Abstract
Background
Surgical site infections are common and expensive infections that can cause fatalities or poor patient outcomes. To prevent these infections, antibiotic prophylaxis is used. However, excessive antibiotic use is related to higher costs and the emergence of antimicrobial resistance.
Objectives
The present meta-analysis aimed to compare the effectiveness of a single dosage versus several doses of antibiotics in preventing the development of surgical site infections.
Methods
PubMed was used to find clinical trials evaluating the effectiveness of a single dosage versus several doses of antibiotics in avoiding the development of surgical site infections. The study included trials that were published between 1984 and 2022. Seventy-four clinical trials were included in the analysis. Odds ratios were used to compare groups with 95% confidence intervals. The data were displayed using OR to generate a forest plot. Review Manager (RevMan version 5.4) was used to do the meta-analysis.
Results
Regarding clean operations, there were 389 surgical site infections out of 5,634 patients in a single dose group (6.90%) and 349 surgical site infections out of 5,621 patients in multiple doses group (6.21%) (OR = 1.11, lower CI = 0.95, upper CI = 1.30). Regarding clean-contaminated operations, there were 137 surgical site infections out of 2,715 patients in a single dose group (5.05%) and 137 surgical site infections out of 2,355 patients in multiple doses group (5.82%) (OR = 0.87, lower CI = 0.68, upper CI = 1.11). Regarding contaminated operations, there were 302 surgical site infections out of 3,262 patients in a single dose group (9.26%) and 276 surgical site infections out of 3,212 patients in multiple doses group (8.59%) (OR = 1.11, lower CI = 0.84, upper CI = 1.47). In general, there were 828 surgical site infections out of 11,611 patients in a single dose group (7.13%) and 762 surgical site infections out of 11,188 patients in multiple doses group (6.81%) (OR = 1.05, lower CI = 0.93, upper CI = 1.20). The difference between groups was not significant.
Conclusion
The present study showed that using a single-dose antimicrobial prophylaxis was equally effective as using multiple doses of antibiotics in decreasing surgical site infections.
Keywords: Antibiotics, Clinical Trials, Multiple Doses, Single Dose, Surgical Site Infections
1. Introduction
Healthcare-associated infections are infections that happen while getting medical care, develop in hospitals or other health centers, and appear forty-eight hours or more after admission to the hospital or within thirty days of receiving medical care (Haque et al., 2018). The US Centers for Disease Control and Prevention says that about 1,700,000 hospitalized patients get healthcare-associated infections every year while being managed for other health problems (Klevens et al., 2007). Patients who have surgery often get infections at the site of the surgery. This is the most prevalent infection caused by medical care (Borchardt and Tzizik, 2018).
Infection at the surgical site can affect any cavity, joint, bone, tissue, or prosthetic that was incised during or after surgery (Hall et al., 2015, Idris et al., 2020). Infections at surgical sites can be stratified into three categories based on the depth of the incision: deep incisional, superficial incisional, and infections in organs and spaces (Borchardt and Tzizik, 2018). Surgical procedures and their incisions are classified as dirty/infected, contaminated, clean-contaminated, or clean. A clean wound is “an uninfected operative wound in which no inflammation is encountered and the respiratory, alimentary, genital, or uninfected urinary tract is not entered”. Clean-contaminated is ”an operative wound in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination”. A contaminated wound is “ an open, fresh, accidental wound. In addition, operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract and incisions in which acute, nonpurulent inflammation is encountered are included in this category”. A dirty wound is “an old traumatic wound with retained devitalized tissue and those that involve an existing clinical infection or perforated viscera“. (CDC, 1999).
If an infection develops within thirty days of surgery, or ninety days if a prosthesis was implanted, it is considered a surgical site infection (SSI) (Seidelman and Anderson, 2021). These infections are some of the most common and expensive ones that are connected to healthcare, and they can cause fatalities or poor patient outcomes (Zabaglo and Sharman, 2023). Local effects of SSI include delayed and ineffective wound healing, osteomyelitis, cellulitis, abscess formation, and the wound becoming worse over time. Systemic effects include bacteremia, which has a chance of distant hematogenous spread, and sepsis (Berríos-Torres et al., 2017).
Since SSIs are a major source of mortality and morbidity, many guidelines and standards have been established to reduce their prevalence (Edwards et al., 2006). To prevent SSIs, antibiotic prophylaxis is used (Engelman et al., 2007, Gordon et al., 1998). Nonetheless, the inappropriate use of antibiotics is one of the most serious global public health threats (Kabrah et al., 2022, Alhomoud et al., 2017, Hussein et al., 2022, Alsugoor et al., 2022, Shaheen et al., 2018, Ahmed et al., 2022a, Ahmed et al., 2022b). Using antibiotics to excess is linked to a greater risk of unpleasant effects, more frequent return visits, and increased use of medical therapy for illnesses that, if left untreated, would generally recover on their own. Furthermore, it is linked to the development of antimicrobial resistance and elevated mortality rates (Harbarth et al., 2000, Llor and Bjerrum, 2014, Kreter and Woods, 1992).
To avoid SSIs and improve postoperative recovery, a single dose of prophylactic antibiotic is recognized as a component of surgical practice in several procedures. The possible clinical advantages of giving the antibiotic in a single dose have drawn more attention in recent years (Bratzler and Houck, 2004, Gilbert et al., 2007, ASHP, 2023, Ahmed et al., 2022a, Ahmed et al., 2022b). Using a single dose of prophylaxis reduces antimicrobial resistance, diminishes superinfections and drug toxicity, and decreases care costs (Edwards et al., 2006).
New guidelines recommended the use of a reduced postoperative course of antibiotics that involves a single dosage or continuance for less than 24 h. According to a study conducted by McDonald et al., there was no discernible superiority between single- or multiple-dose regimens in terms of preventing SSIs. Consequently, they proposed the ongoing utilization of a single-dose antimicrobial prophylaxis for major surgical procedures (McDonald et al., 1998). Igwemadu et al. and Das et al. reported that antibiotic prophylaxis with one dose is just as effective as prophylaxis with several doses while being less expensive and less likely to lead to antibiotic resistance (Igwemadu et al., 2022, Das et al., 2021). Jogdand et al. stated that prophylactic use of combination chemotherapy, which is continued for 5 to 7 days, is the norm in India to prevent SSI. These prolonged treatment periods place a financial burden on the patient or the government without providing the patient with any further benefits, which ultimately results in resource waste (Jogdand et al., 2017). The aim of this meta-analysis was to examine the hypothesis that the efficacy of a single-dose antibiotic regimen is comparable to that of multiple doses of antibiotics in decreasing the incidence of SSIs.
2. Materials and Methods
PubMed was used to find studies evaluating the effectiveness of a single dosage versus several doses of antibiotics in avoiding the occurrence of SSIs. In the advanced search, the terms “surgical site infections,” single dosage,“ and ”antimicrobial“ were used.
The analysis was limited to published clinical research involving human beings. Other studies are not included in the present analysis. The study included clinical trials that compared single doses with multiple doses that were received by patients who had different types of operations. Furthermore, cross-checking of references in individual papers was conducted.
The study included trials that were published between 1984 and 2022. Most of the included trials were published in English, but there were several papers that were written in other languages, but their abstracts in English included the required data. In addition to the overall number of patients who received a single prophylactic antibiotic dose, the number of SSIs among these patients was also gathered. The study also examined the overall number of patients who received multiple prophylactic antibiotic doses and the incidence of SSIs among these patients.
The rate of SSIs in the single-dose and multiple-doses groups was the endpoint of the present study. The numbers and odds ratios of SSIs were compared between these groups. The included studies were categorized according to the types of operations that the patients had into three categories: clean operations, clean-contaminated operations, and contaminated operations.
Odds ratios were used to compare groups with 95% confidence intervals. The data were displayed using OR to generate a forest plot. The heterogeneity of the studies was analyzed using the I2 statistic. A score of 50% or higher for I2 indicated significant heterogeneity among trials. A p value of 0.05 was used as the threshold for statistical significance. Review Manager version 5.4 was used to do the meta-analysis (The Cochrane Collaboration, 2020 Copenhagen, Denmark).
3. Results
Seventy-four clinical trials were included in the analysis. The included trials were published between 1984 and 2022. The study flow chart is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram.
Among the clinical trials that were included in the present study, thirty trials included clean incisions, twenty trials included clean-contaminated incisions, and twenty-four trials included contaminated incisions. Twenty-six studies were published after 2000. Table 1 shows the clinical trials that were included in the study.
Table 1.
The clinical trials that were included in the analysis.
| Incision | Study | Type of surgery |
|---|---|---|
| Clean | Gahm et al., 2022 | Breast reconstruction |
| Igwemadu et al., 2022 | Caesarean section | |
| Sheth et al., 2019 | Dacryocystorhinostomy | |
| Wahab et al., 2013 | Bilateral sagittal split osteotomies | |
| Danda et al., 2010 | Orthognathic surgery | |
| Tamayo et al., 2008 | Cardiac surgery | |
| Hellbusch et al., 2008 | Instrumented lumbar fusion | |
| Lindeboom et al., 2005 | Intraoral bone grafting procedures | |
| Su et al., 2005 | Gynecologic surgery | |
| Lindeboom et al., 2003 | Bilateral sagittal ramus osteotomies | |
| Salminen et al., 1999 | Cardiovascular surgery | |
| Gagey et al., 1999 | Open tibial fracture | |
| Kester et al., 1999 | Vascular surgery | |
| Morimoto and Kinoshita, 1998 | Breast cancer surgery | |
| Hall et al., 1998 | Vascular surgery | |
| Kriaras et al., 1997 | Cardiac surgery | |
| Nooyen et al., 1994 | Coronary artery bypass grafting | |
| Morris, 1994 | Upper abdominal operations | |
| Hall et al., 1993 | Cardiac operations | |
| Galbraith et al., 1993 | Cardiac operations | |
| Wertzel et al., 1992 | Thoracic surgery | |
| Maier and Strutz, 1992 | Head and neck surgery | |
| Nachtkamp et al., 1991 | Abdominal surgery | |
| Olak et al., 1991 | Thoracic surgery | |
| Buckley et al., 1990 | Hip fracture surgery | |
| Karachalios et al., 1990 | Peritrochanteric fractures | |
| Hall et al., 1989 | Abdominal surgery | |
| Periti et al., 1988 | Gynaecological and obstetric surgery | |
| Oostvogel et al., 1987 | General operations | |
| Periti et al., 1984 | Gynecologic and obstetrical surgery | |
| Clean-Contaminated | Loozen et al., 2017 | Cholecystitis |
| Westen et al., 2015 | Cesarean section | |
| Lyimo et al., 2013 | Caesarean section | |
| Alekwe et al., 2008 | Cesarean section | |
| Sakura et al., 2008 | Prostatectomy | |
| Mohri et al., 2007 | Gastric cancer surgery | |
| Kayihura et al., 2003 | Biliary surgery | |
| Hotz et al., 1994 | Maxillofacial surgery | |
| Meijer and Schmitz, 1993 | Biliary surgery | |
| Hjortrup et al., 1991 | Biliary surgery | |
| Galask et al., 1988 | Cesarean section | |
| Roy et al., 1988 | Hysterectomy | |
| McGregor et al., 1988 | Cesarean section | |
| Fabian et al., 1988 | Biliary surgery | |
| Berkeley et al., 1988 | Hysterectomy | |
| El Mufti and Glessa, 1988 | Cholecystectomy | |
| Gall & Hill, 1987 | Cesarean operation | |
| Roy et al., 1984 | Hysterectomy | |
| Maki et al., 1984 | Biliary tract operations or hysterectomy | |
| Kellum et al., 1984 | biliary operations | |
| Contaminated | Espin Basany et al., 2020 | Colon surgery |
| Rafiq et al., 2013 | Appendectomy | |
| Ishibashi et al., 2014 | Rectal cancer surgery | |
| Ahn et al., 2013 | Colorectal surgery | |
| Oshima et al., 2013 | Proctocolectomy | |
| Fujita et al., 2007 | Colorectal surgery | |
| Mui et al., 2005 | Appendicitis | |
| Li et al., 2003 | Colorectal resection | |
| Zelenitsky et al., 2000 | Colorectal surgical | |
| Håkansson et al., 1993 | Colorectal surgery | |
| Elusoji, 1992 | Appendectomy | |
| Tsang et al., 1992 | Appendectomy | |
| Cuthbertson et al., 1991 | Colorectal surgery | |
| Rowe-Jones et al., 1990 | Colorectal surgery | |
| Hershman et al., 1990 | Colorectal surgery | |
| Periti et al., 1989 | Colorectal surgery | |
| Bittner et al., 1989 | Colorectal surgery | |
| Jagelman et al., 1988 | Colorectal surgery | |
| Juul et al., 1987 | Colorectal surgery | |
| Stubbs et al., 1987 | large bowel surgery | |
| Fabian et al., 1984 | Colorectal surgery or small bowel obstruction | |
| Göransson et al., 1984 | Colorectal surgery | |
| Lohr et al., 1984 | Colorectal surgery | |
| Viitanen et al., 1984 | Appendectomy |
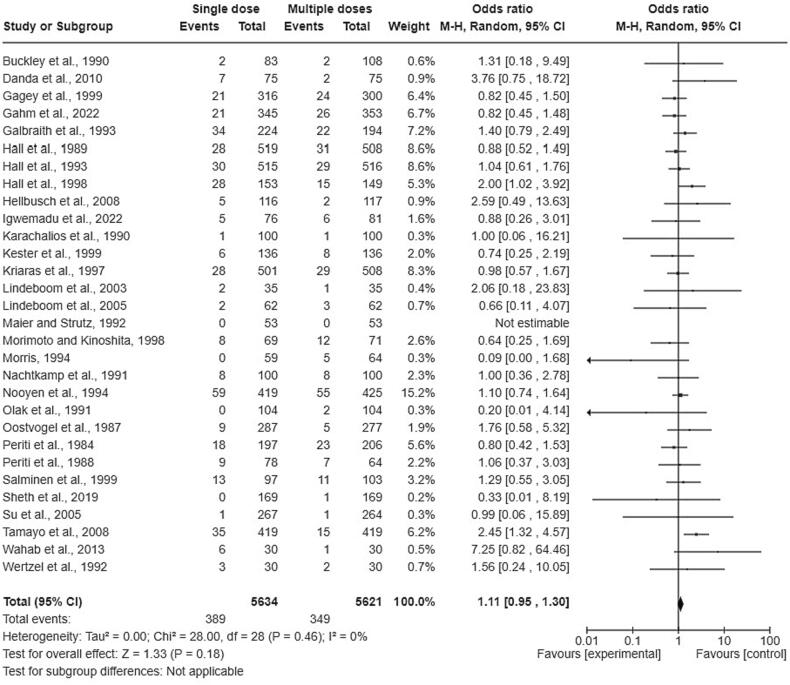
Regarding clean operations, there were 389 SSIs out of 5,634 patients in a single dose group (6.90%) and 349 SSIs out of 5,621 patients in multiple doses group (6.21%) (OR = 1.11, lower CI = 0.95, upper CI = 1.30). Fig. 2 shows the forest plot of the clinical trials that included clean operations. There was no significant difference observed in the incidence of SSIs between individuals who received a single dosage and those who received repeated doses of antibiotics (P = 0.18).
Fig. 2.
The forest plot of the clinical trials that included clean operations.
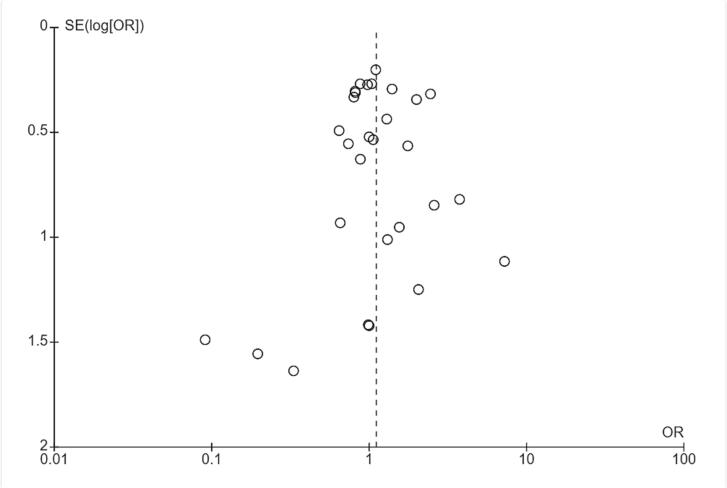
The heterogeneity of the trials that included clean operations was low, as shown in Fig. 3. Furthermore, the heterogeneity I2 was 0, and the p value of the heterogeneity was not significant (p = 0.46).
Fig. 3.
Funnel plot of the trials that included clean operations.
Regarding clean-contaminated operations, there were 137 SSIs out of 2,715 patients in a single dose group (5.05%) and 137 SSIs out of 2,355 patients in multiple doses group (5.82%) (OR = 0.87, lower CI = 0.68, upper CI = 1.11). Fig. 4 shows the forest plot of the clinical trials that included clean-contaminated operations. As shown in the figure, the difference between the efficacy of a single-dose group and a multiple-doses group was not statistically significant (P = 0.27).
Fig. 4.
The forest plot of the clinical trials that included clean-contaminated operations.
The heterogeneity of the trials that included clean-contaminated operations was low, as shown in Fig. 5. Furthermore, the heterogeneity I2 was 0, and the p value of the heterogeneity was not significant (p = 0.79).
Fig. 5.
Funnel plot of the trials that included clean-contaminated operations.
Regarding contaminated operations, there were 302 SSIs out of 3,262 patients in a single dose group (9.26%) and 276 SSIs out of 3,212 patients in multiple doses group (8.59%) (OR = 1.11, lower CI = 0.84, upper CI = 1.47). Fig. 6 shows the forest plot of the clinical trials that included clean-contaminated operations. As shown in the figure, the difference between the efficacy of a single-dose group and a multiple-doses group was not statistically significant (P = 0.44).
Fig. 6.
The forest plot of the clinical trials that included contaminated operations.
Fig. 7 shows the funnel plot of the trials that included contaminated operations. The heterogeneity of the trials was high, as shown in the funnel plot, and the heterogeneity of I2 was more than 50% and p = 0.002.
Fig. 7.
Funnel plot of the trials that included contaminated operations.
A sensitivity analysis was conducted to decrease the heterogeneity. After that, the Håkansson et al. study was removed from the analysis. The I2 decreased to 34% after deleting the study (Fig. 8). Regarding contaminated operations, there were 283 SSIs out of 2,975 patients in a single dose group (9.51%) and 232 SSIs out of 2,932 patients in a multiple doses group (7.91%) (OR = 1.21, lower CI = 0.95, upper CI = 1.54). The difference between the efficacy of a single-dose group and a multiple-doses group was not statistically significant (P = 0.13).
Fig. 8.
The forest plot of the clinical trials that included contaminated operations after conducting sensitivity analysis.
Fig. 9 shows the forest plot of the clinical trials that included all of the operations (clean, clean-contaminated, and contaminated operations). There were 828 SSIs out of 11,611 patients in a single dose group (7.13%) and 762 SSIs out of 11,188 patients in multiple doses group (6.81%) (OR = 1.05, lower CI = 0.93, upper CI = 1.20). As shown in the figure, the difference between the efficacy of a single-dose group and a multiple-doses group was not statistically significant (P = 0.44).
Fig. 9.
The forest plot of the clinical trials that included all of the operations.
Fig. 10 shows the funnel plot of the seventy-four clinical trials that were included in the analysis. In general, the included studies were sufficiently homogeneous (I2 less than 50%, p = 0.06).
Fig. 10.
Funnel plot of the seventy-four clinical trials.
4. Discussion
Surgical antimicrobial prophylaxis is a recognized component of surgical practice in specific operations to reduce SSIs and improve postoperative recovery. When people take too many antibiotics before surgery, they are more likely to have side effects, have to come back more often and get treatment for infections that would go away on their own. It is also linked to the development of bacteria that are resistant to antibiotics. For operations where there is evidence of benefit, using a single dose of antibiotic before the surgical incision is usually sufficient. Calderwood et al. (2023) reported that antimicrobial prophylaxis should be discontinued at the time of surgical closure in the operating room. Nonetheless, repeat intraoperative doses are indicated for lengthy procedures where a short-acting medication is used or if remarkable blood loss occurs.
Our study showed that the difference in the rate of SSIs between the single-dose group and the multiple-doses group was insignificant. Similar to the result of the present study, previous studies found that a single-dose prophylaxis is as effective as multiple-dosage antibiotic prophylaxis in reducing the occurrence of SSIs (Akkour et al., 2020, McDonald et al., 1998, Gahm et al., 2022, Vathana and Muhunthan, 2018, Mugisa et al., 2018, Slobogean et al., 2010, Igwemadu et al., 2022, Kannan et al., 2021, Koirala et al., 2019, Bhatnagar et al., 2017, Salkind and Rao, 2011; Basany et al., 2020; Ahn and Lee, 2013, Ishibashi et al., 2014). Furthermore, Das et al. reported that there are no notable differences between single-dose and multiple-doses antibiotic prophylaxis to prevent SSIs in patients undergoing elective clean-contaminated and clean operations and that a single dosage is more cost-effective (Das et al., 2021). Pooja et al. reported that the use of a single-dose antibiotic regimen should be advocated to reduce antibiotic resistance while also being cost-efficient (Pooja et al., 2021). Moreover, Kannan et al. reported that using extra doses of cefazolin after surgery provides no benefit over the use of a single dose and that a single-dose regimen has the advantages of reduced resistance emergence, fewer allergies or toxicity, and lower cost (Kannan et al., 2021).
Previous meta-analyses compared the use of single-dose vs. multiple-dose regimens. Similar to our results, they found no significant differences between single-dose and multiple-dose regimens in reducing SSIs. Several meta-analyses included clean incisions. They failed to show that multiple-dose prophylaxis was superior to a single-dose method in terms of lowering the SSI rate (Slobogean et al., 2008, Morrison et al., 2012, Ryan et al., 2019, Gillespie and Walenkamp, 2010, Costa and Krauss-Silva, 2004, Barker, 1994, Barker, 2002). Meijer et al. and Zhang et al. included clean-contaminated incisions in their meta-analyses. They evaluated wound infection rates between single-dose and multiple-dose regimens and found no significant difference (Meijer et al., 1990, Zhang et al., 2013). Furthermore, two meta-analyses included contaminated incisions. When comparing single-dose to multiple-dosage antibiotics, they found no statistically significant differences (Nelson et al., 2009, Nelson et al., 2014).
The present study showed that there was no remarkable difference between multiple doses and a single dose of antibiotic prophylaxis in preventing SSIs. Nonetheless, the patients should receive more than one dose for prolonged operative duration or in the case of severe blood loss. According to the Scottish Intercollegiate Guideline Network, there is consistent evidence that prophylaxis for the duration of the surgery alone is sufficient. Prophylaxis for longer periods had no remarkable benefit (SIGN, 2014). According to Munckhof et al., a single dosage of antibiotic is normally sufficient if the surgery lasts 4 h or less (Munckhof et al., 2005). Dehne et al. reported that surgery duration of more than 4 h or predicted blood losses of more than 1.5 L necessitate repeat intraoperative dosing of antibiotics (Dehne et al., 2001). Prophylactic antibiotics should be stopped within twenty-four hours, according to Crader and Varacallo (Crader and Varacallo, 2023). The Saudi Ministry of Health informed that the length of antimicrobial prophylaxis after surgery should be limited to less than twenty-four hours, regardless of the existence of indwelling catheters, drains, or prostheses (MOH, 2021). Ongom et al. (2013) reported that for prophylaxis duration, a shorter course of antibiotics after surgery is recommended. Even if there are indwelling drains and intravascular devices, the duration of prophylaxis should be less than 24 h (Ongom et al., 2013).
Our findings indicated that the administration of a single dose of prophylactic antibiotics significantly reduces the risk of infection, without the need for additional doses. This approach is not only more convenient for patients, but it also reduces the risk of antibiotic resistance and associated side effects. Therefore, we highly recommend the use of single-dose prophylaxis for all patients undergoing surgical procedures, regardless of the classification of their wounds.
The main strength of the present study was that it included seventy-four clinical trials. There were several previous meta-analyses that compared the use of single doses vs. multiple doses, but they focused on specific operations and included only a few trials. Nonetheless, there are several limitations to the current study. The first limitation of the study was that the antibiotics employed in the various studies were not standardized for all of the clinical trials that were included in the analysis. The remarkable discrepancy between the number of cases in the control and intervention groups in several trials was the second limitation. This would have an impact on the odd ratio comparisons. Furthermore, the quality of the studies included in the meta-analysis can vary, leading to potential bias and affecting the overall results. The fourth limitation was that several trials were available as abstracts only and didn’t contain the required information in the abstract, so they were excluded from the study. Limited data availability or incomplete reporting can limit the ability to conduct a comprehensive meta-analysis. Moreover, SSI trials that do not include prospective, direct observation can be biased because relying on patient self-reporting or retrospective chart review may lead to underreporting of infections.
5. Conclusion
The present study showed that single-dose antimicrobial prophylaxis was equally effective as multiple-dose antimicrobial prophylaxis in decreasing the occurrence of SSIs. So, a single-dose antibiotic regimen can be safely practiced before clean, clean-contaminated, and contaminated incisions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFP22UQU4290073DSR133
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Nehad J. Ahmed, Email: pharmdnehadjaser@yahoo.com, n.ahmed@psau.edu.sa.
Abdul Haseeb, Email: amhaseeb@uqu.edu.sa.
Manal AlGethamy, Email: mmalgethamy@moh.gov.sa.
Ahmad J. Mahrous, Email: ajmahrous@uqu.edu.sa.
Amer H. Khan, Email: dramer2006@gmail.com.
References
- Ahmed N.J., Almalki Z.S., Alfaifi A.A., Alshehri A.M., Alahmari A.K., Elazab E., et al. implementing an antimicrobial stewardship programme to improve adherence to a perioperative prophylaxis guideline. Healthcare. 2022;10:464. doi: 10.3390/healthcare10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N., Balaha M., Haseeb A., Khan A. Antibiotic usage in surgical prophylaxis: A retrospective study in the surgical ward of a governmental hospital in Riyadh Region. Healthcare. 2022;10:387. doi: 10.3390/healthcare10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B.K., Lee K.H. Single-dose antibiotic prophylaxis is effective enough in colorectal surgery. ANZ J. Surg. 2013;83(9):641–645. doi: 10.1111/j.1445-2197.2012.06244.x. [DOI] [PubMed] [Google Scholar]
- Akkour K.M., Arafah M.A., Alhulwah M.M., Badaghish R.S., Alhalal H.A., Alayed N.M. A comparative study between a single-dose and 24-hour multiple-dose antibiotic prophylaxis for elective hysterectomy. J. Infect. Dev. Ctries. 2020;14(11):1306–1313. doi: 10.3855/jidc.13034. [DOI] [PubMed] [Google Scholar]
- Alekwe L.O., Kuti O., Orji E.O., Ogunniyi S.O. Comparison of ceftriaxone versus triple drug regimen in the prevention of cesarean section infectious morbidities. J. Matern. Fetal Neonatal Med. 2008;21(9):638–642. doi: 10.1080/14767050802220490. [DOI] [PubMed] [Google Scholar]
- Alhomoud F., Aljamea Z., Almahasnah R., Alkhalifah K., Basalelah L., Alhomoud F.K. Self-medication and self-prescription with antibiotics in the Middle East—do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Int. J. Infect. Dis. 2017;57:3–12. doi: 10.1016/j.ijid.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Alsugoor M.H., Alsuhaymi N., Alshahrani Y., Alsagoor Y.H., Alghamdi A.M., Alalawi S.M., et al. Prevalence of self-medication among students of Umm Al-Qura and AlBaha universities in Saudi Arabia. Med. Sci. 2022;26:ms388e2461. [Google Scholar]
- ASHP. Available online: https://www.ashp.org/surgical-guidelines (accessed on 03 July 2023).
- Barker F.G. Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35(3):484–492. doi: 10.1227/00006123-199409000-00017. [DOI] [PubMed] [Google Scholar]
- Barker F. G., 2nd., 2002. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 51(2), 391–401. [PubMed]
- Berkeley A.S., Orr J.W., Cavanagh D., Freedman K.S., Ledger W.J., Pastorek J.G., 2nd, et al. Comparative effectiveness and safety of cefotetan and cefoxitin as prophylactic agents in patients undergoing abdominal or vaginal hysterectomy. Am. J. Surg. 1988;155(5A):81–85. doi: 10.1016/s0002-9610(88)80219-8. [DOI] [PubMed] [Google Scholar]
- Berríos-Torres, S.I., Umscheid, C.A., Bratzler, D.W., Leas, B., Stone, E.C., Kelz, R.R., et al., 2017. Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA. Surg. 152(8), 784-791. https://doi.org/10.1001/jamasurg.2017.0904. [DOI] [PubMed]
- Bhatnagar N., Sural S., Arora S., Lingaiah P., Dhal A. Efficacy of single dose versus multiple dose injectable antibiotics in hip joint surgery. J. Orthop. Trauma Surg. Rel. Res. 2017;12(2):62–65. [Google Scholar]
- Bittner R., Butters M., Rampf W., Kapfer X. Duration of the preventive use of antibiotics in colorectal surgery-single administration versus short-term prevention. Langenbecks Arch. Chir. 1989;374(5):272–279. doi: 10.1007/BF01261469. [DOI] [PubMed] [Google Scholar]
- Borchardt R.A., Tzizik D. Update on surgical site infections: The new CDC guidelines. JAAPA. 2018;31(4):52–54. doi: 10.1097/01.JAA.0000531052.82007.42. [DOI] [PubMed] [Google Scholar]
- Bratzler D.W., Houck P.W. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin. Infect. Dis. 2004;38(12):1706–1715. doi: 10.1086/423621. [DOI] [PubMed] [Google Scholar]
- Buckley R., Hughes G.N., Snodgrass T., Huchcroft S.A. Perioperative cefazolin prophylaxis in hip fracture surgery. Can. J. Surg. 1990;33(2):122–127. [PubMed] [Google Scholar]
- Calderwood M.S., Anderson D.J., Bratzler D.W., Dellinger E.P., Garcia-Houchins S., Maragakis L.L., et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023;44(5):695–720. doi: 10.1017/ice.2023.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 1999. Available online: https://stacks.cdc.gov/view/cdc/7160 (accessed on 03 July 2023).
- Costa R.J., Krauss-Silva L. Systematic review and meta-analysis of antibiotic prophylaxis in abdominal hysterectomy. Cad. Saude Publica. 2004;20(2):S175–S189. doi: 10.1590/s0102-311x2004000800013. [DOI] [PubMed] [Google Scholar]
- Crader, M.F., Varacallo, M., 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442032 (accessed on 03 July 2023).
- Cuthbertson A.M., McLeish A.R., Penfold J.C., Ross H. A comparison between single and double dose intravenous Timentin for the prophylaxis of wound infection in elective colorectal surgery. Dis. Colon Rectum. 1991;34(2):151–155. doi: 10.1007/BF02049990. [DOI] [PubMed] [Google Scholar]
- Danda A.K., Wahab A., Narayanan V., Siddareddi A. Single-dose versus single-day antibiotic prophylaxis for orthognathic surgery: a prospective, randomized, double-blind clinical study. J. Oral Maxillofac. Surg. 2010;68(2):344–346. doi: 10.1016/j.joms.2009.09.081. [DOI] [PubMed] [Google Scholar]
- Das S., Kundu R., Chattopadhyay B.P. Comparison of single dose versus multiple doses of antibiotic prophylaxis for prevention of surgical site infection. Int. Surg. J. 2021;9(1):129–132. doi: 10.18203/2349-2902.isj20215144. [DOI] [Google Scholar]
- Dehne M.G., Mühling J., Sablotzki A., Nopens H., Hempelmann G. Pharmacokinetics of antibiotic prophylaxis in major orthopedic surgery and blood-saving techniques. Orthopedics. 2001;24(7):665–669. doi: 10.3928/0147-7447-20010701-15. [DOI] [PubMed] [Google Scholar]
- Edwards F.H., Engelman R.M., Houck P., Shahian D.M., Bridges C.R. Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part i: duration. Ann. Thorac. Surg. 2006;81(1):397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- El Mufti M.B., Glessa A. Single-dose clavulanate-potentiated amoxycillin versus three-dose cefotaxime in the prevention of wound infection following elective cholecystectomy: a prospective randomized study. J. Int. Med. Res. 1988;16(2):92–97. doi: 10.1177/030006058801600203. [DOI] [PubMed] [Google Scholar]
- Elusoji S.O. Comparative study of single dose and five days dose of gentamicin as prophylaxis in appendicectomy. J. Pak. Med. Assoc. 1992;42(6):135–136. [PubMed] [Google Scholar]
- Engelman R., Shahian D., Shemin R., Guy T.S., Bratzler D., Edwards F., et al. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part II: antibiotic choice. Ann. Thorac. Surg. 2007;83(4):1569–1576. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Espin Basany E., Solís-Peña A., Pellino G., Kreisler E., Fraccalvieri D., Muinelo-Lorenzo M., et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020;5(8):729–738. doi: 10.1016/S2468-1253(20)30075-3. [DOI] [PubMed] [Google Scholar]
- Fabian T.C., Mangiante E.C., Boldreghini S.J. Prophylactic antibiotics for elective colorectal surgery or operation for obstruction of the small bowel: a comparison of cefonicid and cefoxitin. Rev. Infect. Dis. 1984;6(4):S896–S900. doi: 10.1093/clinids/6.supplement_4.s896. [DOI] [PubMed] [Google Scholar]
- Fabian T.C., Zellner S.R., Gazzaniga A., Hanna C., Nichols R.L., Waxman K. Multicenter open trial of cefotetan and cefoxitin in elective biliary surgery. Am. J. Surg. 1988;155(5A):77–80. doi: 10.1016/s0002-9610(88)80218-6. [DOI] [PubMed] [Google Scholar]
- Fujita S., Saito N., Yamada T., Takii Y., Kondo K., Ohue M., et al. Randomized, multicenter trial of antibiotic prophylaxis in elective colorectal surgery: single dose vs 3 doses of a second-generation cephalosporin without metronidazole and oral antibiotics. Arch. Surg. 2007;142(7):657–661. doi: 10.1001/archsurg.142.7.657. [DOI] [PubMed] [Google Scholar]
- Gagey O., Doyon F., Dellamonica P., Carsenti-Etesse H., Desplaces N., Tancrède C., et al. Infection prophylaxis in open leg fractures. Comparison of a dose of pefloxacin and 5 days of cefazolin-oxacillin. A randomized study of 616 cases. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1999;85(4):328–336. [PubMed] [Google Scholar]
- Gahm J., Ljung Konstantinidou A., Lagergren J., Sandelin K., Glimåker M., Johansson H., et al. Effectiveness of single vs multiple doses of prophylactic intravenous antibiotics in implant-based breast reconstruction: A randomized clinical trial. JAMA Netw. Open. 2022;5(9):e2231583. doi: 10.1001/jamanetworkopen.2022.31583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galask R.P., Benigno B.B., Cunningham F.G., Elliott J.P., Makowski E., McGregor J.A., et al. A. Results of a multicenter comparative study of single-dose cefotetan and multiple-dose cefoxitin as prophylaxis in patients undergoing cesarean section. Am. J. Surg. 1988;155(5A):86–90. doi: 10.1016/s0002-9610(88)80220-4. [DOI] [PubMed] [Google Scholar]
- Gall S.A., Hill G.B. Single-dose versus multiple-dose piperacillin prophylaxis in primary cesarean operation. Am. J. Obstet. Gynecol. 1987;157(2):502–506. doi: 10.1016/S0002-9378(87)80203-X. [DOI] [PubMed] [Google Scholar]
- Gilbert, N.D., Moellering, R.C., Eliopoulos, G.M., Sande, M.A. The Sanford guide to antimicrobial therapy. Antimicrobial Therapy, Inc, Hyde Park, Vt; 2007.
- Gillespie W.J., Walenkamp G.H. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst. Rev. 2010;2010(3):CD000244. doi: 10.1002/14651858.CD000244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson G., Nilsson-Ehle I., Olsson S.A., Petersson B.G., Bengmark S. Single versus multiple dose doxycycline prophylaxis in elective colorectal surgery. Acta Chir. Scand. 1984;150(3):245–249. [PubMed] [Google Scholar]
- Gordon S.M., Serkey J.M., Keys T.F., Ryan T., Fatica C.A., Schmitt S.K., et al. Secular trends in nosocomial bloodstream infections in a 55-bed cardiothoracic intensive care unit. Ann. Thorac. Surg. 1998;65(1):95–100. doi: 10.1016/s0003-4975(97)01039-4. [DOI] [PubMed] [Google Scholar]
- Håkansson T., Raahave D., Hansen O.H., Pedersen T. Effectiveness of single dose prophylaxis with cefotaxime and metronidazole compared with three doses of cefotaxime alone in elective colorectal surgery. Eur. J. Surg. 1993;159(3):177–180. [PubMed] [Google Scholar]
- Hall C., Allen J., Barlow G. Antibiotic prophylaxis. Surgery. 2015;33(11):542–549. doi: 10.1016/j.mpsur.2015.08.005. [DOI] [Google Scholar]
- Hall J.C., Watts J.M., Press L., O'Brien P., Turnidge J., McDonald P. Single-dose antibiotic prophylaxis in contaminated abdominal surgery. Arch. Surg. 1989;124(2):244–247. doi: 10.1001/archsurg.1989.01410020118020. [DOI] [PubMed] [Google Scholar]
- Hall J.C., Christiansen K., Carter M.J., Edwards M.G., Hodge A.J., Newman M.A., et al. Antibiotic prophylaxis in cardiac operations. Ann. Thorac. Surg. 1993;56(4):916–922. doi: 10.1016/0003-4975(93)90355-l. [DOI] [PubMed] [Google Scholar]
- Hall J.C., Christiansen K.J., Goodman M., Lawrence-Brown M., Prendergast F.J., Rosenberg P., et al. Duration of antimicrobial prophylaxis in vascular surgery. Am. J. Surg. 1998;175(2):87–90. doi: 10.1016/S0002-9610(97)00270-5. [DOI] [PubMed] [Google Scholar]
- Haque M., Sartelli M., McKimm J., Abu Bakar M. Health care-associated infections - an overview. Infect. Drug. Resist. 2018;11:2321–2333. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S., Samore M.H., Lichtenberg D., Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101(25):2916–2921. doi: 10.1161/01.cir.101.25.2916. [DOI] [PubMed] [Google Scholar]
- Hellbusch L.C., Helzer-Julin M., Doran S.E., Leibrock L.G., Long D.J., Puccioni M.J., et al. Single-dose vs multiple-dose antibiotic prophylaxis in instrumented lumbar fusion–a prospective study. Surg. Neurol. 2008;70(6):622–627. doi: 10.1016/j.surneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Hershman M.J., Swift R.I., Reilly D.T., Logan W.A., Sackier J.M., Gompertz H., et al. Prospective comparative study of cefotetan with piperacillin for prophylaxis against infection in elective colorectal surgery. J. R. Coll. Surg. Edinb. 1990;35(1):29–32. [PubMed] [Google Scholar]
- Hjortrup A., Moesgaard F., Jensen F., Johansen C., Nielsen R. Antibiotic prophylaxis in high risk biliary surgery: one dose of ceftriaxone compared with two doses of cefuroxime. Eur. J. Surg. 1991;157(6–7):403–405. [PubMed] [Google Scholar]
- Hotz G., Novotny-Lenhard J., Kinzig M., Soergel F. Single-dose antibiotic prophylaxis in maxillofacial surgery. Chemotherapy. 1994;40(1):65–69. doi: 10.1159/000239173. [DOI] [PubMed] [Google Scholar]
- Hussein R.R., Rabie A.S.I., Bin Shaman M., Shaaban A.H., Fahmy A.M., Sofy M.R., et al. Antibiotic consumption in hospitals during COVID-19 pandemic: A comparative study. J. Infect. Dev. Ctries. 2022;16:1679–1686. doi: 10.3855/jidc.17148. [DOI] [PubMed] [Google Scholar]
- Idris K.J., Bamoosa E.M., Alsabbah R.S., Faidah G.H., Alharbi S.J., Bar A.A., et al. Awareness and level of knowledge of surgical site infection among surgical staff in King Abdullah Medical City during Hajj 2019: A cross-sectional study. Int. J. Med. Dev. Ctries. 2020;4:1873–1878. [Google Scholar]
- Igwemadu, G.T., Eleje, G.U., Eno, E.E., Akunaeziri, U.A., Afolabi, F.A., Alao, A.I., et al., 2022. Single-dose versus multiple-dose antibiotics prophylaxis for preventing caesarean section postpartum infections: A randomized controlled trial. Womens Health. 18, 17455057221101071. https://doi.org/10.1177/17455057221101071. [DOI] [PMC free article] [PubMed]
- Ishibashi K., Ishida H., Kuwabara K., Ohsawa T., Okada N., Yokoyama M., et al. Short-term intravenous antimicrobial prophylaxis for elective rectal cancer surgery: results of a prospective randomized non-inferiority trial. Surg. Today. 2014;44:716–722. doi: 10.1007/s00595-013-0695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagelman D.G., Fabian T.C., Nichols R.L., Stone H.H., Wilson S.E., Zellner S.R. Single-dose cefotetan versus multiple-dose cefoxitin as prophylaxis in colorectal surgery. Am. J. Surg. 1988;155(5A):71–76. doi: 10.1016/s0002-9610(88)80217-4. [DOI] [PubMed] [Google Scholar]
- Jogdand S.D., Shinde R.K., Chandrakar N. Outcome of evidence-based allocation of single-dose antibiotic extended to three-dose antibiotic prophylaxis in surgical site infection. Int. J. Recent. Surg. Med. Sci. 2017;3(2):79–84. doi: 10.5005/jp-journals-10053-0046. [DOI] [Google Scholar]
- Juul P., Klaaborg K.E., Kronborg O. Single or multiple doses of metronidazole and ampicillin in elective colorectal surgery. A randomized trial. Dis. Colon. Rectum. 1987;30(7):526–528. doi: 10.1007/BF02554782. [DOI] [PubMed] [Google Scholar]
- Kabrah A., Bahwerth F., Alghamdi S., Alkhotani A., Alahmadi A., Alhuzali M., et al. Antibiotics usage and resistance among patients with severe acute respiratory syndrome coronavirus 2 in the intensive care unit in Makkah. Saudi Arabia. Vaccines. 2022;10:2148. doi: 10.3390/vaccines10122148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan A., Ravichandran M., Sundaramurthi S., Win M., Tara A., Ruo S.W., et al. Is single-dose antimicrobial prophylaxis sufficient to control infections in gastrointestinal oncological surgeries? Cureus. 2021;13(8):e16939. doi: 10.7759/cureus.16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachalios T., Lyritis G.P., Hatzopoulos E. Antibiotic prophylaxis in the surgical treatment of peritrochanteric fractures: a comparative trial between two cephalosporins. Chemotherapy. 1990;36(6):448–453. doi: 10.1159/000238803. [DOI] [PubMed] [Google Scholar]
- Kayihura V., Osman N.B., Bugalho A., Bergström S. Choice of antibiotics for infection prophylaxis in emergency cesarean sections in low-income countries: a cost-benefit study in Mozambique. Acta Obstet. Gynecol. Scand. 2003;82(7):636–641. doi: 10.1034/j.1600-0412.2003.00205.x. [DOI] [PubMed] [Google Scholar]
- Kellum J.M., Jr., Gargano S., Gorbach S.L., Talcof C., Curtis L.E., Weiner B., et al. Antibiotic prophylaxis in high-risk biliary operations: multicenter trial of single preoperative ceftriaxone versus multidose cefazolin. Am. J. Surg. 1984;148(4A):15–18. [PubMed] [Google Scholar]
- Kester R.C., Antrum R., Thornton C.A., Ramsden C.H., Harding I. A comparison of teicoplanin versus cephradine plus metronidazole in the prophylaxis of post-operative infection in vascular surgery. J. Hosp. Infect. 1999;41(3):233–243. doi: 10.1016/s0195-6701(99)90022-1. [DOI] [PubMed] [Google Scholar]
- Klevens, R.M., Edwards, J.R., Richards, C.L., 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health. Rep. 122(2), 160–166. https://doi.org/10.1177/003335490712200205. [DOI] [PMC free article] [PubMed]
- Koirala A., Thakur D., Agrawal S., Chaudhary B.L. Single dose versus multiple dose antibiotics in laparoscopic cholecystectomy: A prospective comparative single blind study. J. Soc. Anesthesiol. Nepal. 2019;5(1):11–15. [Google Scholar]
- Kreter B., Woods M. Antibiotic prophylaxis for cardiothoracic operations. Meta-analysis of thirty years of clinical trials. J. Thorac. Cardiovasc. Surg. 1992;104(3):590–599. [PubMed] [Google Scholar]
- Kriaras I., Michalopoulos A., Michalis A., Palatianos G., Economopoulos G., Anagnostopoulos C., et al. Antibiotic prophylaxis in cardiac surgery. J. Cardiovasc. Surg. 1997;38(6):605–610. [PubMed] [Google Scholar]
- Li Z.L., Tong S.X., Yu B.M., Tang W.S., Wu Z.Y., Wang S.B. Single-dose ceftriaxone versus multiple-dose cefuroxime for prophylaxis of surgical site infection. Zhonghua Wai Ke Za Zhi. 2003;41(5):372–374. [PubMed] [Google Scholar]
- Lindeboom J.A., Baas E.M., Kroon F.H. Prophylactic single-dose administration of 600 mg clindamycin versus 4-time administration of 600 mg clindamycin in orthognathic surgery: A prospective randomized study in bilateral mandibular sagittal ramus osteotomies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;95(2):145–149. doi: 10.1067/moe.2003.54. [DOI] [PubMed] [Google Scholar]
- Lindeboom J.A., Tuk J.G., Kroon F.H., van den Akker H.P. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone grafting procedures: single-dose clindamycin versus 24-hour clindamycin prophylaxis. Mund Kiefer Gesichtschir. 2005;9(6):384–388. doi: 10.1007/s10006-005-0650-4. [DOI] [PubMed] [Google Scholar]
- Llor C., Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5(6):229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr J., Wagner P.K., Rothmund M. Perioperative antibiotic prophylaxis (single or multiple dose) in elective colorectal surgery. A randomized study. Chirurg. 1984;55(8):512–514. [PubMed] [Google Scholar]
- Loozen C.S., Kortram K., Kornmann V.N., van Ramshorst B., Vlaminckx B., Knibbe C.A., et al. Randomized clinical trial of extended versus single-dose perioperative antibiotic prophylaxis for acute calculous cholecystitis. Br. J. Surg. 2017;104(2):e151–e157. doi: 10.1002/bjs.10406. [DOI] [PubMed] [Google Scholar]
- Lyimo F.M., Massinde A.N., Kidenya B.R., Konje E.T., Mshana S.E. Single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post-caesarean infection at Bugando Medical Centre in Mwanza, Tanzania: a randomized, equivalence, controlled trial. BMC Pregnancy Childbirth. 2013;13:123. doi: 10.1186/1471-2393-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W., Strutz J. Perioperative single dose prevention with cephalosporins in the ENT area. A prospective randomized study. Laryngorhinootologie. 1992;71(7):365–369. doi: 10.1055/s-2007-997316. [DOI] [PubMed] [Google Scholar]
- Maki D.G., Lammers J.L., Aughey D.R. Comparative studies of multiple-dose cefoxitin vs. single-dose cefonicid for surgical prophylaxis in patients undergoing biliary tract operations or hysterectomy. Rev. Infect. Dis. 1984;6(4):S887–S895. doi: 10.1093/clinids/6.supplement_4.s887. [DOI] [PubMed] [Google Scholar]
- McDonald M., Grabsch E., Marshall C., Forbes A. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. ANZ J. Surg. 1998;68(6):388–396. doi: 10.1111/j.1445-2197.1998.tb04785.x. [DOI] [PubMed] [Google Scholar]
- McGregor J.A., Gordon S.F., Krotec J., Poindexter A.N. Results of a randomized, multicenter, comparative trial of a single dose of cefotetan versus multiple doses of cefoxitin as prophylaxis in cesarean section. Am. J. Obstet. Gynecol. 1988;158(3 Pt 2):701–706. doi: 10.1016/s0002-9378(16)44530-8. [DOI] [PubMed] [Google Scholar]
- Meijer W.S., Schmitz P.I. Prophylactic use of cefuroxime in biliary tract surgery: randomized controlled trial of single versus multiple dose in high-risk patients. Galant Trial Study Group. Br. J. Surg. 1993;80(7):917–921. doi: 10.1002/bjs.1800800742. [DOI] [PubMed] [Google Scholar]
- Meijer W.S., Schmitz P.I.M., Jeekel J. Meta-analysis of randomized, controlled clinical trials of antibiotic prophylaxis in biliary tract surgery. Br. J. Surg. 1990;77:283–290. doi: 10.1002/bjs.1800770315. [DOI] [PubMed] [Google Scholar]
- MOH, 2021. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/Protocol-015.pdf (accessed on 02 11 2022).
- Mohri, Y., Tonouchi, H., Kobayashi, M., Nakai, K., Kusunoki, M., Mie Surgical Infection Research Group., 2007. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br. J. Surg. 94(6), 683-8. https://doi.org/10.1002/bjs.5837. [DOI] [PubMed]
- Morimoto K., Kinoshita H. Once-daily use of ofloxacin for prophylaxis in breast cancer surgery. Chemotherapy. 1998;44(2):135–141. doi: 10.1159/000007105. [DOI] [PubMed] [Google Scholar]
- Morris W.T. Effectiveness of ceftriaxone versus cefoxitin in reducing chest and wound infections after upper abdominal operations. Am. J. Surg. 1994;167(4):391–395. doi: 10.1016/0002-9610(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Morrison S., White N., Asadollahi S., Lade J. Single versus multiple doses of antibiotic prophylaxis in limb fracture surgery. ANZ J. Surg. 2012;82(12):902–907. doi: 10.1111/j.1445-2197.2012.06143.x. [DOI] [PubMed] [Google Scholar]
- Mugisa G.A., Kiondo P., Namagembe I. Single dose ceftriaxone and metronidazole versus multiple doses for antibiotic prophylaxis at elective caesarean section in Mulago hospital: A randomized clinical trial. AAS Open. Research. 2018;1:11. doi: 10.12688/aasopenres.12849.1. [DOI] [Google Scholar]
- Mui L.M., Ng C.S., Wong S.K., Lam Y.H., Fung T.M., Fok K.L. Optimum duration of prophylactic antibiotics in acute non-perforated appendicitis. ANZ J. Surg. 2005;75(6):425–428. doi: 10.1111/j.1445-2197.2005.03397.x. [DOI] [PubMed] [Google Scholar]
- Munckhof W. Antibiotics for surgical prophylaxis. Aust. Prescr. 2005;28:38–40. doi: 10.18773/austprescr.2005.030. [DOI] [Google Scholar]
- Nachtkamp J., Peiper C., Schumpelick V. Prospective randomized study of the comparison of the effectiveness of cefoxitin (triple dose) and cefuroxime (single shot) in preventive perioperative use of antibiotics in abdominal surgery. Klin. Wochenschr. 1991;69(26):61–66. [PubMed] [Google Scholar]
- Nelson R.L., Glenny A.M., Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst. Rev. 2009;1:CD001181. doi: 10.1002/14651858.CD001181.pub3. [DOI] [PubMed] [Google Scholar]
- Nelson R.L., Gladman E., Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst. Rev. 2014;2014(5):CD001181. doi: 10.1002/14651858.CD001181.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyen S.M., Overbeek B.P., Brutel de la Rivière A., Storm A.J., Langemeyer J.J. Prospective randomised comparison of single-dose versus multiple-dose cefuroxime for prophylaxis in coronary artery bypass grafting. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13(12):1033–1037. doi: 10.1007/BF02111823. [DOI] [PubMed] [Google Scholar]
- Olak J., Jeyasingham K., Forrester-Wood C., Hutter J., al-Zeerah M., Brown E. Randomized trial of one-dose versus six-dose cefazolin prophylaxis in elective general thoracic surgery. Ann. Thorac. Surg. 1991;51(6):956–958. doi: 10.1016/0003-4975(91)91014-m. [DOI] [PubMed] [Google Scholar]
- Ongom P.A., Kijjambu S.C. Antibiotic prophylaxis in colorectal surgery: evolving trends. J. Mol. Pharm. Org. Process Res. 2013;1(3):109. doi: 10.4172/2329-9053.1000109. [DOI] [Google Scholar]
- Oostvogel H.J., van Vroonhoven T.J., van der Werken C., Lenderink A.W. Single-dose v. short-term antibiotic therapy for prevention of wound infection in general surgery. A prospective, randomized double-blind trial. Acta Chir. Scand. 1987;153(10):571–575. [PubMed] [Google Scholar]
- Periti P., Mazzei T., Lamanna S., Mini E. Single-dose ceftriaxone versus multi-dose cefotaxime antimicrobial prophylaxis in gynecologic and obstetrical surgery. Preliminary results of a multicenter prospective randomized study. Chemioterapia. 1984;3(5):299–304. [PubMed] [Google Scholar]
- Periti P., Mazzei T., Periti E. Prophylaxis in gynaecological and obstetric surgery: a comparative randomised multicentre study of single-dose cefotetan versus two doses of cefazolin. Chemioterapia. 1988;7(4):245–252. [PubMed] [Google Scholar]
- Periti P., Mazzei T., Tonelli F. Single-dose cefotetan vs. multiple-dose cefoxitin–antimicrobial prophylaxis in colorectal surgery. Results of a prospective, multicenter, randomized study. Dis. Colon Rectum. 1989;32(2):121–127. doi: 10.1007/BF02553824. [DOI] [PubMed] [Google Scholar]
- Pooja P., Hadi V., Rao S., Mallapur A., Katageri G. Single dose single antibiotic versus multiple doses multiple antibiotic prophylaxis in caesarean section, at a tertiary care centre. New Indian J. Obgyn. 2021;7(2):123–218. doi: 10.21276/obgyn.2021.7.2.3. [DOI] [Google Scholar]
- Rafiq M.S., Khan M.M., Oshima T., Takesue Y., Ikeuchi H., Matsuoka H., et al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis. Colon Rectum. 2013;56(10):1149–1155. doi: 10.1097/DCR.0b013e31829f71a0. [DOI] [PubMed] [Google Scholar]
- Rowe-Jones D.C., Peel A.L., Kingston R.D., Shaw J.F., Teasdale C., Cole D.S. Single dose cefotaxime plus metronidazole versus three dose cefuroxime plus etronidazole as prophylaxis against wound infection in colorectal surgery: multicentre prospective randomised study. Br. Med. J. 1990;300(6716):18–22. doi: 10.1136/bmj.300.6716.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Wilkins J. Single-dose cefotaxime versus 3 to 5 dose cefoxitin for prophylaxis of vaginal or abdominal hysterectomy. J. Antimicrob. Chemother. 1984;14(B):217–221. doi: 10.1093/jac/14.suppl_b.217. [DOI] [PubMed] [Google Scholar]
- Roy S., Wilkins J., Hemsell D.L., March C.M., Spirtos N.M. Efficacy and safety of single-dose ceftizoxime vs. multiple-dose cefoxitin in preventing infection after vaginal hysterectomy. J. Reprod. Med. 1988;33(1):149–153. [PubMed] [Google Scholar]
- Ryan S.P., Kildow B.J., Tan T.L., Parvizi J., Bolognesi M.P., Seyler T.M., et al. Is there a difference in infection risk between single and multiple doses of prophylactic antibiotics? A meta-analysis. Clin. Orthop. Relat. Res. 2019;477(7):1577–1590. doi: 10.1097/CORR.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura M., Kawakami S., Yoshida S., Masuda H., Kobayashi T., Kihara K. Prospective comparative study of single dose versus 3-day administration of antimicrobial prophylaxis in minimum incision endoscopic radical prostatectomy. Int. J. Urol. 2008;15(4):328–331. doi: 10.1111/j.1442-2042.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- Salkind A.R., Rao K.C. Antibiotic prophylaxis to prevent surgical site infections. Am. Fam. Physician. 2011;83(5):585–590. [PubMed] [Google Scholar]
- Salminen U.S., Viljanen T.U., Valtonen V.V., Ikonen T.E., Sahlman A.E., Harjula A.L. Ceftriaxone versus vancomycin prophylaxis in cardiovascular surgery. J. Antimicrob. Chemother. 1999;44(2):287–290. doi: 10.1093/jac/44.2.287. [DOI] [PubMed] [Google Scholar]
- Seidelman J., Anderson D.J. Surgical site infections. Infect. Dis. Clin. North Am. 2021;35(4):901–929. doi: 10.1016/j.idc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Shaheen M.H., Siddiqui M.I., Jokhdar H.A., Hassan-Hussein A., Garout M.A., Hafiz S.M., et al. Prescribing patterns for acute respiratory infections in children in primary health care centers, Makkah Al Mukarramah. Saudi Arabia. J. Epidemiol. Glob. Health. 2018;8:149. doi: 10.2991/j.jegh.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth J., Rath S., Tripathy D. Oral versus single intravenous bolus dose antibiotic prophylaxis against postoperative surgical site infection in external dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction - A randomized study. Indian J. Ophthalmol. 2019;67(3):382–385. doi: 10.4103/ijo.IJO_616_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGN, 2014. Available online: https://www.nationalelfservice.net/dentistry/oral-and-maxillofacial-surgery/antibiotic-prophylaxis-in-surgery-sign-guideline-104/ (accessed on 02 11 2022).
- Slobogean G.P., Kennedy S.A., Davidson D., O'Brien P.J. Single- versus multiple-dose antibiotic prophylaxis in the surgical treatment of closed fractures: a meta-analysis. J. Orthop. Trauma. 2008;22(4):264–269. doi: 10.1097/BOT.0b013e31816b7880. [DOI] [PubMed] [Google Scholar]
- Slobogean G.P., O'Brien P.J., Brauer C.A. Single-dose versus multiple-dose antibiotic prophylaxis for the surgical treatment of closed fractures. Acta Orthop. 2010;81(2):256–262. doi: 10.3109/17453671003587119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs R.S., Griggs N.J., Kelleher J.P., Dickinson I.K., Moat N., Rimmer D.M. Single dose mezlocillin versus three dose cefuroxime plus metronidazole for the prophylaxis of wound infection after large bowel surgery. J. Hosp. Infect. 1987;9(3):285–290. doi: 10.1016/0195-6701(87)90126-5. [DOI] [PubMed] [Google Scholar]
- Su H.Y., Ding D.C., Chen D.C., Lu M.F., Liu J.Y., Chang F.Y. Prospective randomized comparison of single-dose versus 1-day cefazolin for prophylaxis in gynecologic surgery. Acta Obstet. Gynecol. Scand. 2005;84(4):384–389. doi: 10.1111/j.0001-6349.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- Tamayo E., Gualis J., Flórez S., Castrodeza J., Eiros Bouza J.M., Alvarez F.J. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J. Thorac. Cardiovasc. Surg. 2008;136(6):1522–1527. doi: 10.1016/j.jtcvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration, 2020. Copenhagen, Denmark. Available at revman.cochrane.org. (accessed on 03 July 2023).
- Tsang T.M., Tam P.K., Saing H. Antibiotic prophylaxis in acute non-perforated appendicitis in children: single dose of metronidazole and gentamicin. J. R. Coll. Surg. Edinb. 1992;37(2):110–112. [PubMed] [Google Scholar]
- Vathana M., Muhunthan K. A randomized control trial of single dose versus multiple doses of IV antibiotic prophylaxis in caesarean delivery. Sri Lanka J. Obstet. Gyn. 2018;40(4):92–100. doi: 10.4038/sljog.v40i4.7871. [DOI] [Google Scholar]
- Viitanen J., Tunturi T., Auvinen O., Pessi T. Tinidazole prophylaxis in appendicectomies. A controlled study of single-dose versus 3-day therapy. Scand. J. Gastroenterol. 1984;19(1):111–115. [PubMed] [Google Scholar]
- Wahab P.U., Narayanan V., Nathan S., Madhulaxmi Antibiotic prophylaxis for bilateral sagittal split osteotomies: a randomized, double-blind clinical study. Int. J. Oral Maxillofac. Surg. 2013;42(3):352–355. doi: 10.1016/j.ijom.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Wertzel H., Swoboda L., Joos-Würtemberger A., Frank U., Hasse J. Perioperative antibiotic prophylaxis in general thoracic surgery. Thorac. Cardiovasc. Surg. 1992;40(6):326–329. doi: 10.1055/s-2007-1020174. [DOI] [PubMed] [Google Scholar]
- Westen E.H., Kolk P.R., van Velzen C.L., Unkels R., Mmuni N.S., Hamisi A.D., et al. Single-dose compared with multiple day antibiotic prophylaxis for cesarean section in low-resource settings, a randomized controlled, noninferiority trial. Acta Obstet. Gynecol. Scand. 2015;94(1):43–49. doi: 10.1111/aogs.12517. [DOI] [PubMed] [Google Scholar]
- Zelenitsky S.A., Silverman R.E., Duckworth H., Harding G.K. A prospective, randomized, double-blind study of single high dose versus multiple standard dose gentamicin both in combination with metronidazole for colorectal surgical prophylaxis. J. Hosp. Infect. 2000;46(2):135–140. doi: 10.1053/jhin.2000.0814. [DOI] [PubMed] [Google Scholar]
- Zhang C.D., Zeng Y.J., Li Z., Chen J., Li H.W., Zhang J.K., et al. Extended antimicrobial prophylaxis after gastric cancer surgery: a systematic review and meta-analysis. World J. Gastroenterol. 2013;19(13):2104–2109. doi: 10.3748/wjg.v19.i13.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabaglo, M., Sharman, T., 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560533 (accessed on 03 July 2023).