Abstract
The U.S. Food and Drug Administration authorized use of mRNA COVID-19 bivalent booster vaccines on August 31, 2022. Currently, CDC’s clinical guidance states that COVID-19 and other vaccines may be administered simultaneously. At time of authorization and recommendations, limited data existed describing simultaneous administration of COVID-19 bivalent booster and other vaccines. We describe simultaneous influenza and mRNA COVID-19 bivalent booster vaccine administration between August 31–December 31, 2022, among persons aged ≥6 months in the Vaccine Safety Datalink (VSD) by COVID-19 bivalent booster vaccine type, influenza vaccine type, age group, sex, and race and ethnicity. Of 2,301,876 persons who received a COVID-19 bivalent booster vaccine, 737,992 (32.1%) received simultaneous influenza vaccine, majority were female (53.1%), aged ≥18 years (91.4%), and non-Hispanic White (55.7%). These findings can inform future VSD studies on simultaneous influenza and COVID-19 bivalent booster vaccine safety and coverage, which may have implications for immunization service delivery.
Keywords: mRNA covid-19 bivalent booster vaccine, Seasonal influenza vaccine, Simultaneous vaccination, Co-administration
1. Introduction
On August 31, 2022, the U.S. Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for COVID-19 vaccine bivalent formulations of BNT162b2 (Pfizer-BioNTech) for persons aged ≥ 12 years and mRNA-1273 (Moderna) for adults aged ≥ 18 years for use as a single booster dose ≥ 2 months after completion of the mRNA COVID-19 primary series or receipt of a monovalent booster vaccine [1-2]. Subsequently, on October 12, 2022, FDA expanded the EUAs to include persons aged 5–11 years for Pfizer-BioNTech and 6–17 years for Moderna, and on December 8, 2022, to include children aged ≥ 6 months for both products [1-2]. This was followed by a Centers for Disease Control and Prevention (CDC) recommendation that all persons aged ≥ 6 months receive an age-appropriate bivalent COVID-19 vaccine dose [3]. Current CDC clinical guidance states that COVID-19 vaccines and other vaccines may be administered simultaneously (i.e., on the same day or during the same visit) to children, adolescents, and adults, provided that no specific contraindications exist at time of vaccination [4].
There are published data that describe simultaneous vaccination of influenza vaccines with monovalent COVID-19 primary series and monovalent booster vaccines [5-6]. However, to the best of our knowledge, data describing simultaneous administration of seasonal influenza and mRNA COVID-19 bivalent booster vaccines have not yet appeared in the published literature. This study describes simultaneous administration of influenza and mRNA COVID-19 bivalent booster vaccines between August 31–December 31, 2022, among persons aged ≥ 6 months in the Vaccine Safety Datalink (VSD) by mRNA COVID-19 bivalent booster vaccine type, influenza vaccine type, age group, sex, and race and ethnicity. Results of this study may inform future studies that aim to assess the safety and coverage of simultaneous influenza and mRNA COVID-19 bivalent booster vaccine administration.
2. Methods
The VSD is a long-standing collaboration between the CDC and several integrated health care organizations (sites) to monitor and assess the safety of licensed and authorized vaccines in the United States [7]. As of September 2022, 13 VSD sites provide clinical and methodological expertise and contribute data [7]. VSD sites capture healthcare information on their members such as insurance enrollment, demographic characteristics, medical diagnoses, and receipt of COVID-19 and other vaccines, including COVID-19 vaccines administered outside of the healthcare system (e.g., retail pharmacies, mass vaccination clinics, workplaces) [8-11]. The eight VSD sites whose data were included in this study, located in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin, insure a combined population of over 12.5 million persons annually, representing 3.6% of the U.S. population [12]. Among this population, 20% are < 18 years old and 16% are ≥ 65 years old. This study was approved by the Institutional Review Boards of the eight participating VSD sites and by CDC, and was conducted consistent with applicable federal law and CDC policy.
2.1. Study design & population
This descriptive, retrospective study utilized data obtained from VSD’s COVID-19 vaccine rapid cycle analysis (RCA) surveillance [12]. Source population consisted of persons aged ≥ 6 months who received an age-appropriate mRNA COVID-19 bivalent booster vaccine ≥ 2 months after receipt of a COVID-19 monovalent vaccine and were enrolled in one of the eight VSD sites on day of mRNA COVID-19 bivalent booster vaccination between August 31–December 31, 2022. Among those who received an mRNA COVID-19 bivalent booster vaccine, we examined patterns of administration, as described below.
2.2. Data analysis
Among persons who received an mRNA COVID-19 bivalent booster vaccine, we assessed the overall number and proportion of persons who received: 1) simultaneous (i.e., same day) influenza and mRNA COVID-19 bivalent booster vaccines (with or without additional non-influenza simultaneous vaccines), 2) ≥ 1 simultaneous non-influenza vaccine(s) and mRNA COVID-19 bivalent booster vaccines, and 3) no simultaneous vaccines with the mRNA COVID-19 bivalent booster vaccine. We conducted descriptive analyses of vaccine recipients by mRNA COVID-19 bivalent booster vaccine type received, sex, age group, and race and ethnicity. We also assessed the number of persons who received vaccines across the three groups described above by month, and influenza vaccine type received among those who received a simultaneous influenza and mRNA COVID-19 bivalent booster vaccine, (Supplemental Table 1). In instances where a person received both a known and an “other/unknown” influenza vaccine type simultaneously with the COVID-19 bivalent booster due to errors in recording, we only included known influenza vaccine types in the analyses. If more than one known influenza vaccine type was administered simultaneously with the mRNA COVID-19 bivalent booster vaccine, we counted each of these instances in the respective influenza vaccine type totals, but this was rare and occurred in 0.01% (n = 50 persons) out of all persons who received simultaneous influenza and mRNA COVID-19 bivalent booster vaccines. Additionally, we described the most common non-influenza vaccine types administered to persons who received: 1) simultaneous influenza and ≥ 1 non-influenza vaccines with mRNA COVID-19 bivalent booster vaccine, and 2) ≥ 1 simultaneous non-influenza and mRNA COVID-19 bivalent booster vaccines.
3. Results
Between August 31–December 31, 2022, 2,301,876 persons received an mRNA COVID-19 bivalent booster vaccine in the VSD, of whom 32.1% (n = 737,992) received a simultaneous influenza vaccine (Table 1). Among persons who received an mRNA COVID-19 bivalent booster vaccine (with or without simultaneous administration of other vaccines), a majority (70.0%) received a Pfizer-BioNTech bivalent booster, were female (55.3%), aged ≥ 18 years (92.5%), and non-Hispanic White (50.6%). Among those who received a simultaneous influenza and mRNA COVID-19 bivalent booster vaccine, majority (77.4%) received a Pfizer-BioNTech bivalent booster, were female (53.1%), aged ≥ 18 years (91.4%), and non-Hispanic White (55.7%), similar patterns were observed among persons who received simultaneous non-influenza and mRNA COVID-19 bivalent booster vaccines, and among persons who received an mRNA COVID-19 bivalent booster vaccine alone. Ad-hoc analyses showed that between August 31–December 31, 2022, among all persons who received an mRNA COVID-19 bivalent booster vaccine but not a simultaneous influenza vaccine, approximately 13% received an influenza vaccine before receiving their mRNA COVID-19 bivalent booster vaccine, and approximately 39% received an influenza vaccine after receiving their mRNA COVID-19 bivalent booster vaccine.
Table 1.
Characteristics of persons aged ≥ 6 months who received an mRNA COVID-19 bivalent booster vaccine alone, an mRNA COVID-19 bivalent booster and simultaneous influenza vaccine, or an mRNA COVID-19 bivalent booster and simultaneous non-influenza vaccine in the Vaccine Safety Datalink, August 31, 2022–December 31, 2022.
| No. (column %) | ||||
|---|---|---|---|---|
| Characteristic | mRNA COVID-19 bivalent booster vaccine alone (N = 1,526,663) |
mRNA COVID-19 bivalent booster and simultaneous influenza vaccine (N = 737,992)a |
mRNA COVID-19 bivalent booster and simultaneous non-influenza vaccine (N = 37,221) a |
Total (N = 2,301,876) |
| mRNA COVID-19 bivalent booster vaccine type | ||||
| Moderna | 513,079 (33.6) | 166,810 (22.6) | 10,179 (27.3) | 690,068 (30.0) |
| Pfizer-BioNTech | 1,013,584 (66.4) | 571,182 (77.4) | 27,042 (72.7) | 1,611,808 (70.0) |
| Sex | ||||
| Female | 863,474 (56.6) | 391,521 (53.1) | 18,896 (50.8) | 1,273,891 (55.3) |
| Male | 663,189 (43.4) | 346,471 (46.9) | 18,325 (49.2) | 1,027,985 (44.7) |
| Age group, years | ||||
| 0–5b | 6,796 (0.4) | 2,101 (0.3) | 64 (0.2) | 8,961 (0.4) |
| 6–11 | 45,868 (3.0) | 21,551 (2.9) | 911 (2.4) | 68,330 (3.0) |
| 12–17 | 52,899 (3.5) | 39,287 (5.3) | 1,306 (3.5) | 93,492 (4.1) |
| 18–49 | 447,739 (29.3) | 255,403 (34.6) | 6,356 (17.1) | 709,498 (30.8) |
| 50–64 | 378,654 (24.8) | 186,227 (25.2) | 13,401 (36.0) | 578,282 (25.1) |
| ≥65 | 594,707 (39.0) | 233,423 (31.6) | 15,183 (40.8) | 843,313 (36.6) |
| Race/Ethnicity (NH = non-Hispanic) c | ||||
| Hispanic or Latino | 270,348 (17.7) | 113,377 (15.4) | 9,745 (26.2) | 393,470 (17.1) |
| American Indian or Alaska Native, NH | 4,039 (0.3) | 2,166 (0.3) | 128 (0.3) | 6,333 (0.3) |
| Asian, NH | 281,059 (18.4) | 103,969 (14.1) | 5,185 (13.9) | 390,213 (17.0) |
| Black or African American, NH | 81,847 (5.4) | 37,923 (5.1) | 3,401 (9.1) | 123,171 (5.4) |
| Native Hawaiian or Other Pacific Islander, NH | 8,234 (0.5) | 3,490 (0.5) | 223 (0.6) | 11,947 (0.5) |
| White, NH | 738,001 (48.3) | 411,136 (55.7) | 15,674 (42.1) | 1,164,811 (50.6) |
| Multiple/Other Race, NH | 61,851 (4.1) | 29,060 (3.9) | 1,505 (4.0) | 92,416 (4.0) |
| Unknown | 81,284 (5.3) | 36,871 (5.0) | 1,360 (3.7) | 119,515 (5.2) |
“mRNA COVID-19 bivalent booster and simultaneous influenza vaccine” category includes persons who received an influenza vaccine (any type) simultaneously with mRNA COVID-19 bivalent booster vaccine, with or without additional simultaneous non-influenza vaccine(s). “mRNA COVID-19 bivalent booster and simultaneous non-influenza vaccine” category includes persons who received ≥ 1 non-influenza vaccine(s) simultaneously with mRNA COVID-19 bivalent booster vaccine, but no simultaneous influenza vaccine(s).
As of December 31, 2022, for children aged 6 months-4 years, only a bivalent formulation for the third dose of the Pfizer-BioNTech primary series was recommended during the study period, but not a bivalent booster vaccine; therefore, bivalent formulations administered to this age group were not included in analyses. Only a Moderna COVID-19 bivalent booster vaccine was authorized for children aged 6 months-5 years during the study period, and these are the only counts included in analyses for this age group.
“Multiple/Other, NH” includes persons with more than 1 non-Hispanic race and ethnicity and all other non-Hispanic races and ethnicities.
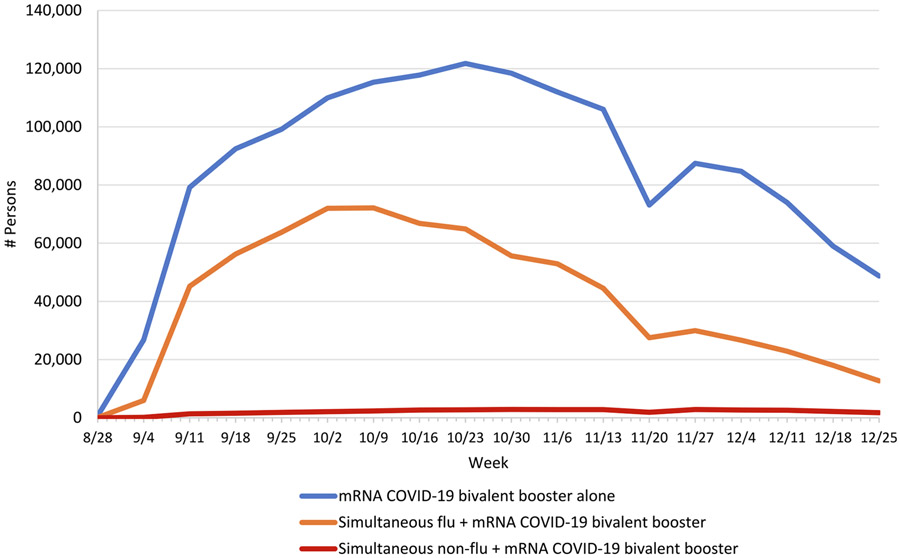
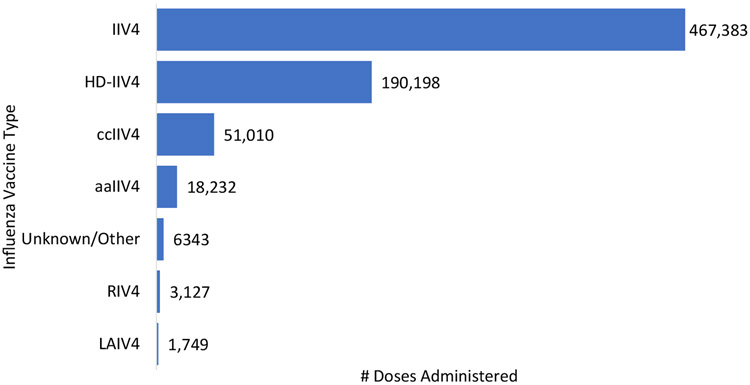
The number of persons who received simultaneous influenza and mRNA COVID-19 bivalent booster vaccines followed the same pattern as peak months of seasonal influenza vaccine administration – increasing in September, peaking during October, and decreasing weekly thereafter (Fig. 1). Among those who received a simultaneous influenza and mRNA COVID-19 bivalent booster vaccine, the most frequently received influenza vaccine types were IIV4 (467,383 doses), HD-IIV4 (190,198 doses), and ccIIV4 (51,010 doses) (Fig. 2). Among persons who received simultaneous influenza and mRNA COVID-19 bivalent booster vaccines, 95.8% (n = 707,159) received only influenza vaccine, while the remainder (n = 30,833) received one influenza plus ≥ 1 other vaccine type(s) simultaneously with the mRNA COVID-19 bivalent booster vaccine. Of those who received one influenza plus ≥ 1 other vaccine type (s) simultaneously with an mRNA COVID-19 bivalent booster vaccine, the top five most frequently administered other vaccine types were herpes zoster (n = 10,390 doses), tetanus, diphtheria and acellular pertussis (Tdap; n = 8,001 doses), pneumococcal (n = 6,363 doses), human papillomavirus (HPV; n = 3,674 doses), and meningococcal (n = 2,741 doses) vaccines (data not shown). Among persons who received a non-influenza and mRNA COVID-19 bivalent booster vaccine, the top five most frequently administered non-influenza vaccine types were herpes zoster (n = 19,114 doses), pneumococcal (n = 7,328 doses), Tdap (n = 7,074), HPV (n = 1,942), and hepatitis B (n = 1,775) vaccines (data not shown).
Fig. 1.
Number of persons who received an mRNA COVID-19 bivalent booster vaccine alone, a simultaneous influenza and an mRNA COVID-19 bivalent booster vaccine, or a simultaneous non-influenza and an mRNA COVID-19 bivalent booster vaccine, by week in the Vaccine Safety Datalink, August 31, 2022–December 31, 2022aa Weekly data starts on Sunday and ends on Saturday of each respective week. “Simultaneous flu + mRNA COVID-19 bivalent booster” category includes persons who received an influenza vaccine (any type) simultaneously with mRNA COVID-19 bivalent booster vaccine, with or without additional simultaneous non-influenza vaccine(s). “Simultaneous non-flu + mRNA COVID-19 bivalent booster” category includes persons who received ≥ 1 non-influenza vaccine simultaneously with mRNA COVID-19 bivalent booster vaccine, but no simultaneous influenza vaccine(s).
Fig. 2.
Number of simultaneous influenza vaccine doses administered with mRNA COVID-19 bivalent booster vaccines, by influenza vaccine type in the Vaccine Safety Datalink, August 31, 2022–Deeember 31, 2022a A person’s record may have indicated more than one influenza vaccine type administered simultaneously with an mRNA COVID-19 bivalent booster vaccine but this was rare; in those instances, all influenza vaccine types were included (n = 50 persons). Abbreviations: aIIV4 = Adjuvanted inactivated influenza vaccine, quadrivalent; ccIIV4 = Cell culture-based inactivated influenza vaccine, quadrivalent; HD-IIV4 = High-dose inactivated influenza vaccine, quadrivalent; IIV4 = Inactivated influenza vaccine, quadrivalent; LAIV4 = Live attenuated influenza vaccine, quadrivalent; RIV4 = Recombinant influenza vaccine, quadrivalent.
4. Discussion
In the VSD, 2,301,876 persons received an mRNA COVID-19 bivalent booster vaccine between August 31–December 31, 2022, of whom 32.1% received a simultaneous influenza vaccine. Most persons who received an mRNA COVID-19 bivalent booster vaccine alone or who received simultaneous influenza and mRNA COVID-19 bivalent booster vaccines received Pfizer-BioNTech, and were female, aged ≥ 18 years, and non-Hispanic White, which reflects the general demographics of the VSD population. Simultaneous administration of influenza and mRNA COVID-19 bivalent booster vaccines in sufficient numbers may facilitate VSD’s ability to conduct vaccine safety surveillance on persons who received simultaneous vaccination. However, it still remains methodologically challenging to disentangle the effect of simultaneous vaccinations and its relationship with medically attended events of interest and emphasizes the importance of assessing the same relationship in individuals who received each vaccination alone as well.
In a study assessing data from v-safe, a voluntary smartphone-based U.S. safety surveillance system, simultaneous COVID-19 mRNA monovalent booster and influenza vaccine administration among respondents aged ≥ 12 years was 9.4%, lower than the 32.1% we observed in VSD for simultaneous mRNA COVID-19 bivalent booster and influenza vaccine administration [6]. The 32.1% we observed was also much higher than VSD’s COVID-19 vaccine data on simultaneous vaccination (any vaccine type) with COVID-19 monovalent primary series vaccines administered between December 11, 2020–May 21, 2022. In that study, only 0.7% of 8,455,037 persons received simultaneous vaccine(s) with COVID-19 monovalent vaccine dose 1, and only 0.3% of 7,787,013 persons received simultaneous vaccine(s) with COVID-19 monovalent vaccine dose 2. Influenza vaccine (any type) was the most frequently administered simultaneous vaccine type [5]. Various factors may have contributed to a higher proportion of persons receiving simultaneous influenza and mRNA COVID-19 bivalent booster vaccines. Initially, when the COVID-19 vaccination program began in December 2020, ACIP recommended that COVID-19 vaccines be administered alone out of an abundance of caution [13]. Subsequently, in May 2021, CDC updated its clinical considerations stating that COVID-19 and other vaccines could be administered simultaneously [14]. In the VSD, 72% of persons received their mRNA COVID-19 primary series vaccines between December 2020–April 2021, prior to CDC’s updated clinical considerations for simultaneous vaccination [5]. This likely contributed to a lower overall proportion of simultaneous vaccination with COVID-19 primary series vaccines. Additionally, when the mRNA COVID-19 bivalent booster vaccines were authorized during months of traditionally high influenza vaccine uptake, COVID-19 mass vaccination sites were rare, leading people to seek COVID-19 vaccines at medical encounters or pharmacies, where depending on vaccine availability, the opportunity for getting more than one vaccine during the same visit was more likely.
CDC encourages getting both influenza and COVID-19 vaccines during the fall and winter months, as both viruses could be in circulation at the same time and vaccination against both may help prevent or reduce severity of illness [15]. CDC also promotes administration of both influenza and COVID-19 vaccines during the same visit if a person is due for both, noting that simultaneous administration (i.e., coadministration) is a common medical practice and can ensure people get all their recommended vaccines as soon as possible and reduce missed opportunities [16]. Studies assessing the safety of simultaneous influenza and COVID-19 bivalent booster vaccine administration may provide additional insight for medical practitioners and the public. In the VSD study assessing frequency of health outcomes following receipt of simultaneous mRNA COVID-19 monovalent primary series and other vaccines, only a small proportion received simultaneous vaccines, and a small number of outcomes were observed among this population, limiting ability to conduct statistical analyses of potential health outcomes [5]. In the current study, given the larger proportion of persons who received influenza vaccine simultaneously with mRNA COVID-19 bivalent booster vaccines, particularly among persons aged 12 years and older, it may be possible for VSD to conduct vaccine safety assessments on a wider range of health outcomes following simultaneous influenza and mRNA COVID-19 bivalent booster vaccine receipt in the future.
This study has several limitations. First, study findings may not be generalizable to all populations in the United States. Second, vaccine administration data may be incomplete or misclassified if individuals received vaccines outside of the participating healthcare systems. However, VSD sites capture COVID-19 vaccines administered in external settings, including vaccines recorded in pharmacy claims and immunization information systems [11]. Third, vaccines may be inaccurately recorded in a patient’s medical record at time of vaccine administration. Fourth, COVID-19 bivalent booster vaccine EUAs were expanded for persons aged 5–17 years on October 12, 2022, and among children aged 6 months-4 years on December 8, 2022 and did not span the entire study period of August 31-December 31, 2022.
5. Conclusion
Our findings describe simultaneous administration of influenza and mRNA COVID-19 bivalent booster vaccines among persons aged ≥ 6 months in the VSD by mRNA COVID-19 bivalent booster vaccine type, influenza vaccine type, age group, sex, and race and ethnicity. Among all persons who received an mRNA COVID-19 bivalent booster vaccine during the study period, 32.1% received a simultaneous influenza vaccine. With nearly one-third of persons receiving simultaneous influenza vaccines, the VSD may be better able to assess vaccine safety in this population. Additionally, future studies assessing COVID-19 bivalent booster coverage may provide valuable information on gaps and disparities. Combined, these studies may have implications for immunization service delivery, particularly during months of traditionally high influenza vaccine uptake.
Supplementary Material
Acknowledgements
We would like to thank the Vaccine Safety Datalink data managers and project managers for their contributions.
Funding/support
This work was supported by the Centers for Disease Control and Prevention (CDC). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. See, for example, 45 C.F.R. part 46.102(1)(2), 21 C.F.R. part 56; 42 U.S.C. §241 (d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Footnotes
CRediT authorship contribution statement
Tat’Yana A. Kenigsberg: Conceptualization, Data curation, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Kristin Goddard: Conceptualization, Project administration, Writing – review & editing. Kayla E. Hanson: Conceptualization, Project administration, Writing – review & editing. Ned Lewis: Writing – review & editing. Nicola Klein: Writing – review & editing. Stephanie A. Irving: Project administration, Writing – review & editing. Allison L. Naleway: Writing – review & editing. Bradley Crane: Writing – review & editing. Tia L. Kauffman: Project administration, Writing – review & editing. Stanley Xu: Writing – review & editing. Matthew F. Daley: Writing – review & editing. Laura P. Hurley: Writing – review & editing. Robyn Kaiser: Writing – review & editing. Lisa A. Jackson: Writing – review & editing. Amelia Jazwa: Project administration, Writing – review & editing. Eric S. Weintraub: Investigation, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kayla E. Hanson reports financial support was provided by Seqirus Srl. Nicola P. Klein reports financial support was provided by Pfizer. Nicola P. Klein reports financial support was provided by Sanofi Pasteur Inc. Nicola P. Klein reports financial support was provided by Merck & Co Inc. Nicola P. Klein reports financial support was provided by GlaxoSmithKline Inc. Allison L. Naleway reports financial support was provided by Pfizer. Allison L. Naleway reports financial support was provided by Vir Biotechnology Inc. Lisa A. Jackson reports financial support was provided by Pfizer.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.08.023.
Data availability
The authors do not have permission to share data.
References
- [1].Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/150386/download. [Google Scholar]
- [2].Food and Drug Administration. Moderna COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/144636/download. [Google Scholar]
- [3].Centers for Disease Control and Prevention. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 6 Months through 5 Years [Press release]; 2022, December 9. https://www.cdc.gov/media/releases/2022/s1209-covid-vaccine.html.
- [4].Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. US Department of Health and Human Services, CDC; 2022. Accessed February 17, 2023, from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. [Google Scholar]
- [5].Kenigsberg TA, Hanson KE, Klein NP, Zerbo O, Goddard K, Xu S, et al. Safety of simultaneous vaccination with COVID-19 vaccines in the Vaccine Safety Datalink. Vaccine 2023;41:4658–65. 10.1016/j.vaccine.2023.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hause AM, Zhang B, Yue X, Marquez P, Myers TR, Parker C, et al. Reactogenicity of simultaneous COVID-19 mRNA booster and influenza vaccination in the US. JAMA Netw Open 2022;5(7):e2222241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. Vaccine Safety Datalink (VSD). Accessed February 27, 2023, from https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html.
- [8].Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(Suppl 1):S45–53. 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- [9].McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32(42):5390–8. 10.1016/j.vaccine.2014.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): a comparison with the United States population. Vaccine 2015;33(36):4446–50. 10.1016/j.vaccine.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Groom HC, Crane B, Naleway AL, Weintraub E, Daley MF, Wain K, et al. Monitoring vaccine safety using the vaccine safety Datalink: assessing capacity to integrate data from Immunization Information systems. Vaccine 2022;40(5):752–6. 10.1016/j.vaccine.2021.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. J Am Med Assoc 2021;326 (14): 1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].ACIP COVID-19 Vaccines Work Group Interpretations of Data. Accessed March 23, 2023, from https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-01/10-COVID-Oliver.pdf.
- [14].Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. US Department of Health and Human Services, CDC; 2022. Accessed October 10, 2022, from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. [Google Scholar]
- [15].Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2021-22 Influenza Season. Accessed February 24, 2023, from: https://www.cdc.gov/flu/fluvaxview/coverage-2022estimates.htm.
- [16].Centers for Disease Control and Prevention. Getting a Flu Vaccine and a COVID-19 Vaccine at the Same Time. Accessed February 24, 2023, from: https://www.cdc.gov/flu/prevent/coadministration.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.