Abstract
The protozoan parasites Cryptosporidium parvum, Cyclospora cayetanensis, and Toxoplasma gondii are major causes of waterborne and foodborne diseases worldwide. The assessment of their removal or inactivation during water treatment and food processing remains challenging, partly because research on these parasites is hindered by various economical, ethical, methodological, and biological constraints. To address public health concerns and gain new knowledge, researchers are increasingly seeking alternatives to the use of such pathogenic parasites. Over the past few decades, several non-pathogenic microorganisms and manufactured microparticles have been evaluated as potential surrogates of waterborne and foodborne protozoan parasites. Here, we review the surrogates that have been reported for C. parvum, C. cayetanensis, and T. gondii oocysts, and discuss their use and relevance to assess the transport, removal, and inactivation of these parasites in food and water matrices. Biological surrogates including non-human pathogenic Eimeria parasites, microorganisms found in water sources (anaerobic and aerobic spore-forming bacteria, algae), and non-biological surrogates (i.e. manufactured microparticles) have been identified. We emphasize that such surrogates have to be carefully selected and implemented depending on the parasite and the targeted application. Eimeria oocysts appear as promising surrogates to investigate in the future the pathogenic coccidian parasites C. cayetanensis and T. gondii that are the most challenging to work with.
Keywords: Surrogate, Oocyst, Protozoa, Cryptosporidium parvum, Cyclospora cayetanensis, Toxoplasma gondii
Graphical abstract
Highlights
-
•
Protozoan parasites are significant waterborne and foodborne pathogens.
-
•
Exposure assessment to protozoan parasites is hampered by numerous constraints
-
•
Surrogates can help to assess removal and/or inactivation in water and food.
-
•
Suitable surrogate should be selected according to the protozoa and the application.
-
•
Eimeria oocysts are promising surrogates for coccidian protozoan pathogens to humans.
1. Introduction
Foodborne and waterborne infectious diseases present a major public health issue. Besides the direct health impact on infected individuals, they can impair socioeconomic development at regional and national levels and can spread worldwide due to increasing travel opportunities and expanding international trade (Robertson et al., 2014). Foodborne and waterborne pathogens include bacteria, viruses, fungi, and parasites. Foodborne parasites are recognized as a neglected pathogen group (Robertson et al., 2018). Nevertheless, over the last decades, parasitic protozoa have been identified as a significant cause of waterborne and foodborne diseases (Dawson, 2005) with significant numbers of cases and exposed persons (Efstratiou et al., 2017; Guzman-Herrador et al., 2015; Ma et al., 2022; Wittler, 2023). A report from a FAO/WHO Expert Committee based on multi-criteria decision analysis identified 24 foodborne parasites of high global priorities (FAO/WHO, 2014). Among them, Cryptosporidium parvum, Cyclospora cayetanensis, and Toxoplasma gondii have been highlighted as parasitic protozoa of greatest concern in food production in Europe and worldwide (Bouwknegt et al., 2018; FAO/WHO, 2014).
These protozoa are coccidian parasites belonging to Apicomplexa, T. gondii and C. cayetanensis being the most phylogenetically closely related (Relman et al., 1996). C. parvum and T. gondii are zoonotic while C. cayetanensis is human-restricted (Votýpka et al., 2017). They exist as environmentally resistant oocyst stages, which originate from the feces of infected hosts and are transmitted to humans through the fecal-oral route, either directly (person-to-person or animal-to-person contact) or indirectly (through contaminated waters or foods). Due to the numerous services that water renders to humans (e.g. recreational activities, food and drinking water production), its quality is crucial. Nevertheless, it is the receptacle of multiple contaminants, originating from human activities, terrestrial contaminations (such as animal waste) and/or inadequate wastewater treatment (Dufour et al., 2012; United Nations World Water Assessment Program, 2017). The zoonotic parasites Cryptosporidium spp. and T. gondii are known to be widely spread in the environment by infected animals and can disseminate in aquatic environments (Li et al., 2022; Ziemer et al., 2010; Hutchison et al., 2005). As enteric pathogens, Cryptosporidium spp. and C. cayetanensis are widespread in wastewater (Almeria et al., 2019; Zahedi et al., 2018). Hence, these three parasites are of particular concern in terms of water quality for food and water production. Global changes and extreme weather events, especially intense raining, increase the probability of water contamination by these parasites through terrestrial runoff in the watersheds and/or wastewater treatment plant bypasses. C. parvum, C. cayetanensis, and T. gondii oocysts do not replicate in food and water but they can persist and survive for long periods under favorable environmental conditions, e.g. low temperatures and exposure to sunlight, and no desiccation (Ortega et al., 1998; Robertson et al., 1992; Shapiro et al., 2019; King et al., 2005, King et al., 2008). Although these parasites can be inactivated by certain UV and heat treatments, they may be resistant to: i) chemicals typically used as disinfectant in potable water or food industries, or ii) to some freezing conditions and non-thermal processes used by food processors (Erickson and Ortega, 2006; Gérard et al., 2019; Mirza Alizadeh et al., 2018; Hijnen et al., 2006). Hence, these three parasites constitute an issue for prevention and control that needs to be considered by all the stakeholders of food and water sectors (producers, processors, regulators, public health authorities) (Robertson et al., 2020).
During the last decades, research on foodborne and waterborne protozoan parasites has led to a significant improvement of the methods to detect and characterize them in contaminated sources (Bartosova et al., 2021; Berrouch et al., 2020; Fradette et al., 2022; Slana et al., 2021). Unfortunately, human exposure to these parasites through food and water remains poorly known because data on the fate and behavior of their oocysts in contaminated sources are relatively scarce. Such data are crucial to enable risk managers to make a relevant assessment of the risk associated with these pathogens. However, they are difficult to obtain because studies on protozoan oocysts are hampered by economical, ethical, methodological, and biological constraints. To address these issues, efforts are increasing to propose surrogates to protozoan parasites for research and development purposes.
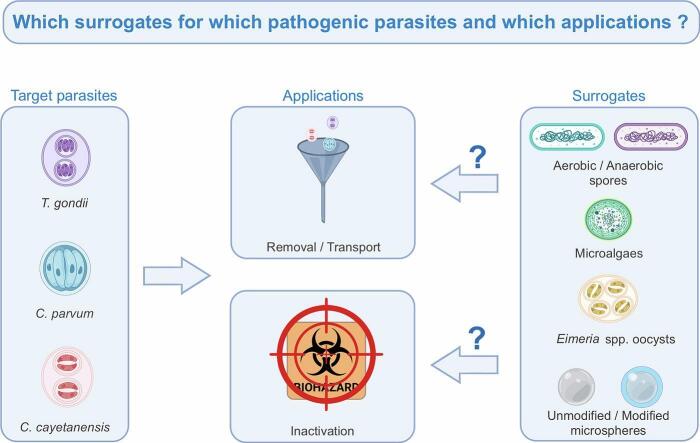
Surrogates are used in research as accessible, convenient, and tractable systems to study a particular area or a biology question in bioterrorism, water quality, occupational health/infection control, food safety, water microbiology, and industrial microbiology (Russell et al., 2017; Sinclair et al., 2012). A surrogate can be defined as a non-pathogenic organism, particle, or substance used to study the fate, removal or inactivation of a target pathogen in a specific environment or to define performance of specific process. Surrogates can be divided into two distinct groups: biological surrogates and non-biological ones. Biological surrogates are live organisms that are relatively well-characterized and for which viability can be easily assessed. They can be plants, microorganisms (e.g. bacteria, yeasts), or animals (e.g., flies, fishes, worms, and rodents) (Russell et al., 2017). Example of non-biological surrogates are inert microspheres. Turbidity, which can be used as an indicator of water quality regarding protozoan contamination (Cryptosporidium), is not addressed in this review for two key reasons: i) turbidity includes different particles with ill-defined characteristics in terms of size, shape and charge; ii) turbidity values do not provide a direct measurement of treatment efficiency. Hence, turbidity is not considered as a suitable surrogate for understanding the behavior of parasites and assessing treatment performance. Regarding their target(s), surrogates are typically chosen because (i) they can be readily produced in larger quantities and are ideally commercially available at low cost, (ii) they are non-pathogenic to humans and safe to handle, (iii) they display similar physical and/or biochemical features (e.g. size, shape, density, surface properties), (iv) they behave similarly to the target and/or show similar inactivation kinetics under given conditions (Montagnes et al., 2012; Russell et al., 2017). Non-biological surrogates are typically used to assess the transport or removal of the parasites while biological ones are intended to evaluate their survival and efficiency of inactivation treatments. Surrogates have been mainly evaluated in water, and there is a notable lack of research in more complex matrices such as food. Several types of surrogates have been described for C. parvum oocysts, which have received more attention than C. cayetanensis and T. gondii oocysts.
The main purpose of this review is to describe and discuss the features and applications of different surrogates for the foodborne and waterborne oocysts of C. parvum, C. cayetanensis and T. gondii. This review could serve as a guide for researchers in the selection of surrogates for protozoan parasites depending on the intended applications.
2. Target parasites
2.1. Cryptosporidium parvum
Cryptosporidium spp. are widespread intestinal parasites that can be transmitted to humans through the fecal-oral route and responsible for cryptosporidiosis. Human infections have been reported all around the world and are mainly due to C. parvum, which has both human and animal reservoirs (zoonotic species), and C. hominis, which appears to be more restricted to humans. Human contamination occurs through ingestion of oocysts that are usually excreted in high numbers in the feces of infected hosts and are immediately infectious upon excretion. The infective dose is low, ranging from 100 to 300 oocysts for C. parvum in immunocompetent people (Dupont et al., 1995; Okhuysen et al., 2002). Cryptosporidiosis is an enteric disease that is self-limiting in immunocompetent individuals. The infection is characterized by diarrhea and other symptoms like abdominal pain, weight loss, nausea, vomiting, fever, and malaise (Chalmers and Davies, 2010). These symptoms can be life-threatening in immunocompromised, very young, and elderly patients. Infection rates vary from 0.6 to 2% in industrialized countries and from 4 to 32% in low-middle income countries. Numerous waterborne outbreaks have been reported in humans, with one of the largest incidents occurring in 1993 in Milwaukee, USA, resulting in over 400,000 infections and 69 deaths (Costa et al., 2020, Costa et al., 2022; Mac Kenzie et al., 1994). Furthermore, outbreaks of C. parvum have been traced back to swimming pools or recreational facilities (Chalmers, 2012). Foodborne outbreaks have been notified, mainly due to the consumption of fresh produce (Nasser, 2022), unpasteurized cider or cow's milk (Costa et al., 2022; Ma et al., 2022). Cryptosporidium spp. oocysts have also been reported in the gills and tissues of edible molluscan shellfish, including clams, cockles, mussels, and oysters worldwide (Hohweyer et al., 2013). This suggests that the parasite can be transmitted to humans by eating raw or undercooked shellfish, but to date, no infection due to contaminated shellfish has been reported. In the water field, Cryptosporidium oocysts are of key concern to both water utilities and public health authorities due to i) their significant prevalence in surface and treated wastewaters (Moulin et al., 2010), ii) their ability to survive for long periods in aquatic environments (Daraei et al., 2021; Farrell et al., 2021), iii) their resistance to drinking water treatments such as chlorination (Korich et al., 1990), iv) their ability to escape filtration (Wood et al., 2019), and v) the lack of effective drugs to treat cryptosporidiosis (Khan and Witola, 2023). C. parvum oocysts are spherical and measure 4 to 6 μm in diameter. They contain four potential infectious sporozoites surrounded by the oocyst wall (Table 1). The oocyst wall (50–80 nm thick) has a multilayer structure that plays a crucial role in the oocyst resistance to environmental factors and inactivation treatments (Jenkins et al., 2010). Laboratory-scale experiments usually rely on C. parvum oocysts from commercial providers or academic laboratories, which produce oocysts in experimentally contaminated calves. The number of provided oocysts varies between 106 and 1011 per gram of stool (Fayer et al., 1997), which are suitable quantities for most studies. A few in vitro oocyst production systems exist; however, they are not reliable alternatives to animal infection due to their much lower oocyst yield (Ramírez-Flores et al., 2022). Mouse bioassay was traditionally considered as the gold standard method for assessing C. parvum oocyst infectivity. However, alternative in vitro cell culture methods have been developed to avoid the constraints and limits associated with animal models. These in vitro methods are now frequently used for testing drugs and decontamination treatments, and evaluating oocyst survival in environmental and food matrices (Kubina et al., 2021; Rousseau et al., 2018; Feix et al., 2023). Viability assays based on mRNA reverse-transcriptase qPCR assays have been also proposed, but they led to overestimation of the viability (Rousseau et al., 2018; Travaillé et al., 2016; Kim et al., 2021).
Table 1.
Main characteristics of Cryptosporidium parvum, Cyclospora cayetanensis, and Toxoplasma gondii oocysts.
| Oocysts | Cryptosporidium parvum | Cyclospora cayetanensis | Toxoplasma gondii |
|---|---|---|---|
| Shedding hosts | Mainly ruminant & humans | Humans | Felids |
| Transmission route to humans | Fecal–oral | Fecal–oral | Fecal–oral |
| Shape | Spherical | Spherical | Subspherical to ellipsoidal |
| Size [Length/width ratio] | 4–6 μm [∼1] | 8–10 μm [∼1] | 11 × 13 μm [1.2] |
| Content | 4 sporozoites | 2 sporocysts × 2 sporozoites | 2 sporocysts × 4 sporozoites |
| Characteristics of the outermost surface ▪ Structure [Thickness] ▪ Main composition of the outermost layer |
▪ Trilayered oocyst wall [50–80 nm] ▪ Carbohydratesa >proteins > lipids |
▪ Bilayered oocyst wall [∼110 nm] ▪ No published data |
▪ Bilayered oocyst wall [50–100 nm] ▪ Proteinsb > lipids>carbohydrates |
| Zeta potential | −25.0 mV in deionized H2O −12.0 mV in tap water |
No published data | −43.7 mV in ultrapure H2O −16.1 mV in freshwater −1.8 mV in estuarine water −2.8 mV in seawater |
| Specific gravity | 1.009–1.08 | No published data | 1.050–1.100 |
| Settling velocity (μm/s) | 0.35–1.31 | No published data | No published data |
| Reliable oocyst production systems | Bioassay in calves | None | Bioassay in cats |
| Infectivity/viability assays | Mouse bioassay/In vitro cell culture | Sporulation rate | Mouse bioassay/In vitro cell culture |
Glucose-rich glycocalyx.
Cys- and Tyr-rich proteins.
2.2. Cyclospora cayetanensis
C. cayetanensis is a human-specific coccidian parasite that causes cyclosporiasis, a disease that is becoming a global issue and a significant cause of morbidity and mortality, especially in immunocompromised individuals. Although the infectious dose of C. cayetanensis is not well established, epidemiological data have suggested that it is low, as few as ten oocysts (Sterling and Ortega, 1999; Villena, 2023). Infected persons excrete low to modest numbers of oocysts in their stool, which further require days to weeks to become infectious (Strausbaugh and Herwaldt, 2000; personal communication). Thus, direct person-to-person transmission is unlikely and transmission to humans is mainly indirect, though the consumption of contaminated food. The parasite leads to mild to moderate, self-limiting diarrhea in immunocompetent people but can cause severe intestinal disease and prolonged diarrhea in immunocompromised individuals (Almeria et al., 2019). Although C. cayetanensis occurs worldwide, it is most common in tropical and subtropical areas. Endemic cases of cyclosporiasis occur mainly seasonally all around the world, with most cases recorded from May to August (Centers for Disease Control and Prevention, 2022). Most outbreaks were reported in the United States and Canada and have been linked to the consumption of imported fresh produce such as berries, mesclun lettuce, snow peas, basil, and cilantro from endemic areas. More occasionally, some outbreaks were related to travelers from regions where the parasite is endemic (Hadjilouka and Tsaltas, 2020). Overall, the true prevalence of cyclosporiasis remains largely unknown due to the possibility of asymptomatic infections or unreported cases or undiagnosed since cyclosporiasis is unrecognized by physicians and needs specific stain coloration for microscopic analyses.
The oocysts of C. cayetanensis are spherical and measure between 8 and 10 μm in diameter (Table 1). They become infective through sporulation in 7–14 days under optimal conditions of temperature, oxygenation, and hygrometry, leading to two sporocysts, each enclosing two infective sporozoites (Ortega et al., 1993, Ortega et al., 1994). The oocyst wall is bilayered and ∼ 110 nm thick (Ortega et al., 1994). It probably contributes to the oocyst survival under harsh conditions including disinfection treatments (Mansfield and Gajadhar, 2004; Rabold et al., 1994; Siński and Behnke, 2004; Soave and Johnson, 1995). As C. cayetanensis is strictly limited to humans (Bartosova et al., 2021), access to oocysts for laboratory investigations is very difficult. The most reliable method to assess the viability of the parasite is the determination of the oocyst sporulation rate, which can only be obtained within a short timeframe following the shedding of oocysts.
2.3. Toxoplasma gondii
T. gondii is a protozoan parasite with a worldwide distribution. Approximately 30% of the human population are infected although T. gondii seroprevalence varies significantly across regions and countries (Dubey, 2010). Very few outbreaks have been reported to date because most infections are asymptomatic and remain therefore undiagnosed. Severe illness is mainly reported in fetuses and people with weakened immune system such as those with AIDS, cancer, or transplant surgery history (Robert-Gangneux and Darde, 2012). The parasite has a complex life cycle, with sexual reproduction occurring only in felids, and asexual phases in both felids and other warm-blooded animals including humans (Vanwormer et al., 2013). Humans can become infected by ingesting: i) oocysts either directly by contact with felid waste or indirectly by consuming vegetables, fruits, and water contaminated with sporulated oocysts; ii) by eating raw or undercooked meat containing tissue cysts of the parasite (Meireles et al., 2015). While transmission to humans through tissue cysts is well known and can be easily prevented with proper meat cooking and kitchen hygiene, the transmission of oocysts through food and water remains underestimated and challenging to prevent due to: i) their large distribution in aquatic environments; ii) their resistance to common inactivation procedures. Though the infectious dose of T. gondii oocysts in humans is not established, studies in rodent models suggest that it might be between 1 and 10 oocysts (Dubey, 2009). In this context, T. gondii oocysts pose a significant public health concern and their management in food and water industries is challenging.
Oocysts released into the environment are unsporulated and therefore non-infectious (Dubey, 1998). Oocysts then acquire infectivity through sporulation, usually within a week, under appropriate conditions of temperature, humidity, and oxygenation (Frenkel and Dubey, 1972; Tenter, 2009). The sporulated oocyst has a subspherical to ellipsoidal shape, measures approximately 11 × 13 μm (Freppel et al., 2019) and possesses a resilient, bilayered wall measuring 50–100 nm in thickness (Table 1). This wall shields two sporocysts, each enclosing four infective sporozoites, which are protected from harsh environmental conditions and commonly used disinfectants (Dubey, 1998; Freppel et al., 2019; Vanwormer et al., 2013). The T. gondii oocyst wall is thought to be similar in structure and biochemical composition to the wall of other coccidian oocysts including Eimeria parasites and probably C. cayetanensis (Belli et al., 2006).
In addition to the potential biohazard of handling T. gondii oocysts, oocyst production requires the infection of laboratory cats. Besides ethical concerns and the complexity of implementing such assays, the quantity of oocysts a cat can produce depends on several factors including its age and immune status at the time of the infection (Dubey and Beattie, 1988), and the strain and infectious stage of the parasite (Dubey, 1995, Dubey, 2009). Recent efforts to produce oocysts in vitro or in “felinised” mice do not give similar oocyst yields as obtained in cats (Martorelli Di Genova et al., 2019). To date, mouse bioassay remains the most reliable method over in vitro cell culture techniques to assess the infectivity of oocysts from environmental matrices. However, it requires specific facilities and authorization, and also raises ethical concerns (Rousseau et al., 2018). As for C. parvum oocysts, viability assays based on mRNA detection were also described (Rousseau et al., 2018; Kim et al., 2021). All these drawbacks render investigations on T. gondii oocysts challenging.
3. Biological surrogates
Many studies have sought to identify microorganisms that are ubiquitous in the environment and can be easily produced and/or cultured in laboratories to serve as surrogates for protozoan parasites (Table 2). Among them, the anaerobic and aerobic spore-forming bacteria including Clostridium and Bacillus genera respectively, are the most studied as Cryptosporidium oocysts surrogates. To a lesser extent, microalgae and oocysts of the non-human pathogenic Eimeria parasites have also been proposed as surrogates for Cryptosporidium, T. gondii and/or C. cayetanensis oocysts.
Table 2.
Main characteristics of surrogates described in this review.
| Surrogate types | Species [host] - Materials (for microspheres) | Pathogenicity to humans | Stage & Shape | Size | Characteristics of the outermost surface ▪ Structure [Thickness] ▪ Main composition of the outermost layer |
Production | Methods to measure removal (R) and/or inactivation (I) |
|---|---|---|---|---|---|---|---|
| Anaerobic SFB | Clostridium perfringens | Y/N a | Spheric spores | 1–1.5 μm c | ▪ Monolayered coat [10–100 nm] ▪ Proteins > lipids = carbohydrates |
Culture | R/I: Culture; Spectrophotometry |
| Aerobic SFB | Bacillus subtilis | N | Ellipsoid spores | 1.0–1.6 × 0.7–1 μm |
▪ Bilayered coat [10–100 nm] ▪ Proteins > > carbohydrates |
Culture | R/I: Culture; Spectrophotometry |
| Microalgae Cyanobacteriota |
Microcystis viridis Microcystis aeruginosa |
N b N b |
Spheric cells |
3–8 μm c 2–5 μm c |
▪ Multiple layered membrane [20–40 nm] ▪ Carbohydrates |
Culture |
R: Microscopy |
| Chlorophyta | Selenastrum capricornutum | N | Crescent cells | 11 μm x 2.5 μm |
▪ Bilayered wall [200 nm] ▪ Proteins > carbohydrates > lipids |
Culture | |
| Diatoms |
Stephanodiscus hantzschii Nitzchia spp. |
N N |
Spheric cells Linear cells |
4–7 μm c 3–5 μm x 15–70 μm |
▪ Monolayered wall [15–20 nm] ▪ Silica |
Culture | |
| Eimeria spp. coccidia | Eimeria acervulina [chicken] | N | Ovoid oocysts | 17 × 21 μm | ▪ Bilayered wall [200–290 nm] ▪ Proteins > lipids > carbohydrates |
Chicken infection | R: Real-time PCR based methods; microscopy I: Chicken bioassays |
| Eimeria papillata [mouse] | N | Ovoid oocysts | 22 × 26 μm | ▪ Bilayered wall [180–240 nm] ▪ Proteins > lipids > carbohydrates |
Mouse infection | R: Real-time PCR based methods; microscopy I: Mouse bioassays |
|
| Microspheres | Latex/polystyrene | N | Spheric beads | 1–10 μm c | ▪ Not applicable ▪ Uncoated or protein-coated surface |
Manufacturing | R: EM and/or solid-phase/flow cytometry |
Y/N, i.e. depending on the strain.
Microcystis species can release toxins for humans and animals upon disruption and death.
Dimensions correspond to the diameter. SFB: spore-forming bacteria. EM: Epifluorescence microscopy.
3.1. Anaerobic spore-forming bacteria
Clostridium spp. are Gram-positive anaerobic bacteria that produce spores in natural conditions (soil and intestine). More than 300 species of Clostridium have been described (List of prokaryotic names with standing in nomenclature – genus Clostridium, https://lpsn.dsmz.de/search?word=clostridium), with the majority of them being harmless. However, a few species have significant impact on public health, causing severe diseases such as tetanus (C. tetani), botulism (C. botulinum) and gastrointestinal illnesses (C. difficile, C. perfringens) (Mehdizadeh Gohari et al., 2021; Gibbs, 2009). Spores are shed in feces and can persist for long periods in waters (Stelma, 2018). Consequently, spores of Clostridium spp., and more specifically of C. perfringens (Table 2), are considered as fecal indicators of enteric pathogens in water resources, including Cryptosporidium oocysts (Table 3). In raw surface waters used for drinking water production, C. perfringens spores were equally or more frequently detected than Cryptosporidium oocysts (Chauret et al., 1995; Nieminski et al., 2000; Payment and Franco, 1993; Ribas et al., 2000). However, only one study found a positive correlation between the concentrations of oocysts and Clostridium spores in raw waters (Payment and Franco, 1993). In most studies, C. perfringens spores were 1 to 3 log10 more abundant than Cryptosporidium oocysts and no correlation was observed (Chauret et al., 1995; Galofré et al., 2004; Mazoua and Chauveheid, 2005; Nieminski et al., 2000; Ribas et al., 2000). The specificities of the sampling sites, the seasonal presence of Cryptosporidium oocysts in the environment and the performances of the different methods applied to detect oocysts in waters could explain discrepancies between studies. Overall, these data demonstrate that C. perfringens spores are not reliable indicators to predict the contamination of raw waters by Cryptosporidium oocysts. C. perfringens spores range between 1 and 1.5 μm in diameter and are able to resist some physical treatments (wet and dry heat, desiccation, UV and γ-radiation), and chemical factors such as enzymes, genotoxic agents, oxidizing agents, aldehydes, acids, and alkalis (Setlow, 2014). Because of their structural features, they have been proposed as surrogates of Cryptosporidium oocysts to assess drinking water treatment performances, and more specifically during removal treatments (Table 3). However, comparative studies performed on industrial sites and based on indigenous concentrations of the spores and oocysts showed contradictory results: C. perfringens spores were either removed at higher levels than Cryptosporidium oocysts (1.5 to 4 times higher decimal reduction, (Chauret et al., 1995; Galofré et al., 2004; Payment and Franco, 1993), or at lower levels (1 to 1.8 times lower decimal reduction; Chauret et al., 1995; Ribas et al., 2000). These different results are probably due to the large diversity of applied drinking water treatments (settling, filtration and disinfection modes and numbers). The low and/or non-detectable concentrations of both microorganisms in raw and treated waters respectively, also represent a limit to accurately measure a decimal reduction and could contribute to such opposite results. To circumvent these drawbacks, other studies assessed slow sand filtration by spiking the two microorganisms at controlled high doses (103 to 105/mL) in the influent waters of a mature pilot plant filter or sand columns. In these conditions, decimal reduction ranging from 2.3 to 3.9 log10 for C. perfringens spores and from 5.3 to >6.5 log10 for Cryptosporidium oocysts could be measured (Hijnen et al., 2004, Hijnen et al., 2007), demonstrating that spores were less efficiently removed than the parasites. Such findings were also found for C. sporogenes spores that showed up to 2.5 log10 removal following conventional treatment with dual media filtration at pilot-scale, while up to 4 log10 were observed for C. parvum oocysts (Monis et al., 2017). Hence, Clostridium spp. spores could be relevant surrogates of Cryptosporidium oocysts to assess removal performance in controlled conditions but appeared not recommended for evaluation on industrial sites.
Table 3.
Surrogates of Cryptosporidium parvum, Cyclospora cayetanensis, and Toxoplasma gondii oocysts used in various applications.
| Target parasite | Surrogate | Applications [surrogate's species if specified] | Surrogate detection methods | References |
|---|---|---|---|---|
| Cryptosporidium parvum | BIOLOGICAL - Spores of anaerobic spore-forming bacteria |
Raw water monitoring | Culture | Chauret et al., 1995 |
| Removal assessment at drinking water treatment plant | Culture | Chauret et al., 1995; Hijnen et al., 2004*; Galofré et al., 2004; Payment and Franco, 1993; Ribas et al., 2000; Schijven et al., 2003* | ||
| Conventional treatment removal a | Culture | Monis et al., 2017 | ||
| Filtration removal (Slow sand) | Culture | Hijnen et al., 2004, Hijnen et al., 2007 | ||
| Transport (granular porous media) | Culture | Hijnen et al., 2005 | ||
| Chemical's inactivation (chlorine dioxide, mixed-oxidant disinfectant, free chlorine, ozone) |
Culture | Chauret et al., 2001; Hijnen et al., 2002; Venczel et al., 1997; Verhille et al., 2003* | ||
| BIOLOGICAL - Spores of aerobic spore-forming bacteria |
Raw water monitoring | Culture | Headd and Bradford, 2016 | |
| Removal assessment at drinking water treatment plant | Culture | Brown and Cornwell, 2007*; Cornwell et al., 2003*; Galofré et al., 2004; Mazoua and Chauveheid, 2005; Nieminski et al., 2000; Rice et al., 1994* | ||
| Conventional treatment removal | Culture | Rice et al., 1994* | ||
| Filtration removal (granular media) [B. subtilis] |
Culture | Emelko, 2001 | ||
| Transport (granular porous media) [B. subtilis] |
Spectrophotometry | Bradford et al., 2016; Kim et al., 2010 | ||
| Chemical's inactivation (ozone) – [B. subtilis] |
Culture | Facile, 2000* | ||
| Physical's inactivation (pulsed-UV light) [B. megaterium, B. cereus, B. pumilus] |
Culture | Garvey et al., 2013 | ||
| BIOLOGICAL -Microalgae | Removal assessment at drinking water treatment plant [Total blue/green/brown algae] |
Microscopy | Akiba et al., 2002* | |
| Conventional treatment removal – [Total microalgae] [Microcystis viridis, M. aeruginosa, Selenastrum capricornutum] |
Flow cytometry Microscopy |
Monis et al., 2017 Akiba et al., 2002 |
||
| Filtration removal (slow sand) - [Stephanodiscus hantzschii] | Microscopy | Hijnen et al., 2007 | ||
| BIOLOGICAL – Eimeria oocysts | Inactivation in oysters (accumulation/depuration) [E. acervulina] |
Poultry bioassays | Lee and Lee, 2003* | |
| Control of oocysts & DNA extraction [E. papillata] |
Real-time qPCR | Lalonde and Gajadhar, 2016a, Lalonde and Gajadhar, 2016b* | ||
| NON BIOLOGICAL - FCM | Filtration removal - (dual media) | EM | Brown and Emelko, 2009; Emelko et al., 2003 | |
| (conventional and biological media) | Amburgey et al., 2005; | |||
| (conventional and sand media) | Emelko and Huck, 2004 | |||
| Transport (granular porous media) | Flow cytometry and/or EM | Dai and Hozalski, 2003; Harvey et al., 2008; Mohanram et al., 2010; Pang et al., 2012; Metge et al., 2007; Tufenkji et al., 2004; Tufenkji and Elimelech, 2005 | ||
| Chemical's inactivation (ozone) | Flow cytometry | Tang et al., 2005*; Mariñas et al., 1999* | ||
| NON BIOLOGICAL - F-GC-CM | Conventional treatment removal | EM | Monis et al., 2017 | |
| Coagulation and filtration removal | EM | Liu et al., 2019a* | ||
| Filtration removal (granular media) | EM and/or solid-phase/flow cytometry | Liu et al., 2019b; Pang et al., 2012, Pang et al., 2022*; Stevenson et al., 2015; Zhang et al., 2017* | ||
| Domestic filtration removal | EM | Pang et al., 2021* | ||
| NON BIOLOGICAL - F-BC-CM | Filtration removal (granular media) | EM and/or solid-phase cytometry | Pang et al., 2012; Stevenson et al., 2015 | |
| Cyclospora cayentanensis | BIOLOGICAL - Eimeria oocysts |
Physical's inactivation (HPP, UV radiation, washing, freezing, heating, gamma irradiation) [E. acervulina] |
Poultry bioassays | Kniel et al., 2007*; Lee and Lee, 2001* |
| Control of oocysts & DNA extraction [E. papillata] |
Real-time qPCR | Lalonde and Gajadhar, 2016a, Lalonde and Gajadhar, 2016b* | ||
| Toxoplasma gondii | BIOLOGICAL -Eimeria oocysts | Inactivation in oysters (accumulation/depuration) [E. acervulina] |
Poultry bioassays | Lee and Lee, 2003* |
| Control of oocysts & DNA extraction [E. papillata] |
Real-time qPCR | Lalonde and Gajadhar, 2016a, Lalonde and Gajadhar, 2016b* | ||
| NON BIOLOGICAL –FCM | Transport [water] | Flow cytometry and/or EM | Shapiro et al., 2010a* | |
| Development of filtration-based concentration method | Shapiro et al., 2010b |
Conventional treatment, i.e. coagulation/sedimentation/granular media filtration. * No simultaneous comparison was made between the target and the surrogate. FCM: fluorescent carboxylated microspheres. F-GC/BC-CM: fluorescent carboxylated microspheres coated with glycoproteins (G) or biotin (B). HPP: High hydrostatic pressure. EM: Epifluorescence microscopy.
The features of C. perfringens spores also led some authors to evaluate their potential to address the transport of C. parvum oocysts in two different soil types (Table 3): gravel aquifer and fine dune sand (Hijnen et al., 2005). They showed that the elimination rate of C. parvum oocysts was higher than C. perfringens spores in the gravel soil with a removal of >6.7 log10 and 2.4 log10, respectively. Conversely, in the sandy soil, C. parvum showed an elimination rate >3 log10 while C. perfringens spores removal was >4.5 log10. Hence, depending on the soil type, C. perfringens can act as a conservative surrogate for C. parvum oocyst transport. This transport/retention behavior difference is not surprising considering that the size, the surface properties of the microorganisms and the soil characteristics govern such mechanisms. Therefore, using surrogates to model the pathogen transports needs to be carefully evaluated, to ensure that the surrogate is suitable for this application.
Clostridium spp. spores have finally been evaluated as surrogates of C. parvum oocysts in oxidant-induced inactivation studies at laboratory scale (Table 3). Spores of Clostridium spp. and specifically C. sporogenes were found to be 3 to 100,000 times more sensitive than C. parvum oocysts to chlorine dioxide (Chauret et al., 2001; Verhille et al., 2003). It was also shown that the spores of C. perfringens were more sensitive than oocysts to 5 mg/L free chlorine exposure for 4 h (1.4 log10 inactivation vs. no inactivation respectively), but displayed similar high levels of inactivation (>3 log10) following application of mixed-oxidant solution in the same conditions (Venczel et al., 1997). In addition, Hijnen et al. (2002) revealed that ozone at CT (concentration x exposure time) ranging between 0 and 15 mg.min.L−1 was effective in inactivating both C. parvum oocysts and C. perfringens spores, with similar inactivation rates observed at +10 °C (Chick-Watson modeling, k constant = 0.14 ± 0.014; P < 0.001 and 0.25 ± 0.01; P < 0.001 respectively). These findings suggest that spores of Clostridium perfringens could be a suitable surrogate to assess inactivation of C. parvum oocysts by ozone and mixed-oxidant solution. However, they appear to be irrelevant for other oxidant treatments such as free chlorine and chlorine dioxide and the question remains to be addressed for other inactivation treatments.
3.2. Aerobic spore-forming bacteria
The aerobic spore-forming bacteria (ASFB) are Gram-positive bacteria that can be found in a wide range of environmental niches. Among them, bacteria from the Bacillus genera are usually harmless for humans. Bacillus spp. are easily cultured in laboratories and produce ovoid or ellipsoid spores (up to 109 spores/mL) of 1.0–1.6 μm length and 0.7–1 μm width depending on the species and sporulation conditions (Table 2). These spores are known to resist heat, cold, radiation, desiccation, and disinfectants (Carrera et al., 2007; Turnbull, 1996; Xu Zhou et al., 2017).
Due to their ubiquitous nature, spores of ASFB (ASFBsp) occur in almost all surface waters, usually at high concentrations. These features enable monitoring 4 to 5 log10 removal in drinking water treatment plants, hence making them the most relevant direct indicator for such assessment (Brown and Cornwell, 2007; Nieminski et al., 2000; Rice et al., 1994). Their ability to be used as surrogates of Cryptosporidium oocysts to identify contaminated water resources has been previously discussed (Headd and Bradford, 2016). It was concluded that not all ASFBsp appear suitable to mimic oocysts' environmental fate and that a single ASFBsp would probably not allow to answer all questions. Besides, as it has been generally observed for spores of Clostridium spp., ASFBsp are more frequently present at higher concentrations (3 to 6 log10 times) than Cryptosporidium spp. oocysts in raw waters, and AFSBsp-oocyst correlation has not been demonstrated (Galofré et al., 2004; Mazoua and Chauveheid, 2005; Nieminski et al., 2000). In the few studies that compared ASFBsp to Cryptosporidium oocyst removals in drinking water plants (Table 3), contradictory results were obtained: Galofré et al. (2004) showed lower removal of ASFBsp than oocysts (2.44 vs. 3.28 log10 reduction) while other studies found a higher reduction (2.0 to 4.0 vs. 1.5 to 3.8 log10 reduction; Mazoua and Chauveheid, 2005; Nieminski et al., 2000). The log10 reductions measured for ASFBsp in these latter studies are consistent with other works that assessed only ASFBsp removal in water treatment plants based on indigenous ASFBsp concentrations throughout the process (1.68 to 4.89 log10 reduction; Brown and Cornwell, 2007, Cornwell et al., 2003, Rice et al., 1994). Such results were also obtained in laboratory- and pilot-scales experiments (1.0 to 2.42 log10 reduction; Rice et al., 1994). Hence, as previously discussed for Clostridium spores, the higher reduction rates observed for ASFBsp compared to Cryptosporidium oocysts in studies performed on industrial sites are probably linked to the low level of Cryptosporidium oocysts across the process and the limit of the detection method that enables to calculate reliable log10 reduction for oocysts. Such limits in the log10 removal interpretations have been highlighted at pilot- and full-scale plants, demonstrating an underestimation of Cryptosporidium oocysts removal during conventional (flocculation-sedimentation-filtration) and direct-filtration (i.e. w/o sedimentation) treatments (Nieminski and Ongerth, 1995). One study evaluated the correlation between Bacillus subtilis spores (Table 3) and C. parvum oocysts removal by granular media filtration in pilot plant experiments and showed no relationship (r2 < 0.5) indicating that these spores were not suitable surrogates to provide reliable quantitative values of oocyst removal (Emelko, 2001). However, due to their natural presence at measurable levels throughout the drinking water treatment process, ASFBsp (including B. subtilis) represent interesting conservative indicators to inform on Cryptosporidium oocyst removal at industrial sites (worst-case scenario): if a process can achieve a significant log10 reduction (>3 log10) of spores, it is likely effective against Cryptosporidium oocysts (Brown and Cornwell, 2007). Bacillus subtilis spores were also demonstrated to be a conservative surrogate for C. parvum oocyst transport and retention over a range of physicochemical conditions (Bradford et al., 2016; Kim et al., 2010).
Garvey et al. (2013) compared the inactivation of endospores of different Bacillus species and of C. parvum oocysts submitted to increasing doses of pulsed-UV (PUV) (Table 3). On one hand, they showed that irrespective of the PUV dose, B. pumilus spores were always more sensitive than B. megaterium and B. cereus spores, and C. parvum oocysts. On the other hand, for PUV doses ranging from 6.48 to 12.96 μJ/cm2, B. megaterium and B. cereus spores displayed similar or lower inactivation levels (4.03 to 5.65 log10) than C. parvum oocysts (4.2 to 5.9 log10). Therefore, ASFBsp are interesting surrogates of C. parvum oocysts to safely investigate the performance of PUV treatments. By comparing the CT values they obtained for B. subtilis spores to published data for C. parvum, Facile (2000) suggested that bacterial spores could be used as a surrogate to assess inactivation of oocysts induced by ozone. Hence, depending on the species, the strain and their age, the spores of Bacillus spp. display different features (size, surface properties, resistance) (Headd and Bradford, 2016; La Carbona, personal communication) that will make them good surrogates or not, to assess physical and chemical treatments. Consequently, the selection of Bacillus spores species as a surrogate of oocysts for a given inactivation treatment needs to be documented before use. Their relevance as surrogates for other inactivation treatments remains to be addressed.
3.3. Microalgae
Microalgae encompass a diverse group of non-pathogenic prokaryotic and eukaryotic microorganisms showing a high adaptability capacity (Table 2). Prokaryotic microalgae consist of a single phylum formerly known as cyanobacteria (i.e. blue algae) but recently recalled cyanobacteriota (Oren et al., 2022). Eukaryotic microalgae were traditionally classified in three main taxonomic groups based on pigmentation characteristics: green, red, and brown algae. Microalgae are widely distributed on the planet, including fresh and marine aquatic ecosystems, and play a major role in carbon dioxide sequestration. Their easy culture in laboratories and low production cost make them used in diverse applications in aquaculture, cosmetic, food, pharmaceutic and energy fields (Bougaran and Saint-Jean, 2014). Considering these features, the microalgae have been tested as potential surrogates of Cryptosporidium oocysts (Table 3).
In a field survey conducted for 5 years in eight different drinking water treatment plants applying a conventional treatment, diatomeae (brown algae) were found to be the most frequently algal cells detected in raw waters (87%) followed by chlorophyta (green algae, 11%) and cyanobacteriota (i.e. cyanophyta, blue algae, 2%) (Akiba et al., 2002). The removal rates measured for these microorganisms in these plants ranged between 2.33 and 3.47 log10, diatomeae being the most efficiently removed. This range appeared to be similar to removal rates measured for Cryptosporidium oocysts in other plants applying equivalent treatment (Galofré et al., 2004; Nieminski and Ongerth, 1995; Ribas et al., 2000). However, it's important to note that in the study conducted by Akiba et al. (2002), diatomeae that were mainly found in raw water belonged to Nitzschia spp., which has a long shape. So, if these brown algae can easily be quantified in raw waters and throughout the process making them interesting candidates to assess global water quality on industrial sites, their distinct shape from Cryptosporidium oocysts questions their relevance to mimic Cryptosporidium fate in these conditions. Besides, it was demonstrated that Stephanodiscus hantzschii, another diatomeae that displays closer features to Cryptosporidium oocysts (Table 1, Table 2), was much less removed in slow sand filtration experiments (1.8 log10 removal) compared to Cryptosporidium oocysts (5.3 log10 removal) (Hijnen et al., 2007). One can hypothesize that the lower removal of algae could be due to the ability of algal cells to replicate during the treatment. However, it is worth considering that even in wastewaters naturally rich in many nutrients, the C:N ratio is not sufficient to meet the algal growth requirements (Molazadeh et al., 2019). Therefore, the probability of microalgae growth during the treatment is probably low. Due to their difference in shape and/or the fact that they are too conservative indicators, brown algae appear irrelevant as surrogates of Cryptosporidium oocysts to assess drinking water treatment performance, either at plant or laboratory scales. In laboratory spiking experiments, Akiba et al. (2002) demonstrated that, in the same conditions, the cyanobacteriota Microcystis viridis and M. aeruginosa, and the chlorophyta Selenastrum capricornutum displayed similar removal rates (1.15 to 2.05 log10) to C. parvum oocysts (1.49 log10) after coagulation, S. capricornutum being the closer (1.51 log10). When testing after the filtration step, S. capricornutum was again the microalga that behaves most like C. parvum oocysts, exhibiting similar removal rates of approximately 3 log10 at the highest removal period. The authors concluded that S. capricornutum exhibited similar coagulation and filtration characteristics to C. parvum oocysts and could consequently be an appropriate surrogate to assess removal of oocysts upon conventional drinking water treatment. However, this species was not the most predominant from the chlorophyta group in raw waters (Akiba et al., 2002) and strongly differs from Cryptosporidium oocysts in size and shape (Table 1, Table 2). Therefore, the implementation of S. capricornutum as a surrogate to assess Cryptosporidium oocyst removal at industrial scale remains questionable. Finally, in a pilot-scale experiment assessing conventional treatment using dual media filtration, the total microalgae were shown to be 1 log10 removed compared to 4 log10 for C. parvum oocysts in the same condition (Monis et al., 2017), being highly conservative. Overall, the potential of microalgae as surrogates of C. parvum oocysts is quite limited.
3.4. Eimeria oocysts
Eimeria are coccidian parasites that infect numerous host species, including poultry, cattle, mice, rabbits, and many other species. Each species of Eimeria is host-specific but none of them is pathogenic to humans, and therefore these parasites are safe to handle. Depending on the species, Eimeria oocysts range from 11 to 84 μm in length and 8 to 56 μm in width and share structural and molecular features with T. gondii and C. cayetanensis (Table 1, Table 2). Following sporulation (in 1–2 days), oocysts of Eimeria consist of 4 sporocysts, each enclosing two infective sporozoites. They are transmitted to their host by the fecal-oral route at a low infectious dose (e.g. five sporulated E. acervulina oocysts (Velkers et al., 2010)). The rodent and poultry Eimeria species can be relatively easily produced, although requiring specific facilities and authorizations, at high rate (e.g. total of 1.5 × 106 E. acervulina oocysts per infected host (Velkers et al., 2010)) and at a reasonable cost. As with T. gondii and probably C. cayetanensis, sporulated oocysts of Eimeria can remain infective following some treatments applied in conditions that would be lethal for most other microorganisms, including bleach treatment, ultraviolet irradiation, and high-pressure treatment (Belli et al., 2006; Fitzgerald, 1968; Kniel et al., 2007; Shearer et al., 2007).
Due to their phylogenetic proximity to C. cayetanensis, Eimeria spp. appear as relevant surrogates for this challenging pathogen. More specifically, E. acervulina oocysts (Table 2) that infect poultry (Pieniazek and Herwaldt, 1997; Relman et al., 1996) represent the most interesting alternative to investigate control measures regarding Cyclospora in foods (recently reviewed by Tucker et al., 2022; Table 3). E. acervulina oocysts have also been used as surrogates of T. gondii and C. parvum oocysts to explore the bioaccumulation of coccidian oocysts by eastern oysters (Crassostrea virginica) and the ability of bioaccumulated parasites to retain their infectivity (Table 3; Lee and Lee, 2003). The authors found that oysters can quickly concentrate Eimeria oocysts (i.e., in 6 h). Oysters recovered 24 h after contamination of the waters still displayed infective oocysts but very few after 48 h, indicating that the oocysts passed through the shellfish in one day. Depending on fecal contamination of the production zone (based on E. coli criteria), shellfish can be directly sold for human raw consumption or require a depuration step before being commercialized (European regulation CE 854/2004). This depuration process consists in immersing the shellfish in clean water for short (usually 48 h) to long periods (according to the contamination level). Considering these depuration practices and their results obtained with infective Eimeria oocysts, the authors extrapolated that the risk of human contamination is limited regarding coccidian parasites in general in raw oysters. It is worth noting that in another study, C. parvum could still be detected by immunofluorescence assay in C. virginica 7 days after exposure (Willis et al., 2014). Similarly, C. virginica oysters submitted to water contaminated by T. gondii oocysts were still infective to mice 3 days post-water contamination (Lindsay et al., 2001). Hence, even if the experimental sets differed between these studies, as well as the measured parameters (i.e. viable Eimeria and T. gondii oocysts, viable and/or dead C. parvum oocysts), these findings strongly suggest that extrapolation from surrogates should be carefully made. To address the risk linked to the presence of coccidian parasites in live bivalves by using Eimeria surrogates, simultaneous comparisons of the behavior of Eimeria oocysts and target coccidian parasites should be first conducted to validate the surrogate. Finally, Eimeria papillata oocysts (Table 2), which infect mice, were used as surrogates for coccidia of public concern to optimize and define the performance of methods to isolate oocysts and detect their DNA in fresh produce (Lalonde and Gajadhar, 2016a; Table 3). Although the comparison of the detection of E. papillata oocysts and other coccidian parasites in these matrices has not been strictly done, E. papillata oocysts have been further successfully used as a positive extraction control in a Canadian survey in leafy green vegetables (Lalonde and Gajadhar, 2016b). E. papillata was also implemented as a surrogate to assess the fate of parasites in general on field lettuce, during process for ready-to-eat lettuce and in packaged lettuce, demonstrating overall their ability to persist (Delaquis, 2014).
4. Non-biological surrogates: microspheres
Microspheres have been used as abiotic substitutes for protozoan oocysts because they can be easily (and cheaply) manufactured with sizes and surface properties similar to those of the target parasites (Table 2). They include unmodified fluorescent carboxylated polystyrene or latex microspheres (FCM), and modified fluorescent carboxylated microspheres coated with glycoproteins (F-GC-CM) or biotin (F-BC-CM) (Harvey et al., 2017). The use of microspheres as surrogates of oocysts offers several advantages. Microspheres are inert, enabling them to maintain their physical and chemical properties under certain harsh conditions (i.e. high temperatures, extreme pH levels, exposure to strong solvents). Furthermore, they can exhibit a negative surface charge on the same order of magnitude as the oocyst surface charge (Table 1). FCM surface can be modified through coating to mimic the molecules (glycoproteins) and properties of the oocyst surface (e.g. zeta potential, isoelectric point, hydrophobicity) (Table 1). Additionally, fluorescent microspheres can be easily detected and quantified by automated counting techniques such as epifluorescence microscopy, solid-phase cytometry and flow cytometry (Table 2). Microspheres are available in a wide range of size (typically 1 to 10 μm), encompassing the size of protozoan oocysts. Their length/width ratio (1.03) and buoyant density (1.05 g/cm3) are also comparable to those of oocysts (1–1.2 length/width ratio and 1.03–1.07 g/cm3 buoyant density) (Table 1). Moreover, microspheres have been proven to be non-hazardous, in terms of human toxicology and ecotoxicology, thus ensuring the safety of researchers handling them (Behrens et al., 2001). Most studies have evaluated microspheres as C. parvum oocyst surrogates to assess water treatments, mainly filtration, and characterize the transport of the parasites in water or porous media (Table 3).
4.1. Unmodified microspheres
Studies using FCM to address C. parvum oocyst removal during water treatment showed that these microspheres can be reliable surrogates at pilot-scale when assessing different types of filters (conventional/biological/dual media filters; Table 3) (Amburgey et al., 2005; Emelko et al., 2003; Emelko and Huck, 2004). Emelko et al. (2003) showed that both FCM and oocysts achieved >4.5 log10 removal in pilot-scale sand-anthracite dual-media filters under optimal conditions. Moreover, Amburgey et al. (2005) reported that FCM and oocysts demonstrated similar trends, with approximately 1.96 and 1.84 log10 reductions respectively in both conventional (with coagulant) and biological filtration methods. When coagulation conditions became sub-optimal (due to increased temperature), FCM showed higher removal rates than oocysts (2.2 vs. 1.6 log10). Brown and Emelko (2009) concluded that FCM might be reliable surrogates of C. parvum oocyst removal by in-line filtration preceded by alum and FeCl3 coagulation, but not chitosan coagulation. All these studies indicated that FCM can be reliable surrogates of C. parvum oocysts depending on variations in filter media properties and/or coagulation regime. More recently, new fluorescent microspheres (polymethyl acrylate-based) were successfully used to investigate C. parvum oocysts removal by sintered metal membrane in drinking water treatment plant (Li et al., 2020).
Unmodified microspheres have also been used as surrogates of C. parvum oocysts to study their transport in porous media in laboratory- and field-scale experiments (Table 3). Mohanram et al. (2010) and Tufenkji et al. (2004) demonstrated that FCM and oocysts had similar transport and attachment behaviors through granular porous media. These findings were consistent with Dai and Hozalski's (2003) study, in which it was also observed similar transport behavior through glass bead filters between oocysts (16.3 ± 1.5% of removal) and FCM (14 ± 1.7%) in the presence of natural organic matter. However, in the absence of natural organic matter, the system removed fewer microspheres (40.3 ± 1.5%) compared to oocysts (49.7 ± 2.9%) and underestimated the retention of oocysts when coagulant was added. Other studies by Pang et al. (2012) and Tufenkji and Elimelech (2005) also found that FCM underestimated oocyst retention in sand media columns and glass beads, respectively. On the other hand, Harvey et al. (2008) and Metge et al. (2007) demonstrated that FCM can overestimate oocyst removal in other types of porous media (karst limestone and alluvial aquifer, respectively). Hence, the behavior of non-modified microspheres relative to C. parvum oocysts is highly variable depending on the tested media and suggest that this surrogate is not appropriate to address the transport of C. parvum oocysts in porous media.
To our knowledge, studies by Shapiro et al., 2009, Shapiro et al., 2010a, Shapiro et al., 2010b were the only ones that assessed microspheres as potential surrogates for T. gondii oocysts (Table 3). They evaluated two types of FCM: Dragon Green (DG, 10.35 μm diameter) and Glacial Blue (GB, 8.6 μm diameter) microspheres (Table 3). DG were purchased as unwashed suspensions containing surfactant (Shapiro et al., 2009). The authors observed that, following washing and elimination of the surfactant, DG tended to become hydrophobic, leading to a shorter transport potential than oocysts. Conversely, GB microspheres were hydrophilic and could remain stable in suspension without the need of surfactant (Tween20), suggesting that GB have a longer transport potential than oocysts. Therefore, it may be advisable to use a combination of microspheres representing different sizes and surface properties to finely characterize the transport behavior and interactions of oocysts with the environmental matrices. This approach proved successful in the development of techniques to concentrate T. gondii oocysts from different water types by capsule filtration (Shapiro et al., 2010b), as well as to assess the transport of oocysts in wetlands (Shapiro et al., 2010a).
As microspheres are biologically inert, it is assumed they cannot provide information on inactivation of the protozoan oocysts they intend to mimic. A study conducted by Tang et al. (2005) used FCM to evaluate the effects of ozone treatments in inactivating C. parvum oocysts (Table 3). A mathematical model previously validated in a pilot-scale experiment (Kim et al., 2002a, Kim et al., 2002b) was applied to predict ozone performance in inactivating oocysts based on changes in FCM fluorescence intensity. For this, FCM were analyzed for fluorescence using flow cytometry as described by Mariñas et al. (1999). Changes in their fluorescence intensity were correlated with the inactivation kinetics of C. parvum oocysts by selecting an appropriate fluorescence intensity threshold. Though this approach appears simple and accurate, it does not provide information on how ozone at different CT affects the viability of the sporozoites within the oocyst wall.
4.2. Modified microspheres
The relevance of unmodified FCM can truly be questioned for filtration and transport applications, because, under similar chemical conditions (type of soil, water, pH, …) and using similar counting methods (flow cytometry, epifluorescence microscopy), FCM exhibit a higher electronegativity compared to oocysts, which can therefore affect interactions between FCM and the porous filter media and soils. To address this, the surface of FCM can be modified by grafting covalently glycoproteins (F-GC-CM) and biotin (F-BC-CM) to mimic the surface molecules and properties of the target parasites, and therefore consider their interaction capacities with molecules when studying removal by coagulation, settling and filtration (Table 3). Stevenson et al. (2015) found that F-GC-CM and F-BC-CM matched better the retention of C. parvum than FCM in filtration processes by granular limestone media. Such observations were also made when assessing sand filtration, with F-GC-CM and F-BC-CM showing 2 log10 and 2.1 log10 removal respectively, relative to 2.7 to 3.0 log10 reduction of C. parvum oocysts (Pang et al., 2012). These findings were in line with Liu et al. (2019b) who assessed retention in granular silica sand media, although they demonstrated that copolymers-modified microspheres appeared to be more relevant surrogates in these conditions. Consistent with this, F-GC-CM were used as Cryptosporidium surrogates to study removal in different filtration systems (sand, anthracite, pumice, engineered ceramic sand, household filters) and in different operating conditions (Pang et al., 2021, Pang et al., 2022; Zhang et al., 2017).
Overall, studies highlight the potential of microspheres as promising surrogates for studying oocyst removal through different water treatments rather on a pilot scale, as their use on a full scale (on industrial sites) may prove cost-prohibitive due to the substantial quantities required. They can provide conservative or comparable estimates and allow to investigate the interactions between the parasites and water/porous media systems, encompassing processes such as adsorption/desorption, retention, and transport. Proteins at the microsphere surface can play a critical role in their attachment to solid surfaces such as soil grains and sediments, underscoring the importance of using functionalized over unmodified microspheres to better understand the parasite behavior in different media. Their applications to the food matrix remain to be documented, but their relevance for such application is questionable since food processes mainly aim at inactivating pathogens.
5. Conclusions and future directions
To improve risk assessment linked to the presence of C. parvum, C. cayetanensis and T. gondii oocysts in food and water, it is required to increase knowledge on human exposure to these matrices. To progress on these questions, researchers are facing challenges to work with these three parasites at different levels of difficulty. For C. parvum, the main barrier relies on its pathogenicity that requires laboratories with sufficient biosafety level. Concerning T. gondii, beside its pathogenicity, ethical and practical concerns linked to the implementation of in vivo assays (cats for production of oocysts and mice to assess their infectivity) are important constraints. Finally, C. cayetanensis is the most complex parasite to work with, due to the difficulties to obtain oocysts (exclusively shed by humans) and the lack of approaches to characterize their infective potential. For these reasons, researchers are seeking alternatives, i.e. surrogates of these pathogens, to hasten progress in exposure assessment. Most of the literature on surrogates concern Cryptosporidium oocysts and drinking water treatment performances (removal, Table 3), in line with the large Milwaukee outbreak in 1993 (Mac Kenzie et al., 1994). However, recent methodological advances for detection and infectivity assessment in water and food matrices make the need for alternatives of Cryptosporidium oocysts less essential. On the other hand, works evaluating surrogates for T. gondii and C. cayetanensis oocysts are scarcer and more recent (Table 3). Nevertheless, considering the increasing need to gain new knowledge in exposure assessment for these parasites, identifying relevant surrogates appears essential to overcome the constraints and limits associated to each of these pathogens.
Recent efforts focused on development of methods to detect the three parasites in food and water in order to conduct surveillance studies (Bartosova et al., 2021; Berrouch et al., 2020; Fradette et al., 2022; Slana et al., 2021). Significant advances concern molecular-based methods that allow to detect the DNA of the parasites and that are considered specific, sensitive, rapid, and now routinely used in laboratories. To conclude from surveillance analyses using these methods in food, it's crucial to be able to guarantee that no problem happened during the procedure. To that aim, extraction control can be used that should be different from the target pathogen(s) but should mimic its behavior during the procedure. E. papillata oocysts have been proposed as relevant extraction control of C. parvum, T. gondii and C. cayetanensis oocysts, in different red berries and leafy greens (Table 3, Table 4). Simultaneous comparison of recovery and molecular detection of oocysts E. acervulina and T. gondii from leafy greens indicated that this Eimeria species could also be used as a control process in this matrix (Augendre, unpublished work). Hence, oocysts of both Eimeria species represent valuable surrogates for validating the extraction processes from food matrices, for coccidia of public health importance. Future research should focus on validating the use of E. acervulina in other relevant food matrices that may require distinct extraction procedures.
Table 4.
Proposed surrogate or recommendations for each target depending on the targeted application.
| Application | Target parasites | Relevant surrogates based on current knowledge | Need for surrogates | Promising surrogates to investigate in the future (if yes) or recommendations (if no) |
|---|---|---|---|---|
| Control of oocysts and DNA extraction for molecular-based detection of pathogens in food | C. parvum | E. papillata | Yes | E. papillata, E. acervulina |
| C. cayetanensis | E. papillata | Yes | E. papillata, E. acervulina | |
| T. gondii | E. papillata | Yes | E. papillata, E. acervulina | |
| Identification of water resources contamination by the target parasites | C. parvum | To date, no relevant indicator identified | No | Direct detection of each targeted parasite in the resource a |
| C. cayetanensis | No published data | No | ||
| T. gondii | No published data | No | ||
| Assessment of removal performance in water at industrial scale | C. parvum | Spores of aerobic-SFB as worst case-scenario | Yes | Spores of aerobic-SFB as the worst-case scenario for all protozoa |
| C. cayetanensis | No published data | Yes | ||
| T. gondii | No published data | Yes | ||
| Assessment of inactivation in water at laboratory scale | C. parvum | Oxydants b: spores of anaerobic-SFB; Pulsed-UV light: spores of aerobic-SFB (B. megaterium and B. cereus) |
No | Assess inactivation in water directly on C. parvum oocysts |
| C. cayetanensis | E. acervulina | Yes | E. acervulina and other Eimeria spp. oocysts | |
| T. gondii | No published data | Yes | E. acervulina and other Eimeria spp. oocysts | |
| Assessment of inactivation in food at laboratory scale | C. parvum |
E. acervulina (Accumulation/depuration in oysters) |
No | Assess inactivation in food directly on C. parvum oocysts. |
| C. cayetanensis | E. acervulina | Yes | E. acervulina and other Eimeria spp. oocysts | |
| T. gondii |
E. acervulina (Accumulation/depuration in oysters) |
Yes | E. acervulina and other Eimeria spp. oocysts | |
| Assessment of parasite transport in terrestrial and aquatic environments | C. parvum | F-GC/BC-CM | No | F-GC/BC-CM |
| C. cayetanensis | No published data | Yes | Modified microspheres; in cocktail. | |
| T. gondii | Cocktail of FCM | Yes | Cocktail of FCM |
By molecular-based methods.
Mixed oxidant solution, ozone. SFB: spore-forming bacteria. FCM: fluorescent carboxylated microspheres. F-GC/BC-CM: fluorescent carboxylated microspheres coated with glycoproteins (G) or biotin (B).
To address the question of occurrence in raw matrices and of contamination routes, the increasing availability of molecular-based methods in routine laboratories, will probably lower the need for surrogates for such application in the future. This is particularly welcoming considering that neither E. coli, the spores of anaerobic/aerobic-spores forming bacteria nor microalgae appear relevant to identify water resources contaminated by Cryptosporidium oocysts (Table 4, Sylvestre et al., 2021), while no surrogates have been proposed to monitor water contamination by C. cayetanensis and T. gondii oocysts (Table 4). Hence, from our point of view there is no need to further investigate surrogates to characterize the contamination of water resources.
Food and water processes are key steps to reduce the contamination in raw matrices, and to eliminate the risk for consumers to be exposed to pathogens. To validate control measures, their performance have to be evaluated in terms of removal and/or inactivation (loss of viability/infectivity) of the target microorganism. This requires having sufficient initial levels of pathogens in raw water or foods to measure the reduction across the process, which is rarely the case for C. parvum, C. cayetanensis and T. gondii oocysts. For this reason and the fact that pathogens cannot obviously be intentionally introduced in industrial sites, it is not possible to assess processes directly in plants for these parasites without the use of safe surrogates. In drinking water industries, plant-scale assessment is the most common strategy to assess removal efficiency and relies on indirect approaches based on the use of indigenous performance indicators. These indicators must be at least as efficiently removed as the target pathogens and should not undergo re-growth during treatment. For Cryptosporidium oocysts, our literature review indicates that the spores of aerobic-spore forming bacteria are the most relevant indicator to assess removal efficiency at plant-scale as the “worst-case scenario” (Table 4), as recommended by the United States Environmental Protection Agency (USEPA, 2010). No surrogates have been identified to date for C. cayetanensis and T. gondii oocysts, possibly due to their lesser significance in drinking water production. However, considering data on Cryptosporidium, the spores of aerobic-spore forming bacteria appeared the most promising surrogate to be investigated for those latter. This should be validated at least for T. gondii in laboratory-scale experiments, before considering the spores of aerobic-spore forming bacteria as the surrogate to assess drinking water treatment performance on all protozoan parasites, in a worst-case scenario approach.
Evaluation of inactivation efficiency of water/food industrial processes for most pathogens is usually performed at laboratory- or pilot-scales in safety facilities. In this case, the pathogens are spiked in the targeted matrix at high levels to be able to measure sufficient inactivation induced by the process (i.e. removal of viable pathogens). Spore-forming bacteria have been tested as surrogates of C. parvum oocysts to assess chemical- (oxidant, Clostridium spp.) or physical- (pulsed-UV light, Bacillus spp.) based disinfection in water. We caution that the selection of the Bacillus strain should be carefully made depending on the inactivation treatment, since it can exert a significant influence on the fate of the spores vs. the oocysts (Headd and Bradford, 2016). In food, E. acervulina oocysts have been proposed as surrogates to assess the inactivation of C. parvum during bioaccumulation/depuration process of oysters. However, one can discuss the real need of surrogate for C. parvum in laboratory experiments considering that: i) it's quite easy to access large numbers of C. parvum oocysts (commercially available); and ii) CC-qPCR assays are available and accessible to laboratories to measure inactivation (Table 4). The best recommendation, if suitable facilities are available, would be to assess inactivation in water and food matrices directly on C. parvum oocysts to gain the most relevant information. In contrast, very few studies have addressed the use of surrogates to assess the inactivation of C. cayetanensis and T. gondii oocysts in water or food matrices (Table 3, Table 4). Nevertheless, the constraints associated with the obtaining of these oocysts and measuring inactivation for both parasites make the need for surrogates essential for such assessments. For C. cayetanensis, a recent review highlighted the relevance of E. acervulina oocysts as surrogates (Tucker et al., 2022). Current work at our lab suggests that E. acervulina oocysts are also interesting surrogates of T. gondii oocysts to measure inactivation induced by different physical and chemical treatments in water (Augendre et al., manuscript in preparation). Altogether, E. acervulina oocysts appear to be promising surrogates to assess inactivation of C. cayetanensis and T. gondii oocysts (Table 4). Future research works are required to validate this approach. Besides, the need for poultry bioassays to measure infectivity will eventually represent a limit that will need to be addressed. To date, only one study described a cell-culture system for E. acervulina oocysts (Taha et al., 2021) but its applicability in inactivation studies remains to be demonstrated. As an alternative, other Eimeria species such as E. tenella or E. papillata could be evaluated as surrogates too. Although they are larger than C. cayetanensis and T. gondii oocysts (Table 1, Table 2), in vitro assays have been described and successfully used (Abdel-Tawab et al., 2020; Felici et al., 2023; Mattig Frank et al., 1995; Thabet et al., 2017).
Modified (F-GC-CM, F-BC-CM) and unmodified (FCM) microspheres were the most studied surrogates to address the filtration efficiency and the transport of C. parvum and T. gondii oocysts respectively (Table 3). The easy production, ability to be prepared as cocktails, the flexibility in terms of size and surface characteristics and the absence of risk for handlers make the modified microspheres the most relevant surrogates for all parasites in transport applications (Table 4). The interest of modified microspheres rather than unmodified ones for T. gondii oocysts still need to be validated though. For C. cayetanensis, no data are available but modified microspheres should be investigate as the most relevant potential surrogate.
Inactivated oocysts could also be used as surrogates but were not included in this review. If they are the closest to the target pathogens and are safe to handle, they don't circumvent the problems linked to the obtaining of oocysts and they don't allow to assess inactivation performance. Besides, depending on the method used to inactivate the oocysts, the structure may be differentially affected (Dumètre et al., 2012), and consequently biased the behavior of oocysts upon removal treatments and transport and not accurately represent live oocysts (Kuznar and Elimelech, 2005; Ongerth and Pecoraro, 1995; Zhang et al., 2017). Furthermore, researchers have recognized that numerous factors can potentially alter or damage the oocyst wall (oocyst source, production methods, use of antibiotics, exposure to high ionic strength solutions, extensive washing), particularly its surface molecules, thus impacting its surface charge, viability, or capacity to be stained by viability probes (Brush et al., 1998; Butkus et al., 2003).
To conclude, as we could expect, no single surrogate is the best for all protozoa and all applications. Each surrogate has strengths and weaknesses, and researchers need to consider these depending on the research questions. This review aimed at guiding the users to select the most suitable surrogate for a specific protozoan parasite and a specific application. Eimeria spp. oocysts emerge as promising surrogates for studying human pathogenic coccidian parasites that are challenging to work with (C. cayetanensis and T. gondii) in diverse applications. Besides, multiple surrogates in cocktails may be the most relevant approach to cover larger panels of parasites and/or applications. But, this strategy has to be evaluated and validated in studies comparing the surrogates relative to oocysts in the same laboratory conditions and ideally, by assessing the same parameter (i.e., detection and or infectivity). In the food/water safety domains, careful selection of surrogates is crucial to avoid underestimation of the exposure to C. parvum, C. cayetanensis and T. gondii oocysts. In the most secure approach, surrogates should at least lead to overestimation of the exposure to oocysts in raw matrices and underestimation of the control measures efficiency. Despite the fact that surrogates may not fully represent the behavior of live protozoa, their use could contribute to modeling oocyst transmission and developing strategies to limit contamination and reduce human infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully thank Angélique Goodwin for improving the English editing. This work was supported by the UMT ACTIA PROTORISK 2 (Ministry of Agriculture and Food Sovereignty) and the University of Reims Champagne-Ardenne. Laure Augendre PhD thesis was funded by ACTALIA within a CIFRE agreement between ACTALIA and the French Ministry of Higher Education, Research and Innovation, and by the ANR grant BreakingTheWall (ANR-22-CE35-0008). The graphical abstract was created with icons from BioRender.com.
References
- Abdel-Tawab H.R., Abdel-Baki A.A., El-mallah A., Al-Quraishy S., Abdel-Haleem H.M. In vivo and in vitro anticoccidial efficacy of Astragalus membranaceus against Eimeria papillata infection. J. King Saud Univ.-Sci. 2020 doi: 10.1016/j.jksus.2020.03.016. [DOI] [Google Scholar]
- Akiba M., Kunikane S., Kim H.-S., Kitazawa H. Algae as surrogate indices for the removal of Cryptosporidium oocysts by direct filtration. Water Supply. 2002;2:73–80. doi: 10.2166/ws.2002.0087. [DOI] [Google Scholar]
- Almeria S., Cinar H.N., Dubey J.P. Cyclospora cayetanensis and Cyclosporiasis: an update. Microorg. 2019;7:317. doi: 10.3390/microorganisms7090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amburgey J.E., Amirtharajah A., York M.T., Brouckaert B.M., Spivey N.C., Arrowood M.J. Comparison of conventional and biological filter performance for Cryptosporidium and microsphere removal. J. Am. Water Works Assoc. 2005;97:77–91. doi: 10.1002/j.1551-8833.2005.tb07542.x. [DOI] [Google Scholar]
- Bartosova B., Koudela B., Slana I. Detection of Cyclospora cayetanensis, Echinococcus multilocularis, Toxocara spp. and microsporidia in fresh produce using molecular methods: – a review. Food Waterborne Parasitol. 2021;23 doi: 10.1016/j.fawpar.2021.e00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens H., Beims U., Dieter H., Dietze G., Eikmann T., Grummt T., et al. Toxicological and ecotoxicological assessment of water tracers. Hydrogeol. J. 2001;9:321–325. doi: 10.1007/s100400100126. [DOI] [Google Scholar]
- Belli S.I., Smith N.C., Ferguson D.J.P. The coccidian oocyst: a tough nut to crack! Trends Parasitol. 2006;22:416–423. doi: 10.1016/j.pt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Berrouch S., Escotte-Binet S., Amraouza Y., Flori P., Aubert D., Villena I., et al. Cryptosporidium spp., Giardia duodenalis and Toxoplasma gondii detection in fresh vegetables consumed in Marrakech. Morocco. Afr. H. Sci. 2020;20:1669–1678. doi: 10.4314/ahs.v20i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougaran G., Saint-Jean B. Microalgues: de petits végétaux aux grandes promesses! Biofutur December 2014. Issue. 2014;360:28–31. http://archimer.ifremer.fr/doc/00252/36321/ [Google Scholar]
- Bouwknegt M., Devleesschauwer B., Graham H., Robertson L.J., van der Giessen J.W., The Euro-Fbp workshop participants Prioritisation of food-borne parasites in Europe, 2016. Eur.o Surveill. 2018;23(9) doi: 10.2807/1560-7917.ES.2018.23.9.17-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford S.A., Kim H., Headd B., Torkzaban S. Evaluating the transport of Bacillus subtilis spores as a potential surrogate for Cryptosporidium parvum oocysts. Environ. Sci. Technol. 2016;50:1295–1303. doi: 10.1021/acs.est.5b05296. [DOI] [PubMed] [Google Scholar]
- Brown R.A., Cornwell D.A. Using spore removal to monitor plant performance for Cryptosporidium removal. J. Am. Water Works Assoc. 2007;99:95–109. doi: 10.1002/j.1551-8833.2007.tb07892.x. [DOI] [Google Scholar]
- Brown T.J., Emelko M.B. Chitosan and metal salt coagulant impacts on Cryptosporidium and microsphere removal by filtration. Water Res. 2009;43:331–338. doi: 10.1016/j.watres.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Brush C.F., Walter M.F., Anguish L.J., Ghiorse W.C. Influence of pretreatment and experimental conditions on electrophoretic mobility and hydrophobicity of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 1998;64(11) doi: 10.1128/AEM.64.11.4439-4445.1998. 44394445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkus M.A., Bays J.T., Labere M.P. Influence of surface characteristics on the stability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 2003;69:3819–3825. doi: 10.1128/AEM.69.7.3819-3825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera M., Zandomeni R.O., Fitzgibbon J., Sagripanti J.L. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2007;102:303–312. doi: 10.1111/j.1365-2672.2006.03111.x. [DOI] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention, 2017. Parasites-Cyclosporiasis (Cyclospora Infection) Surveillance & Outbreaks. 2022. https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2022/seasonal/index.html (accessed mars 2023)
- Chalmers R.M. Waterborne outbreaks of cryptosporidiosis. Ann. dell’Istit. Super. San. 2012;48:429–446. doi: 10.4415/ANN_12_04_10. [DOI] [PubMed] [Google Scholar]
- Chalmers R.M., Davies A.P. Minireview: clinical cryptosporidiosis. Exp. Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Chauret C., Armstrong N., Fisher J., Sharma R., Springthorpe S., Sattar S. Correlating Cryptosporidium and Giardia with microbial indicators. J. Am. Water Works Assoc. 1995;87:76–84. doi: 10.1002/j.1551-8833.1995.tb06453.x. [DOI] [Google Scholar]
- Chauret C.P., Radziminski C.Z., Lepuil M., Creason R., Andrews R.C. Chlorine dioxide inactivation of Cryptosporidium parvum oocysts and bacterial spore indicators. Appl. Environ. Microbiol. 2001;67:2993–3001. doi: 10.1128/AEM.67.7.2993-3001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell D.A., MaCphee M.J., Brown R.A., Via S.H. Demonstrating Cryptosporidium removal using spore monitoring at lime-softening plants. J. Am. Water Works Assoc. 2003;95:124–133. doi: 10.1002/j.1551-8833.2003.tb10367.x. [DOI] [Google Scholar]
- Costa D., Razakandrainibe R., Valot S., Vannier M., Sautour M., Basmaciyan L., et al. Epidemiology of cryptosporidiosis in France from 2017 to 2019. Microorg. 2020;8(9):1358. doi: 10.3390/microorganisms8091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D., Razakandrainibe R., Basmaciyan L., Raibaut J., Delaunay P., Morio F., et al. A summary of cryptosporidiosis outbreaks reported in France and overseas departments, 2017–2020. Food Waterborne Parasitol. 2022;27 doi: 10.1016/j.fawpar.2022.e00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Hozalski R.M. Evaluation of microspheres as surrogates for Cryptosporidium parvum oocysts in filtration experiments. Environ. Sci. Technol. 2003;37:1037–1042. doi: 10.1021/es025521w. [DOI] [PubMed] [Google Scholar]
- Daraei H., Oliveri Conti G., Sahlabadi F., Thai V.N., Gholipour S., Turki H., et al. Prevalence of Cryptosporidium spp. in water: a global systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021;28:9498–9507. doi: 10.1007/s11356-020-11261-6. [DOI] [PubMed] [Google Scholar]
- Dawson D. Foodborne protozoan parasites. Int. J. Food Microbiol. 2005;103:207–227. doi: 10.1016/j.ijfoodmicro.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Delaquis P. Defence R&D Canada; Canada: 2014. Mitigating Dissemination of Bioterrorisms Agents in Canadian Food Distribution Systems. [Google Scholar]
- Dubey J. Second edition. CRC Press, Boca Rotan; 2010. Toxoplasmosis of Animals and Humans. [DOI] [Google Scholar]
- Dubey J.P. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J. Parasitol. 1995;81(3):410–415. doi: 10.2307/3283823. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasma gondii oocyst survival under defined temperatures. J. Parasitol. 1998;84(4):862–865. doi: 10.2307/3284606. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009;39:877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Beattie C.P. CRC Press; Boca Raton, FL: 1988. Toxoplasmosis of Animals and Man. [DOI] [Google Scholar]
- Dufour A., Bartram J., Robert B., Gannon V. WHO, IWA Publishing; London, UK: 2012. Animal Waste, water quality and Human Health. [DOI] [Google Scholar]
- Dumètre A., Aubert D., Puech P.H., Hohweyer J., Azas N., Villena I. 2012. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 2012 Feb;78(4):905–912. doi: 10.1128/AEM.06488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont H.L., Chappell C.L., Sterling C.R., Okhuysen P.C., Rose J.B., Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- Efstratiou A., Ongerth J.E., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011-2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Emelko M. Univ of Waterloo; Waterloo, Ontario: 2001. Removal of Cryptosporidium parvum by Granular Media Filtration.http://hdl.handle.net/10012/614 PhD. [Google Scholar]
- Emelko M.B., Huck P.M. Microspheres as surrogates for Cryptosporidium filtration. J. Am. Water Works Assoc. 2004;96:94–105. doi: 10.1002/j.1551-8833.2004.tb10577.x. [DOI] [Google Scholar]
- Emelko M.B., Huck P.M., Douglas I.P. Cryptosporidium and microsphere removal during late in-cycle filtration. J. Am. Water Works Assoc. 2003;95:173–182. doi: 10.1002/j.1551-8833.2003.tb10371.x. [DOI] [Google Scholar]
- Erickson M.C., Ortega Y.R. 2006. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006 Nov;69(11):2786–2808. doi: 10.4315/0362-028X-69.11.2786. [DOI] [PubMed] [Google Scholar]
- Facile N. Evaluating bacterial aerobic spores as a surrogate for Giardia and Cryptosporidium inactivation by ozone. Water Res. 2000;34:3238–3246. doi: 10.1016/S0043-1354(00)00086-5. [DOI] [Google Scholar]
- FAO/WHO, 2014. Multicriteria-based ranking for risk management of food-borne parasites. Microbiol. Risk Assess. Ser. 23. ISBN: 978-92-4-156470-0.
- Farrell M.L., Joyce A., Duane S., Fitzhenry K., Hooban B., Burke L.P., et al. Evaluating the potential for exposure to organisms of public health concern in naturally occurring bathing waters in Europe: a scoping review. Water Res. 2021;206 doi: 10.1016/j.watres.2021.117711. [DOI] [PubMed] [Google Scholar]
- Fayer R., Speer C.A., Dubey J.P. Cryptosporidium and Cryptosporidiosis. CRC Press; Boca Raton, FL: 1997. The general biology of Cryptosporidium; pp. 1–41. [Google Scholar]
- Feix A.S., Cruz-Bustos T., Ruttkowski B., Joachim A. In vitro cultivation methods for coccidian parasite research. Int. J. Parasitol. 2023;53:477–489. doi: 10.1016/j.ijpara.2022.10.002. [DOI] [PubMed] [Google Scholar]
- Felici M., Tugnoli B., Ghiselli F., Baldo D., Ratti C., Piva A., et al. Investigating the effects of essential oils and pure botanical compounds against Eimeria tenella in vitro. Poult. Sci. 2023;102898 doi: 10.1016/j.psj.2023.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.R. Effects of ionising radiation from cobalt-60 on oocysts of Eimeria bovis. J. Parasitol. 1968;54:233–240. doi: 10.2307/3276927. [DOI] [PubMed] [Google Scholar]
- Fradette M.S., Culley A.I., Charette S.J. Detection of Cryptosporidium spp. and Giardia spp. in environmental water samples: a journey into the past and new perspectives. Microorg. 2022;10(6):1175. doi: 10.3390/microorganisms10061175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J.K., Dubey J.P. Toxoplasmosis and its prevention in cats and man. J. Infect. Dis. 1972;126(6):664–673. doi: 10.1093/infdis/126.6.664. [DOI] [PubMed] [Google Scholar]
- Freppel W., Ferguson D.J.P., Shapiro K., Dubey J.P., Puech P.-H., Dumètre A. Structure, composition, and roles of the Toxoplasma gondii oocyst and sporocyst walls. Cell Surf. 2019;5 doi: 10.1016/j.tcsw.2018.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galofré B., Israel S., Dellundé J., Ribas F. Aerobic bacterial spores as process indicators for protozoa cysts in water treatment plants. Water Sci. Technol. 2004;50:165–172. doi: 10.2166/wst.2004.0049. [DOI] [PubMed] [Google Scholar]
- Garvey M., Clifford E., O’Reilly E., Rowan N.J. Efficacy of using harmless Bacillus endospores to estimate the inactivation of Cryptosporidium parvum oocysts in water. J. Parasitol. 2013;99:448–452. doi: 10.1645/12-48.1. [DOI] [PubMed] [Google Scholar]
- Gérard C., Franssen F., La Carbona S., Monteiro S., Cozma-Petruţ A., Utaaker, et al. Inactivation of parasite transmission stages: efficacy of treatments on foods of non-animal origin. Trends Food Sci. Technol. 2019;91:12–23. doi: 10.1016/j.tifs.2019.06.015. [DOI] [Google Scholar]
- Gibbs P.A. Woodhead Publishing; 2009. Pathogenic Clostridium species. Foodborne Pathogens; pp. 820–843. [DOI] [Google Scholar]
- Guzman-Herrador B., Carlander A., Ethelberg S., Freiesleben de Blasio B., Kuusi M., Lund V., et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2015;20 doi: 10.2807/1560-7917.es2015.20.24.21160. [DOI] [PubMed] [Google Scholar]
- Hadjilouka A., Tsaltas D. Cyclospora Cayetanensis—major outbreaks from ready to eat fresh fruits and vegetables. Foods. 2020;9:1703. doi: 10.3390/foods9111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.W., Metge D.W., Shapiro A.M., Renken R.A., Osborn C.L., Ryan J.N., et al. Pathogen and chemical transport in the karst limestone of the Biscayne aquifer: 3. Use of microspheres to estimate the transport potential of Cryptosporidium Parvum oocysts. Water Resour. Res. 2008;44(8):1–12. doi: 10.1029/2007WR006060. [DOI] [Google Scholar]
- Harvey R.W., Metge D.W., Le Blanc D. Microbial-sized, carboxylate-modified microspheres as surrogate tracers in a variety of subsurface environments: an overview. Proc. Earth Planetary Sci. 2017;17:372–375. doi: 10.1016/j.proeps.2016.12.094. [DOI] [Google Scholar]
- Headd B., Bradford S.A. Use of aerobic spores as a surrogate for Cryptosporidium oocysts in drinking water supplies. Water Res. 2016;90:185–202. doi: 10.1016/j.watres.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A.M., Van der Veer A.J., Van Beveren J., Medema G.J. Spores of sulphite-reducing clostridia (SSRC) as surrogate for verification inactivation capacity of full-scale ozonation for Cryptosporidium. Water Sci. Technol. Water Supply. 2002;2:163–170. doi: 10.2166/ws.2002.0021. [DOI] [Google Scholar]
- Hijnen W.A.M., Schijven J.F., Bonné P., Visser A., Medema G.J. Elimination of viruses, bacteria and protozoan oocysts by slow sand filtration. Water Sci. Technol. 2004;50:147–154. doi: 10.2166/wst.2004.0044. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A.M., Brouwer-Hanzens A.J., Charles K., Medema G.J. Transport of MS2 phage, Escherichia coli, Clostridium perfringens, Cryptosporidium parvum and Giardia intestinalis in a gravel and a sandy soil. Environ. Sci. Technol. 2005;39:7860–7868. doi: 10.1021/es050427b. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A.M., Dullemont Y.J., Schijven J.F., Hanzens-Brouwer A.J., Rosielle M., Medema G. Removal and fate of Cryptosporidium parvum, Clostridium perfringens and small-sized centric diatoms (Stephanodiscus hantzschii) in slow sand filters. Water Res. 2007;41:2151–2162. doi: 10.1016/j.watres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Hohweyer J., Dumètre A., Aubert D., Azas N., Villena I. Tools and methods for detecting and characterizing Giardia, Cryptosporidium, and Toxoplasma parasites in marine mollusks. J. Food Prot. 2013;76:1649–1657. doi: 10.4315/0362-028X.JFP-13-002. [DOI] [PubMed] [Google Scholar]
- Hutchison M.L., Walters L.D., Moore T., Thomas D.J.I., Avery S.M. Fate of pathogens present in livestock wastes spread onto fescue plots. Appl. Environ. Microbiol. 2005;71:691–696. doi: 10.1128/AEM.71.2.691-696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.B., Eaglesham B.S., Anthony L.C., Kachlany S.C., Bowman D.D., Ghiorse W.C. Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 2010;76:1926–1934. doi: 10.1128/AEM.02295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.M., Witola W.H. Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: a comprehensive review. Front. Cell. Infect. Microbiol. 2023;13:1115522. doi: 10.3389/fcimb.2023.1115522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.N., Walker S.L., Bradford S.A. Coupled factors influencing the transport and retention of Cryptosporidium parvum oocysts in saturated porous media. Water Res. 2010;2010(44):1213–1223. doi: 10.1016/j.watres.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Tomiak R.B., Mariñas B.J. Inactivation of Cryptosporidium oocysts in a pilot-scale ozone bubble-diffuser contactor. I: model development. J. Environ. Eng. 2002;128:514–521. doi: 10.1061/(ASCE)0733-9372(2002)128:6(514). [DOI] [Google Scholar]
- Kim J.-H., Rennecker J.L., Tomiak R.B., Mariñas B.J., Miltner R.J., Owens J.H. Inactivation of Cryptosporidium oocysts in a pilot-scale bubble-diffuser contactor - II.: model validation and application. ASCE J. Environ. Eng. Div. 2002;128(6):522532. doi: 10.1061/(ASCE)0733-9372(2002)128:6(52. [DOI] [Google Scholar]
- Kim M., Shapiro K., Rajal V.B., Packham A., Aguilar B., Rueda L., et al. Quantification of viable protozoan parasites on leafy greens using molecular methods. Food Microbiol. 2021;99 doi: 10.1016/j.fm.2021.103816. [DOI] [PubMed] [Google Scholar]
- King B.J., Keegan A.R., Monis P.T., Saint C.P. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 2005;71:3848–3857. doi: 10.1128/AEM.71.7.3848-3857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.J., Hoefel D., Daminato D.P., Fanok S., Monis P.T. Solar UV reduces Cryptosporidium parvum oocyst infectivity in environmental waters. J. Appl. Microbiol. 2008;104:1311–1323. doi: 10.1111/j.1365-2672.2007.03658.x. [DOI] [PubMed] [Google Scholar]
- Kniel K.E., Shearer A.E.H., Cascarino J.L., Wilkins G.C., Jenkins M.C. High hydrostatic pressure and UV light treatment of produce contaminated with Eimeria acervulina as a Cyclospora cayetanensis surrogate. J. Food Prot. 2007;70:2837–2842. doi: 10.4315/0362-028X-70.12.2837. [DOI] [PubMed] [Google Scholar]
- Korich D.G., Mead J.R., Madore M.S., Sinclair N.A., Sterling C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990;56:1423–1428. doi: 10.1128/AEM.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubina S., Costa D., Favennec L., Gargala G., Rousseau A., Villena I., et al. Detection of infectious Cryptosporidium parvum oocysts from lamb's lettuce: CC-qPCR's intake. Microorganism. 2021;9:215. doi: 10.3390/microorganisms9020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznar Z.A., Elimelech M. Role of surface proteins in the deposition kinetics of Cryptosporidium parvum oocysts. Langmuir. 2005;21(January):710–716. doi: 10.1021/la047963m. [DOI] [PubMed] [Google Scholar]
- Lalonde L.F., Gajadhar A.A. Optimization and validation of methods for isolation and real-time PCR identification of protozoan oocysts on leafy green vegetables and berry fruits. Food Waterborne Parasitol. 2016;2:1–7. doi: 10.1016/j.fawpar.2015.12.002. [DOI] [Google Scholar]
- Lalonde L.F., Gajadhar A.A. Detection of Cyclospora cayetanensis, Cryptosporidium spp., and Toxoplasma gondii on imported leafy green vegetables in Canadian survey. Food Waterborne Parasitol. 2016;2:8–14. doi: 10.1016/j.fawpar.2016.01.001. [DOI] [Google Scholar]
- Lee M.B., Lee E.-H. Coccidial contamination of raspberries: mock contamination with Eimeria acervulina as a model for decontamination treatment studies. J. Food Prot. 2001;64:1854–1857. doi: 10.4315/0362-028X-64.11.1854. [DOI] [PubMed] [Google Scholar]
- Lee M.B., Lee E.-H. Passage of a coccidial parasite (Eimeria acervulina) through the eastern oyster (Crassostrea virginica) J. Food Prot. 2003;66:679–681. doi: 10.4315/0362-028X-66.4.679. [DOI] [PubMed] [Google Scholar]
- Li M.-Y., Kang Y.-H., Sun W.-C., Hao Z.-P., Elsheikha H.M., Cong W. Terrestrial runoff influences the transport and contamination levels of Toxoplasma gondii in marine organisms. Sci. Total Environ. 2022;851 doi: 10.1016/j.scitotenv.2022.158168. [DOI] [PubMed] [Google Scholar]
- Li W., Qi W., Chen J., Zhou W., Li Y., Sun Y., Ding K. Effective removal of fluorescent microparticles as Cryptosporidium parvum surrogates in drinking water treatment by metallic membrane. J. Membr. Sci. 2020;594 doi: 10.1016/j.memsci.2019.117434. [DOI] [Google Scholar]
- Lindsay D.S., Phelps K.K., Smith S.A., Flick G., Sumner S.S., Dubey J.P. Removal of Toxoplasma gondii oocysts from sea water by eastern oysters (Crassostrea virginica) J. Eukaryot. Microbiol. 2001;2001(Suppl):197S–198S. doi: 10.1111/j.1550-7408.2001.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang Y., Craik S., James W., Shu Z., Narain R., et al. Removal of Cryptosporidium surrogates in drinking water direct filtration. Colloids Surf. B: Biointerfaces. 2019;181:499–505. doi: 10.1016/j.colsurfb.2019.05.065. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang Y., Narain R., Liu Y. Functionalized polystyrene microspheres as Cryptosporidium surrogates. Colloids Surf. B: Biointerfaces. 2019;175:680–687. doi: 10.1016/j.colsurfb.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Ma J.Y., Li M.Y., Qi Z.Z., Fu M., Sun T.F., Elsheikha H.M., Cong W. Waterborne protozoan outbreaks: an update on the global, regional, and national prevalence from 2017 to 2020 and sources of contamination. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150562. [DOI] [PubMed] [Google Scholar]
- Mac Kenzie W.R., Hoxie N.J., Proctor M.E., Gradus M.S., Blair K.A., Peterson D.E., et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Mansfield L.S., Gajadhar A.A. Cyclospora cayetanensis, a food - and waterborne parasite. Vet. Parasitol. 2004;126:73–90. doi: 10.1016/j.vetpar.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Mariñas B.J., Rennecker J.L., Teefy S., Rice E.W. Assessing ozone disinfection with nonbiological surrogates. J. Am. Water Works Assoc. 1999;91:79–89. doi: 10.1002/j.1551-8833.1999.tb08699.x. [DOI] [Google Scholar]
- Martorelli Di Genova B., Wilson S.K., Dubey J.P., Knoll L.J. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattig Frank R., Chobotar Bill, Rolf Entzeroth. Vol. 2491. Faculty Publications; 1995. Light and Electron Microscopy Study of First Generation Development of Eimeria papillata (Apicomplexa: Eimeriidae) in Polarized MDCK-cells in vitro. [DOI] [Google Scholar]
- Mazoua S., Chauveheid E. Aerobic spore-forming bacteria for assessing quality of drinking water produced from surface water. Water Res. 2005;39:5186–5198. doi: 10.1016/j.watres.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh Gohari I., Navarro M., Li J., Shrestha A., Uzal F., McClane B. Pathogenicity and virulence of Clostridium perfringens. Virulence. 2021 Dec;12(1):723–753. doi: 10.1080/21505594.2021.1886777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles L.R., Ekman C.C., Andrade H.F., Jr., Luna E.J. Human toxoplasmosis outbreaks and the agent infecting form. Findings from a systematic review. Rev. Inst. Med. Trop. Sao Paulo. 2015 Sep-Oct;57(5):369–376. doi: 10.1590/S0036-46652015000500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metge D.W., Harvey R.W., Anders R., Rosenberry D.O., Seymour D., Jasperse J. Use of carboxylated microspheres to assess transport potential of Cryptosporidium parvum oocysts at the Russian river water supply facility, Sonoma County, California. Geomicrobiol J. 2007;24:231–245. doi: 10.1080/01490450701456867. [DOI] [Google Scholar]
- Mirza Alizadeh A., Jazaeri S., Shemshadi B., Hashempour-Baltork F., Sarlak Z., Pilevar Z., et al. A review on inactivation methods of Toxoplasma gondii in foods. Pathog. Glob. Health. 2018;112:306–319. doi: 10.1080/20477724.2018.1514137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanram A., Ray C., Harvey R.W., Metge D.W., Ryan J.N., Chorover J., et al. Comparison of transport and attachment behaviors of Cryptosporidium parvum oocysts and oocyst-sized microspheres being advected through three minerologically different granular porous media. Water Res. 2010;44:5334–5344. doi: 10.1016/j.watres.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Molazadeh M., Ahmadzadeh H., Pourianfar H.R., Lyon S., Rampelotto P.H. The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 2019;7:42. doi: 10.3389/fbioe.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis P., Lau M., Harris M., Cook D., Drikas M. Risk-based management of drinking water safety in Australia: implementation of health based targets to determine water treatment requirements and identification of pathogen surrogates for validation of conventional filtration. Food Waterborne Parasitol. 2017;8–9:64–74. doi: 10.1016/j.fawpar.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnes D., Roberts E., Lukeš J., Lowe C. The rise of model protozoa. Trends Microbiol. 2012;20:184–191. doi: 10.1016/j.tim.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Moulin L., Richard F., Stefania S., Goulet M., Gosselin S., Gonçalves A., et al. Contribution of treated wastewater to the microbiological quality of Seine River in Paris. Water Res. 2010;44:5222–5231. doi: 10.1016/j.watres.2010.06.037. [DOI] [PubMed] [Google Scholar]
- Nasser A.M. Transmission of Cryptosporidium by fresh vegetables. J. Food Prot. 2022;85:1737–1744. doi: 10.4315/JFP-22-152. [DOI] [PubMed] [Google Scholar]
- Nieminski E.C., Ongerth J.E. Removing Giardia and Cryptosporidium by conventional treatment and direct filtration. J. Am. Water Works Assoc. 1995;87:96–106. doi: 10.1002/j.1551-8833.1995.tb06426.x. [DOI] [Google Scholar]
- Nieminski E.C., Bellamy W.D., Moss L.R. Using surrogates to improve plant performance. J. Am. Water Works Assoc. 2000;92:67–78. doi: 10.1002/j.1551-8833.2000.tb08910.x. [DOI] [Google Scholar]
- Okhuysen P.C., Rich S.M., Chappell C.L., Grimes K.A., Widmer G., Feng X., et al. Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-g knockout mice. J. Infect. Dis. 2002;2002(185):1320–1325. doi: 10.1086/340132. [DOI] [PubMed] [Google Scholar]
- Ongerth J., Pecoraro J. Removing Cryptosporidium using multimedia filters. J. Am. Water Works Assoc. 1995;87(12):83. doi: 10.1002/j.1551-8833.1995.tb06468.x. [DOI] [Google Scholar]
- Oren A., Mareš J., Rippka R. Validation of the names Cyanobacterium and Cyanobacterium stanieri, and proposal of Cyanobacteriota. Phyl. Nov. Int. J. Syst. Evol. Microbiol. 2022;72:5528. doi: 10.1099/ijsem.0.005528. [DOI] [PubMed] [Google Scholar]
- Ortega Y.R., Sterling C.R., Gilman R.H., Cama V.A., Diaz F. Cyclospora species - a new protozoan pathogen of humans. New Engl. J. Med. 1993;328:1308–1312. doi: 10.1056/NEJM199305063281804. [DOI] [PubMed] [Google Scholar]
- Ortega Y.R., Sterling C.R., Gilman R.H. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J. Parasitol. 1994;80:625–629. doi: 10.2307/3283201. [DOI] [PubMed] [Google Scholar]
- Ortega Y.R., Sterling C.R., Gilman R.H. Cyclospora cayetanensis. Adv. Parasitol. 1998;40:399–418. doi: 10.1016/S0065-308X(08)60128-1. [DOI] [PubMed] [Google Scholar]
- Pang L., Nowostawska U., Weaver L., Hoffman G., Karmacharya A., Skinner A., et al. Biotin- and glycoprotein-coated microspheres: potential surrogates for studying filtration of Cryptosporidium parvum in porous media. Environ. Sci. Technol. 2012;46:11779–11787. doi: 10.1021/es302555n. [DOI] [PubMed] [Google Scholar]
- Pang L., Lin S., Premaratne A., Tham A., Abraham P., Nokes C. Cryptosporidium surrogate removal in five commonly used point-of-use domestic filters. J. Water Process Eng. 2021;44 doi: 10.1016/j.jwpe.2021.102390. [DOI] [Google Scholar]
- Pang L., Tham A., Nilprapa P., Cocker A., MacDonald P., Adams R., et al. Cryptosporidium surrogate removal in pilot-scale rapid sand filters comprising anthracite, pumice or engineered ceramic granular media, and its correlation with turbidity. J. Water Process. Eng. 2022;46 doi: 10.1016/j.jwpe.2022.102614. [DOI] [Google Scholar]
- Payment P., Franco E. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl. Environ. Microbiol. 1993;59:2418–2424. doi: 10.1128/aem.59.8.2418-2424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieniazek N.J., Herwaldt B.L. Reevaluating the molecular taxonomy: is human-associated Cyclospora a mammalian Eimeria species? Emerg. Infect. Dis. 1997;3(3):381–383. doi: 10.3201/eid0303.970319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabold J.G., Hoge C.W., Shlim D.R., Kefford C., Rajah R., Echeverria P. Cyclospora outbreak associated with chlorinated drinking water. Lancet. 1994;344:1360–1361. doi: 10.1016/S0140-6736(94)90716-1. [DOI] [PubMed] [Google Scholar]
- Ramírez-Flores C.J., Tibabuzo Perdomo A.M., Gallego-López G.M., Knoll L.J. Transcending dimensions in apicomplexan research: from two-dimensional to three-dimensional in vitro cultures. Microbiol. Mol. Biol. Rev. 2022;86(2):e0002522. doi: 10.1128/mmbr.00025-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D.A., Schmidt T.M., Gajadhar A., Sogin M., Cross J., Yoder K., et al. Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 1996;173 doi: 10.1093/infdis/173.2.440. [DOI] [PubMed] [Google Scholar]
- Ribas F., Bernal A., Perramón J. Elimination of Giardia cysts, Cryptosporidium oocysts, turbidity and particles in a drinking water treatment plant with clarification and double filtration. Water Sci. Technol. 2000;41:203–211. doi: 10.2166/wst.2000.0134. [DOI] [Google Scholar]
- Rice E.W., Fox K.W., Miltner R.J., Lytle D.A., Johnson C.H. A microbiological surrogate for evaluating treatment efficiency, in Water quality technology conference, San Francisco, CA, November 6-10, 1994. Am. Water Works Ass. 1994:2035–2045. [Google Scholar]
- Robert-Gangneux F., Darde M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012;25:264–296. doi: 10.1128/cmr.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L.J., Campbell A.T., Smith H.V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L.J., Sprong H., Ortega Y.R., van der Giessen J.W., Fayer R. Impacts of globalisation on foodborne parasites. Trends Parasitol. 2014;30(1):37–52. doi: 10.1016/j.pt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Robertson L.J., Torgerson P.R., van der Giessen J. Foodborne parasitic diseases in Europe: social cost-benefit analyses of interventions. Trends Parasitol. 2018;34:919–923. doi: 10.1016/j.pt.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Robertson L.J., Lalle M., Paulsen P. Why we need a European focus on foodborne parasites. Exp. Parasitol. 2020;214 doi: 10.1016/j.exppara.2020.107900. [DOI] [PubMed] [Google Scholar]
- Rousseau A., La Carbona S., Dumètre A., Robertson L.J., Gargala G., Escotte-Binet S., Favennec L., et al. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite. 2018;25:14. doi: 10.1051/parasite/2018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.J., Theriot J.A., Sood P., Marshall W.F., Landweber L.F., Fritz-Laylin L., et al. Non-model model organisms. BMC Biol. 2017;15 doi: 10.1186/s12915-017-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijven J.F., de Bruin H.A.M., Hassanizadeh S.M., de Roda Husman A.M. Bacteriophages and Clostridium spores as indicator organisms for removal of pathogens by passage through saturated dune sand. Water Res. 2003;37:2186–2194. doi: 10.1016/S0043-1354(02)00627-9. [DOI] [PubMed] [Google Scholar]
- Setlow P. Spore resistance properties. Microbiol. Spectr. 2014;2:2.5.11. doi: 10.1128/microbiolspec.TBS-0003-2012. [DOI] [PubMed] [Google Scholar]
- Shapiro K., Largier J., Mazet J.A.K., Bernt W., Ell J.R., Melli A.C., Conrad P.A. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Appl. Environ. Microbiol. 2009;75:1185–1191. doi: 10.1128/AEM.02109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K., Bahia-Oliveira L., Dixon B., Dumètre A., de Wit L.A., VanWormer E., Villena I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food and Waterborne Parasitology. 2019;15 doi: 10.1016/j.fawpar.2019.e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K., Conrad P.A., Mazet J.A.K., Wallender W.W., Miller W.A., Largier J.L. Effect of estuarine wetland degradation on transport of Toxoplasma gondii surrogates from land to sea. Appl. Environ. Microbiol. 2010;76:6821–6828. doi: 10.1128/AEM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K., Mazet J.A.K., Schriewer A., Wuertz S., Fritz H., Miller W.A., et al. Detection of Toxoplasma gondii oocysts and surrogate microspheres in water using ultrafiltration and capsule filtration. Water Res. 2010;44:893–903. doi: 10.1016/j.watres.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Shearer A.E.H., Wilkins G.C., Jenkins M.C., Kniel K.E. Effects of high hydrostatic pressure on Eimeria acervulina pathogenicity, immunogenicity and structural integrity. Innovative Food Sci. Emerg. Technol. 2007;8:259–268. doi: 10.1016/j.ifset.2007.01.004. [DOI] [Google Scholar]
- Sinclair R.G., Rose J.B., Hashsham S.A., Gerba C.P., Haas C.N. Criteria for selection of surrogates used to study the fate and control of pathogens in the environment. Appl. Environ. Microbiol. 2012;78:1969–1977. doi: 10.1128/AEM.06582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siński E., Behnke J.M. Apicomplexan parasites: environmental contamination and transmission. Pol. J. Microbiol. 2004;53:67–73. (PMID: 15787200) [PubMed] [Google Scholar]
- Slana I., Bier N., Bartosova B., Marucci G., Possenti A., Mayer-Scholl A., et al. Molecular methods for the detection of Toxoplasma gondii oocysts in fresh produce: an extensive review. Microorg. 2021;2021(9):167. doi: 10.3390/microorganisms9010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soave R., Johnson W.D. Cyclospora: conquest of an emerging pathogen. Lancet. 1995;345:667–668. doi: 10.1016/S0140-6736(95)90863-3. [DOI] [PubMed] [Google Scholar]
- Stelma G.N. Use of bacterial spores in monitoring water quality and treatment. J. Water Health. 2018;16:491–500. doi: 10.2166/wh.2018.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling C.R., Ortega Y.R. Cyclospora: an enigma worth unraveling. Emerg. Infect. Dis. 1999;5(1):48–53. doi: 10.3201/eid0501.990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M.E., Blaschke A.P., Toze S., Sidhu J.P.S., Ahmed W., van Driezum I.H., et al. Biotin- and glycoprotein-coated microspheres as surrogates for studying filtration removal of Cryptosporidium parvum in a granular limestone aquifer medium. Appl. Environ. Microbiol. 2015;81:4277–4283. doi: 10.1128/AEM.00885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L.J., Herwaldt B.L. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 2000;31(4):1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- Sylvestre E., Dorner S., Burnet J.-B., Smeets P., Medema G., Cantin P., et al. Changes in Escherichia coli to enteric protozoa ratios in rivers: implications for risk-based assessment of drinking water treatment requirements. Water Res. 2021;205:10. doi: 10.1016/j.watres.2021.117707. [DOI] [PubMed] [Google Scholar]
- Taha S., Nguyen-Ho-Bao T., Daugschies A., Rentería-Solís Z. In vitro infection of Madin-Darby bovine kidney (MDBK) cells with Eimeria acervulina sporozoites: quantitative analysis of parasite cellular invasion and replication using real-time polymerase chain reaction (PCR) Parasitol. Res. 2021 Jul;120(7):2689–2693. doi: 10.1007/s00436-021-07211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Adu-Sarkodie K., Kim D., Kim J.-H., Teefy S., Shukairy H.M., et al. Modeling Cryptosporidium parvum oocyst inactivation and bromate formation in a full-scale ozone contactor. Environ. Sci. Technol. 2005;39:9343–9350. doi: 10.1021/es050345n. [DOI] [PubMed] [Google Scholar]
- Tenter A.M. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz. 2009;104(2):364–369. doi: 10.1590/S0074-02762009000200033. [DOI] [PubMed] [Google Scholar]
- Thabet A., Zhang R., Alnassan A.-A., Daugschies A., Bangoura B. Anticoccidial efficacy testing: in vitro Eimeria tenella assays as replacement for animal experiments. Vet.Parasitol. 2017;233:86–96. doi: 10.1016/j.vetpar.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Travaillé E., La Carbona S., Gargala G., Aubert D., Guyot K., Dumètre A., et al. Development of a qRT-PCR method to assess the viability of Giardia intestinalis cysts, Cryptosporidium spp. and Toxoplasma gondii oocysts. Food Control. 2016;59:359–365. doi: 10.1016/j.foodcont.2015.06.007. [DOI] [Google Scholar]
- Tucker M.S., Khan A., Jenkins M.C., Dubey J.P., Rosenthal B.M. Hastening progress in Cyclospora requires studying Eimeria surrogates. Microorg. 2022;10:1977. doi: 10.3390/microorganisms10101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufenkji N., Elimelech M. Spatial distributions of Cryptosporidium oocysts in porous media: evidence for dual mode deposition. Environ. Sci. Technol. 2005;39:3620–3629. doi: 10.1021/es048289y. [DOI] [PubMed] [Google Scholar]
- Tufenkji N., Miller G.F., Ryan J.N., Harvey R.W., Elimelech M. Transport of Cryptosporidium oocysts in porous media: role of straining and physicochemical filtration. Environ. Sci. Technol. 2004;38(November):5932–5938. doi: 10.1021/es049789u. [DOI] [PubMed] [Google Scholar]
- Turnbull P.C.B. In: Medical Microbiology. 4th ed. Baron S., editor. Chapter 15. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Bacillus. PMID: 21413260. [Google Scholar]
- United Nations World Water Assessment Program . The United Nations world water development report; UNESCO, Paris: 2017. Wastewater: The Untapped Resource. [Google Scholar]
- USEPA, United States Environmental Protection Agency . 2010. Long Term 2 Enhanced Surface Water Treatment Rule Toolbox Guidance Manual. [Google Scholar]
- Vanwormer E., Fritz H., Shapiro K., Mazet J.A., Conrad P.A. Molecules to modeling: Toxoplasma gondii oocysts at the human-animal-environment interface. Comp. Immunol. Microbiol. Infect. Dis. 2013 doi: 10.1016/j.cimid.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkers F.C., Bouma A., Graat E.A.M., Klinkenberg D., Stegeman J.A., Jong M.C.M.D. Eimeria acervulina: the influence of inoculation dose on transmission between broiler chickens. Exp. Parasitol. 2010;125:286–296. doi: 10.1016/j.exppara.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Venczel L.V., Arrowood M., Hurd M., Sobsey M.D. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine 63. Appl. Environ. Microbiol. 1997;1598-601 doi: 10.1128/aem.63.11.4625-4625b.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhille S., Hofmann R., Chauret C., Andrews R. Indigenous bacterial spores as indicators of Cryptosporidium inactivation using chlorine dioxide. J. Water Health. 2003;1:91–100. doi: 10.2166/wh.2003.0011. [DOI] [PubMed] [Google Scholar]
- Villena I. Parasites et aliments, surveillance et moyens de maîtrise en France. Rev. Fr. Lab. 2023;2023:53–65. doi: 10.1016/S1773-035X(23)00055-2. [DOI] [Google Scholar]
- Votýpka J., Modrý D., Oborník M., Šlapeta J., Lukeš J. In: Handbook of the Protists. Archibald J.M., Simpson A.G.B., Slamovits C.H., editors. Springer Int. Publishing; Cham: 2017. Apicomplexa; pp. 567–624. [DOI] [Google Scholar]
- Willis J.E., McClure J.T., McClure C., Spears J., Davidson J., Greenwood S.J. Bioaccumulation and elimination of Cryptosporidium parvum oocysts in experimentally exposed eastern oysters (Crassostrea virginica) held in static tank aquaria. Int. J. Food Microbiol. 2014;173:72–80. doi: 10.1016/j.ijfoodmicro.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Wittler R.R. Foodborne and waterborne illness. Pediatr. Rev. 2023;44(2):81–91. doi: 10.1542/pir.2022-005621. [DOI] [PubMed] [Google Scholar]
- Wood M., Simmonds L., MacAdam J., Hassard F., Jarvis P., Chalmers R.M. Role of filtration in managing the risk from Cryptosporidium in commercial swimming pools – a review. J. Water Health. 2019;17:357–370. doi: 10.2166/wh.2019.270. [DOI] [PubMed] [Google Scholar]
- Xu Zhou K., Wisnivesky F., Wilson D.I., Christie G. Effects of culture conditions on the size, morphology and wet density of spores of Bacillus cereus 569 and Bacillus megaterium QM B1551. Lett. Appl. Microbiol. 2017;65:50–56. doi: 10.1111/lam.12745. [DOI] [PubMed] [Google Scholar]
- Zahedi A., Gofton A.W., Greay T., Monis P., Oskam C., Ball A., et al. Profiling the diversity of Cryptosporidium species and genotypes in wastewater treatment plants in Australia using next generation sequencing. Sci. Total Environ. 2018;644:635–648. doi: 10.1016/j.scitotenv.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Zhang H., Seaman J., Wang Y., Zeng H., Narain R., Ulrich A., et al. Filtration of glycoprotein-modified carboxylated polystyrene microspheres as Cryptosporidium oocysts surrogates: effects of flow rate, alum, and humic acid. J. Environ. Eng. 2017;143 doi: 10.1061/(ASCE)EE.1943-7870.0001201. 04017032. [DOI] [Google Scholar]
- Ziemer C.J., Bonner J.M., Cole D., Vinjé J., Constantini V., Goyal S., et al. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. J. Anim. Sci. 2010;88:E84–E94. doi: 10.2527/jas.2009-2331. [DOI] [PubMed] [Google Scholar]