.
On a nanoplate (Bruker, Bremen, Germany) coupled to a timsTOF Pro (Bruker, Bremen, Germany) with a CaptiveSpray source, peptide samples (200 ng) blended with iRT were analysed. On a 25 cm × 75 mm analytical column with 1.6 mm C18 beads and a packed emitter tip (IonOpticks, Australia), peptides were separated. Using an integrated oven (Sonation GmbH, Germany), the column temperature was kept at 50 °C. Before the sample was placed in 100 % buffer A with 99.9 % Milli-Q water and 0.1 % FA, the column was equilibrated using four column volumes. Both equilibration and sample loading steps were performed at 800 bar. With a linear gradient starting at 2 % buffer B (99.9 % ACN, 0.1 % FA) and increasing to 25 % buffer B over the course of 90 min, the samples were separated at a flow rate of 300 nL/min. The gradient was then ramped up to 37 % buffer B for 10 min, then increased again to 80 % buffer B for 10 min, and then maintained at 80 % buffer B for 10 min.
The following parameters were used to operate the timsTOF Pro (Bruker, Bremen, Germany) in PASEF mode: mass range 100–1700 m/z, 1/K0 start 0.75 V s/cm2, End 1.4 V s/cm2, ramp time 100 ms, lock duty cycle set to 100 %, capillary voltage at 1500 V, dry gas at 3 l/min, and dry temp at 180 °C. PASEF settings included ten MS/MS scans with a total cycle time of 1.16 s, a charge range of 0–5, active exclusion for 0.4 min, a scheduling target intensity of 20,000, an intensity threshold of 2500, and a CID collision energy of 42 eV.
To create an initial target list, it was necessary to process and examine original DDA data using Spectronaut (Biognosys AG, Switzerland) with default parameters. For fixed modification, carbamidomethyl (C) was employed. The two different modifications were acetylation (Protein N-term) and oxidation (M). The precursor's and protein levels' Q-value (FDR) cutoffs were both fixed at 1 %.
To execute the data-independent isolation of multiple precursor windows inside a single TIMS separation for diaPASEF, we adapted the instrument firmware [10]. Two windows were employed in each 100 ms diaPASEF scan in our procedure. With narrow 25 m/z precursor windows, 18 scans covered the diagonal scan line for doubly and triple-charged peptides.
Spectronaut (Biognosys AG, Switzerland) was applied to process and analyse the raw DIA data using the default parameters. The dynamic iRT retention time prediction type was set. Based on comprehensive mass calibration, Spectronaut determined the data extraction. Spectronaut dynamically determines the ideal extraction window depending on the iRT calibration and gradient stability. The precursor's and protein levels' Q-value (FDR) cutoffs were both set at 1 %. The generation of decoys was set to mutate, similar to the scrambled generation, but with only a random number of AA position swamps (min = 2, max = length/2). For quantification, all chosen precursors that made it through the filter were used. Except for the three least interfering ions, all interfering fragment ions were eliminated by the MS2 interference. The number of major groups was determined by averaging the first three filtered peptides that passed the 1 % Q cutoff.
2.3. Bioinformatics analyses
All differentially expressed proteins' GO biological processes (BPs), cellular components (CC), and molecular function (MF) annotations were examined. The linked signal pathway of differentially expressed proteins was examined using the KEGG pathway analysis. A Venn diagram was used to detect co-differentially expressed proteins in the placenta and plasma. In the cluster analysis process, the clustering algorithm classifies two dimensions of samples and quantitative information of proteins, which further helps test the rationality of the screened target proteins.
2.4. Correlation analysis of plasma and placental proteins
Sequencing data for quantitative proteomics of placental and plasma samples were log-transformed. Pearson's correlation analysis was applied to explore whether there was a correlation between the expression levels of differentially expressed proteins in the plasma and placental samples. Finally, the targeted proteins with a correlation coefficient greater than 0.5 were selected for further experimental verification.
2.5. Immunohistochemistry
Paraffin blocks of placental tissue from 40 participants, including 20 ICP patients and 20 healthy pregnant women, were fabricated using tissue microarray (TMA). The TMA sections were then prepared for IHC staining. The primary antibodies used in this study were anti-NECTIN1 (1:100 dilution, Cat. 24713-1-AP, ProteinTech), anti-SPINT1 (1:100 dilution, Cat. ab189511; Abcam), anti-SERPINA7 (1:300 dilution; Cat. 66454-1-Ig; ProteinTech), anti-CD320 (1:300 dilution; Cat. 10343-1-AP, ProteinTech), anti-ITIH3 (1:100 dilution, Cat. DF14157, Affinity), anti-ENPP2 (1:50 dilution, Cat. 14243-1-AP, ProteinTech), and anti-SERPIND1 (1:25 dilution, Cat. ab232859; Abcam, Cambridge, UK). DAB and a counterstain of hematoxylin were used to visualize antibody staining. Two experienced individual pathologists assessed the TMA staining data. Simultaneously, the expression levels of NECTIN1, SPINT1, SERPINA7, CD320, ITIH3, ENPP2, and SERPIND1 were assessed based on the scores of immunoreactivity.
2.6. ELISA
Based on the requirements of the manufacturer, the levels of the NECTIN1 (Cat. ab289649; Abcam, Cambridge, UK), SPINT1 (cat. ab288176; Abcam, Cambridge, UK), SERPPINA7 (Cat. ab289642; Abcam), CD320 (Cat. DL-CD320-Hu, Develop), ITIH3 (Cat. RD-ITIH3-Hu, R&D Systems), ENPP2 (Cat. DL-ENPP2-Hu, Develop), and SERPIND1 (Cat. ab277402, Abcam) in all 60 maternal peripheral plasma samples were detected using their respective ELISA kits. By employing a Multifunctional Microplate Reader, the optical densities of the samples in each well were determined at 450 nm (Thermo Fisher Scientific).
2.7. Statistical analysis
For statistical analysis and figure presentation, we utilized GraphPad Prism 9 and SPSS 25.0. The proteomic data were log-transformed to fit a normal distribution. Student's t-test or non-parametric Mann–Whitney U test was employed in this study. They were applied to compare the differences in clinical indicators and expression levels of biomarker proteins between the ICP and control groups. Pearson's correlation or Spearman's correlation was used to analyse the correlation between sequencing data and experimental results and the correlation between experimental results and clinical data. ROC curve analysis was performed to assess the diagnostic value of the biomarkers for ICP. Sensitivities and specificities (with 95 % confidence intervals [CIs]) were calculated based on the area under the curves (AUCs). An AUC of more than 0.7 is regarded as an acceptable level of discrimination. Binary logistic regression analysis was adopted to produce ROC curves for protein combinations containing two or more proteins. All data are presented as means ± standard error (SEs). For all comments, when a P value was of <0.05, it was considered statistically significant and labelled with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3. Results
3.1. Clinical characteristics of pregnant women with ICP and healthy pregnant controls
Table 1 highlights the main clinical data and perinatal outcomes of the 20 pregnant women with ICP and the 20 controls. For the two groups, maternal age, alkaline phosphatase, total bilirubin, direct bilirubin, and newborn weight were not remarkably varied. However, compared to controls, female participants with ICP at the pregnancy stage had significantly greater levels of TBA, alanine transaminase (ALT), and aspartate transaminase (AST) (Table 1). When compared to controls, ICP patients had remarkably shorter gestational ages at birth (Table 1).
Table 1.
Clinical characteristics of pregnant women with ICP and healthy pregnant controls.
| Variable | Screening Samples |
Validation Samples |
||||
|---|---|---|---|---|---|---|
| Control(n = 8) | ICP(n = 8) | P value | Control(n = 20) | ICP(n = 20) | P value | |
| Maternal age(years) | 32.4 ± 4.9 | 31.4 ± 5.0 | 1.000 | 32.5 ± 5.6 | 31.6 ± 4.7 | 0.570 |
| Delivery weeks | 39.1 ± 0.4 | 37.0 ± 1.1 | 0.024* | 39.3 ± 0.7 | 37.0 ± 1.6 | 0.004* |
| TBA(μmol/L) | 2.7 ± 0.8 | 65.2 ± 48.2 | 0.005* | 4.0 ± 2.0 | 53.0 ± 39.1 | 0.000* |
| ALT(IU/L) | 9.1 ± 1.6 | 102.0 ± 69.5 | 0.000* | 11.1 ± 7.0 | 102.0 ± 85.5 | 0.000* |
| AST(IU/L) | 16.4 ± 3.4 | 67.8 ± 39.4 | 0.004* | 18.2 ± 4.6 | 73.3 ± 61.7 | 0.000* |
| ALP(IU/L) | 153.7 ± 65.2 | 236.1 ± 36.2 | 0.334 | 155.9 ± 57.4 | 216.8 ± 56.0 | 0.889 |
| TBIL(μmol/L) | 8.3 ± 3.1 | 14.8 ± 12.2 | 0.156 | 9.1 ± 3.1 | 12.3 ± 8.3 | 0.142 |
| DBIL(μmol/L) | 2.2 ± 1.0 | 7.8 ± 9.8 | 0.063 | 2.3 ± 0.9 | 5.6 ± 6.4 | 0.058 |
| Newborn weight(g) | 3391.3 ± 563.6 | 2914.4 ± 453.5 | 0.451 | 3579.5 ± 484.1 | 2975.0 ± 461.3 | 0.983 |
3.2. Screening and identification of differentially proteins
In the end, 290 proteins were expressed differently in the plasma of female participants with ICP at the pregnancy stage. Among them, 49 were downregulated and 241 were upregulated (Fig. 1A). The placental tissues of ICP patients also showed differential expression of 713 proteins. Among them, 663 proteins were upregulated and 50 proteins were downregulated (Fig. 1B).
Fig. 1.
Screening of co-differentially expressed proteins via proteomics quantitative analysis. (A) Volcano plot showing the analysis of differentially expressed proteins in plasma between ICP patients and healthy pregnant women (control). (B) Volcano plot displaying analysis of the differentially expressed proteins in the placenta between the two groups. (C) Venn diagram analysis of co-differentially expressed proteins between placental and plasma samples. (D, E) Clustering analysis of the top nine co-differentially expressed proteins with correlation coefficients between plasma and placental samples. ICP, intrahepatic cholestasis of pregnancy.
3.3. Gene ontology enrichment and pathway enrichment analyses of differentially expressed proteins
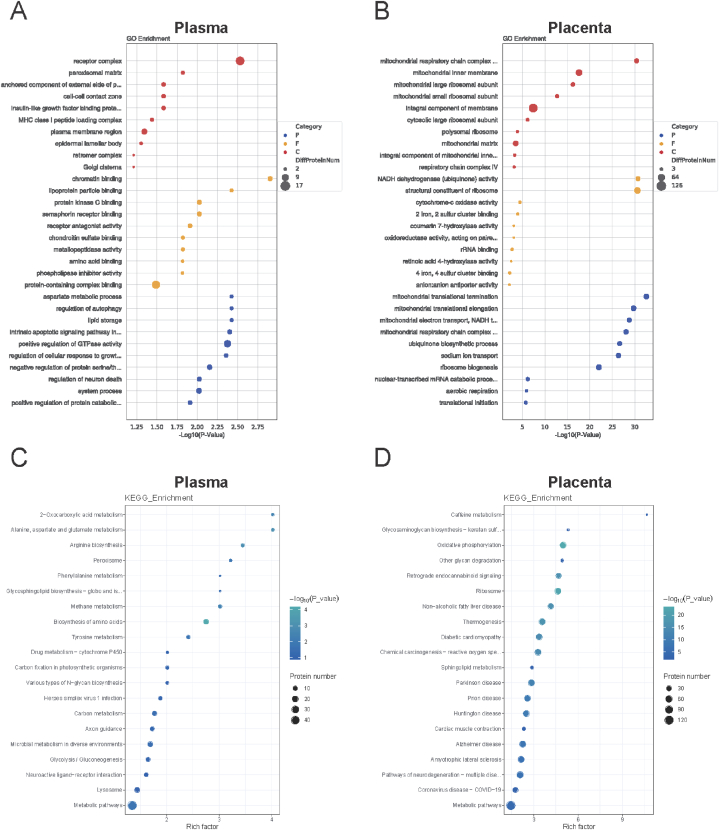
The GO enrichment-linked differently expressed proteins in plasma were identified. These proteins are related to many BPs, such as aspartate metabolism, autophagy regulation, and lipid storage. Proteins play a role in a variety of CC processes, including the receptor complex, peroxisomal matrix, and anchored components of the external side of the plasma membrane. These proteins are engaged in numerous MF-related processes, including chromatin, lipoprotein particles, and protein kinase C binding (Fig. 2A). The differentially expressed proteins were involved in metabolic pathways, 2oxo carboxylic acid metabolism, alanine metabolism, and aspartate and glutamate metabolism, according to KEGG pathway analysis (Fig. 2C).
Fig. 2.
Bioinformatics analysis of differentially expressed proteins. (A) GO analysis of the differentially expressed proteins in plasma samples. (B) GO analysis of the differentially expressed proteins in placental samples. (C) KEGG pathway analysis of the differentially expressed proteins in plasma samples. (D) KEGG pathway analysis of the differentially expressed proteins in placental samples. GO, gene ontology; KEGG, Kyoto Encyclopaedia of Genes and Genomes.
In addition, GO enrichment-linked differently expressed proteins in the placenta were identified. These proteins participate in various BPs. These processes contain mitochondrial electron transport, mitochondrial electron elongation, and mitochondrial translation termination. Proteins are also involved in many CC activities, including mitochondrial respiratory chain complex I, the mitochondrial inner membrane, and an integral component of the membrane. These proteins play important roles in various IF processes. They comprises ribosome structural elements, cytochrome-c oxidase activity, and NADH dehydrogenase (ubiquinone) activity (Fig. 2B). The KEGG pathway was then examined. The findings showed that the differentially expressed proteins played a role in sphingolipid metabolism, oxidative phosphorylation, and other metabolic pathways (Fig. 2D).
3.4. Identification of Co-differentially expressed proteins and correlation analysis
In the placenta and plasma, 17 proteins were shown to be co-differentially expressed by using a Venn diagram(Fig. 1C). These included CD177, ENPP2, GM2A, PODXL, NECTIN1, RPS7, FOLR2, ITIH3, NECTIN3, CD320, CD59, SPINT1, CD55, MMRN1, SERPINA7, SERPIND1, and FMOD. According to Pearson correlation analysis, CD177, GM2A, PODXL, FOLR2, CD59, CD55, MMRN1, and FMOD were not significantly correlated with placental or plasma expression levels (Fig. S1A). Additionally, we discovered a positive correlation between the expression levels of RPS7, NECTIN3, NECTIN1, SPINT1, SERPINA7, CD320, ITIH3, ENPP2, and SERPIND1 in the placenta and their plasma levels (r = 0.5077, P = 0.0447; r = 0.5464, P = 0.0285; r = 0.6130 and P = 0.0116; r = 0.6756 and P = 0.0041; r = 0.6922 and P = 0.0030; r = 0.7339 and P = 0.0012; r = 0.7385 and P = 0.0011, r = 0.7778 and P = 0.0004, and r = 0.7957 and P = 0.0002, respectively; Table 2, Fig. 3A–I). Nine proteins had considerably different expression levels between ICP patients and healthy pregnant controls, according to cluster analysis (Fig. 1D and E). Cluster analysis could aid in distinguishing subsets of proteins with different expression patterns from the protein ensembles.
Table 2.
Associated co-expressed differential proteins in placenta and plasma among pregnant women with ICP and healthy pregnant women.
| Gene | Protein | Placenta |
Plasma |
|||
|---|---|---|---|---|---|---|
| Fold Change | P value | Fold Change | P value | Trend | ||
| RPS7 | 40S ribosomal protein S7 | 1.33 | 0.004 | 2.88 | 0.013 | UP |
| NECTIN3 | Nectin-3 | 1.26 | 0.048 | 2.33 | 0.026 | UP |
| NECTIN1 | Nectin-1 | 1.35 | 0.009 | 1.28 | 0.015 | UP |
| SPINT1 | Kunitz-type protease inhibitor 1 | 1.22 | 0.022 | 1.49 | 0.002 | UP |
| SERPINA7 | Thyroxine-binding globulin | 0.77 | 0.008 | 0.66 | 0.000 | DOWN |
| CD320 | CD320 antigen | 1.24 | 0.025 | 4.00 | 0.006 | UP |
| ITIH3 | Inter-alpha-trypsin inhibitor heavy chain H3 | 1.28 | 0.038 | 1.39 | 0.025 | UP |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 | 1.61 | 0.022 | 1.81 | 0.004 | UP |
| SERPIND1 | Heparin cofactor 2 | 0.74 | 0.036 | 0.67 | 0.040 | DOWN |
Fig. 3.
Correlation analysis of co-differentially expressed protein levels between placental and plasma samples. (A) Correlation analysis of RPS7 between placental and plasma samples. (B) Correlation analysis of NECTIN3 between placental and plasma samples. (C) Correlation analysis of NECTIN1 between placental and plasma samples. (D) Correlation analysis of SPINT1 between placental and plasma samples. (E) Correlation analysis of SERPINA7 between placental and plasma samples. (F) Correlation analysis of CD320 between placental and plasma samples. (G) Correlation analysis of ITIH3 between placental and plasma samples. (H) Correlation analysis of ENPP2 between placental and plasma samples. (I) Correlation analysis of SERPIND1 between placental and plasma samples.
3.5. Correlation between proteomic expression values and ELISA assay results
Seven candidate proteins with a correlation coefficient greater than 0.7 between the placenta and plasma were identified in the proteomics data analysis. Subsequently, we analysed the correlation between quantitative proteomic data and the ELISA results. There was a significant correlation between the sequencing data and ELISA experimental data for NECTIN1, SPINT1, SERPINA7, ITIH3, and SERPIND1 (r = 0.6045, P = 0.0131; r = 0.5207, P = 0.0387; r = 0.5838, P = 0.0176; r = 0.7118, P = 0.0027; and r = 0.6586, P = 0.0055; respectively; Fig. 4D and E). CD320 and ENPP2 showed no significant correlation between the sequencing and experimental data (Figs. S1B–C).
Fig. 4.
Correlation analysis between ELISA and sequencing results and the validation of plasma differential proteins using ELISA. (A–E) The correlation analysis of SPINT1, SERPINA7, NECTIN1, SERPIND1, and ITIH3 between ELISA and sequencing results. (F) NECTIN1 levels in plasma samples obtained from ICP and control groups (P < 0.0001). (G) SPINT1 levels in plasma samples obtained from ICP and control groups (P < 0.0001). (H) ITIH3 levels in plasma samples obtained from ICP and control groups (P = 0.0001). (I) ENPP2 levels in plasma samples obtained from ICP and control groups (P < 0.0001). (J) SERPIND1 levels in plasma samples obtained from ICP and control groups (P < 0.0001); (****P < 0.0001, ***P < 0.001). ELISA, enzyme-linked immunosorbent assay; ICP, intrahepatic cholestasis of pregnancy.
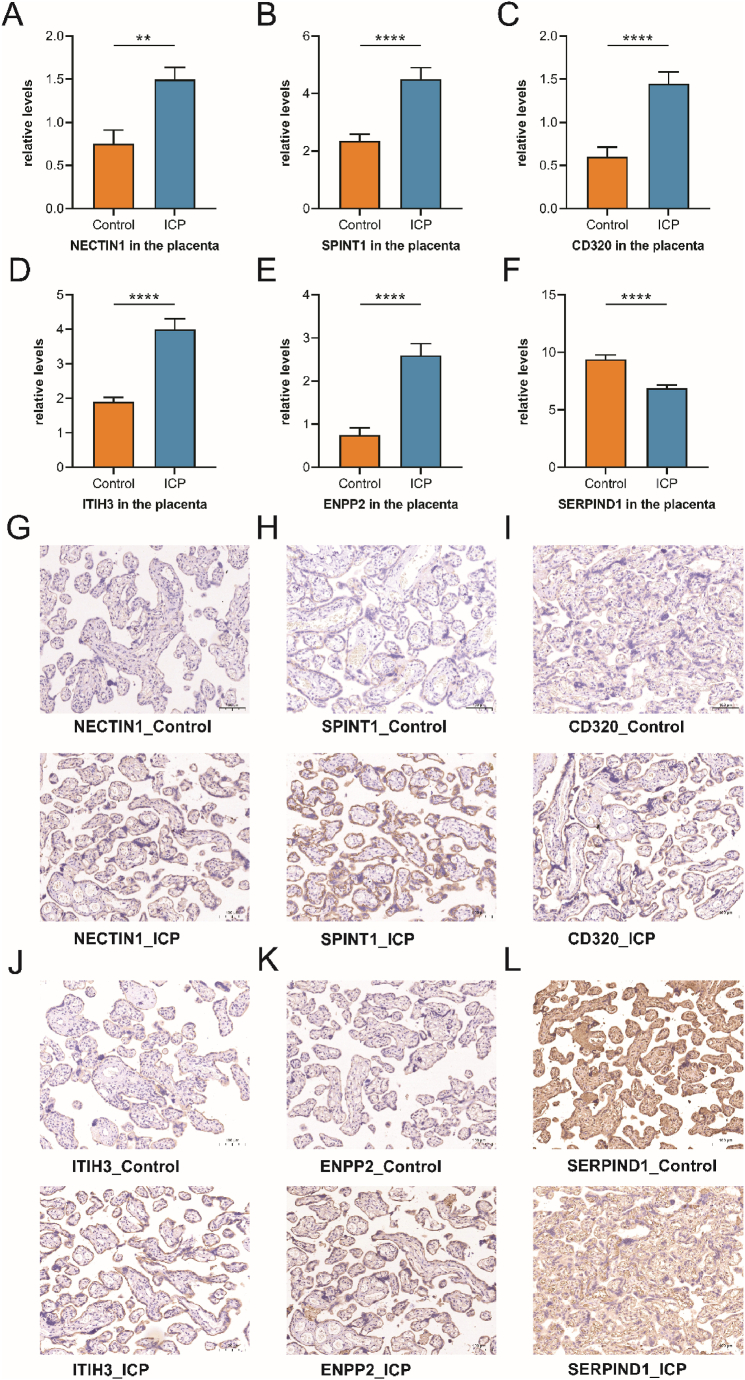
3.6. Validation of differentially expressed proteins in placenta (IHC)
IHC was used to validate our proteomic findings and further verify the differential expression of NECTIN1, SPINT1, SERPINA7, CD320, ITIH3, ENPP2, and SERPIND1 in the placentas of ICP patients and controls. CD320 was expressed in the cytomembrane (Fig. 5I), whereas NECTIN1 was expressed in both the cell membrane and secretion (Fig. 5G). SPINT1, SERPINA7, ITIH3, ENPP2, and SERPIND1 showed secreted protein staining in placental villous trophoblasts (Fig. 5H, Fig. S1D, Fig. 5J-L). Compared with the control group, the mean positive signal intensities of NECTIN1, SPINT1, CD320, ITIH3, and ENPP2 in the placenta of patients with ICP was significantly higher (P = 0.0022, P < 0.0001, P < 0.0001, P < 0.0001, and P < 0.0001; Fig. 5A–E). In addition, the IHC experiment indicated that ICP patients' placental SERPIND1 expression level was considerably lower than that of the controls(P < 0.0001; Fig. 5F). Additionally, we discovered that there was no remarkable difference between the two groups in terms of SERPINA7 expression (Fig. S1D).
Fig. 5.
Validation of placenta differential proteins using IHC. (A) NECTIN1 levels in the placental samples obtained from ICP and control groups (P = 0.0022). (B) SPINT1 levels in the placental samples obtained from ICP and control groups (P < 0.0001). (C) CD320 levels in the placental samples obtained from ICP and control groups (P < 0.0001). (D) ITIH3 levels in the placental samples obtained from ICP and control groups (P < 0.0001). (E) ENPP2 levels in the placental samples obtained from ICP and control groups (P < 0.0001). (F) SERPIND1 levels in the placental samples obtained from ICP and control groups (P < 0.0001); (****P < 0.0001, **P < 0.01). (G–L) IHC staining for NECTIN1, SPINT1, CD320, ITIH3, ENPP2, and SERPIND1 in the placental samples obtained from ICP and control groups ( × 200). IHC, immunohistochemistry; ICP, intrahepatic cholestasis of pregnancy.
3.7. Validation of differentially expressed proteins in plasma (ELISA)
Similar to the IHC experimental verification, NECTIN1, SPINT1, SERPINA7, CD320, ITIH3, ENPP2, and SERPIND1 levels in maternal plasma were determined using ELISA. After filtering these proteins, we found that NECTIN1, SPINT1, and ENPP2 levels were remarkably elevated in ICP patients compared with those controls (P < 0.0001; Fig. 4F–G, I). The ITIH3 level was also remarkably elevated in the ICP group compared with that in the control group (P = 0.0001; Fig. 4H). The SERPIND1 level was significantly lowered in the plasma of patients with ICP compared with that in standard samples (P < 0.0001; Fig. 4J). We found, however, that there was no remarkable variation in the expression of SERPINA7 or CD320 between patients with ICP and the control group.
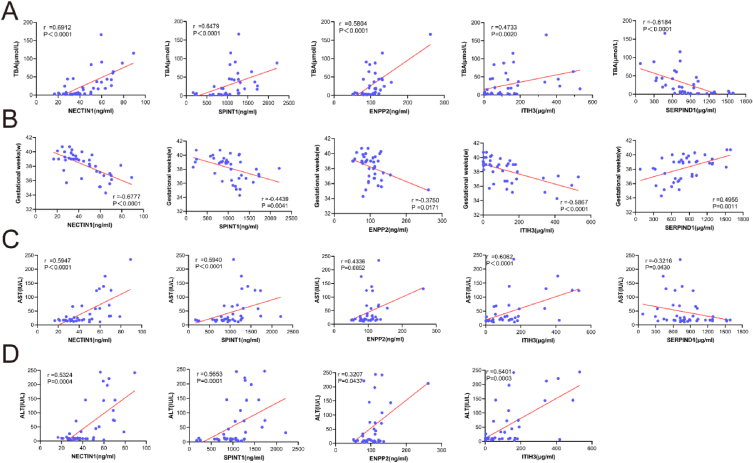
3.8. Correlation analysis of plasma differential proteins with TBA, ALT, AST, and gestational week
The levels of NECTIN1, SPINT1, ITIH3, and ENPP2 had a positive relationship with TBA levels (r = 0.6912, P < 0.0001; r = 0.6479, P < 0.0001; r = 0.4733, P = 0.0020; and r = 0.5804, P < 0.0001, respectively; Fig. 6A). SERPIND1 was negatively correlated with TBA (r = −0.6184 and P < 0.0001; Fig. 6A). CD320 and SERPINA7 expression was not significantly associated with TBA (Fig. S1E). We also found that the expression levels of NECTIN1, SPINT1, ITIH3, and ENPP2 were linked to ALT levels in a positive manner (r = 0.5324, P = 0.0004; r = 0.5653, P = 0.001; r = 0.5401, P = 0.0003; and r = 0.3207, P = 0.0437; respectively; Fig. 6D). The remaining three proteins did not correlate significantly with ALT levels (Fig. S2A). In addition, the results exhibited that NECTIN1, SPINT1, ITIH3, and ENPP2 positively correlated with AST levels (r = 0.5947, P < 0.0001; r = 0.5940, P < 0.0001; r = 0.6062, P < 0.0001; and r = 0.4336, P = 0.0052, respectively; Fig. 6C). SERPIND1 levels were associated with AST levels in a negative manner(r = 0.3216, P = 0.0430; Fig. 6C). We also found that SERPINA7 and CD320 levels were not significantly associated with AST levels (Fig. S2B). Moreover, we also analysed the correlation between the expression levels of these seven proteins and gestational week, suggesting that the expression levels of NECTIN1, SPINT1, ITIH3, and ENPP2 were negatively related to the gestational week (r = −0.6777, P < 0.0001; r = −0.4439, P = 0.0041; r = −0.586, P < 0.0001; and r = −0.3750, P = 0.0171; respectively; Fig. 6B), whereas SERPIND1 levels were correlated with the gestational week in a positive manner(r = 0.4955, P = 0.0011; Fig. 6B). The expression of CD320 and SERPINA7 did not correlate with the gestational age (Fig. S1F).
Fig. 6.
Correlations between co-differentially expressed proteins and clinical indicators (TBA, gestational weeks, AST, and ALT) in pregnant women. (A) The correlation analysis between TBA and expression levels of NECTIN1, SPINT1, ENPP2, ITIH3, and SERPIND1. (B) The correlation analysis between gestational weeks and expression levels of NECTIN1, SPINT1, ENPP2, ITIH3, and SERPIND1. (C) The correlation analysis between AST and expression levels of NECTIN1, SPINT1, ENPP2, ITIH3, and SERPIND1. (D) The correlation analysis between ALT and expression levels of NECTIN1, SPINT1, ENPP2, and SERPIND1. TBA, total bile acid; AST, aspartate transaminase; ALT, alanine transaminase.
3.9. Diagnostic utility of plasma differential protein levels
To assess the diagnostic efficacy of ICP, ROC curves were generated. On top of that, the corresponding AUCs were calculated. The AUCs for NECTIN1, SPINT1, ITIH3, and SERPIND1 were 0.7925, 0.8313, 0.8163, and 0.9075, respectively (Fig. 7A–E). The diagnostic utility of NECTIN1, SPINT1, ITIH3, and SERPIND1 at cutoff values of 50.12, 1064, 45.31, and 724.4 was evaluated; according to the Youden index, these cutoffs were optimal. The sensitivities and specificities of NECTIN1, SPINT1, ITIH3, and SERPIND1 were 55 % and 100 %, 72.5 % and 90 %, 92.5 % and 65 %, and 77.5 % and 95 %, respectively (Table 3). Although SERPINA7, CD320, and ENPP2 did not perform well, their AUCs were 0.6013, 0.6688, and 0.6650, respectively (Fig. S2C).
Fig. 7.
Diagnostic utility of single and combined secreted protein levels in maternal plasma for pregnant women with ICP. (A–E) The ROC curve of NECTIN1, SPINT1, ITIH3, and SERPIND1. (F–L) The ROC curve of SERPIND1/NECTIN1, SERPIND1/SPINT1, SERPIND1/ITIH3, SERPIND1/NECTIN1/SPINT1, SERPIND1/NECTIN1/ITIH3, SERPIND1/SPINT1/ITIH3, and SERPIND1/NECTIN1/SPINT1/ITIH3. ICP, intrahepatic cholestasis of pregnancy; ROC, receiver operator characteristics.
Table 3.
Diagnostic value analysis of plasma proteins in ICP.
| Plasma Protein | AUC | 95%CI | Sensitivity | Specificity | Cutoff Value |
|---|---|---|---|---|---|
| NECTIN1 (ng/ml) | 0.7925 | 0.6814 to 0.9036 | 0.55 | 1 | 50.12 |
| SPINT1(ng/ml) | 0.8313 | 0.7263 to 0.9362 | 0.725 | 0.9 | 1064 |
| SERPINA7(μg/ml) | 0.6013 | 0.4569 to 0.7456 | 0.45 | 0.9 | 5.773 |
| CD320(ng/ml) | 0.6688 | 0.5251 to 0.8124 | 0.8 | 0.45 | 2.593 |
| ITIH3(μg/ml) | 0.8163 | 0.6978 to 0.9347 | 0.925 | 0.65 | 45.31 |
| ENPP2(ng/ml) | 0.6650 | 0.5281 to 0.8019 | 0.6 | 0.75 | 94.47 |
| SERPIND1(μg/ml) | 0.9075 | 0.8327 to 0.9823 | 0.775 | 0.95 | 724.4 |
Following binary logistic regression analyses, it can be concluded the combined biomarker data produced AUCs of 0.9375, 0.9288, 0.9288, 0.9438, 0.9388, 0.9300, and 0.9425 for SERPIND1/NECTIN1, SERPIND1/SPINT1, SERPIND1/ITIH3, SERPIND1/NECTIN1/SPINT1, SERPIND1/NECTIN1/ITIH3, SERPIND1/SPINT1/ITIH3, and SERPIND1/NECTIN1/SPINT1/ITIH3, respectively (Fig. 7F-L). The sensitivities and specificities of the seven combinations were 90 % and 85 %; 77.5 % and 95 %; 92.5 % and 85 %; 90 % and 85 %; 90 % and 85 %; 90 % and 80 %; and 92.5 % and 80 %, respectively (Table 4). Based on the above results, it can be well established that combining these secreted proteins is a viable option. Furthermore, this novel biomarker elevates the compound's value in terms of its diagnostic power.
Table 4.
Diagnostic utility of combined plasma proteins in ICP.
| Plasma Protein | AUC | 95%CI | Sensitivity | Specificity |
|---|---|---|---|---|
| SERPIND1/NECTIN1 | 0.9375 | 0.8791 to 0.9959 | 0.9 | 0.85 |
| SERPIND1/SPINT1 | 0.9288 | 0.8655 to 0.9920 | 0.775 | 0.95 |
| SERPIND1/ITIH3 | 0.9288 | 0.8572 to 1.000 | 0.925 | 0.85 |
| SERPIND1/NECTIN1/SPINT1 | 0.9438 | 0.8901 to 0.9974 | 0.9 | 0.85 |
| SERPIND1/NECTIN1/ITIH3 | 0.9388 | 0.8818 to 0.9957 | 0.9 | 0.85 |
| SERPIND1/SPINT1/ITIH3 | 0.9300 | 0.8672 to 0.9928 | 0.9 | 0.8 |
| SERPIND1/NECTIN1/SPINT1/ITIH3 | 0.9425 | 0.8882 to 0.9968 | 0.925 | 0.8 |
4. Discussion
ICP features maternal pruritus and elevated serum TBA concentrations and triggers a series of adverse pregnancy outcomes, such as the staining of amniotic fluid, spontaneous preterm labour, and unexpected intrauterine death. In the current study, it was discovered that females with ICP at the pregnancy stage had considerably lower gestational ages than pregnant women in the control group. This suggests that patients with ICP have a higher risk of premature birth. Taking this into consideration, early ICP diagnosis and prediction have significant clinical significance. However, the current clinical diagnosis of ICP is mainly based on TBA levels, and its sensitivity and specificity are limited. Patients with other hepatobiliary disorders may also have increased TBA levels, according to Williamson et al. [11]. This also includes hepatic fibrosis or cirrhosis, primary biliary cirrhosis, acute fatty liver, and hepatitis C infection during pregnancy. A total of 5073 articles were found by Manzotti et al. [12]. Their findings showed that the sensitivity and specificity ranges of TBA were 0.71–0.98 and 0.81 to 0.97, respectively. Consequently, it is important to find useful biomarkers. This may aid in improving the specificity and sensitivity for ICP prediction and diagnosis.
Secreted proteins are defined as those that are actively transported out of the cell. Proteins secreted by cells play crucial roles in many physiological processes and are essential for intercellular communication [13]. The secreted proteins can be identified in primary tissues and secreted into the blood as candidate biomarkers. Therefore, secreted proteins have been widely used in mechanical research as potential clinical molecular biomarkers for gynaecological and obstetrical diseases. According to Cocco et al. [14], patients with endometrioid endometrial cancer had a remarkably higher expression level of serum amyloid A (SAA), a protein released by the liver, than that in normal endometrial tissue. They also discovered that SAA might be a new biomarker for endometrioid cancer. The recurrence of the disease and the effectiveness of treatment could be monitored using this marker. Klotz et al. [15]found that the secreted protein Dickkopf-1 (DKK1) can suppress the differentiation and bone-building activity of osteoblasts by acting as an inhibitor of Wnt signalling, and soluble DKK1 can be used as a potential therapy-monitoring marker in ovarian cancer patients. A pre-eclamptic placental meta-signature made up of 40 annotated gene transcripts and 17 miRNAs was discovered based on the findings of Kleinrouweler et al. [16]. In addition, they discovered that a protein produced by the cell was transcribed by at least half of the mRNA transcripts. This finding suggests that it might be used as a biomarker. There have not been many studies on secreted proteins as non-invasive indicators for ICP, as far as we know. As a result, we jointly examined differentially expressed proteins in the placenta and plasma of females with ICP at the pregnancy stage for the first time.
NECTIN1 is a member of the Nectins family, a new class of cell-adhesion molecules that play an essential role in tumourigenesis and disease progression. NECTIN1 is involved in mechanical adhesive puncta and adherent junctions of synapses. Yamada et al. [17] found that diffuse NECTIN1 expression in the cancer-associated fibroblasts of pancreatic ductal adenocarcinoma patients was associated with invasion, metastasis, and shorter survival. NECTIN1 was expressed at a higher level in liver hepatocellular carcinoma (HCC) than in paracancerous tissues, according to Wang et al. [18]. NECTIN1 may be a promising novel molecular marker for prognostic evaluation and therapeutic target of HCC, according to the aforementioned study. NECTIN1 is significant because it can enhance hepatocellular carcinoma cell proliferation and migration. Additionally, it can differentiate between the prognosis of HCC at various stages and grades. In our study, the NECTIN1 level was remarkably higher in ICP patients compared with that in controls. In addition, it was negatively correlated with gestational week (delivery), suggesting that NECTIN1 has excellent potential as a marker for the diagnosis and prognosis of ICP. The AUC of NECTIN1 was 0.7925. Its sensitivity and specificity were 55 % and 100 %, respectively, suggesting that this secreted protein has high specificity and accuracy in the diagnosis of ICP. However, the sensitivity of this secreted protein is low. This means that it must be used in combination with the other three secreted proteins to increase the sensitivity.
SPINT1 is a membrane-bound cell-surface protease that was initially identified for its ability to inhibit hepatocyte growth factor activators. Prostasin, matriptase, and hepsin are among the cell surface proteases that SPINT1 targets. It was worth noting that Szabo et al. [19] discovered that Spint1 loss led to embryonic lethality in genetic mouse knockout tests. Additionally, it was linked to labyrinthine dysplasia and severe placental anomalies. Low circulating levels of SPINT1 may be a sign of real placental insufficiency, according to Kaitu'u-Lino et al.’s findings [20]. This could be a biomarker for pregnancies with poor placental function and foetal growth restriction. Nevertheless, the expression level of SPINT1 was negatively correlated with gestational age in our study. This finding seems inconsistent with the results of previous studies. The value of future research may lie more in elucidating its molecular mechanisms. Determining if the ICP is being caused by a driver or a bystander is also crucial. SPINT1's AUC was 0.8313. It had a sensitivity of 72.5 % and a specificity of 90 %, respectively. SPINT1 alone has a low sensitivity. In order to predict and diagnose ICP, various indications must be included.
ITIH3 is a member of the α-trypsin inhibitor family and is enriched in the extracellular matrix of various organs, including blood circulation. A well-known function of this protein family is to covalently bind hyaluronic acid to stabilise the extracellular matrix. Plasma expression levels of ITIH3 were higher in gastric cancer patients than in healthy controls, according to Chong et al. [21]. ITIH3 may therefore be a crucial biomarker for the early detection of gastric cancer. In a recent study, Liu et al. [22] found that the level of ITIH3 was 2.04 times higher in a castration-resistant prostate cancer (CRPC) group than in a prostate cancer group, and ITIH3 had the potential to become a CRPC marker. However, ITIH3 has rarely been studied in obstetric diseases. De Almeida et al. [23] revealed that plasma ITIH3 may be a biomarker of inflammation that helps monitor the onset and progression of preeclampsia and gestational hypertension. The AUC of ITIH3 is 0.8163. Its sensitivity and specificity are 92.5 % and 65, respectively. Although these two values are relatively high, the protein specificity is low. Therefore, it is necessary to use it in combination with three other proteins. This will enhance its specificity.
SERPIND1 belongs to the serine protease inhibitor (SERPINS) superfamily. SERPIND1 is mainly secreted by the hepatocytes and circulates in the blood system [24]. Liao et al. [25] revealed that SERPIND1 is a novel metastasis enhancer that is overexpressed in non-small cell lung cancer (NSCLC) and may be used as a prognostic predictor in NSCLC treated with heparin. Ikeda et al. [26] found that SERPIND1 is involved in the regulation of angiogenesis and is a novel therapeutic target for the treatment of patients with peripheral circulatory insufficiency. Rau et al. [27] showed that the expression level of SERPIND1 was elevated during pregnancy, contributing to the maintenance of placental hemostasis. Nevertheless, we found that SERPIND1 levels were downregulated in ICP patients than in controls. Therefore, we speculated that the increased probability of postpartum haemorrhage in the ICP group might be related to the downregulation of SERPIND1 expression, indicating that SERPIND1 has great potential as a biomarker for ICP. The AUC of SERPIND1 was 0.9075, which was the highest among all candidate markers. The sensitivity and specificity of SERPIND1 were 77.5 % and 95 %, respectively. Therefore, different combinations of SERPIND1 and three other secreted proteins were used to identify a more stable diagnostic biomarker.
5. Conclusions
Through preliminary sequencing of plasma and placental quantitative proteomic data filtering, we verified seven candidate protein markers using ELISA and IHC experiments and eventually identified NECTIN1, SPINT1, ITIH3, and SERPIND1 as promising biomarkers for the diagnosis of ICP, with an AUC of 0.9425. However, this is preliminary work; the diagnostic performance of the four secreted proteins combined still requires more convincing validation with large sample sizes in future research. We will further investigate the biological functions of these four secreted proteins to explore the pathogenesis of ICP.
Data availability statement
Data will be made available on request.
Funding
This study was kindly supported by many projects. They are the Major Project of the Wuxi Science and Technology Bureau (N20201006), Wuxi Double-Hundreds Talent Fund Project (BJ2020076), Wuxi Health Commission Precision Medicine Project (J202106), Jiangsu Provincial Maternal and Child Health Research Project (F202034), and Jiangsu Provincial Six Talent Peaks Project (No. YY-124).
Ethics statement
This study was reviewed and approved by [ Wuxi Maternal and Child Health Hospital Ethical Review], with the approval number:[ 2020-01-0310-08 ]. All patients provided informed consent to participate in the study. All patients provided informed consent for the publication of their anonymised case details and images.
CRediT authorship contribution statement
Lingyan Chen: Writing – review & editing, Writing – original draft, Data curation. Jingyang Li: Methodology, Formal analysis. Yilan You: Investigation, Conceptualization. Zhiwen Qian: Visualization, Formal analysis. Jiayu Liu: Formal analysis. Ying Jiang: Validation, Software. Ying Gu: Supervision, Project administration. Jianping Xiao: Supervision, Project administration. Yan Zhang: Supervision, Resources, Project administration, Funding acquisition.
Conflict of interest statement
The authors declare no competing interests.
Acknowledgments
We would like to express our sincere gratitude to all participants for their dedication and cooperation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21616.
Contributor Information
Lingyan Chen, Email: chenlingyan9711@163.com.
Jingyang Li, Email: sunshineljy666@163.com.
Yilan You, Email: youyilan@126.com.
Zhiwen Qian, Email: qian99022021@163.com.
Jiayu Liu, Email: joey_ljy7193@163.com.
Ying Jiang, Email: 13771361998@163.com.
Ying Gu, Email: 13861870460@163.com.
Jianping Xiao, Email: jianpingx999@126.com.
Yan Zhang, Email: fuyou2007@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Society for Maternal-Fetal Medicine (Smfm) Electronic address: pubs@smfm.org, R.H. Lee, null mara greenberg, T.D. Metz, C.M. Pettker, society for maternal-fetal medicine consult series #53: intrahepatic cholestasis of pregnancy: replaces consult #13, april 2011. Am. J. Obstet. Gynecol. 2021;224:B2–B9. doi: 10.1016/j.ajog.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Roediger R., Fleckenstein J. Intrahepatic cholestasis of pregnancy: natural history and current management. Semin. Liver Dis. 2021;41:103–108. doi: 10.1055/s-0040-1722264. [DOI] [PubMed] [Google Scholar]
- 3.Beuers U., Wolters F., Oude Elferink R.P.J. Mechanisms of pruritus in cholestasis: understanding and treating the itch. Nat. Rev. Gastroenterol. Hepatol. 2023;20:26–36. doi: 10.1038/s41575-022-00687-7. [DOI] [PubMed] [Google Scholar]
- 4.Sentilhes L., Verspyck E., Pia P., Marpeau L. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2006;107:458–460. doi: 10.1097/01.AOG.0000187951.98401.f7. [DOI] [PubMed] [Google Scholar]
- 5.Dong R., Ye N., Zhao S., Wang G., Zhang Y., Wang T., Zou P., Wang J., Yao T., Chen M., Zhou C., Zhang T., Luo L. Studies on novel diagnostic and predictive biomarkers of intrahepatic cholestasis of pregnancy through metabolomics and proteomics. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.733225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou S., Dong R., Wang J., Liang F., Zhu T., Zhao S., Zhang Y., Wang T., Zou P., Li N., Wang Y., Chen M., Zhou C., Zhang T., Luo L. Use of data-independent acquisition mass spectrometry for comparative proteomics analyses of sera from pregnant women with intrahepatic cholestasis of pregnancy. J. Proteonomics. 2021;236 doi: 10.1016/j.jprot.2021.104124. [DOI] [PubMed] [Google Scholar]
- 7.Meier F., Brunner A.-D., Frank M., Ha A., Bludau I., Voytik E., Kaspar-Schoenefeld S., Lubeck M., Raether O., Bache N., Aebersold R., Collins B.C., Röst H.L., Mann M. diaPASEF: parallel accumulation-serial fragmentation combined with data-independent acquisition. Nat. Methods. 2020;17:1229–1236. doi: 10.1038/s41592-020-00998-0. [DOI] [PubMed] [Google Scholar]
- 8.Gharesi-Fard B., Zolghadri J., Kamali-Sarvestani E. Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta. 2010;31:121–125. doi: 10.1016/j.placenta.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 10.Demichev V., Szyrwiel L., Yu F., Teo G.C., Rosenberger G., Niewienda A., Ludwig D., Decker J., Kaspar-Schoenefeld S., Lilley K.S., Mülleder M., Nesvizhskii A.I., Ralser M. dia-PASEF data analysis using FragPipe and DIA-NN for deep proteomics of low sample amounts. Nat. Commun. 2022;13:3944. doi: 10.1038/s41467-022-31492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson C., Geenes V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2014;124:120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 12.Manzotti C., Casazza G., Stimac T., Nikolova D., Gluud C. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst. Rev. 2019;7:CD012546. doi: 10.1002/14651858.CD012546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Gao Z., Sun T., Zhang S., Yang S., Zheng M., Shen H. Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: a comprehensive review of preclinical and clinical studies. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1098570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocco E., Bellone S., El-Sahwi K., Cargnelutti M., Buza N., Tavassoli F.A., Schwartz P.E., Rutherford T.J., Pecorelli S., Santin A.D. Serum amyloid A: a novel biomarker for endometrial cancer. Cancer. 2010;116:843–851. doi: 10.1002/cncr.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz D.M., Link T., Goeckenjan M., Wimberger P., Poetsch A.R., Jaschke N., Hofbauer L.C., Göbel A., Rachner T.D., Kuhlmann J.D. Evaluation of circulating Dickkopf-1 as a prognostic biomarker in ovarian cancer patients. Clin. Chem. Lab. Med. 2022;60:109–117. doi: 10.1515/cclm-2021-0504. [DOI] [PubMed] [Google Scholar]
- 16.Kleinrouweler C.E., van Uitert M., Moerland P.D., Ris-Stalpers C., van der Post J.A.M., Afink G.B. Differentially expressed genes in the pre-eclamptic placenta: a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada M., Hirabayashi K., Kawanishi A., Hadano A., Takanashi Y., Izumi H., Kawaguchi Y., Mine T., Nakamura N., Nakagohri T. Nectin-1 expression in cancer-associated fibroblasts is a predictor of poor prognosis for pancreatic ductal adenocarcinoma. Surg. Today. 2018;48:510–516. doi: 10.1007/s00595-017-1618-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Xing Z., Chen H., Yang H., Wang Q., Xing T. High expression of nectin-1 indicates a poor prognosis and promotes metastasis in hepatocellular carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.953529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo R., Molinolo A., List K., Bugge T.H. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- 20.Kaitu’u-Lino T.J., MacDonald T.M., Cannon P., Nguyen T.-V., Hiscock R.J., Haan N., Myers J.E., Hastie R., Dane K.M., Middleton A.L., Bittar I., Sferruzzi-Perri A.N., Pritchard N., Harper A., Hannan N.J., Kyritsis V., Crinis N., Hui L., Walker S.P., Tong S. Circulating SPINT1 is a biomarker of pregnancies with poor placental function and fetal growth restriction. Nat. Commun. 2020;11:2411. doi: 10.1038/s41467-020-16346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong P.K., Lee H., Zhou J., Liu S.-C., Loh M.C.S., Wang T.T., Chan S.P., Smoot D.T., Ashktorab H., So J.B.Y., Lim K.H., Yeoh K.G., Lim Y.P. ITIH3 is a potential biomarker for early detection of gastric cancer. J. Proteome Res. 2010;9:3671–3679. doi: 10.1021/pr100192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P., Wang W., Wang F., Fan J., Guo J., Wu T., Lu D., Zhou Q., Liu Z., Wang Y., Shang Z., Chan F.L., Yang W., Li X., Zhao S.-C., Zheng Q., Wang F., Wu D. Alterations of plasma exosomal proteins and motabolies are associated with the progression of castration-resistant prostate cancer. J. Transl. Med. 2023;21:40. doi: 10.1186/s12967-022-03860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Almeida L.G.N., Young D., Chow L., Nicholas J., Lee A., Poon M.-C., Dufour A., Agbani E.O. Proteomics and metabolomics profiling of platelets and plasma mediators of thrombo-inflammation in gestational hypertension and preeclampsia. Cells. 2022;11:1256. doi: 10.3390/cells11081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bano S., Fatima S., Ahamad S., Ansari S., Gupta D., Tabish M., Rehman S.U., Jairajpuri M.A. Identification and characterization of a novel isoform of heparin cofactor II in human liver. IUBMB Life. 2020;72:2180–2193. doi: 10.1002/iub.2361. [DOI] [PubMed] [Google Scholar]
- 25.Liao W.-Y., Ho C.-C., Hou H.-H., Hsu T.-H., Tsai M.-F., Chen K.-Y., Chen H.-Y., Lee Y.-C., Yu C.-J., Lee C.-H., Yang P.-C. Heparin co-factor II enhances cell motility and promotes metastasis in non-small cell lung cancer. J. Pathol. 2015;235:50–64. doi: 10.1002/path.4421. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y., Aihara K., Yoshida S., Iwase T., Tajima S., Izawa-Ishizawa Y., Kihira Y., Ishizawa K., Tomita S., Tsuchiya K., Sata M., Akaike M., Kato S., Matsumoto T., Tamaki T. Heparin cofactor II, a serine protease inhibitor, promotes angiogenesis via activation of the AMP-activated protein kinase-endothelial nitric-oxide synthase signaling pathway. J. Biol. Chem. 2012;287:34256–34263. doi: 10.1074/jbc.M112.353532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau J.C., Mitchell J.W., Fortenberry Y.M., Church F.C. Heparin cofactor II: discovery, properties, and role in controlling vascular homeostasis. Semin. Thromb. Hemost. 2011;37:339–348. doi: 10.1055/s-0031-1276582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.