Abstract
Background & Aims:
The available risk stratification indices for hepatocellular cancer (HCC) have limited applicability. We developed and externally validated a HCC risk stratification index in U.S. cohorts of patients with cirrhosis.
Methods:
We used data from two prospective U.S. cohorts to develop the risk index. Patients with cirrhosis were enrolled from eight centers and followed until development of HCC, death, or 12/31/2021. We identified an optimal set of predictors with the highest discriminatory ability (C-index) for HCC. The predictors were refit using competing risk regression and its predictive performance was evaluated using the area under receiver operating characteristic curve (AUC). External validation was performed in a cohort of 21,550 patients with cirrhosis seen in the U.S Veterans Affairs system between 2018 and 2019 with follow up through 2021.
Results:
We developed the model in 2520 patients (mean age 60, 31% women, 24% cured hepatitis C, 19% alcoholic liver disease, and 28% nonalcoholic fatty liver disease). The selected model had the C-index of 0.77 (95% CI, 0.73–0.81), and the predictors were age, sex, smoking, alcohol use, body mass index, etiology, alpha-fetoprotein, albumin, alanine aminotransferase and platelet levels. The AUC were 0.78 (95% CI=0.71–0.85) at 1 year, 0.78 (95% CI=0.73–0.82) at 2 years, and 0.75 (95% CI=0.70, 0.80) at 3 years and the model was well calibrated. In the external validation cohort, the AUC at 2 years was 0.70 with excellent calibration.
Conclusion:
The risk index, including objective and routinely available risk factors, can differentiate patients with cirrhosis who will develop HCC and help guide discussions regarding HCC surveillance and prevention.
Keywords: Prediction, validation, personalized, liver cancer
BACKGROUND
Benefits of effective risk assessment to target screening efforts are established in cancers such as breast, cervical and colorectal cancer.1–3 In the U.S. hepatocellular cancer (HCC) is a growing cause of cancer deaths and is associated with a dismal 5-year survival rate.4,5 Most HCC cases (>80%) arise in patients with cirrhosis. However, HCC risk in the large and diverse group of patients with cirrhosis is neither uniform nor linear; some patients progress rapidly to HCC, while others progress more slowly, and a large fraction do not develop HCC.
There are currently a few risk stratification models for HCC in cirrhosis.6–10 Many were derived from cohorts of patients with specific etiology and included patients with untreated (i.e., active) HCV or HBV.6,7,10 Therefore, these models are likely less relevant to contemporary clinical practice where most patients are treated for viral hepatitis or have alcohol or metabolic dysfunction associated liver disease. Furthermore, available HCC risk stratification models did not examine diverse population of patients with cirrhosis including women and racial/ethnic minorities. Lack of effective risk stratification hence remains as a major roadblock in developing targeted screening, prevention, and early detection efforts for HCC in patients with cirrhosis.
Using data from two prospective cohort studies of patients with cirrhosis from multiple etiologies seen in routine clinical care, we developed and validated a HCC risk index to predict future risk of progression to HCC. Because our objective was to create a simple tool that can be translated to a routine clinical setting, we considered factors that can be assessed using reproducible methods including simple history, physical examination, and/or objective assessment of liver disease etiology and severity. We used a large multisite dataset of patients with cirrhosis with diverse racial/ethnic and etiology representation to externally validate the HCC risk index.
METHODS
Derivation Cohorts
We used data from two prospective cohort studies of patients with cirrhosis: the Texas Hepatocellular Carcinoma Consortium Cohort (THCCC) and the Houston Veterans Administration Cirrhosis Surveillance Cohort (HVASC).11–13
In the THCCC, we prospectively recruited patients with cirrhosis from eight liver clinics at institutions in four cities (Michael E DeBakey VA Medical Center and Baylor St. Luke’s Medical Center in Houston; University of Texas Southwestern, Parkland Health & Hospital System, and two clinical sites within Baylor Scott & White Research Institute in Dallas; Doctor’s Hospital at Renaissance in McAllen; and Texas Liver Institute in San Antonio, Texas). Recruitment started on December 2016 and is still ongoing.
Cirrhosis diagnosis was based on predefined criteria for liver histology, radiology, or elastography, or serum biomarkers (eTable 1). Patients with uncontrolled hepatic decompensation, history of HCC, or non-hepatic cancer were excluded. Data were collected using surveys and electronic medical records (EMR).11 Patients were scheduled for 6-month visits and followed until HCC diagnosis, liver transplantation, or death. For the current analysis, we included THCCC participants enrolled between December 2016 and April 2020, with follow up until December, 2021.
The HVASC is a cohort of patients with cirrhosis in active HCC surveillance recruited from hepatology clinics at the Michael E DeBakey VA Medical Center between August 2014 and December 2016 with follow-up until June 2021. HVASC used similar eligibility, inclusion and exclusion criteria, and recruitment procedures as those for THCCC.
We harmonized data from THCCC and HVASC into one common dataset to use for the current analysis.14 We used the source documents and EMR for both cohorts to complete missing data items as needed.
Primary Outcome
Our primary outcome was incident HCC that developed after one month of enrollment. The overall adherence to HCC surveillance was high. In total, 80% of participants underwent at least one HCC surveillance imaging during follow up. EMR reviews were conducted for all participants at 3-month intervals to capture incident HCCs, liver transplantation and death dates. We defined HCC according to AASLD criteria including histological or radiological diagnosis using characteristic appearance (arterial enhancement and delayed washout of contrast) on triple phase CT or MRI (LI-RAD 5) or those with suspicious lesions (LI-RAD 4) that were reviewed in multidisciplinary tumor boards and treated as HCC. All study sites have multidisciplinary HCC tumor boards. For our analysis, we used the date of final confirmation as the date of HCC diagnosis.
Possible HCC Predictor Variables
We based the selection of potential predictor variables on a priori hypotheses guided by literature as well as easy and cost-efficient availability as part of routine care.
Sociodemographic variables included age at enrollment and sex. We did not include race or ethnicity to minimize the chances of any unintended consequences of risk stratification (e.g., prioritization for care or lack thereof) in patients belonging to certain racial and ethnic groups. Liver disease specific variables included etiology and severity of liver disease. We defined hepatitis C virus infection (HCV) based on a positive HCV ribonucleic acid test. Definition of cured HCV was based on documentation of sustained virological response (SVR) in the EMR.15 We defined alcohol-associated cirrhosis based on a combination of clinician recorded diagnosis of alcoholic liver disease and patients’ self-report of former heavy or any current use of alcohol.11 We defined NAFLD as the possible etiology of cirrhosis for patients without HCV (active/untreated or cured HCV), HBV, moderate/high alcohol, or other etiological risk factors, including few patients who were classified as having cryptogenic cirrhosis. Other etiologies were defined as described before.11
Other clinical variables included history of smoking, alcohol use, and presence of metabolic traits. We defined smoking status as never, past, and current smoker based on self-report. We used a validated survey for ascertaining alcohol use that classified alcohol use status as lifetime abstention (never), former light to moderate use, former heavy use, current light to moderate and current heavy use, as defined by the Centers for Disease Control and Prevention. We used height and weight values at enrollment to calculate body mass index (BMI). We defined diabetes and dyslipidemia based on patient’s medical history (survey or electronic medical review, EMR) or self-reported treatment with diabetes medications, anti-hypertensives, or treatments for dyslipidemia at any time before enrollment.11
We extracted data for serum levels of bilirubin, sodium, and creatinine and international normalized ratio at enrollment. Other liver disease factors included serum levels of albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), platelet count, and alpha-fetoprotein (AFP). We chose the individual components of MELD-Na and FIB-4 scores in lieu to using the composite scores in the model development stage.
External Validation Cohort.
For external validation, we used data from a separate cohort of patients with cirrhosis who were seen in ambulatory clinics at 130 VA hospitals from 01/1/2018 and 12/31/2019 with follow up through 12/31/2020. Patients were included if they had at least 2 instances of cirrhosis or at least 1 code for cirrhosis with at least 1 filled prescription of spironolactone (≥100 mg for ascites), rifaximin, or lactulose (for encephalopathy). This definition was found to have high positive predictive value (86%−93%) in medical records.16,17 We selected the first clinic visit after meeting cohort entry criteria as the index date for follow-up. We excluded patients younger than 18 or older than 90 years, patients who received a liver transplant, who had a diagnosis of HCC or metastatic cancer, or those who had Child Class C cirrhosis before the index date. We acquired data through December 31, 2020, to ascertain HCC, liver transplantation, and death. HCC was defined based on 2 or more instances of HCC codes, or any instance of HCC recorded in the VA Cancer Registry.15,18 All-cause mortality data was obtained from the VA Vital Status File. Definitions of the predictor variables are included in eTable 2.
Statistical methods
We checked distributions of all continuous predictors, and took the log transformation for ALT, AFP, FIB4 and platelets in the model development because of their right-skewed distributions. We used two approaches for model development: the least absolute shrinkage and selection operator (Lasso)19 and stepwise regression20 to remove less contributive predictors. We then compared the performance of the two panels selected by these methods to identify an optimal panel of predictors associated with the higher C-index, reflecting higher discriminatory performance. We refitted the predictors included in the best-performing panel using the Fine-Gray competing risk model,21 that accounted for the competing risks of liver transplantation and death, to construct the Texas HCC risk index (THCC-RI).
Performance measures
We assessed model performance through discrimination, calibration, and the Brier score. We calculated the area under receiver operating characteristic curve (AUC) at 1, 2 and 3 years to evaluate predictive discrimination of the HCC risk index. We evaluated calibration with visual assessments of calibration curves.22,23 As an overall measure of model performance, we calculated the Brier score, a scoring rule affected by both discrimination and calibration, with lower scores indicating better model accuracy.24 We examined time-specific cumulative risk of HCC in cirrhosis patients based on deciles of predicted risk scores and compared the cumulative incidence function curves and hazard ratios across three groups (highest 20th percentile, lowest 20th percentile and intermediate risk).
Validation
Bootstrap validation provides nearly unbiased estimates of model performance compared to split-sample techniques.25,26 We used 200 bootstrap samples to evaluate the predictive performance of the THCC-RI. We externally validated the final prediction model by examining model performance in the VA cirrhosis cohort. We estimated time specific AUC values and constructed calibration plots.
RESULTS
Patient Characteristics
The harmonized THCCC and the HVASC cohorts involved 2520 patients with cirrhosis with a mean age of 60 years (standard deviation, SD 9.9 years) and 791 (31.4%) of whom were women. The cohort included 49.6% non-Hispanic white, 27.8% Hispanics, and 19.8% non-Hispanic Blacks. HCV was the leading etiological risk factor of cirrhosis (43.4%) followed by NAFLD (28.3%) and alcohol-related liver disease (19.0%). In total, 19.0% had active HCV and 24.4% had cured HCV. More than 23.0% of patients were current tobacco smokers, while 6.8% reported current heavy alcohol use, 43.4% had diabetes and most (79.1%) were overweight or obese (BMI ≥25 kg/m2). Most patients (63.6%) had a baseline Child-Pugh Class A, and only 4.4% had Child Pugh C class. During a total of 8187 person-years of follow up (median 3.0 years, interquartile range 2.0–4.2 years), 156 patients developed incident HCC at an annual incidence rate of 1.91% (95% CI 1.62% to 2.23%), 63 patients received transplantation, and 350 died during follow-up.
The external validation cohort included 21,550 patients with cirrhosis. There were some differences in patient characteristics compared with the derivation cohort. Patients in the validation cohort were older (mean age 64 years, SD 7.65years) and most were men. The cohort was racially diverse with 61.0% whites, 25.0% Blacks, and 6.3% Hispanics. Liver disease severity and laboratory data were not different, although greater proportions of patients had HCV as the etiological risk factor and more patients reported current smoking and heavy drinking than in the derivation cohort. During a total of 47,191 person-years of follow up (median 2.4 years, interquartile range 1.7–2.7 years), 900 patients developed incident HCC. The annual incidence rate of HCC (1.91%, 95% CI 1.79% to 2.04%) was similar to those in the derivation cohort. Cumulative incidence function curves from the derivation and validation cohorts are shown in eFigure1.
THCC-RI Development
The model containing a panel of predictors selected by the Lasso approach had the highest C-index (0.77, 95% CI, 0.73–0.81 vs. 0.74 using backward selection). Given better performance characteristics of the Lasso method, we used the variables selected by this approach to construct the Fine-Gray Model and the THCC-RI.
The selected panel included age, current or past history of smoking, current heavy use of alcohol, BMI, active or cured HCV, AFP, serum albumin, ALT and platelet count. Table 2 displays the multivariable-adjusted hazard ratios in the Fine-Gray model. See eTable 3 for the equation for THCC-RI.
Table 2.
Multivariable-adjusted hazard ratios for variables included in the final HCC prediction model (Texas HCC Risk Index) for ambulatory patients with cirrhosis.
| Variable | Adjusted hazard ratio (95% CI) |
|---|---|
| Age in years | 1.04 (1.02, 1.06) |
| Gender (ref: female) | |
| Male | 1.70 (1.08, 2.65) |
| Alcohol use (ref: never) | |
| Current heavy | 1.32 (0.72, 2.43) |
| Other alcohol use (past history or current non-heavy) | 0.76 (0.51, 1.14) |
| Smoking (ref: never) | |
| Past or Current | 1.32 (0.89, 1.94) |
| Metabolic traits | |
| Body mass index (kg/m2) | 1.02 (1.00, 1.05) |
| Etiology of liver disease (ref: non-HCV) | |
| HCV (active or cured) | 1.72 (1.17, 2.52) |
| Laboratory data | |
| Serum albumin (g/dL) | 0.52 (0.39, 0.69) |
| Alanine aminotransferase (IU/L) log10 | 0.75 (0.39, 1.43) |
| Platelet count (106/mL) log10 | 0.30 (0.16, 0.56) |
| Alpha fetoprotein (ng/mL) log10 | 2.54 (1.60, 4.03) |
Model Performance in Internal and External Validation
After bootstrap internal validation, the AUC values were 0.78 (95% CI, 0.71–0.85) at 1 year, 0.78 (95% CI, 0.73–0.82) at 2 years, and 0.75 (95% CI, 0.70, 0.80) at 3 years respectively suggesting good overall discrimination.27 Figure 1 displays the predicted vs. observed risk of HCC at the 2 years across deciles of predicted risk. This showed that the model was well calibrated with good agreement between observed and predicted risk. The Brier score was 0.036, (95% CI, 0.027–0.045), indicating good estimation accuracy.
Figure 1.
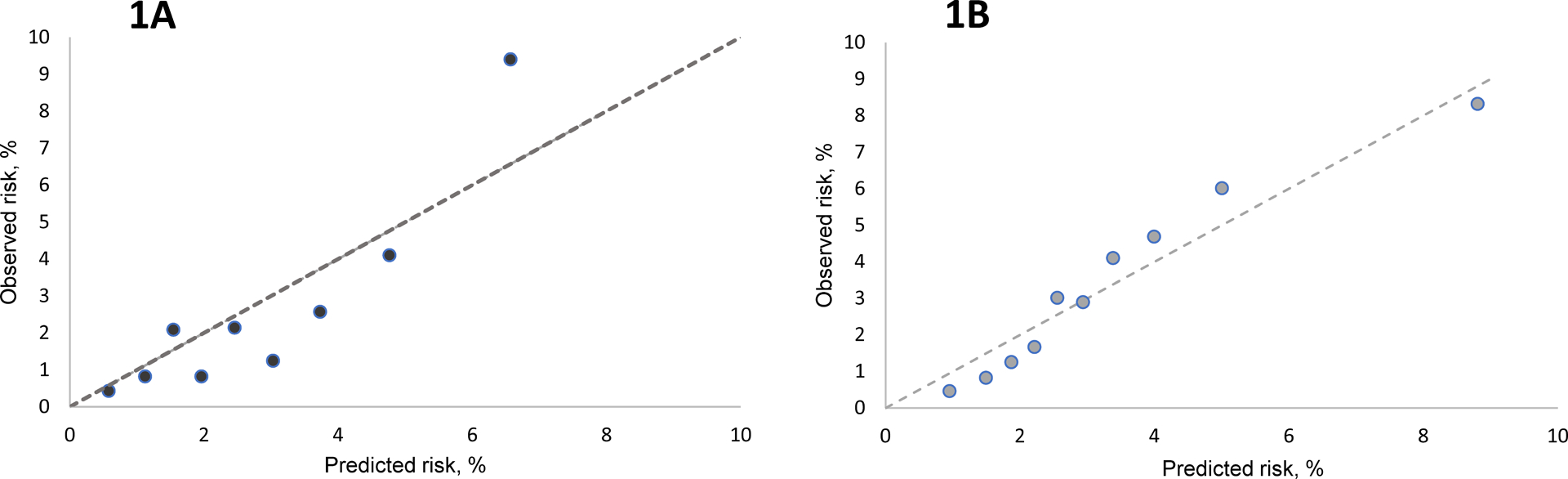
Calibration plots for the HCC prediction model in the derivation (Texas HCC Consortium and Houston VA Surveillance Cohort, [1A]) and external validation cohort (VA cirrhosis cohort [1B]).
On external validation, the time-specific AUC values were 0.70 (95% CI, 0.67–0.74) at 1 year and 0.70 (95% CI, 0.68–0.73) at 2 years (Table 3). Calibration was similar if not better than in the derivation cohort (Figure 1).
Table 3.
Performance of Texas HCC Risk Index in the Texas Hepatocellular Carcinoma Consortium (THCCC) and the Houston Veterans Administration Cirrhosis Surveillance Cohorts (HVASC) [Derivation Cohort] and the Veterans Health Administration Cirrhosis Cohort [Validation Cohort].
| Timeframe | No. at the end of the year in the harmonized THCCC and HVASC cohorts | AUROC in THCCC and HVASC cohorts after internal validation (95% CI) | No. at the end of the year in the VA cirrhosis cohort | AUROC in VA cirrhosis cohort (95% CI) |
|---|---|---|---|---|
| 1 year | 2271 | 0.78 (0.71, 0.85) |
19617 | 0.70 (0.67–0.74) |
| 2 years | 1927 | 0.78 (0.73, 0.83) |
14760 | 0.70 (0.68–0.73) |
| 3 years | 1293 | 0.75 (0.70, 0.80) |
NR | NR |
The AUC at 3 years is not reported for the external validation because the time period of observation was not long enough to obtain reliable estimates at 3 years
Table 4 shows each decile of predicted incidence and mean observed HCC incidence for a given decile in the derivation cohort. To further illustrate the discriminatory ability of the THCC-RI, we split the cohort into low (below 20th percentile), intermediate (20th to 80th), and high-risk subgroup (higher than 80th percentile) based on visual inspection of risk distribution (Table 4). These cut-offs differentiated patients in the internal and external validation cohorts (Figure 2, eTable 4). There were no HCC cases in the low-risk group in the derivation cohort. Compared to the medium-risk group, the high-risk group had 4.71-fold (HR=4.71, 95% CI=2.80–7.92) higher risk of HCC In the external validation cohort, compared to the low-risk group, the hazard ratios for the medium-risk and high-risk groups were 4.98 (95% CI=2.98–8.47) and 11.49 (95% CI=6.75–19.55) respectively.
Table 4.
Distribution of HCC risk based on the THCC Risk Index in the Texas Hepatocellular Carcinoma Consortium (THCCC) and the Houston Veterans Administration Cirrhosis Surveillance Cohorts (HVASC) [Derivation Cohort] and the Veterans Health Administration Cirrhosis Cohort [Validation Cohort].
| Cumulative HCC incidence (95% confidence interval) | ||
|---|---|---|
| Year 1 | Year 2 | Year 3 |
| 0 (0, 0) | 0.4 (0, 1.2) | 0.4 (0, 1.2) |
| 0.4 (0, 1.2) | 0.8 (0, 2.0) | 0.8 (0, 2.0) |
| 1.2 (0, 2.6) | 2.1 (0.3, 3.9) | 3.3 (0.8, 5.8) |
| 0.4 (0, 1.2) | 0.8 (0, 2.0) | 2.7 (0.3, 5.1) |
| 1.7 (0.1, 3.3) | 2.2 (0.2, 4.2) | 3.9 (1.2, 6.6) |
| 0.8 (0, 2.0) | 1.3 (0, 2.7) | 4.3 (1.4, 7.2) |
| 0.9 (0, 2.1) | 2.7 (0.5, 4.9) | 2.7 (0.5, 4.9) |
| 3.3 (0.9, 5.7) | 4.2 (1.7, 6.7) | 6.7 (3.2, 10.2) |
| 3.4 (1.0, 5.8) | 10.0 (6.1, 13.9) | 11.9 (7.4, 16.4) |
| 8.9 (5.2, 12.6) | 17.1 (12, 22.2) | 24.0 (17.7, 30.3) |
Figure 2.
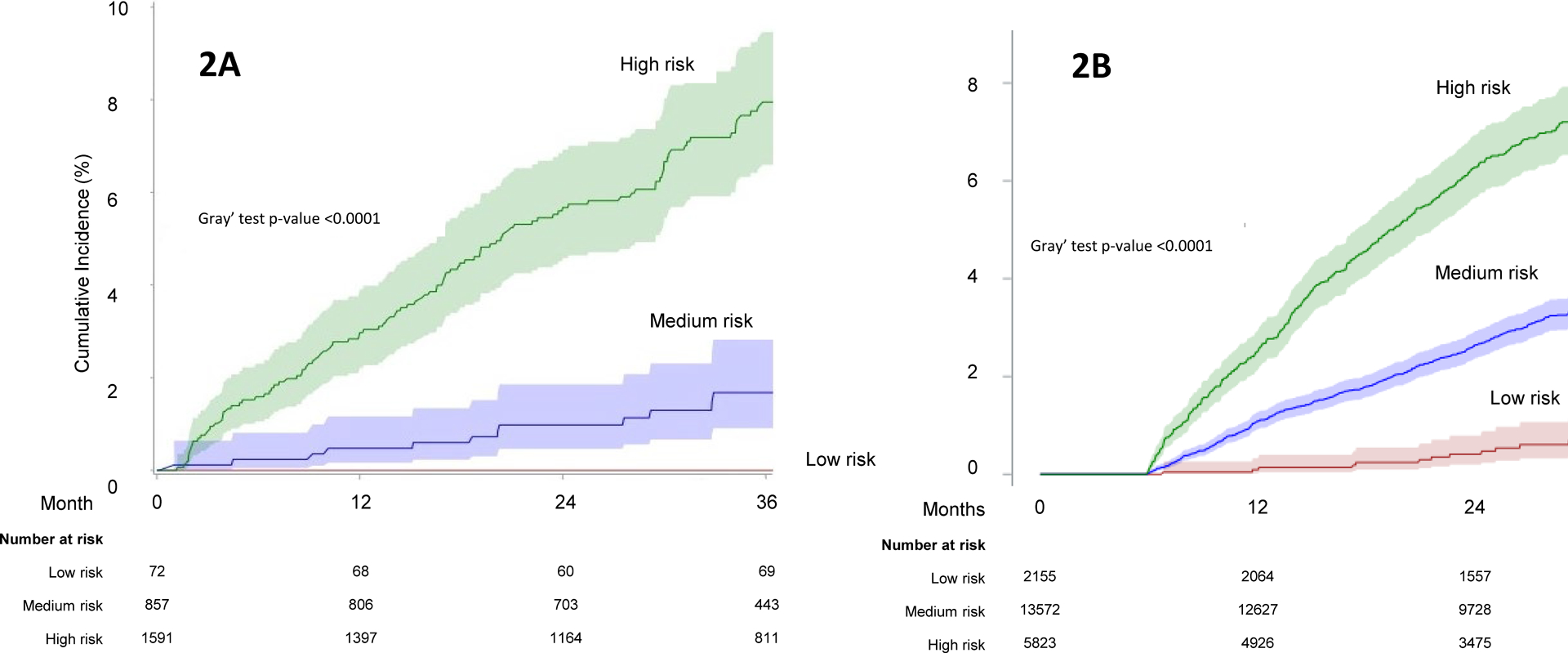
Cumulative incidence of HCC according to the Texas HCC Risk Index in the derivation (2A) and external validation (2B) cohorts.
DISCUSSION
Using data from two prospective cohort studies of patients with cirrhosis in clinical care, we derived and externally validated an HCC risk index to predict future risk of HCC in patients with cirrhosis across diverse etiologies. We included variables that have face validity and are readily obtainable in clinical practice. Measures of model performance, including discrimination and calibration, were good and exceeded the discrimination of previous HCC prediction models in patients with cirrhosis.6–10 Our model provides accurate prognostic estimates for a heterogenous population of ambulatory patients with cirrhosis that may be used to inform decision making about HCC surveillance and prevention.
Our study addresses the limitations of previous prediction models in cirrhosis. Lok et al. used data from the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) cohort to develop a model that predicted risk of HCC.6 However, the HALT-C cohort included patients with active HCV with limited applicability to contemporary clinical practices that mostly include for patients with cured HCV. Several risk models can predict future risk of HCC among patients with HBV-infection.7 In a recent analysis of 11 prospective studies, aMAP (age, male, albumin-bilirubin, and platelets) predicted the risk of HCC among patients with viral hepatitis.10 However, the discriminatory ability of this score was suboptimal in patients with cirrhosis, with estimates below 0.75 in most cohorts. Most models have included Asian or European patients,7, 10 limiting their use for risk stratification of patients with cirrhosis with diverse background and etiologies seen in the U.S. Few U.S. based models also exist. Using data from U.S. Veterans Health Administration, Ioannou and colleagues developed models estimating HCC risk in men with cured HCV-cirrhosis, NAFLD-cirrhosis or alcoholic-cirrhosis; each has limitations related the use of single-etiology cohorts and limited generalizability to non-veterans, especially women with cirrhosis.6, 28 The large size of the present study cohorts from different centers, broad representation of patients in clinical care, with few exclusion criteria overcome these limitations and ensure our model is broadly generalizable.
We believe THCC-RI can impact clinical care. Using the empirically derived cut-offs (Table 4, Figure 2), clinicians can identify patients who are at the highest risk of progression for more intensive surveillance strategies such as MRI-based surveillance to improve early detection. This escalation of surveillance tailored to patients’ risk is likely to be more cost-effective than using MRI-based surveillance in all at-risk patients.29 The THCC-RI can also facilitate future clinical trial design by allowing targeted recruitment of high-risk patients. The model can also be used for translational research to test the incremental prediction benefit of adding novel biomarkers, such as metabolic biomarkers and/or genomic signatures, to the fully specified model in eTable 3. This will allow the use of smaller cohorts than would be needed for de-novo model derivation, thus providing a critical tool for biomarker validation in cirrhosis.
The variables selected by simple machine learning were consistent with those in the published literature. Our study highlighted the importance of etiological risk factors in HCC.11 Beyond the contribution of traditional risk factors, such as age, sex, liver biochemistry and AFP, risky health behaviors resulted in significant improvement in the prediction performance metrics. These variables are potentially modifiable. Thus, our tool could provide a personalized HCC risk assessment to patients including how this risk may change over time with treatment or lifestyle changes.
Our derivation cohorts are unique in that they enrolled large numbers of cirrhosis patients from racial and ethnic minority groups that are both at high HCC risk as well as growing in number in the U.S. External validation using a separate cohort is also a strength. The validation cohort included cirrhosis patients with different sex and racial composition and frequency of etiological and behavioral risk factors. This strengthens our analysis because prediction models that perform well in an external cohort that differs from the derivation cohort provide greater generalizability to other populations with cirrhosis.
Our study has limitations. The index does not include other risk factors such as information on genomic or metabolomic markers. More specialized indices comprising these measures would require additional laboratory assays but can use our simple office-based model as the foundation.30 We did not include race and ethnicity in the model based on concerns that encoding racial/ethnic differences may reinforce discrimination and health disparities.31 We used empirically derived cut-offs (Table 4, eTable4) to illustrate the full spectrum of HCC risk. However, further research is needed to determine the optimal risk threshold for decision making that accounts for the risks and benefits of HCC surveillance. The follow-up duration in our cohorts precluded assessment of model performance at 5 or 10 years. Given that model performance was relatively similar at 1, 2 and 3 years, it is likely that our estimates will remain reliable over longer term follow up.
In conclusion, we developed and validated Texas HCC risk index that provides accurate risk stratification for HCC in patients with cirrhosis using objective, reliable and easily measurable factors. The model is suitable for implementation in routine clinical practice to identify patients at high risk for HCC who could be candidates for resource intensive HCC surveillance. Further refinement could be achieved by evaluating novel clinical, radiomic and genomic risk markers. The THCC-RI holds the promise to further improve risk stratification by identifying low risk patients, who may be candidates for de-escalation of HCC surveillance.
Supplementary Material
Table 1.
Cohort characteristics of patients with cirrhosis in the harmonized Texas Hepatocellular Carcinoma Consortium (THCCC) and the Houston Veterans Administration Cirrhosis Surveillance Cohorts (HVASC) [Derivation Cohort] and the Veterans Health Administration Cirrhosis Cohort [Validation Cohort].
| Characteristics, N % | Derivation Cohort | Validation Cohort |
|---|---|---|
| Age in years, mean (SD) | 60.0 (9.92) | 64.4 (7.65) |
| Gender | ||
| Male | 1729 (68.6) | 20742 (96.3) |
| Female | 791 (31.4) | 808 (3.7) |
| Race/Ethnicity | ||
| Non-Hispanic (NH) white | 1250 (49.6) | 13134 (61.0) |
| NH-Black | 500 (19.8) | 5382 (25.0) |
| Hispanic | 701 (27.8) | 1366 (6.3) |
| Other | 69 (2.7) | 1668 (7.7) |
| Alcohol use | ||
| Never | 775 (30.8) | 8097 (37.6) |
| Current heavy | 173 (6.8) | 4528 (21.0) |
| Other alcohol use (past history or current non-heavy) | 1572 (62.4) | 8925 (41.4) |
| Smoking | ||
| Never | 968 (38.4) | 5167 (24.0) |
| Current | 580 (23.0) | 9165 (42.5) |
| Past | 972 (38.6) | 7218 (33.5) |
| Metabolic traits | ||
| Diabetes | 1094 (43.4) | 12363 (57.4) |
| Body mass index (kg/m2) | 30.9 (6.9) | 30.0 (6.3) |
| Etiology of liver disease | ||
| Active HCV | 479 (19.0) | 1617 (7.5) |
| Cured HCV | 615 (24.4) | 9884 (45.9) |
| Alcohol related liver disease | 419 (19.0) | 6812 (31.6) |
| Nonalcoholic fatty liver disease | 714 (28.3) | 3237 (15.0) |
| Laboratory data, mean (SD) | ||
| Serum bilirubin (mg/dL) | 1.33 (1.5) | 1.02 (1.2) |
| Serum albumin (g/dL) | 3.74 (0.6) | 3.84 (0.6) |
| Alanine aminotransferase (IU/L) | 41.2 (38.6) | 37.5 (43.0) |
| Aspartate aminotransferase (IU/L) | 50.0 (40.9) | 44.1 (70.1) |
| International normalized ratio | 1.22 (0.5) | 1.40 (1.5) |
| Platelet count (106/mL) | 137.3 (70.1) | 158.7 (72.7) |
| Alpha fetoprotein (ng/mL) | 6.83 (14.4) | 10.8 (69.7) |
In total, 1078 (5.0) of patients in the external validation cohort had missing information on race.
Grant Support:
This work was supported by the National Cancer Institute (NCI U01 CA230997, U01 CA230694, and R01CA186566), Cancer Prevention & Research Institute of Texas grant (RP150587), and in part by Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338). Drs. Kanwal and El-Serag are investigators at the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, Texas.
Abbreviations:
- HCC
hepatocellular cancer
- NAFLD
nonalcoholic fatty liver disease
- HR
hazard ratio
- CI
confidence interval
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- THCCC
Texas Hepatocellular Carcinoma Consortium Cohort
- HVASC
Houston Veterans Administration Cirrhosis Surveillance Cohort
- EMR
electronic medical record
- SVR
sustained virological response
- BMI
body mass index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AFP
alpha-fetoprotein
- AUROC
area under receiver operating characteristic curve
- THCC-RI
Texas HCC risk index
Footnotes
Conflicts of Interest: No conflicts of interest to disclose.
Analytic methods will be made available to other researchers. Select data can be made available with specific request to the Texas HCC Consortium Study.
References
- 1.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. Jan 2003;12(1):4–13. [PubMed] [Google Scholar]
- 2.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. May 4 2005;97(9):675–83. [DOI] [PubMed] [Google Scholar]
- 3.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. Oct 20 2004;96(20):1518–27. [DOI] [PubMed] [Google Scholar]
- 4.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. May 20 2016;34(15):1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaderi S, Kanwal F. Changing epidemiology of hepatocellular cancer in the United States: Winning the battle but it is not over yet. Hepatology. Apr 7 2022; [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. Jan 2009;136(1):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, Yu X, Kramer J, et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J Hepatol. Feb 2022;76(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. Nov 2018;69(5):1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. Nov 15 2014;120(22):3485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. Dec 2020;73(6):1368–1378. [DOI] [PubMed] [Google Scholar]
- 11.Kanwal F, Khaderi S, Singal AG, et al. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology. Mar 1 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Marrero JA, Khaderi S, et al. Design of the Texas Hepatocellular Carcinoma Consortium Cohort Study. Am J Gastroenterol. Mar 2019;114(3):530–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. Feb 3 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolland B, Reid S, Stelling D, et al. Toward Rigorous Data Harmonization in Cancer Epidemiology Research: One Approach. Am J Epidemiol. Dec 15 2015;182(12):1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. Jan 2020;71(1):44–55. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan DE, Dai F, Aytaman A, et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. Dec 2015;13(13):2333–41 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. Jul 2012;143(1):70–7. [DOI] [PubMed] [Google Scholar]
- 18.Moon AM, Weiss NS, Beste LA, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. Oct 2018;155(4):1128–1139 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibshirani R The lasso method for variable selection in the Cox model. Stat Med. Feb 28 1997;16(4):385–95. doi: [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z Variable selection with stepwise and best subset approaches. Ann Transl Med. Apr 2016;4(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. Nov 30 2017;36(27):4391–4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Cortese G, Combescure C, et al. Overview of model validation for survival regression model with competing risks using melanoma study data. Ann Transl Med. Aug 2018;6(16):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Calster B, McLernon DJ, van Smeden M, et al. Calibration: the Achilles heel of predictive analytics. BMC Med. Dec 16 2019;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graf E, Schmoor C, Sauerbrei W, Schumacher M. Assessment and comparison of prognostic classification schemes for survival data. Stat Med. 1999;18(17–18):2529–45. [DOI] [PubMed] [Google Scholar]
- 25.Mondol MH, Rahman MS. A comparison of internal validation methods for validating predictive models for binary data with rare events Journal of Statistical Research 2018;doi: 10.47302/jsr.2017510203. [DOI] [Google Scholar]
- 26.Steyerberg EW, Harrell FE Jr., Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. Aug 2001;54(8):774–81. doi: 10.1016/s0895-4356(01)00341-9 [DOI] [PubMed] [Google Scholar]
- 27.Bowers AJ. Receiver Operating Characteristic (ROC) Area Under the Curve (AUC): A Diagnostic Measure for Evaluating the Accuracy of Predictors of Education Outcomes. Journal of Education for Students Placed at Risk. 2019;29(1):20–46. [Google Scholar]
- 28.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. Sep 2019;71(3):523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin Gastroenterol Hepatol. Jan 2022;20(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara N, Kobayashi M, Fobar AJ, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (N Y). Jul 9 2021;2(7):836–850 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. Oct 25 2019;366(6464):447–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


