Abstract
Soybean (Glycine max) and pea (Pisum sativum) differ in the transport of fixed nitrogen from nodules to shoots. The dominant nitrogen transport compounds for soybean are ureides, while amides dominate in pea. A possible enzymic basis for this difference was examined.
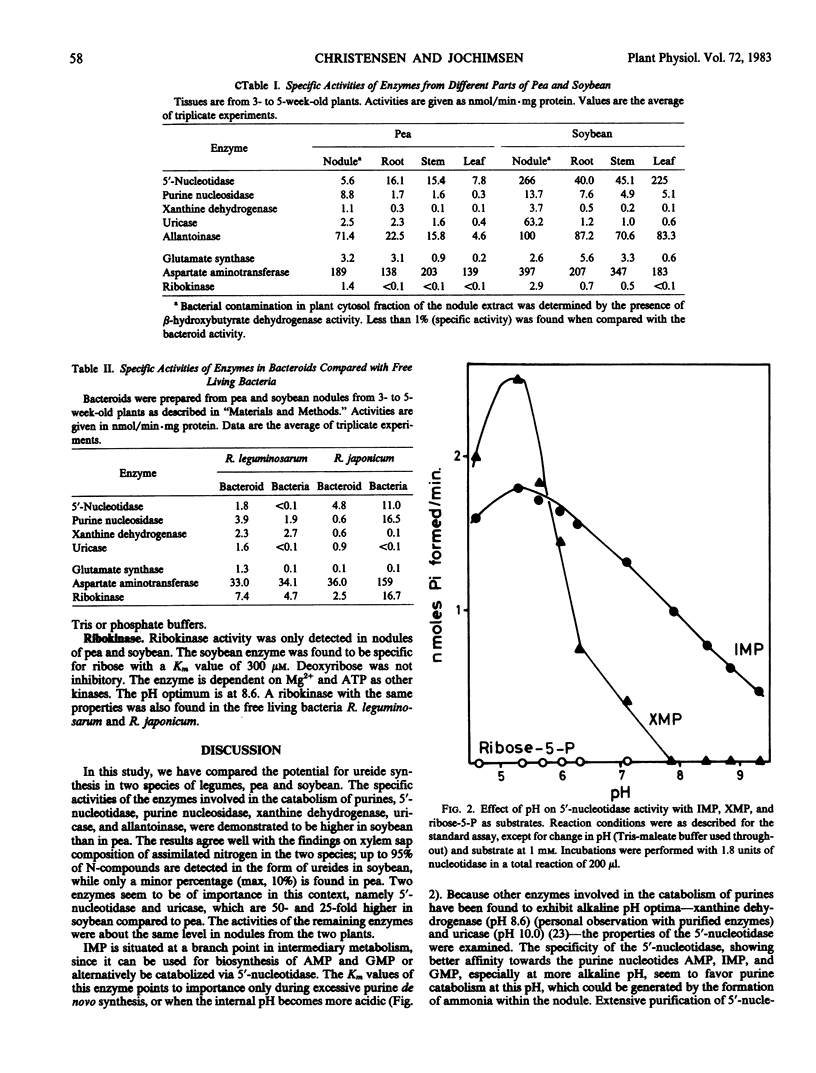
The level of enzymes involved in the formation of the ureides allantoin and allantoic acid from inosine 5′-monophosphate (IMP) was compared in different tissues of pea and soybean. Two enzymes, 5′-nucleotidase and uricase, from soybean nodules were found to be 50- and 25-fold higher, respectively, than the level found in pea nodules. Other purine catabolizing enzymes (purine nucleosidase, xanthine dehydrogenase, and allantoinase) were found to be at the same level in the two species. From comparison of enzyme activities in nodules with those from roots, stems, and leaves, two enzymes were found to be nodule specific, namely uricase and xanthine dehydrogenase. The level of enzymes found in the bacteroids indicated no significant contribution of Rhizobium japonicum purine catabolism in the overall formation of ureides in the soybean nodule. The presence in the nodules of purine nucleosidase and ribokinase activities makes a recirculation of the ribose moiety possible. In concert with phosphoribosylpyrophosphate synthetase, ribose becomes available for a new round of purine de novo synthesis, and thereby ureide formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland M. J., Benny A. G. Enzymes of nitrogen metabolism in legume nodules. Purification and properties of NADH-dependent glutamate synthase from lupin nodules. Eur J Biochem. 1977 Oct 3;79(2):355–362. doi: 10.1111/j.1432-1033.1977.tb11816.x. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Fujihara S., Yamaguchi M. Asparagine formation in soybean nodules. Plant Physiol. 1980 Jul;66(1):139–141. doi: 10.1104/pp.66.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A. Purine catabolism in plants : purification and some properties of inosine nucleosidase from yellow lupin (lupinus luteus L.) seeds. Plant Physiol. 1982 Aug;70(2):344–349. doi: 10.1104/pp.70.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludwig R. A., Signer E. R. Glutamine synthetase and control of nitrogen fixation in Rhizobium. Nature. 1977 May 19;267(5608):245–248. doi: 10.1038/267245a0. [DOI] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Genetic and metabolic control of the purine catabolic enzymes of Neurospora crasse. Mol Gen Genet. 1975 Aug 5;139(1):39–55. doi: 10.1007/BF00267994. [DOI] [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Woo K. C. Ureide Synthesis in a Cell-Free System from Cowpea (Vigna unguiculata [L.] Walp.) Nodules : STUDIES WITH O(2), pH, AND PURINE METABOLITES. Plant Physiol. 1981 Jun;67(6):1156–1160. doi: 10.1104/pp.67.6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]


