Abstract
We report a patient with anorexia nervosa without bronchiectasis and cystic fibrosis who developed acute pneumonia caused by Exophiala dermatitidis (E. dermatitidis). The black fungus found in multiple sputum cultures was determined to be E. dermatitidis using mass spectrometry and identified using genetic analysis. Although the initiation of antifungal therapy was late, the pneumonia gradually improved with long-term treatment. This case highlights the need for early diagnosis and effective long-term treatment of the fungal etiologic agent.
Keywords: Exophiala dermatitidis, Anorexia nervosa, Opportunistic infection, Early diagnosis
1. Introduction
Exophiala dermatitidis (E. dermatitidis) is a black fungus that can be isolated from natural environments, such as wet environments, including dishwashers, humidifiers, and bathtubs [1]. E. dermatitidis is commonly reported to be a pathogen isolated from the skin and subcutaneous tissue [2], whereas E. dermatitidis pneumonia has rarely been reported in approximately 6 % of patients with bronchiectasis and cystic fibrosis [3]. E. dermatitidis is increasingly detected as an opportunistic infection in patients with cancer or in an immunosuppressive state, which causes fatal invasive infections of the skin, eye, liver, and central nervous system [4]. Here, we report the diagnosis and clinical treatment of E. dermatitidis isolated from a young patient with acute pneumonia who was considered immunocompromised due to a psychiatric illness with extreme anorexia nervosa (AN).
2. Case presentation
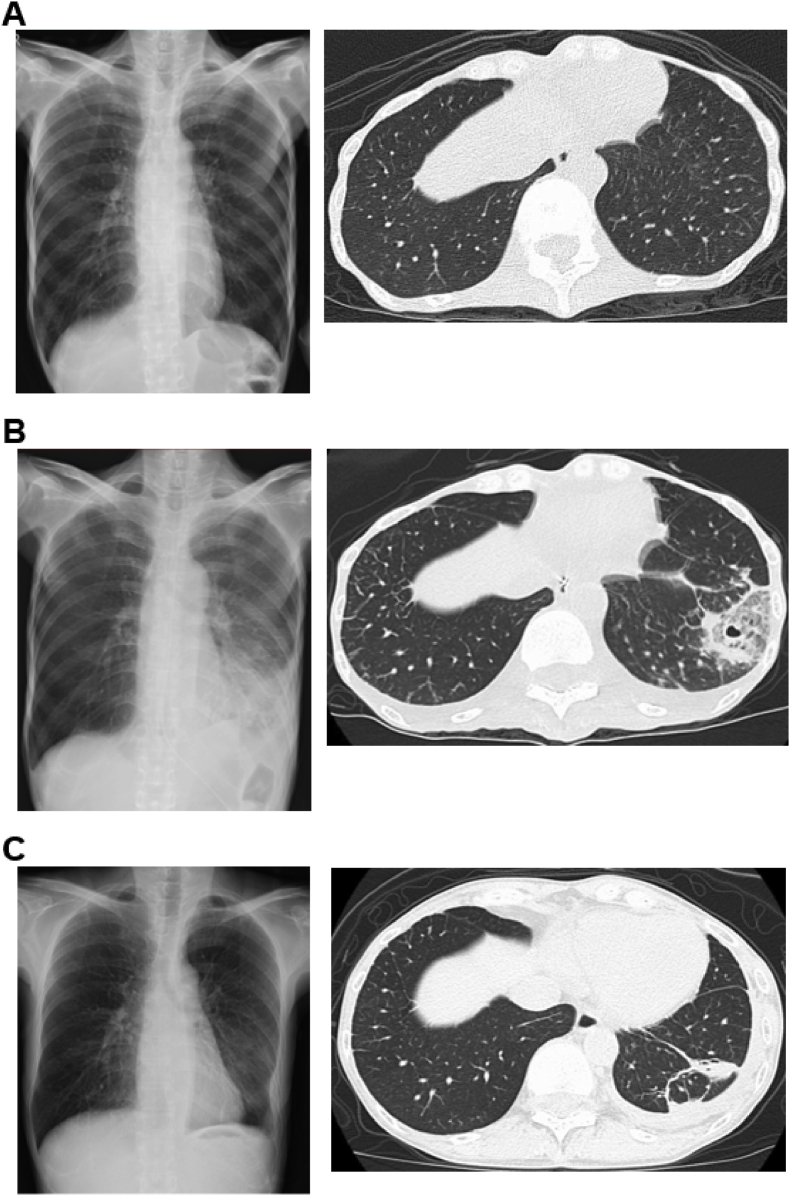
A young, extremely thin patient (body mass index, BMI: 10.1) was admitted to our hospital for the treatment of psychiatric illness, depression, and AN (laboratory results are shown in Table S1). The patient had no history of smoking and no family history of pneumonia or dust exposure. Chest radiography and computed tomography (CT) before hospitalization are shown in Fig. 1A depicting her healthy condition at that time.
Fig. 1.
Image findings of the patient. The chest radiography and CT were taken (A) before hospitalization, (B) on day 10 of hospitalization, and (C) on day 127 before discharge.
On day 7 of hospitalization, the patient suddenly presented with fever and pain in the left chest. Laboratory results revealed a white blood cell count (WBC) of 9600 cells/μL, neutrophils of 8200 cells/μL, lymphocytes of 800 cells/μL, monocytes of 200 cells/μL, and c-reactive protein (CRP) level of 8.27 mg/dL. Chest radiography and CT showed consolidation with a small cavity in the left lower lobe (Fig. 1B).
A nasogastric tube was inserted to provide nutritional support and administer medications. A course of antibiotics, including tazobactam/piperacillin and clarithromycin did not improve inflammation. Additional laboratory tests were performed to investigate potential causes of atypical pneumonia, such as fungal and tuberculosis infections. However, the results indicated a negative level of β-D-glucan, and negative findings for serum Aspergillus Ag (not galactomannan antigen) detected by enzyme-linked immunosorbent assay, MAC Ab, HIV-1 Ab, and T-spot TB. Multiple sputum examinations were performed repeatedly to identify the pathogenic bacteria responsible for pneumonia. Small black colonies were barely detected and were observed on Sabouraud dextrose agar (SDA) after 3 days. The patient refused to undergo a bronchoscopy.
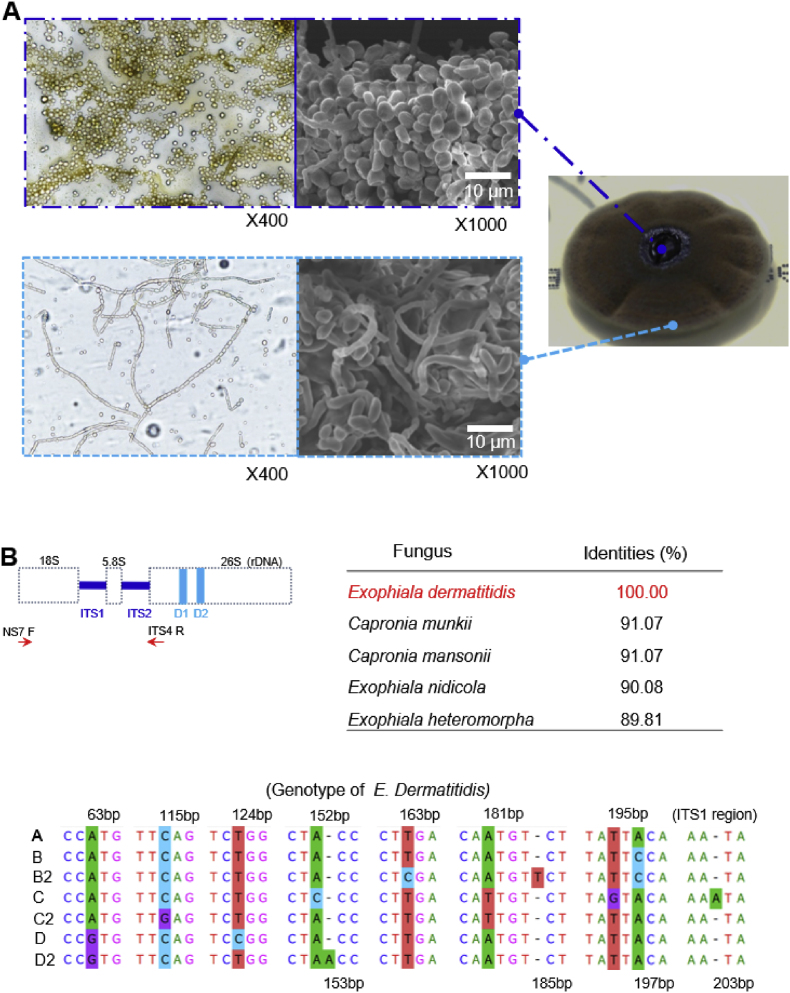
Using VITEK MS V3., matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS), the isolated small colonies were consistent with E. dermatitidis. The slide culture method was used to observe the morphology of giant colonies under aerobic incubation on SDA for 2 weeks. Fungal details were analyzed using the slide culture method and scanning electron microscopy (SEM) (Fig. 2A), suggesting that the morphology of the colonies was also consistent with E. dermatitidis.
Fig. 2.
The morphology and gene identification of E. dermatitidis (A) Specimen images of the central and outer regions stained with lactophenol cotton blue, × 400 using the slide culture method, and × 1000 using the SEM. The black, jelly-like central area was dominated by mycelia, whereas the brown outer area contained a mixture of septate mycelia, mycelial-forming cells, and mycelia. (B) The genomic DNA sequence of E. dermatitidis was amplified using NS7 F and ITS4 R primers (Table S2). The top five identities (%) are shown. E. dermatitidis is classified into seven genomic types based on its gene sequences. The isolated E. dermatitidis was identified as genotype A.
Genomic DNA was extracted from the colonies, and the sequences of internal transcribed spacer 1, 5.8S ribosomal RNA, and internal transcribed spacer 2 were compared by gene homology analysis using the Basic Local Alignment Search Tool on the NCBI website. The isolated DNA was 100 % homologous to these regions in E. dermatitidis (Fig. 2B) (Table S2). Finally, we made a definitive diagnosis of E. dermatitidis genotype A, which is the most frequently reported in Japan [5].
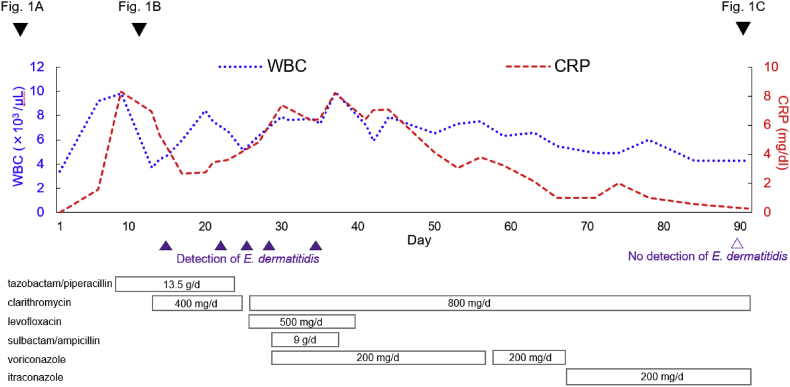
According to recommended treatments for E. dermatitidis [6,7], voriconazole 200 mg/d was started on day 27. The left pleural effusion, infiltrating consolidation in the left lower lung, and CRP gradually improved. On day 63, photophobia was observed and the voriconazole was changed to itraconazole 200 mg/d. However, we did not monitor the voriconazole concentration. Clarithromycin treatment was continued to prevent bacterial pneumonia until her nutritional status improved and the nasogastric tube was removed. After the improvement in nutritional status, laboratory results (Table S1), and chest imaging (Fig. 1C), the patient was discharged on day 133 and continued on oral itraconazole for 3 months. The course of treatment during hospitalization is shown in Fig. 3.
Fig. 3.
Course of patient data and treatment during hospitalization. Laboratory data (WBC and CRP levels), medications used for treatment, dates of chest radiography and CT, and results of multiple sputum samples collected at different times are shown.
We also investigated the susceptibility of the isolated E. dermatitidis to clinically available antifungal agents according to CLSI M38, 3rd edition, to confirm that voriconazole is an effective antifungal agent (Table 1). Voriconazole and posaconazole had the most potent antifungal activity against E. dermatitidis. Itraconazole, isavuconazole, and amphotericin B also showed good antifungal activity, whereas micafungin and fluconazole showed poor activity against E. dermatitidis, which is consistent with previous reports [8].
Table 1.
Antifungal susceptibility testing of E. dermatitidis.
| drugs | MIC50 (μg/ml) | MIC (μg/ml) |
|---|---|---|
| fluconazole | 4 | 16 |
| voriconazole | 0.063 | 0.13 |
| isavuconazole | 0.13 | 1 |
| itraconazole | 0.25 | 0.25 |
| posaconazole | 0.063 | 0.13 |
| amphotericin B | 0.063 | 0.25 |
| micafungin | 8 | 16 |
3. Discussion
We encountered a case of severe AN (BMI was 10.1) complicated by a rare fungal pneumonia. Despite many studies on the specific abnormalities and changes in the immune system of AN patients, these changes in the immune system remain controversial and inconclusive [9]. The incidence of infection in the AN group has been reported to be identical to that in controls [10]. In contrast, morbidity and mortality were higher in the AN group than in the control group [11]. Interest in the AN immune system has been renewed by recent reports on COVID-19 infection and concomitant AN [12].
In this case, we concluded that the patient was in an immunocompromised state with cachexia, depression, severe low weight, frequent hypoglycemic episodes, and hepatic dysfunction, as typical of severe AN. We initially suspected that a bacterial infection caused the pneumonia. However, the inflammatory findings did not improve by some antibiotics, and E. dermatitidis was detected in all sputum samples. Voriconazole was started on day 27 of hospitalization and was changed to itraconazole. The patient's body weight increased after feeding through a nasogastric tube, and the inflammatory findings gradually improved with long-term voriconazole and itraconazole treatment.
MALDI-TOF MS analysis cannot provide perfect detection; however, it is a powerful tool that can contribute to the early diagnosis and appropriate antifungal treatment [13]. E. dermatitidis has a slower growth rate than other fungi. We observed very small colonies of E. dermatitidis after incubation for 3 days on the SDA culture plate, suggesting that laboratory technicians may miss the presence of E. dermatitidis on the culture plate in the case of short incubation.
E. dermatitidis pneumonia requires a relatively long treatment [6]. The prognosis of systemic and invasive infections has been reported to be almost fatal [4]. Therefore, when treating immunocompromised patients, potent fungicidal activity and tissue migration of antifungals to the site of infection are required. A report on tissue concentrations in biopsy specimens obtained at autopsy from seven patients receiving posaconazole prophylaxis showed that posaconazole concentrations in all organs examined, except the brain, were higher than those in plasma [14]. The formulation of posaconazole is mixed with hydroxy-β-cyclodextrin, which improves its pharmacokinetics by enhancing its solubility and oral bioavailability [15], as is the oral solution of itraconazole [16]. These results suggested that posaconazole, with better tissue migration, may be a therapeutic option for invasive E. dermatitidis infections. Unfortunately, we were not able to use posaconazole to treat E. dermatitidis because it is not covered by health insurance in Japan.
AN complicated by fungal pneumonia has been reported in some cases, such as pulmonary aspergillosis [17,18], here we reported the first case of AN complicated by E. dermatitidis pneumonia. In this case, we concluded that treatment with antifungal drugs (voriconazole and itraconazole) and improved nutritional status by nasogastric tube feeding contributed to the cure of E. dermatitidis fungal pneumonia in AN.
Ethical form
Written informed consent was obtained from the patient or legal guardian(s) for the publication of this case report and accompanying images. A copy of the written consent form is available for review by the journal's Editor-in-Chief upon request.
As the corresponding author, I declare that I have signed this document.
Funding source
There are none.
Declaration of competing interest
These authors declare no conflict of interest.
Acknowledgements
We thank the staff of the Department of Laboratory Medicine at the Kumamoto University Hospital for technical assistance and Editage (www.editage.jp) for English language editing.
Handling Editor: Dr Adilia Warris
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mmcr.2023.100617.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zupančič J., Babič M.N., Zalar P., Gunde-Cimerman N. The black yeast Exophiala dermatitidis and other selected opportunistic human fungal pathogens spread from dishwashers to kitchens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148166. https://10.1371/journal.pone.0148166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isa-Isa R., García C., et al. Subcutaneous phaeohyphomycosis (mycotic cyst) Clin. Dermatol. 2012;30:425–431. doi: 10.1016/j.clindermatol.2011.09.015. https://10.1016/j.clindermatol.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 3.Lebecque P., Leonard A., Huang D., Reychler G., Boeras A., Leal T., et al. Prevalence and risk factors of exophiala (Wangiella) dermatitis and cystic fibrosis. Med. Mycol. 2010;48:S4–S9. doi: 10.3109/13693786.2010.495731. https://10.3109/13693786.2010.495731 [DOI] [PubMed] [Google Scholar]
- 4.Kirchhoff L., Olsowski M., Rath P.M., Steinmann J. Exophiala dermatitidis: key issues of an opportunistic fungal pathogen. Virulence. 2019;10:984–998. doi: 10.1080/21505594.2019.1596504. https://10.1080/21505594.2019.1596504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alimu Y., Ban S., et al. Molecular phylogenetic study of strains morphologically identified as Exophiala dermatitidis from Clinical and Environmental Specimens in Japan. Med. Mycol. J. 2022;63:1–9. doi: 10.3314/mmj.21-00012. https://10.3314/mmj.21-00012 [DOI] [PubMed] [Google Scholar]
- 6.Mukai Y., Nureki S.I., Hata M., Shigenaga T., Tokimatsu I., Miyazaki E., et al. The E. dermatitidis pneumonia was successfully treated with long-term itraconazole therapy. J. Infect. Chemother. 2014;20:446–449. doi: 10.1016/j.jiac.2014.02.006. https://10.1016/j.jiac.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Bulloch M.N. Treatment of pulmonary Wangiella dermatitidis infections with oral voriconazole. J. Clin. Pharm. Therapeut. 2011;36:433–436. doi: 10.1111/j.1365-2710.2010.01214.x. https://10.1111/j.1365-2710.2010.01214.x [DOI] [PubMed] [Google Scholar]
- 8.Fothergill A.W., Rinaldi M.G., et al. Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole, and voriconazole. Med. Mycol. 2009;47:41–43. doi: 10.1080/13693780802512451. https://10.1080/13693780802512451 [DOI] [PubMed] [Google Scholar]
- 9.Gibson D., Mehler P.S. Anorexia nervosa and the immune system: a narrative review. J. Clin. Med. 2019;8:1915. doi: 10.3390/jcm8111915. https://10.3390/jcm8111915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raevuori A., Lukkariniemi L., Suokas J.T., Gissler M., Suvisaari J.M., Haukka J. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int. J. Eat. Disord. 2016;49:542–552. doi: 10.1002/eat.22497. https://10.1002/eat.22497 [DOI] [PubMed] [Google Scholar]
- 11.Brown R.F., Bartrop R., Birmingham C.L., et al. Immunological disturbances and infectious diseases in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20:117–128. doi: 10.1111/j.1601-5215.2008.00286.x. https://10.1111/j.1601-5215.2008.00286.x [DOI] [PubMed] [Google Scholar]
- 12.DeSarbo J.R., DeSarbo L. Anorexia nervosa and COVID-19. Current Psychiatry. 2020;19:23–28. https://10.12788/cp.0011 [Google Scholar]
- 13.Kondori N., Erhard M., Welinder-Olsson C., Groenewald M., et al. Analysis of black fungi by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS): species-level identification of clinical isolates of Exophiala dermatitidis. FEMS Microbiol. Lett. 2015;362:1–6. doi: 10.1093/femsle/fnu016. https://10.1093/femsle/fnu016 [DOI] [PubMed] [Google Scholar]
- 14.Blennow O., Eliasson E., Pettersson T., Pohanka A., Szakos A., El-Serafi I., et al. Posaconazole concentrations in human tissues after allogeneic stem cell transplantation. Antimicrob. Agents Chemother. 2014;58:4941–4943. doi: 10.1128/AAC.03252-14. https://10.1128/AAC.03252-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greer N.D. vol. 20. Bayl Univ Med Cent Proc; 2007. pp. 188–196.https://10.1080/08998280.2007.11928283 (Posaconazole (Noxafil): A New Triazole Antifungal Agent). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouton J.W., Van Peer A., De Beule K., Van Vliet A., Donnelly J.P., Soons P.A. Pharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple dosing of the novel formulation. Antimicrob. Agents Chemother. 2006;50:4096–4102. doi: 10.1128/AAC.00630-06. https://10.1128/AAC.00630-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogi A., Kosaka T., Yamaki E., Kuwano H. Pulmonary aspergilloma in a patient with anorexia nervosa: a case report. Ann. Thorac. Cardiovasc. Surg. 2012;18:465–467. doi: 10.5761/atcs.cr.11.01728. https://10.5761/atcs.cr.11.01728 [DOI] [PubMed] [Google Scholar]
- 18.Takushima M., Haraguchi S., Hioki M., Endou N., Kawamura J., Yamashita Y., et al. Video-assisted thoracic surgery for pulmonary aspergilloma in a patient with anorexia nervosa. J. Nippon Med. Sch. 2004;71:333–336. doi: 10.1272/jnms.71.333. https://10.1272/jnms.71.333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.