Abstract
Hereditary angioedema due to C1 inhibitor deficiency (C1-INH-HAE) is a rare and life-threatening condition characterized by recurrent localized edema. We conducted a systematic screening of SERPING1 defects in a cohort of 207 Czech patients from 85 families with C1-INH-HAE. Our workflow involved a combined strategy of sequencing extended to UTR and deep intronic regions, advanced in silico prediction tools, and mRNA-based functional assays. This approach allowed us to detect a causal variant in all families except one and to identify a total of 56 different variants, including 5 novel variants that are likely to be causal. We further investigated the functional impact of two splicing variants, namely c.550 + 3A > C and c.686-7C > G using minigene assays and RT-PCR mRNA analysis. Notably, our cohort showed a considerably higher proportion of detected splicing variants compared to other central European populations and the LOVD database. Moreover, our findings revealed a significant association between HAE type 1 missense variants and a delayed HAE onset when compared to null variants. We also observed a significant correlation between the presence of the SERPING1 variant c.-21 T > C in the trans position to causal variants and the frequency of attacks per year, disease onset, as well as Clinical severity score. Overall, our study provides new insights into the genetic landscape of C1-INH-HAE in the Czech population, including the identification of novel variants and a better understanding of genotype–phenotype correlations. Our findings also highlight the importance of comprehensive screening strategies and functional analyses in improving the C1-INH-HAE diagnosis and management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-023-01565-w.
Keywords: HAE, C1-INH-HAE, hereditary angioedema, SERPING1, splicing, genotype–phenotype relationship, time to diagnosis
Introduction
Hereditary angioedema (HAE) is a disorder characterized by recurrent bouts of localized subcutaneous or submucosal edema, typically affecting various organs including limbs, intestinal mucosa, genitals, face or airways. These attacks often cause functional damage, severe pain in the abdominal area, breathing obstructions, and overall quality of life is reduced. The most severe manifestation is life-threatening edema of the larynx.
HAE can be classified into three types based on the immunological findings. HAE-1 is characterized by a reduction in both antigenic and functional C1 inhibitor (C1-INH) levels. Patients with HAE-2 have a normal C1-INH protein concentration but impaired C1-INH function. HAE with normal C1 inhibitor (nC1-INH-HAE) primarily arises from defects in the F12 and PLG. Notably, variants in the F12 gene have been found to predominantly cause HAE in females. Other genes that have been linked to nC1-INH-HAE in few patients include ANGPT, MYOX, KNG1, and HS3ST6 [1–4].
Both HAE-1 and 2 are inherited in an autosomal dominant mode and are caused by pathogenic variants in SERPING1—gene encoding C1-INH and located in the 11q12-q13.1 chromosome. It is composed of eight exons and seven introns. SERPING1 is a naturally alternatively spliced gene, but the role of alternative transcripts still remains unclear [5]. Whereas pathogenic variants disrupt the C1-INH structure and abolish protein production in HAE-1, variants changing the active center of C1-INH cause normal production levels of dysfunctional protein in HAE-2. C1-INH-HAE prevalence is 1/50,000–1/100,000, without known ethnic differences [6].
C1-INH belongs to the serpin family (serine protease inhibitors), and contributes especially to vascular permeability and inflammation regulation. Edema in HAE-1/2 is the result of an incorrectly regulated contact system in the absence of functional C1-INH and consequent production of bradykinin from kininogen. Bradykinin, as a powerful vasodilator, increases capillary permeability and constricts smooth muscles.
C1-INH levels should theoretically be 50% in dominantly inherited HAE; however, C1-INH serum levels are typically less than 35% of normal [7, 8]. Although the underlying mechanism is not fully understood in most pathogenic variants, the generally assumed cause is haploinsufficiency with an additional negative effect from a defective allele product on the normal allele expression [9].
Interestingly, the HAE severity can range from asymptomatic to very severe, irrespective of the disease-causing variant type, as even the members of the same family carrying the same SERPING1 alleles have very distinct disease manifestation [10, 11]. It is thus probable that the HAE phenotype is also influenced by some factors other than the causative variant in SERPING1. In very rare cases, disease severity was more or less convincingly associated with particular variants or other factors, while no association was demonstrated at all in other cases [11–15].
In this study, we describe the clinical phenotype and genotype of Czech patients with HAE, and provide an overview of SERPING1 variants identified in Czech HAE patients involving those published previously as well as some novel variants [16–19]. We evaluate their significance and discuss the impact of some previously published variants.
Material and Methods
Patients
Two hundred seven patients with HAE from 85 unrelated Czech families were recruited retrospectively for this study through extensive collaboration with clinical immunologists from all over the Czech Republic who treated the patients and collected their data. C1-INH-HAE diagnosis was established based on clinical signs and the following complement measurements: serum C1-inhibitor concentration, C1-inhibitor activity, and C4 level.
Complement Testing
Over the last 33 years, methods to detect C4 and C1-INH levels have changed in our country. In the 1980s and 1990s, these levels were detected by radial immunodiffusion and later by immunoprecipitation combined with nephelometry or turbidimetry. Since 1996, C1-INH function has been analyzed by the Enzyme-Linked ImmunoSorbent Assay (Quidel MicroVue C1 InhibitorPlus). The normal C1-INH concentration range was 210–390 mg/L, and the normal values for its functional activity were greater than 68% of the reference value for the standard serum.
Genotyping and Sequencing
DNA was extracted from EDTA-containing whole blood samples using a standard desalting procedure. The variants in SERPING1 coding regions (exons 2–8) and their adjacent sequences were analyzed using standard Sanger sequencing protocols (primers and conditions available on request). Subsequently, multiplex ligation-dependent probe amplification (MLPA) was performed using the SALSA MLPA P243-A2 SERPING1 kit (MRC-Holland, The Netherlands) to search for large deletions and duplications.
When no variant was detected by either coding region sequencing or MLPA, non-coding regions (3′UTR, 5′UTR, proximal part of intron 6) were amplified and Sanger sequencing of these regions was performed.
All obtained sequences were compared to GenBank reference sequences NM_000062.3 and NP_000053.2. Detected variants’ nomenclature follows Human Genome Variation Society recommendations [20].
RNA Analysis
Total RNA was extracted from the peripheral blood, PBMCs and HeLa cells. The extracted RNA was reverse transcribed to cDNA with random hexamers. The subsequent PCR was performed in two steps using primers with sequences situated inside exons. Specific reaction conditions and primer sequences were described previously [17, 19]. Amplicons from the second reaction were checked on 2% agarose gels and then characterized by capillary analysis.
Minigene Assay
Minigene constructs were used to investigate the sequence variant’s effect on RNA splicing. Wild-type and mutant genomic fragments of SERPING1 comprising appropriate exons and at least 150 bp flanking introns were amplified with primers. PCR products were cloned into multiple cloning sites inside the pET01 vector (MoBiTec). Subsequently, HeLa and/or HepG2 cells (European Collection of Authenticated Cell Cultures) were transfected with the minigene construct. RNA was extracted 24 h after transfection and then RT-PCR was performed. The specific procedure conditions and primer sequences were described previously [17, 19].
Restriction Analysis
The presence of c.-21 T > C variant in a patient was established by Sanger sequencing of exon 2 or by AvaII restriction analysis of exon 2 amplification products [18]. The variant’s trans or cis position was determined by analyzing its occurrence among the patient's blood relatives.
Targeted NGS
Patients’ genomic DNA samples were analyzed firstly on a NextSeq Illumina platform (Illumina, San Diego, CA) using the SureSelect QXT (Agilent Technologies, Santa Clara, CA). The targeted NGS panel comprised exon sequences from genes related to primary immunodeficiencies, including all genes associated with HAE. Intronic sequences of SERPING1 were also covered by the analysis, except for highly repetitive deep-intronic parts. Library preparation and sequencing were performed according to the manufacturer’s instructions.
Raw data read quality control was performed using the FastQC program [21]. Alignment to the reference hg19 genome was carried out using BWA-MEM [22]. SAMtools was used to sort and index the alignments [23]. The Picard MarkDuplicates tool [24] was employed to mark and remove duplicates. The Vardict program was used to determine genetic variants [25]. Identified variants were annotated with the Annovar tool [26]. Integrative Genomics Viewer (IGV) was employed to visualize read alignment and detected variants [27].
Databases and Bioinformatics
Interpreting sequence variant impact was based on the criteria established by the American College of Medical Genetics and Genomics (ACMG). Several population and variant databases and bioinformatic tools have been used to annotate variants and estimate variant impact (Supplementary Methods).
Results
There are 4 major centers specialized in HAE patient treatment in the Czech Republic, and the vast majority of genetic testing has been provided by the Molecular Genetic laboratory CKTCH Brno. Several individual cases were reported to the laboratory by individual specialists as well. Data of the patients were collected over a long time period using available technologies at the time. In 2012, a specialized patient database was introduced providing not only the attending physicians but also the patients with the opportunity to report HAE attacks and disease development.
Clinical Evaluation of Laboratory Results
Altogether, 207 patients from 85 families were recorded in the Czech Republic. One hundred seventy-five patients from 74 families (87.1%) were diagnosed with HAE-1, and 32 patients from 11 families (12.9%) with HAE-2. The specific data of all patients can be found in Supplementary (Table S1). The data of our cohort are summarized in Table 1.
Table 1.
Czech HAE patient cohort. (A) Numbers of specific groups of HAE patients. (B) C1-INH and C4 concentration and C1-INH function were crucial to establish diagnosis. The lowest values were considered in case of repeated measurements. C1-INH function and concentration in HAE-1 patients were reduced even in asymptomatic patients, i.e. patients diagnosed before symptom onset, typically blood-related to a proband. There were 9 patients (5%) in the whole cohort that showed normal C1-INH function levels even after repeated testing. Patients with HAE-2 had normal but more often increased C1-INH levels. C4 levels in HAE-1 and HAE-2 symptomatic patients did not differ, with a median 0.05 g/l and 0.06 g/l, respectively. The normal range slightly changed over time, but generally, only 12 out of all 188 (6.4%) patients showed consistently normal C4 levels. Patients with inconclusive complement test results were typically part of families where the variant segregated with the disease and/or was classified unequivocally pathogenic. When the C4 level and C1-INH level and function were considered together, all but one patient (P05505) with available results exhibited at least one abnormal value
| (A) | Number | ||
| Patients | 207 | ||
| Probands | 85 | ||
| HAE-1 patients | 175 | ||
| HAE-1 probands | 74 | ||
| HAE-2 patients | 32 | ||
| HAE-2 probands | 11 | ||
| Females | 109 | ||
| Males | 98 | ||
| (B) | Median | Range | Typical normal values |
| Symptomatic HAE-1 patients | |||
| C1-INH concentration (g/l; n = 146) | 0.06 | 0.018–0.21 | 0.210–0.390 |
| C1-INH function (%; n = 136) | 38 | 0–78 | > 68 |
| C4 concentration (g/l; n = 146) | 0.05 | 0.018–0.23 | 0.100–0.380 |
| Asymptomatic HAE-1 patients | |||
| C1-INH concentration (g/l; n = 14) | 0.089 | 0.03–0.168 | 0.210–0.390 |
| C1-INH function (%; n = 13) | 56 | 20–82 | > 68 |
| C4 concentration (g/l; n = 14) | 0.075 | 0.02–0.11 | 0.100–0.380 |
| Symptomatic HAE-2 patients | |||
| C1-INH concentration (g/l; n = 27) | 0.383 | 0.212–0.765 | 0.210–0.390 |
| C1-INH function (%; n = 27) | 45 | 15–79 | > 68 |
| C4 concentration (g/l; n = 25) | 0.06 | 0.019–0.21 | 0.100–0.380 |
| Asymptomatic HAE-2 patients | |||
| C1-INH concentration (g/l; n = 3) | 0.383 | 0.35–0.414 | 0.210–0.390 |
| C1-INH function (%; n = 3) | 57 | 33–75 | > 68 |
| C4 concentration (g/l; n = 3) | 0.06 | 0.05–0.066 | 0.100–0.380 |
Course of the Disease
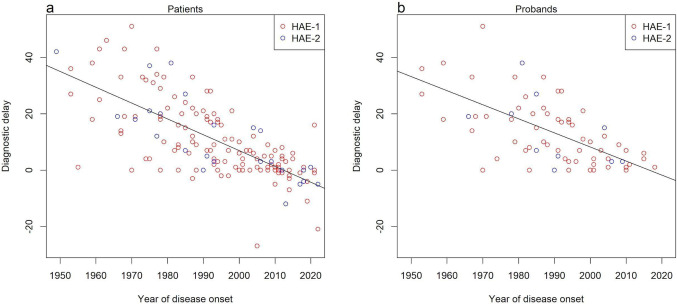
The mean age at onset of clinical symptoms was 14 years (range 1–72 years; n = 167). Seventy-four patients (44.3%) suffered from their first attack before the onset of puberty (before 13 years of age), and the disease started during puberty (13–16 years of age) in 28 patients (16.8%). A causal variant was detected in 37 patients before the onset of HAE symptoms due to testing HAE patients’ relatives with a known SERPING1 variant. In 1 patient (P05505 in Table S1), the age of HAE onset was 68 years. Throughout the years, diagnosing HAE has become more achievable with improving immunologic and genetic tests, and the diagnostic delay between the first symptoms and establishing the diagnosis decreased, as shown in Fig. 1.
Fig. 1.
Diagnostic delay, i.e., difference in time between establishing the diagnosis and disease onset. a Graph for all Czech patients with available data. b Graph for probands of the cohort with available data
Information on HAE patient treatment was collected from the Czech national registry of primary immunodeficiencies, where almost all diagnosed HAE patients in the Czech Republic are registered. The data collected between March 2012 and October 2021 were analyzed. A total of 6317 HAE attacks were recorded in 150 patients. Attack location and their treatment are specified in Tables S2 and S3, respectively. Long-term prophylaxis was used in 95 patients.
We calculated the clinical severity score for each patient in our cohort using available information on age of onset, attack location, and long-term prophylaxis usage, following the method introduced by Bygum et al. [28]. It should be noted that, except for age of onset, the score calculation was based solely on the Czech national registry of primary immunodeficiencies data, capturing information from 2012 till 2021. However, the score considers swelling occurrences at any point in a patient’s lifetime, which would potentially result in higher scores in some patients when calculated based on their complete “lifetime” records.
Genetic Analysis
Several methods were used to find causative sequence defects in SERPING1 in HAE patients. When the HAE genetic diagnostics were introduced, denaturing gradient gel electrophoresis was used to search for a defect in SERPING1 coding parts, followed by Sanger sequencing of regions showing a pattern that differed from the reference control. Later, direct Sanger sequencing of all coding parts and adjacent intronic sequences of the gene was performed. If the causal variant was not found in a patient, MLPA was used to search for large defects in the gene. Nonetheless, causative sequence variants in the gene were still not found in some cases. Then, we tried to gain RNA from blood samples of the patients and their relatives—both healthy and suffering from HAE. To uncover potential splicing defects, we used their cDNA to amplify several SERPING1 mRNA segments overlapping particular exon boundaries, and search for exon inclusion abnormalities using fragment analysis [17].
Generally, we tried to establish and confirm the intronic and splicing variants’ impact independently. Typically, we applied minigene assay to investigate the effect of the detected sequence variant on RNA splicing, as described in the “Material and Methods” section [19].
The workflow of methods currently used to detect and evaluate causal variants by our laboratory is illustrated in Fig. S1. Using this set of methods, we detected a sequence variant that we considered as causative or probably causative in 206 out of 207 in our cohort of Czech patients, i.e. in 84 families out of 85. We found 56 unique pathogenic or likely pathogenic sequence variants.
These variants included 18 different missense, 4 nonsense, 13 frameshift, 16 splicing variants, and 5 copy number variations (CNVs) (Tables 2, 3, 4, 5, and 6).
Table 2.
Missense variants found in the Czech cohort. Variants were evaluated by in silico tools (CADD, Polyphen, and SIFT). The Proof of Pathogenicity column provides information on which the variant evaluation is based. 'MP' indicates multiple published patients (including this study), while 'FP' signifies functional proof of variant impact with referenced articles containing such evidence. The Proof of pathogenicity column provides information on which the variant evaluation is based with “MP” indicating multiple published patients (including this study), and “FP” functional proof of variant impact. The resources are indicated in the References column, and articles containing functional proof are [33, 34, 57]$$$$$$$$$. The numbers of patients and probands of our cohort are given in corresponding columns. # indicates previously published probands/patients, and x in x# indicates the number of them. Two substitutions in the position c.550 were included in the splicing variants subset (Table 5) because their pathomechanism is primarily disruption of mRNA splicing
| Variant cDNA | Variant protein | CADD | Polyphen category | SIFT category | Proof of pathogenicity | ACMG evaluation | Number of probands | Number of patients | References |
|---|---|---|---|---|---|---|---|---|---|
| c.498C > A | p.Asn166Lys | 24 | Probably damaging | Damaging | MP | Pathogenic | 1 | 1 | [12, 29–32] |
| c.503C > A | p.Ala168Asp | 22.9 | Probably damaging | Damaging | MP, FP [33, 34] | Pathogenic | 1 | 3 | [30, 31, 33, 34] |
| c.506 T > C | p.Phe169Ser | 29 | Probably damaging | Damaging | MP | Pathogenic | 1 | 2 | [35, 36] |
| c.548 T > C | p.Leu183Pro | 32 | Probably damaging | Damaging | MP | Pathogenic | 1# | 1# | [17, 37, 38] |
| c.614G > A | p.Cys205Tyr | 24.3 | Benign | Damaging | MP | Pathogenic | 1 | 3 | [37, 39, 40] |
| c.629 T > C | p.Leu210Pro | 24.8 | Probably damaging | Damaging | MP | Pathogenic | 1# | 21# | [17, 38, 41] |
| c.706 T > G | p.Phe236Val | 24.8 | Probably damaging | Damaging | Likely pathogenic | 1# | 21# | [17] | |
| c.722G > C | p.Arg241Pro | 14.82 | Probably damaging | Tolerated | MP, FP [33] | Likely pathogenic | 1 | 2 | [33, 36] |
| c.743C > G | p.Pro248Arg | 23.2 | Probably damaging | Damaging | MP | Likely pathogenic | 1 | 3 | [36, 42] |
| c.793 T > G | p.Trp265Gly | 25.4 | Probably damaging | Damaging | Likely pathogenic | 1# | 31# | [17] | |
| c.1046 T > C | p.Leu349Pro | 26.8 | Probably damaging | Damaging | MP | Pathogenic | 1# | 21# | [17, 43] |
| c.1195C > T | p.Pro399Ser | 23.2 | Probably damaging | Damaging | MP | Pathogenic | 1 | 2 | [36, 38, 44, 45] |
| c.1202 T > A | p.Ile401Asn | 25.5 | Probably damaging | Damaging | MP | Likely pathogenic | 1# | 21# | [17, 46] |
| c.1322 T > A | p.Met441Lys | 25.1 | Probably damaging | Damaging | Likely pathogenic | 1# | 31# | [17] | |
| c.1346 T > C | p.Leu449Pro | 28.9 | Probably damaging | Damaging | MP | Pathogenic | 1 | 1 | [30, 47] |
| c.1361 T > G | p.Val454Gly | 28.2 | Probably damaging | Damaging | MP | Pathogenic | 41# | 81# | [17] |
| c.1396C > T | p.Arg466Cys | 25.3 | Probably damaging | Damaging | MP, FP [57] | Pathogenic | 52# | 155# | [30, 32, 34, 36, 38, 45, 48–60] |
| c.1397G > A | p.Arg466His | 23.4 | Benign | Damaging | MP, FP [57] | Pathogenic | 62# | 174# | [28, 30, 32, 39, 46, 48, 50, 52, 54, 57, 61–64] |
Table 3.
Nonsense variants found in the Czech cohort. The Proof of pathogenicity column provides information on which the variant evaluation is based with “null” indicating that the variant probably results in no gene product and “MP” indicates multiple published patients (including this study). The resources are indicated in the References column. Numbers of patients and probands of our cohort are given in corresponding columns. # indicates previously published probands/patients of our cohort and x in x# indicates the number of them
| Variant cDNA | Variant protein | Proof of pathogenicity | ACMG evaluation | Number of probands | Number of patients | References |
|---|---|---|---|---|---|---|
| c.209C > G | p.(Ser70*) | Null | Pathogenic | 1# | 1# | [18] |
| c.897G > A | p.(Trp299*) | Null, MP | Pathogenic | 1# | 31# | [17, 30, 32] |
| c.1036C > T | p.(Gln346*) | Null, MP | Pathogenic | 1 | 3 | [30, 35, 36] |
| c.1420C > T | p.(Gln474*) | Null, MP | Pathogenic | 1 | 3 | [12, 30, 56] |
Table 4.
Frameshift variants found in the Czech cohort. The column Proof of pathogenicity provides information on which the variant evaluation is based—“null” indicating that the variant probably results in no gene product, 'MP' indicates multiple published patients (including this study), while 'FP' signifies functional proof of variant impact with referenced articles containing such evidence. The resources are indicated in the References column, and articles containing functional proof are [17]. Numbers of patients and probands of our cohort are given in corresponding columns. # indicates previously published probands/patients of our cohort, and x in x# indicates the number of them. The variant c.726_777del included in this table comprises more than 20 bases but because it does not affect the whole exon, it was not included in the CNV subset (Table 6). On the other hand, the deletion c.1225_1249 + 19del was placed in the splicing variant table (Table 5) as it primarily disrupts mRNA splicing
| Variant cDNA | Variant protein | Proof of pathogenicity | ACMG evaluation | Number of probands | Number of patients | References |
|---|---|---|---|---|---|---|
| c.120_121del | p.(Gly41Argfs*16) | Null, MP, FP [17] | Pathogenic | 1# | 3# | [17, 18, 33, 36, 42, 47, 56] |
| c.151_152del | p.(Ser51Glnfs*6) | Null | Pathogenic | 1 | 2 | novel |
| c.160del | p.(Leu54Tyrfs*25) | Null | Pathogenic | 1# | 31# | [18] |
| c.305_317del | p.(Pro102Leufs*42) | Null, FP [17] | Pathogenic | 21# | 141# | [17] |
| c.600dup | p.(Lys201Glnfs*56) | Null, MP | Pathogenic | 1# | 21# | [12, 30, 36, 47, 64] |
| c.650del | p.(Gly217fs*15) | Null, MP | Pathogenic | 1# | 1# | [17, 53] |
| c.726_777del | p.(Leu243Serfs*19) | Null | Pathogenic | 1 | 7 | novel |
| c.795_796delGGinsT | p.(Trp265Cysfs*14) | Null | Pathogenic | 1 | 2 | novel |
| c.855_856del | p.(Arg286Profs*18) | Null | Pathogenic | 1# | 2# | [18] |
| c.1115del | p.(Gln372Argfs*25) | Null | Pathogenic | 1# | 21# | [17] |
| c.1283del | p.(Cys428Leufs*3) | Null, MP | Pathogenic | 1# | 1# | [17, 33] |
| c.1284_1285del | p.(Cys428Trpfs*44) | Null, MP | Pathogenic | 31# | 41# | [18] |
| c.1460_1466del | p.(Lys487Metfs*87) | Null | Pathogenic | 1 | 5 | novel |
Table 5.
Splicing variants found in the Czech cohort. The Proof of Pathogenicity column provides information on which the variant evaluation is based. 'MP' indicates multiple published patients (including this study), while 'FP' signifies functional proof of variant impact with referenced articles containing such evidence. The resources are indicated in the References column, articles containing functional proof are [17, 19, 35, 66, 73]. Numbers of patients and probands of our cohort are given in corresponding columns. # indicates previously published probands/patients of our cohort, and x in x# indicates the number of them. Two substitutions in the position c.550 and the deletion c.1225_1249 + 19del were included in this table because their pathomechanism is primarily disruption of mRNA splicing
| Variant cDNA | Intron | Proof of pathogenicity | ACMG evaluation | Number of probands | Number of patients | References |
|---|---|---|---|---|---|---|
| c.-22-19_-22-4del | 1 | MP | Likely pathogenic | 1 | 2 | [36, 65] |
| c.51 + 5G > A | 2 | MP, FP [35, 66] | Pathogenic | 1 | 1 | [30, 31, 35, 66] |
| c.550G > A | exon 3 | MP, FP [17] | Pathogenic | 1# | 1# | [17, 28, 31, 32, 35, 36, 38–40, 42, 45, 50, 51, 56, 58, 62, 67–70] |
| c.550G > T | exon 3 | MP, FP [17] | Pathogenic | 1# | 1# | [17, 36, 56] |
| c.550 + 3A > C | 3 | FP [this study] | Likely pathogenic | 1 | 1 | novel |
| c.551-2A > G | 3 | MP, FP [17] | Pathogenic | 21# | 111# | [17, 36, 49, 62] |
| c.685 + 1del | 4 | FP [17] | Pathogenic | 1# | 41# | [17] |
| c.685 + 2_685 + 13del | 4 | FP [17] | Pathogenic | 1# | 41# | [17] |
| c.686-12A > G | 4 | MP, FP [17] | Pathogenic | 1# | 1# | [17, 44, 47, 55] |
| c.686-7C > G | 4 | MP, FP [this study] | Pathogenic | 1 | 2 | [36] |
| c.686-1G > T | 4 | MP | Pathogenic | 1 | 1 | [38] |
| c.1029 + 384A > G | 6 | MP, FP [19] | Pathogenic | 31# | 1512# | [19, 34, 71, 72] |
| c.1225_1249 + 19del | 7 | FP [17] | Pathogenic | 1# | 21# | [17] |
| c.1249 + 1G > A | 7 | MP | Pathogenic | 1 | 1 | [37, 43, 49, 61] |
| c.1249 + 2 T > C | 7 | MP | Pathogenic | 1 | 1 | [56] |
| c.1249 + 5G > A | 7 | MP, FP [17, 73] | Pathogenic | 1# | 1# | [17, 36, 65, 73] |
Table 6.
Large deletions and duplication found in the Czech cohort. Numbers of patients and probands of our cohort are given in corresponding columns. The variant c.726_777del was included in the frameshift set (Table 4) even though it comprises more than 20 bases because the deletion does not affect a whole exon
| Variant cDNA | Number of probands | Number of patients |
|---|---|---|
| EX1-6del | 1 | 3 |
| EX1-8del | 2 | 2 |
| EX4del | 9 | 15 |
| EX7del | 2 | 6 |
| EX5-6dup | 1 | 1 |
Missense Variants
Eighteen different missense variants were detected in 30 probands which accounted for 35.3% of all probands (Table 2). The most prevalent variants, p.Arg466His (17 patients in 6 families) and p.Arg466Cys (15 patients in 5 families) in exon 8, were connected to HAE-2 phenotype. Interestingly, the most common missense variant causing HAE-1, p.Val454Gly (8 patients in 4 families), was also located in exon 8. The potential effect of this missense variation was estimated by three different prediction programs, all of them predicting the change to have damaging effects on the protein (Table 2). It has been previously described only once, also in a patient of Czech origin [17]. Now we report the variant in another three probands. In one of the families, the variant was detected in the affected father (P05601) and also in his daughter (P05602) when she was 10 years old. She had not had any HAE attacks but showed low C1-INH concentration and function. It was also found in another family depicted in Fig. 2b.
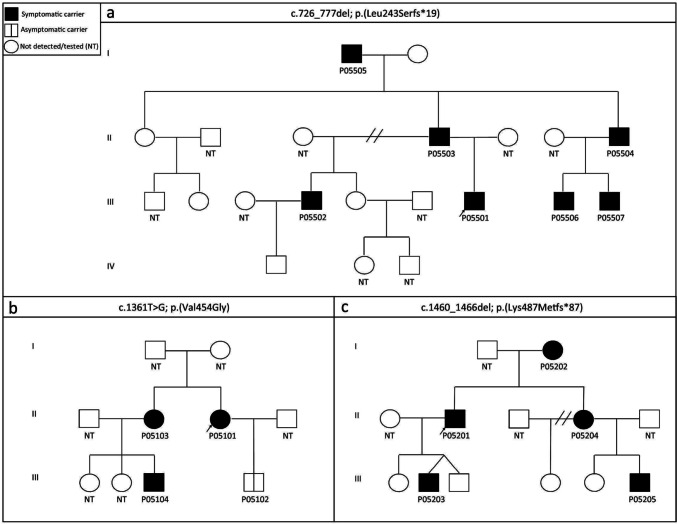
Fig. 2.
Family trees of HAE patients. HAE-affected family members carrying the causal variant are shown in black, Asymptomatic/presymptomatic carriers of causal variants are depicted by a partitioned symbol, and healthy individuals are depicted by a blank symbol. Individuals who were not tested are signified by NT. a The variant c.726_777del was identified in 7 members of one family with HAE-1. The variant was first revealed in a 10-year-old boy (P05501) and his father, who both showed relatively severe HAE symptoms, and their condition started at a young age, at 9 and 7 years, respectively. Further genetic analysis revealed the same variant in another 4 members of the family. Interestingly, the oldest member of the family—an 81-year-old grandfather (P05505) of this 10-year-old boy—suffered from only 2 attacks in his life, both appearing before establishing his diagnosis perioperatively at 68 and 73 years of age. His C1-INH and C4 levels, and C1-INH function were normal. All other members with the detected variant had low C4 and C1-INH levels as well as C1-INH function, and all of them also showed HAE symptoms. 4 asymptomatic members with normal C4 and C1-INH levels were tested, and the variant was not detected in their DNA samples. b The variant p.Val454Gly was among other patients also found in 4 members of the depicted family. Three family members (P05101, P05103 and P05104) suffered from HAE attacks and also showed laboratory HAE symptoms, whereas 29-year-old P05102 showed only C1-INH concentration and function deficit with no clinical symptoms of HAE. c Seven base pair deletion c.1460_1466del was detected in a family with 5 patients. The HAE phenotype in this family segregates with the presence of the variant and the disease course is quite severe in all affected members—in P05205, the attacks appeared as early as 2 years of age. The variant was not found in 4 other asymptomatic family members tested
All other detected missense variants were specific to particular families, although they had been described previously in HAE patients (see references in Table 2). The potential impacts of all these variants were estimated by in silico tools and the variants were evaluated based on ACMG rules. Specific concern was paid to functional studies, which, regrettably, have been published for only four variants to date, and to the number of previously described HAE patients carrying the respective variant (Table 2).
Needless to add, two other substitutions at the c.550 position were detected, but as these variants’ pathomechanism is de facto mRNA splicing disruption [17, 42], they were included in the splicing variant subset.
Nonsense Variants
Four different nonsense variants were identified in 10 patients from 4 families and they comprised 4.7% of all probands. All these variants had been described before as causative for HAE-1 (Table 3).
Frameshift Variants
Detected frameshift variants comprised 11 deletions, 1 duplication, and 1 indel variant. Altogether, they were detected in 48 patients from 16 families and accounted for 18.8% of probands (Table 4).
A novel 2-base deletion, c.151_152del, was identified in exon 3 in a mother with HAE symptoms (P03501) and her infant daughter (P03502). The variant potentially leads to the frameshift and premature stop codon introduction (p.(Ser51Glnfs*6)) in the mRNA. Both the mother and her daughter carrying this variant did not have any other rare SERPING1 variation. They displayed HAE symptoms, and their complement measurements showed a deficient C1-INH level and function as well as below-normal C4 level.
Another novel deletion, c.726_777del; p.(Leu243Serfs*19), was identified in 7 members of one family with HAE-1 (P05501- P05507; Fig. 2a).
In a family with 5 patients (P05201-P05205), we additionally detected 7 base deletion c.1460_1466del; p.(Lys487Metfs*87) in exon 8 (Fig. 2c), which had not been described before.
Furthermore, we found a novel indel variant leading to frameshift c.795_796delGGinsT; p.(Trp265Cysfs*14) in a patient (P05301) and her daughter (P05302) both showing clinical and laboratory signs of HAE.
Some of the detected deletions comprise more than 20 bases [74] and therefore should fall rather into the gross deletion category. However, as they do not affect the whole exon(s) and their pathological consequences are frameshift and introduction of a premature stop codon, we included them in the frameshift category.
To categorize the deletion c.1225_1249 + 19del, the situation is even more complicated because the variant causes primarily splicing defects [17] and was therefore included in the splicing variant subset.
Large Deletions and Duplications
A major part of gross variants has been detected by MLPA; however, two deletions mentioned in the previous paragraph, which technically should be gross deletions, were detected by Sanger sequencing. When these are not taken into account, the other gross deletions of one or several exons were detected in 26 patients from 14 families, which comprise 16.5% of the cohort. A duplication of exons 5–6 was detected in 1 patient (1.2% of the probands).
Splicing Variants
As mentioned before, 2 missense and 1 frameshift variant found in our cohort disrupt mRNA splicing. In addition, we found 13 other splicing variants. Thus, in total, we detected splicing variants in 49 patients from 19 families which account for 22.4% of probands.
Out of the 16 detected variants, 7 disrupted canonical splice site positions, which is a well-established pathogenic mechanism, and additionally, functional studies have been described for 4 of these variants [17]. In our cohort, we identified 9 variants located in non-canonical splice site positions, with 8 of them being previously published. Functional studies have been conducted for 6 of these variants (Table 5). In this study, we performed functional studies on a previously published variant as well as a novel variant that we detected.
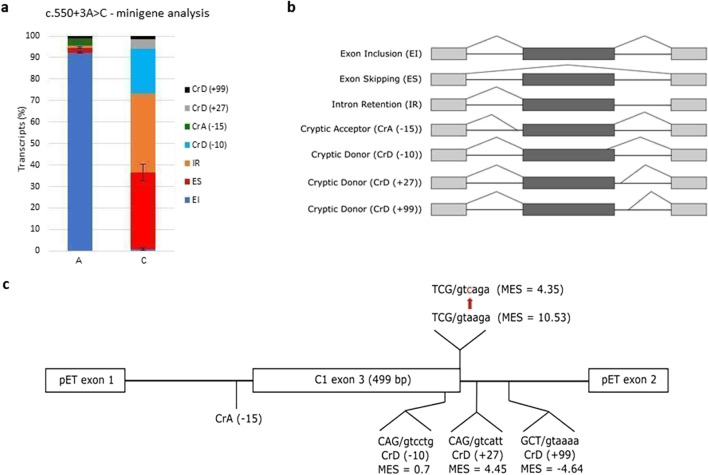
The impact of the substitutions in exon 3’s last nucleotide (position c.550) on splicing was previously functionally evaluated using RNA analysis and was established as pathogenic [75]. In the same splice site, we detected a novel variant, c.550 + 3A > C, in a patient P02201 and as the variant had not been previously reported, we tried to functionally evaluate it. Unfortunately, none of the patient’s family members were available for testing. The variant potentially affecting the donor splice site (5'ss) of exon 3 was analyzed in silico by MaxEnt Score [76], and the ratio between mutated and wild-type 5'ss sequence was 0.41—a number suggesting a substantial effect on splicing. According to Le Guédard-Méreuze et al. [77], substitution + 3A > C is prone to cause a splicing defect even if the donor splice site does not contain any further nucleotide changes. We performed minigene analysis (detailed procedure described in Supplement Methods) to confirm the deleterious effect on splicing and it showed aberrant splicing in nearly 100% of mutated minigene construct transcripts (Fig. 3).
Fig. 3.
Minigene splicing analysis of a novel variant c.550 + 3A > C. The wild-type and mutant genomic fragments of SERPING1 comprising exon 3 and flanking upstream (229 bp) and downstream (255 bp) intron sequences, were cloned into pET01 vector and HepG2 cells were transfected with these minigenes. Capillary electrophoresis of RT-PCR products showed aberrant splicing in nearly 100% of mutated minigene construct transcripts. Several different aberrant transcripts were detected. The most abundant was intron 3 retention followed by exon 3 skipping. Transcripts using cryptic donor splice sites − 10 and + 27 were found in the mutant minigene analysis, whereas they were not detected in the wild type at all. a Minigene analysis results: column A shows transcript proportions resulting from control minigene construct representing c.550 + 3A; column C shows transcript proportions resulting from minigene construct representing c.550 + 3C. b Scheme of the transcripts detected in the minigene analysis. c Scheme of the pET minigene construct. Cryptic splice sites found in the analysis and their exact sequences are displayed. MaxEnt Score values (MES) are specified beneath each splice site
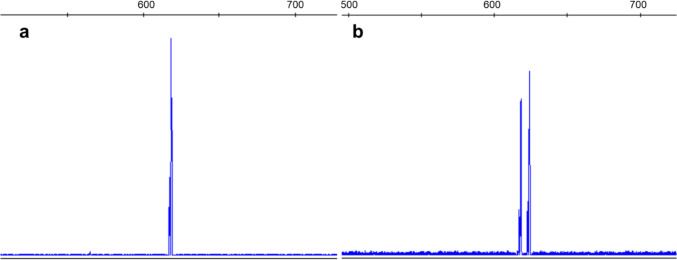
Variant c.686-7C > G was detected in a mother and her daughter (P03001-P03002), both affected with HAE symptoms. This variant had been described before [7], but its impact had not been yet fully evaluated. Therefore, we extracted RNA from both patients’ blood and analyzed samples by RT-PCR and fragment analysis (Supplementary Methods), which showed complete impairment caused by the variant (Fig. 4).
Fig. 4.
mRNA analysis of patients carrying variant c.686-7C > G. Capillary electrophoresis was performed on PCR products amplified by primers annealing to exons 4 and 7. a Healthy control shows only one wild type peak. b Both patient sample results show 2 peaks—reference transcript peak and a peak corresponding to a 6 bp longer transcript using a de novo created acceptor splice site. Aberrant and normal transcript proportion was roughly equal, which corresponds to complete impairment caused by the variant. The variant preserves a reading frame and leads to incorporation of additional two amino acids (proline-alanine) into the polypeptide chain
Variant Type and Course of Disease
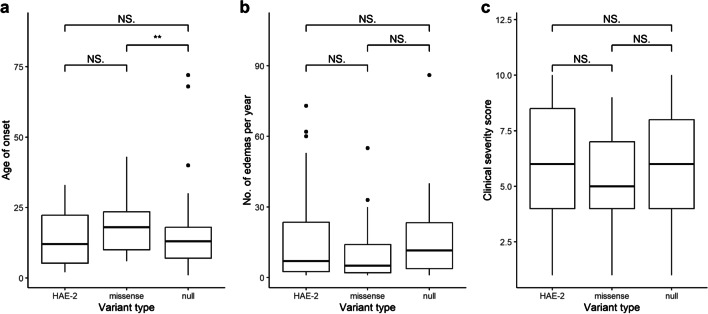
We classified patients based on their genetic variants. Those with variants that prevented the production of a functional transcript were grouped as null variants, while those with a missense defect in Arg466 were classified as HAE-2. Patients with other missense variants were categorized as missense. We then studied the patient groups to determine potential connections between the type of causal variant with the age of onset, the number of disease attacks per year, and the clinical severity score. Although there was no apparent association between the type of causal variant and the frequency of HAE attacks among the groups or clinical severity score, patients with missense variants showed a significantly higher age of HAE onset compared to those with null variants (Kruskal–Wallis; p = 0.023; Fig. 5).
Fig. 5.
Impact of causal variant types on HAE phenotype. HAE Patients were classified into different groups based on their specific causal variant types: HAE-2 variants causing damage to the active center of SERPING1, other missense variants, and null variants preventing C1 inhibitor formation. The primary objective was to investigate the impact of these variant types on various phenotypic characteristics. a The impact of causal variant type on age of HAE onset was analyzed in 26, 32, and 109 patients from HAE-2, missense and null groups, respectively. Analysis showed no significant association in relation to the HAE-2 group. However, null variants in patients were significantly associated with lower age of HAE onset compared to those with the missense variant (Kruskal–Wallis; p = 0.023). b The impact of causal variant type on HAE attack frequency was analyzed in 27, 34, and 108 patients from HAE-2, missense, and null groups, respectively. Analysis revealed no significant associations. c The impact of causal variant type on clinical severity score [28] was analyzed in 20, 31, and 79 patients from HAE-2, missense, and null groups, respectively. No significant association was found
Potential Impact of c.-21 T > C on HAE Course
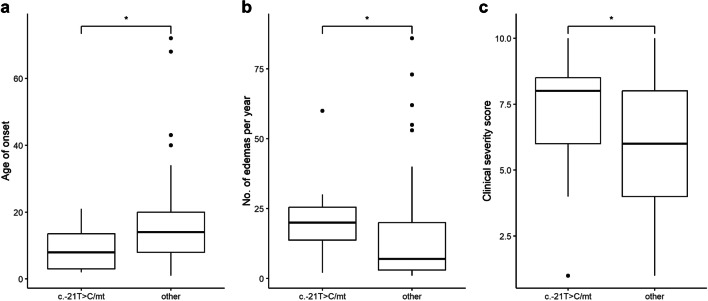
We examined the patients for the presence of the exonic variant c.-21 T > C in trans conformation with the disease-causing variant. It was possible to unambiguously determine c.-21 T > C in trans form in 12 patients in our cohort. Its presence was significantly associated with a lower age of HAE onset (Mann–Whitney; p = 0.024; Fig. 6a), a higher number of attacks per year (Mann–Whitney; p = 0.018; Fig. 6b), and a higher clinical severity score (Mann–Whitney; p = 0.048; Fig. 6c).
Fig. 6.
Influence of the variant c.-21 T > C in trans conformation with the disease-causing variant on disease phenotype. In the analysis, two groups were compared: patients carrying the c.-21 T > C variant in trans conformation with causal variant, depicted in the graph as c.-21 T > C/mt, and patients who did not carry the c.-21 T > C variant or had this variant in cis form, depicted as other a The impact on the age of HAE onset was evaluated by comparing 12 patients carrying c.-21 T > C in trans conformation with causal variant, 148 patients that did not carry c.-21 T > C or have this variant in cis form. The presence of the variant c.-21 T > C in trans conformation was significantly associated with a lower age of HAE onset (Mann–Whitney; p = 0.024). To determine how much this result is affected by the type of causal mutation, we determined the ratio of causal mutation types in the both groups: in the group carrying the c.-21C > T variant the ratio was 1:1:4 and in the other 1:1.1:4.1 (HAE-2:missense:null). b The impact on the HAE attack frequency was evaluated by comparing 12 patients carrying c.-21 T > C in trans conformation with 150 patients that did not carry c.-21 T > C or have this variant in cis form. The presence of the variant c.-21 T > C in trans conformation was significantly associated with a higher number of attacks per year (Mann–Whitney; p = 0.018). c The association of c.-21 T > C in trans conformation with Clinical severity score was evaluated by comparing 11 patients carrying c.-21 T > C in trans conformation with 134 patients that did not carry c.-21 T > C or have this variant in cis form. The presence of the variant c.-21 T > C in trans conformation was significantly associated with a higher Clinical severity score (Mann–Whitney; p = 0.045)
Discussion
Here, we present a report of clinical and genetic data from Czech C1-INH-HAE patients (Table S1), which is an update on the whole historical cohort diagnosed in the past years, including previously published cases [16–19].
Fast and Precise
As we have shown, it took decades to come to conclusive genetic diagnosis in some families [19], but with the advancement of molecular biological techniques, the diagnosis can be reached much faster, with a notable increase in sensitivity. Earlier single-center observations [16] were confirmed in a larger number of individuals from all over the country, as shown on Fig. 1. The time between the first attack of the disease and establishing the diagnosis diminished during the years. Reaching a conclusive diagnosis in the first occurrence in a family of course presents a much more demanding task than when investigating family members, but as seen in Fig. 1b, the diagnostic delay substantially decreased, even in probands. Based on current guidelines, genetic testing is not necessary to establish HAE diagnosis [78]. However, as C1-INH levels and activity, and C4 levels tend to vary between attacks and remissions, it might be essential to identify the disease-causing variant in a patient when C1-INH-HAE is suspected, but the complement test results are inconclusive. It is therefore favorable to confirm the disease genetically in young children where interpreting the complement test results might be especially tricky, and first HAE symptoms could easily be misinterpreted. Also, thanks to genetic counseling and testing for a familial variant the diagnosis can be established in relatives before symptoms emerge, which might prevent them from life-threatening manifestations. Interestingly, one patient in our cohort had a very mild course of disease – the first attack appeared perioperatively at 68 years of age (P05505) and the patient also exhibited normal C4 level, as well as C1-INH level and function. This attack might have not been recognized as an HAE incident, were he not a member of a large family of HAE patients with a formerly established diagnosis.
Only one patient (P01701) from our cohort remained undiagnosed after performing all advanced molecular testing. However, we were able to detect variants classified as pathogenic or potentially pathogenic based on ACMG criteria in all other Czech patients.
Incorporating NGS into the detection method spectrum might be quite useful, specifically, when targeted to intronic and UTR SERPING1 regions and to other previously described genes related to HAE phenotypes. Recently, we also validated targeted NGS to detect large deletions and duplications, and we are able to search for gross rearrangements and intronic/UTR variants in one step.
Variant Spectrum
The diversity of the identified pathogenic or probably pathogenic variants in Czech patients (Fig. S2) confirmed the heterogeneity of causal variants observed in other countries [44, 47–49].
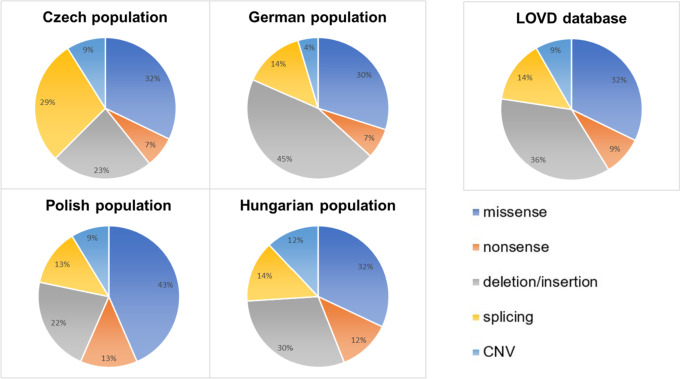
When comparing the proportion of various detected variant types in our cohort with the worldwide dataset (LOVD database [9, 76]), most variant types are of similar amounts. Only in our dataset, the proportion of causal splicing variants is remarkably higher (Fig. 7). This may be due to the higher prevalence of splicing defects in Czech patients, but also because our group focuses specifically on splicing analysis. It might seem to be a result of our specific approach to variant classification; however, the same approach was applied also by Drouet et al. [9] who reviewed data on pathogenic/likely pathogenic SERPING1 variants from the LOVD database, which we compared our data with (Fig. 7).
Fig. 7.
Comparison of HAE causal variant types distribution in Czech cohort, other central European populations, and LOVD worldwide dataset. The data for the Polish population were taken from Obtulowicz et al. [65], and LOVD, while the data for the Hungarian population were taken from Szabó et al. [34]. The data for the German population were taken from LOVD. Regrettably, data for Slovak and Austrian populations, which should be closest to the Czech population from a historical point of view, are not available. The analysis shows that most types of variants were present in similar amounts in all populations studied. However, the proportion of causal splicing variants was remarkably higher in Czech patients than in the other populations and the LOVD dataset
The Importance of Being Causal
In case of SERPING1 nonsense and frameshift variants, prematurely introducing a stop codon and/or nonsense mediated decay is generally the assumed pathomechanism. Similarly, there are no pathomechanism doubts in case of whole exon deletions. However, assessing the impact of missense variants and splicing variants located outside canonical splicing positions (± 1,2) is a more demanding process, as only a few functional studies are available (for variants assessed by functional test(s) see Tables 2, 3, 4, and 5). Therefore, clinically based databases like HGMD [74] or LOVD [76] play crucial roles in providing information on reported cases carrying the same variant which is very important when applying ACMG based variant classification. Further, it is noteworthy that the ClinGen Variant Curation Expert Panel has begun to investigate HAE [79].
Even though mRNA analysis might sometimes be strenuous due to the small extracted quantity of SERPING1 mRNA from the whole blood [19], PCR of cDNA designed to detect a specific splicing defect followed by capillary electrophoresis still presents the first-choice methodology in our hands. Using this procedure, we were able to detect aberrant transcripts in two related patients carrying the c.686-7C > G variant. In this case, the aberrant transcript was not degraded by NMD; however, even in NMD-driven degradation, capillary electrophoresis appears to be sensitive enough to detect the aberrant transcript [19].
Specific splicing in silico prediction tools may help specify the defect and draw attention in the right direction. However, splicing variants’ impact outside canonical GT or AG dinucleotides is sometimes difficult to assess by these tools as, for instance, the MaxEnt Score often does not decrease substantially. Therefore, using a minigene system can provide invaluable information especially if the patient’s RNA is unavailable.
For example, minigene analysis of c.550 + 3A > C confirmed exon 3 splicing disruption. However, it is important to carefully interpret the test results. In this variant, the transcript created by retaining part of intron 3 makes a substantial part of the detected mutant minigene transcripts but a similar transcript would not occur in vivo at all [5]. This difference emerges from simplifying the genomic context in a minigene, where the intron downstream of studied exon is shortened from 1657 to 530 bp only, which makes intron retention more probable compared to real SERPING1. Thus, we would primarily expect exon skipping and cryptic 5'ss use in a patient carrying this variant.
With as many as 20% of causal de novo variants, SERPING1 is regarded as a mutagenic liability, possibly due to its location near the centromeric region and presence of CpG islands in the coding region. Nevertheless, it still might be useful to monitor a particular population even with such a high sequence variation rate. We found few variants that occur specifically in the Czech cohort. The most common variant in our HAE-1 cohort—p.Val454Gly—previously described only in one patient in our other study, was additionally found in three other pedigrees. Similarly, the variant c.1284_1285del, which was previously reported only in one Czech pedigree [18], was discovered in two additional families. Furthermore, another deep intronic variant, c.1029 + 384A > G, was detected in three families, however, this variant’s incidence in other populations might still be underestimated because the variant location is usually not routinely analyzed by Sanger sequencing and targeted or exome NGS [71, 72]. Beside these variants, others were specific to one or two families except for the HAE-2 variants in active center and large deletions.
Severity of HAE
The HAE phenotype severity ranges from asymptomatic to very severe and even members of the same family carrying the same SERPING1 alleles have a very distinct disease. Numerous studies have investigated the correlation between causal variant types and phenotype, adopting diverse approaches for variant classification and phenotype characterization. In several studies, variants were categorized into two groups—first comprising nonsense, frameshift, large deletion/insertions, splicing defects and HAE-2 variants, and second missense variants excluding HAE-2 variants [44, 50, 80], and whereas Andrejevic et al. and Grivčeva-Panovska et al. [44, 80] found that the first group of variants correlated with worse clinical severity score, Maia et al. [50] found no correlation with the phenotype. Similar to our approach, Speletas et al. [12] considered HAE-2 variants a specific entity and compared HAE-1 missense variants to null variants, and similarly to our results, they found association between missense variant and later onset of the HAE.
Duponchel et al. [66] showed that the c.-21 T > C variant causes partial exon 2 skipping. It has been suggested that even though it is not causal in heterozygous carriers, it may still potentially cause mild HAE in a homozygous state [48] and, in trans position to another causal SERPING1 variant, may be linked to a more severe clinical manifestation [35, 51, 81]. We detected no homozygous c.-21 T > C carrier in our cohort. However, we did examine its potential influence on HAE severity and, indeed, found a significant association between c.-21 T > C in trans position with another causal variant and a higher number of attacks per year, a lower age at disease onset, as well as a higher Clinical severity score [28].
Although our study comprises the largest reported number of patients with c.-21 T > C in trans with another causal variant to the best of our knowledge, it would still be useful to collect and analyze data from several databases, preferably in the form of a multicenter international study, to get a clearer picture of the association between this variant and HAE phenotype.
Conclusion
Most of the HAE genetic causes are determined by routinely used approaches, such as direct SERPING1 sequencing of exons, exon/intron boundaries, as well as determining CNVs. When no causal variant is identified by these conventional methods, further molecular genetic techniques should be applied in order to discover the pathogenic alteration in the background of the disease. Primarily, we suggest sequencing intronic and UTR parts of the gene, where pathologic variants have been previously reported, then, analyzing mRNA ideally in several affected and unaffected family members, and/or performing functional minigene tests, if a variant of unknown significance is found. Using targeted panel sequencing, which is becoming standard, we can analyze all the SERPING1 regions, as well as other genes associated with HAE in one step.
As demanding as the procedure of uncovering the possible underlying defect might appear, functional analysis and correct interpretation of the variant pathogenicity often presents an even more substantial challenge. Even though we have provided an experimental insight into the pathomechanism of some splicing variants in previously published studies as well as in this paper, several variants possibly affecting SERPING1 expression and splicing still await functional evidence.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
The study was designed by Tomas Freiberger and Hana Grombirikova. Patients’ data collection and immunological analyses were performed by Roman Hakl, Marta Sobotkova, Radana Zachova, Pavel Kuklinek, Pavlina Kralickova, Irena Krcmova, Jana Hanzlikova, Martina Vachova, Olga Krystufkova, Eva Dankova, Milos Jesenak, and Jiri Litzman. HAE database was established and its data was analyzed by Martina Novackova, Michal Svoboda, and Roman Hakl. Molecular genetic analyses were performed by Hana Grombirikova, Viktor Bily, Premysl Soucek, Michal Kramarek, Lucie Ballonova, Barbora Ravcukova, Dita Ricna, Karolina Kozena, Lucie Kratochvilova and Tomas Freiberger. The first draft of the manuscript was written by Hana Grombirikova, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access publishing supported by the National Technical Library in Prague. The study was supported by grant number NV18-05–00330 from the Ministry of Health of the Czech Republic, and Specific University Research Grant number MUNI/A/1098/2022 provided by the Ministry of Education, Youth and Sports of the Czech Republic.
Data Availability
The datasets analyzed during the current study are available in the Supplement; more detailed information is available from the corresponding author on reasonable request.
Declarations
Ethics Approval
This is an observational study. Therefore, no ethical approval was required.
Consent to Participate
All participants gave their informed written consent for molecular genetic analysis of their samples. In addition, they provided written consent to collect and analyze their data.
Consent for Publication
Informed consent to publish their data was obtained from all individual participants included into the study.
Competing Interests
Authors RH and PK have received speaker and consultant honoraria from Shire and Takeda, and RH served as a principal investigator in the clinical trials supported by BioCryst Pharmaceuticals, Phavaris Netherands, Kalvista, Pharming, and CSL Behring. MaS has received speaker and consultant honoraria from Takeda, Pharming and Kalvista; travel support from CSL Behring and Takeda. RZ has received speaker, and consultant honoraria from CSL Behring and Takeda. MJ has received speaker and consultant honoraria from Takeda, Pharming, CSL Behring, Novartis, Zentiva, SOBI, ALK, Stallergenes-Greer, Chiesi, BerlinChemie Menarini and GSK; travel support from Company Novartis, Takeda, CSL Behring and ALK, and served as a principal investigator in the clinical trials supported by BioCryst Pharmaceuticals, Kalvista, Pharming, and Takeda. TF received speaker honoraria from Takeda. Authors HG, VB, PS, MK, LB, BR, DR, KK, LK, PK, IK, MV, OK, JH, ED, MN, MiS, and JL have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun [Internet]. 2006 [cited 2023 Jun 13];343:1286–9. 10.1016/j.bbrc.2006.03.092 [DOI] [PubMed]
- 2.Bafunno V, Firinu D, D’Apolito M, Cordisco G, Loffredo S, Leccese A, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. J Allergy Clin Immunol [Internet]. 2018 [cited 2023 Jun 13];141:1009–17. 10.1016/j.jaci.2017.05.020 [DOI] [PubMed]
- 3.Bork K, Wulff K, Witzke G, Machnig T, Hardt J. Treatment of patients with hereditary angioedema with the c.988A>G (p.Lys330Glu) variant in the plasminogen gene. Orphanet J Rare Dis [Internet]. 2020 [cited 2023 Jun 13];15:52. 10.1186/s13023-020-1334-8 [DOI] [PMC free article] [PubMed]
- 4.Ariano A, D’Apolito M, Bova M, Bellanti F, Loffredo S, D’Andrea G, et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy [Internet]. 2020 [cited 2023 Jun 13];75:2989–92. 10.1111/all.14454 [DOI] [PubMed]
- 5.Grymová T, Grodecká L, Souček P, Freiberger T. SERPING1 exon 3 splicing variants using alternative acceptor splice sites. Mol Immunol [Internet] 2019;107:91–96. doi: 10.1016/j.molimm.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Internal Med [Internet] 2001;161:2417–2429. doi: 10.1001/archinte.161.20.2417. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med [Internet] 1996;334:1666–1667. doi: 10.1056/NEJM199606203342510. [DOI] [PubMed] [Google Scholar]
- 8.Bowen B, Hawk JJ, Sibunka S, Hovick S, Weiler JM. A review of the reported defects in the human C1 esterase inhibitor gene producing hereditary angioedema including four new mutations. Clin Immunol [Internet]. 2001 [cited 2022 Nov 30];98:157–63. 10.1006/clim.2000.4947 [DOI] [PubMed]
- 9.Drouet C, López-Lera A, Ghannam A, López-Trascasa M, Cichon S, Ponard D, et al. SERPING1 variants and C1-INH biological function: a close relationship with C1-INH-HAE. Front Allergy [Internet]. 2022 [cited 2022 Nov 30];3:835503. 10.3389/falgy.2022.835503 [DOI] [PMC free article] [PubMed]
- 10.Winnewisser J, Rossi M, Späth P, Bürgi H. Type I hereditary angio-oedema. Variability of clinical presentation and course within two large kindreds. J Internal Med [Internet] 1997;241:39–46. doi: 10.1046/j.1365-2796.1997.76893000.x. [DOI] [PubMed] [Google Scholar]
- 11.Freiberger T, Grombiříková H, Ravčuková B, Jarkovský J, Kuklínek P, Kryštůfková O, et al. No evidence for linkage between the hereditary angiooedema clinical phenotype and the BDKR1, BDKR2, ACE or MBL2 gene. Scand J Immunol [Internet] 2011;74:100–106. doi: 10.1111/j.1365-3083.2011.02547.x. [DOI] [PubMed] [Google Scholar]
- 12.Speletas M, Szilagyi A, Psarros F, Moldovan D, Magerl M, Kompoti M, et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol [Internet] 2015;135:570–573.e10. doi: 10.1016/j.jaci.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Loffredo S, Bova M, Suffritti C, Borriello F, Zanichelli A, Petraroli A, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy [Internet] 2016;71:989–996. doi: 10.1111/all.12862. [DOI] [PubMed] [Google Scholar]
- 14.Lung CC, Chan EK, Zuraw BL. Analysis of an exon 1 polymorphism of the B2 bradykinin receptor gene and its transcript in normal subjects and patients with C1 inhibitor deficiency. J Allergy Clin Immunol [Internet] 1997;99:134–146. doi: 10.1016/s0091-6749(97)70310-5. [DOI] [PubMed] [Google Scholar]
- 15.Woodard-Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ. Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics [Internet] 2010;20:532–536. doi: 10.1097/FPC.0b013e32833d3acb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakl R, Kuklínek P, Kadlecová P, Litzman J. Hereditary angio-oedema with C1 inhibitor deficiency: Characteristics and diagnostic delay of Czech patients from one centre. Allergol Immunopathol (Madr) [Internet] 2016;44:241–5. doi: 10.1016/j.aller.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Grodecká L, Hujová P, Kramárek M, Kršjaková T, Kováčová T, Vondrášková K, et al. Systematic analysis of splicing defects in selected primary immunodeficiencies-related genes. Clin Immunol [Internet] 2017;180:33–44. doi: 10.1016/j.clim.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Freiberger T, Kolárová L, Mejstrík P, Vyskocilová M, Kuklínek P, Litzman J. Five novel mutations in the C1 inhibitor gene (C1NH) leading to a premature stop codon in patients with type I hereditary angioedema. Human Mutation [Internet]. 2002 [cited 2022 Nov 30];19:461–461. 10.1002/humu.9029 [DOI] [PubMed]
- 19.Hujová P, Souček P, Grodecká L, Grombiříková H, Ravčuková B, Kuklínek P, et al. Deep intronic mutation in SERPING1 caused hereditary angioedema through pseudoexon activation. J Clin Immunol [Internet]. 2020 [cited 2022 Nov 30];40:435–46. 10.1007/s10875-020-00753-2 [DOI] [PubMed]
- 20.Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. VarSome: the human genomic variant search engine. Bioinformatics [Internet]. 2019 [cited 2022 Nov 30];35:1978–80. 10.1093/bioinformatics/bty897 [DOI] [PMC free article] [PubMed]
- 21.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. 2010; Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ Accessed 30 Nov 2022
- 22.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics [Internet] 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. GigaScience [Internet] 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard MarkDuplicates [Internet]. Available from: https://broadinstitute.github.io/picard/ Accessed 30 Nov 2022
- 25.Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, et al. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res [Internet] 2016;44:e108. doi: 10.1093/nar/gkw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res [Internet]. 2010 [cited 2022 Nov 30];38:e164. 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed]
- 27.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol [Internet]. 2011 [cited 2022 Nov 30];29:24–6. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed]
- 28.Bygum A, Fagerberg CR, Ponard D, Monnier N, Lunardi J, Drouet C. Mutational spectrum and phenotypes in Danish families with hereditary angioedema because of C1 inhibitor deficiency. Allergy [Internet] 2011;66:76–84. doi: 10.1111/j.1398-9995.2010.02456.x. [DOI] [PubMed] [Google Scholar]
- 29.Cagini N, Veronez CL, Constantino-Silva RN, Buzolin M, Martin RP, Grumach AS, et al. New mutations in SERPING1 gene of Brazilian patients with hereditary angioedema. Biol Chem [Internet]. 2016 [cited 2022 Aug 17];397:337–44. 10.1515/hsz-2015-0222 [DOI] [PubMed]
- 30.Loules G, Zamanakou M, Parsopoulou F, Vatsiou S, Psarros F, Csuka D, et al. Targeted next-generation sequencing for the molecular diagnosis of hereditary angioedema due to C1-inhibitor deficiency. Gene [Internet] 2018;667:76–82. doi: 10.1016/j.gene.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Gábos G, Moldovan D, Dobru D, Mihály E, Bara N, Nădăşan V, et al. Mutational spectrum and genotype-phenotype relationships in a cohort of Romanian hereditary angioedema patients caused by C1 inhibitor deficiency. Rev Romana Med Laborator [Internet] 2019;27:255–267. doi: 10.2478/rrlm-2019-0029. [DOI] [Google Scholar]
- 32.Veronez CL, Mendes AR, Leite CS, Gomes CP, Grumach AS, Pesquero JB, et al. The panorama of primary angioedema in the Brazilian population. J Allergy Clin Immunol: In Practice [Internet]. 2021;9:2293–2304.e5. 10.1016/j.jaip.2020.11.039 [DOI] [PubMed]
- 33.Suffritti C, Zanichelli A, Maggioni L, Bonanni E, Cugno M, Cicardi M. High-molecular-weight kininogen cleavage correlates with disease states in the bradykinin-mediated angioedema due to hereditary C1-inhibitor deficiency. Clin Exp Allergy [Internet] 2014;44:1503–1514. doi: 10.1111/cea.12293. [DOI] [PubMed] [Google Scholar]
- 34.Szabó E, Csuka D, Andrási N, Varga L, Farkas H, Szilágyi Á. Overview of SERPING1 variations identified in Hungarian patients with hereditary angioedema. Front Allergy [Internet] 2022;3:836465. doi: 10.3389/falgy.2022.836465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verpy E, Biasotto M, Brai M, Misiano G, Meo T, Tosi M. Exhaustive mutation scanning by fluorescence-assisted mismatch analysis discloses new genotype-phenotype correlations in angiodema. American Journal of Human Genetics [Internet]. 1996;59:308. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1914725/ Accessed 30 Nov 2022 [PMC free article] [PubMed]
- 36.Ponard D, Gaboriaud C, Charignon D, Ghannam A, Wagenaar-Bos IGA, Roem D, et al. SERPING1 mutation update: mutation spectrum and C1 Inhibitor phenotypes. Human Mutation [Internet]. 2020 [cited 2022 Dec 2];41:38–57. 10.1002/humu.23917 [DOI] [PubMed]
- 37.López-Lera A, Garrido S, Roche O, López-Trascasa M. SERPING1 mutations in 59 families with hereditary angioedema. Mol Immunol [Internet] 2011;49:18–27. doi: 10.1016/j.molimm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Hashimura C, Kiyohara C, Fukushi JI, Hirose T, Ohsawa I, Tahira T, et al. Clinical and genetic features of hereditary angioedema with and without C1-inhibitor (C1-INH) deficiency in Japan. Allergy [Internet] 2021;76:3529–3534. doi: 10.1111/all.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuraw BL, Herschbach J. Detection of C1 inhibitor mutations in patients with hereditary angioedema. J Allergy Clin Immunol [Internet] 2000;105:541–6. doi: 10.1067/mai.2000.104780. [DOI] [PubMed] [Google Scholar]
- 40.Haslund D, Ryø LB, Seidelin Majidi S, Rose I, Skipper KA, Fryland T, et al. Dominant-negative SERPING1 variants cause intracellular retention of C1 inhibitor in hereditary angioedema. J Clin Invest [Internet] 2019;129:388–405. doi: 10.1172/JCI98869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yakushiji H, Kaji A, Suzuki K, Yamada M, Horiuchi T, Sinozaki M. Hereditary angioedema with recurrent abdominal pain in a patient with a novel mutation. Intern Med [Internet]. 2016 [cited 2022 Dec 2];55:2885–7. 10.2169/internalmedicine.55.6951 [DOI] [PMC free article] [PubMed]
- 42.Roche O, Blanch A, Duponchel C, Fontán G, Tosi M, López-Trascasa M. Hereditary angioedema: the mutation spectrum of SERPING1/C1NH in a large Spanish cohort. Human Mutation [Internet] 2005;26:135–144. doi: 10.1002/humu.20197. [DOI] [PubMed] [Google Scholar]
- 43.Jindal AK, Rawat A, Kaur A, Sharma D, Suri D, Gupta A, et al. Novel SERPING1 gene mutations and clinical experience of type 1 hereditary angioedema from North India. Pediatr Allergy Immunol [Internet] 2021;32:599–611. doi: 10.1111/pai.13420. [DOI] [PubMed] [Google Scholar]
- 44.Andrejević S, Korošec P, Šilar M, Košnik M, Mijanović R, Bonači-Nikolić B, et al. hereditary angioedema due to C1 inhibitor deficiency in Serbia: two novel mutations and evidence of genotype-phenotype association. PLoS One [Internet]. 2015 [cited 2022 Nov 30];10:e0142174. 10.1371/journal.pone.0142174 [DOI] [PMC free article] [PubMed]
- 45.Kanepa A, Nartisa I, Rots D, Gailite L, Farkas H, Kurjane N. National survey on clinical and genetic characteristics of patients with hereditary angioedema in Latvia. Allergy, Asthma Clin Immunol [Internet]. 2023 [cited 2023 Jun 12];19:28. 10.1186/s13223-023-00783-6 [DOI] [PMC free article] [PubMed]
- 46.Sheikh F, Alajlan H, Albanyan M, Alruwaili H, Alawami F, Sumayli S, et al. Phenotypic and genotypic characterization of hereditary angioedema in Saudi Arabia. J Clin Immunol [Internet] 2022;43:479–484. doi: 10.1007/s10875-022-01399-y. [DOI] [PubMed] [Google Scholar]
- 47.Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol [Internet] 2008;45:3536–3544. doi: 10.1016/j.molimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Rijavec M, Korošec P, Šilar M, Zidarn M, Miljković J, Košnik M. Hereditary angioedema nationwide study in Slovenia reveals four novel mutations in SERPING1 gene. PLoS One [Internet]. 2013 [cited 2022 Nov 30];8:e56712. 10.1371/journal.pone.0056712 [DOI] [PMC free article] [PubMed]
- 49.Gösswein T, Kocot A, Emmert G, Kreuz W, Martinez-Saguer I, Aygören-Pürsün E, et al. Mutational spectrum of the C1INH (SERPING1) gene in patients with hereditary angioedema. Cytogenet Genome Res [Internet] 2008;121:181–188. doi: 10.1159/000138883. [DOI] [PubMed] [Google Scholar]
- 50.Maia LSM, Moreno AS, Ferriani MPL, Nunes FL, Ferraro MF, Dias MM, et al. Genotype-phenotype correlations in Brazilian patients with hereditary angioedema due to C1 inhibitor deficiency. Allergy [Internet]. 2019 [cited 2022 Dec 2];74:1013–6. 10.1111/all.13699 [DOI] [PubMed]
- 51.Pappalardo E, Cicardi M, Duponchel C, Carugati A, Choquet S, Tosi M. Frequent de novo mutations and exon deletions in the Clinhibitor gene of patients with angioedema. J Allergy Clin Immunol [Internet] 2000;106:1147–1154. doi: 10.1067/mai.2000.110471. [DOI] [PubMed] [Google Scholar]
- 52.Skriver K, Radziejewska E, Silbermann JA, Donaldson VH, Bock SC. CpG mutations in the reactive site of human C 1 - inhibitor. J Biol Chem [Internet]. 1989 [cited 2022 Dec 2];264:3066–71. 10.1016/S0021-9258(18)94031-7 [PubMed]
- 53.Nabilou S, Pak F, Alizadeh Z, Fazlollahi MR, Houshmand M, Ayazi M, et al. Genetic study of hereditary angioedema type I and type II (first report from Iranian patients: describing three new mutations). Immunol Investig [Internet]. 2022 [cited 2022 Dec 2];51:170–81. 10.1080/08820139.2020.1817068 [DOI] [PubMed]
- 54.Guryanova I, Suffritti C, Parolin D, Zanichelli A, Ishchanka N, Polyakova E, et al. Hereditary angioedema due to C1 inhibitor deficiency in Belarus: epidemiology, access to diagnosis and seven novel mutations in SERPING1 gene. Clin Mol Allergy [Internet] 2021;19:3. doi: 10.1186/s12948-021-00141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendoza-Alvarez A, Tosco-Herrera E, Muñoz-Barrera A, Rubio-Rodríguez LA, Alonso-Gonzalez A, Corrales A, et al. A catalog of the genetic causes of hereditary angioedema in the Canary Islands (Spain). Front Immunol [Internet]. 2022 [cited 2023 Feb 8];13:997148. 10.3389/fimmu.2022.997148 [DOI] [PMC free article] [PubMed]
- 56.Wang X, Lei S, Xu Y, Liu S, Zhi Y. Mutation update of SERPING1 related to hereditary angioedema in the Chinese population. Hereditas [Internet]. 2022 [cited 2022 Dec 20];159:28. 10.1186/s41065-022-00242-z [DOI] [PMC free article] [PubMed]
- 57.Eldering E, Huijbregts CC, Lubbers YT, Longstaff C, Hack CE. Characterization of recombinant C1 inhibitor P1 variants. J Biol Chem [Internet]. 1992;267:7013–20. Available from: https://pubmed.ncbi.nlm.nih.gov/1551909/. [PubMed]
- 58.Xu Y-Y, Zhi Y-X, Yin J, Wang L-L, Wen L-P, Gu J-Q, et al. Mutational spectrum and geno-phenotype correlation in Chinese families with hereditary angioedema. Allergy [Internet] 2012;67:1430–1436. doi: 10.1111/all.12024. [DOI] [PubMed] [Google Scholar]
- 59.Topyıldız E, Duman Şenol H, Gülen F, Demir E, Mete Gökmen N. Successful treatment of post-pericardiotomy syndrome via c1 inhibitor replacement therapy in a hereditary angioedema patient with marfan syndrome. TurkJPediatr [Internet]. 2023 ;65:338. Available from: 10.24953/turkjped.2022.637 Accessed 12 Jun 2023 [DOI] [PubMed]
- 60.Sim DW, Park KH, Lee J-H, Park J-W. A case of type 2 hereditary angioedema with SERPING1 mutation. Allergy Asthma Immunol Res [Internet]. 2017 [cited 2023 Jun 13];9:96–8. 10.4168/aair.2017.9.1.96 [DOI] [PMC free article] [PubMed]
- 61.Veronez CL, Aabom A, Martin RP, Filippelli-Silva R, Gonçalves RF, Nicolicht P, et al. Genetic variation of Kallikrein-Kinin system and related genes in patients with hereditary angioedema. Front Med [Internet] 2019;6:28. doi: 10.3389/fmed.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Förster TM, Magerl M, Maurer M, Zülbahar S, Zielke S, Inhaber N, et al. HAE patient self-sampling for biomarker establishment. Orphanet J Rare Dis [Internet] 2021;16:399. doi: 10.1186/s13023-021-02021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aulak Ks, Cicardi M, Harrison Ra. Identification of a new P1 residue mutation (444Arg→Ser) in a dysfunctional C1 inhibitor protein contained in a type II hereditary angioedema plasma. FEBS Lett [Internet]. 1990 [cited 2022 Dec 2];266:13–6. 10.1016/0014-5793(90)81494-9 [DOI] [PubMed]
- 64.Ren Z, Zhao S, Li T, Wedner HJ, Atkinson JP. Insights into the pathogenesis of hereditary angioedema using genetic sequencing and recombinant protein expression analyses. J Allergy Clin Immunol [Internet]. 2022;S0091–6749(22)02557-X. 10.1016/j.jaci.2022.11.027 [DOI] [PMC free article] [PubMed]
- 65.Obtulowicz K, Ksiaźek T, Bogdali A, Dyga W, Czarnobilska E, Juchacz A. Genetic variants of SERPING1 gene in Polish patients with hereditary angioedema due to C1 inhibitor deficiency. Cent Eur J Immunol [Internet]. 2020 [cited 2022 Dec 5];45:301–9. 10.5114/ceji.2020.101252 [DOI] [PMC free article] [PubMed]
- 66.Duponchel C, Djenouhat K, Frémeaux-Bacchi V, Monnier N, Drouet C, Tosi M. Functional analysis of splicing mutations and of an exon 2 polymorphic variant of SERPING1/C1NH. Hum Mutat [Internet] 2006;27:295–296. doi: 10.1002/humu.9414. [DOI] [PubMed] [Google Scholar]
- 67.Madsen DE, Hansen S, Gram J, Bygum A, Drouet C, Sidelmann JJ. Presence of C1-inhibitor polymers in a subset of patients suffering from hereditary angioedema. Plos One [Internet]. 2014 [cited 2023 Jun 13];9:e112051. 10.1371/journal.pone.0112051 [DOI] [PMC free article] [PubMed]
- 68.Johnsrud I, Kulseth MA, Rødningen OK, Landrø L, Helsing P, Waage Nielsen E, et al. A nationwide study of Norwegian patients with hereditary angioedema with C1 inhibitor deficiency identified six novel mutations in SERPING1. PLoS One [Internet] 2015;10:e0131637. doi: 10.1371/journal.pone.0131637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de la Cruz RM, López-Lera A, López-Trascasa M. Analysis of SERPING1 expression on hereditary angioedema patients: quantitative analysis of full-length and exon 3 splicing variants. Immunol Lett [Internet] 2012;141:158–164. doi: 10.1016/j.imlet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Kesim B, Uyguner ZO, Gelincik A, Gökmen NM, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol [Internet] 2011;156:443–450. doi: 10.1159/000323915. [DOI] [PubMed] [Google Scholar]
- 71.Germenis AE, Vatsiou S, Csuka D, Zamanakou M, Farkas H. Deep intronic SERPING1 gene variants: ending one odyssey and starting another? J Clin Immunol [Internet]. 2021 [cited 2022 Dec 5];41:248–50. 10.1007/s10875-020-00887-3 [DOI] [PubMed]
- 72.Hida T, Ishikawa A, Okura M, Kishibe M, Uhara H. A Japanese patient with hereditary angioedema caused by deep intron variation in the SERPING1 gene. J Dermatol [Internet]. 2023. 10.1111/1346-8138.16817 [DOI] [PubMed]
- 73.Colobran R, Pujol-Borrell R, Hernández-González M, Guilarte M. A novel splice site mutation in the SERPING1 gene leads to haploinsufficiency by complete degradation of the mutant allele mRNA in a case of familial hereditary angioedema. J Clin Immunol [Internet] 2014;34:521–523. doi: 10.1007/s10875-014-0042-3. [DOI] [PubMed] [Google Scholar]
- 74.Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, Database Mutation The Human Genome (HGMD): towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet [Internet]., [cited, et al. 8];136:665–77. Available from. 2017. 10.1002/humu.10212. [DOI] [PMC free article] [PubMed]
- 75.Germenis AE, Speletas M. Genetics of hereditary angioedema revisited. Clin Rev Allerg Immunol [Internet]. 2016 [cited 2022 Nov 30];51:170–82. 10.1007/s12016-016-8543-x [DOI] [PubMed]
- 76.Fokkema IFAC, Taschner PEM, Schaafsma GCP, Celli J, Laros JFJ, den Dunnen JT. Leiden Open Variation Database (LOVD). Human Mutation [Internet]. 2011 [cited 2022 Nov 30];32:557–63. 10.1002/humu.21438
- 77.Guédard-Méreuze SL, Vaché C, Molinari N, Vaudaine J, Claustres M, Roux A-F, et al. Sequence contexts that determine the pathogenicity of base substitutions at position +3 of donor splice-sites. Human Mutation [Internet]. 2009 [cited 2022 Nov 30];30:1329–39. 10.1002/humu.21070 [DOI] [PubMed]
- 78.Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygören-Pürsün E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy [Internet] 2022;77:1961–1990. doi: 10.1111/all.15214. [DOI] [PubMed] [Google Scholar]
- 79.Clinical Genome Resource [Internet]. [cited 2023 Jan 30]. Available from: https://clinicalgenome.org/affiliation/50128/.
- 80.Grivčeva-Panovska V, Košnik M, Korošec P, Andrejević S, Karadža-Lapić L, Rijavec M. Hereditary angioedema due to C1-inhibitor deficiency in Macedonia: clinical characteristics, novel SERPING1 mutations and genetic factors modifying the clinical phenotype. Ann Med [Internet]. 2018 [cited 2023 Jun 13];50:269–76. 10.1080/07853890.2018.1449959 [DOI] [PubMed]
- 81.Kalmár L, Hegedüs T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive, locus-specific mutation database for the C1 inhibitor gene. Hum Mutat [Internet] 2005;25:1–5. doi: 10.1002/humu.20112. [DOI] [PubMed] [Google Scholar]
- 82.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature [Internet]. 2020 [cited 2022 Nov 30];581:434–43. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed]
- 83.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res [Internet]. 2018 [cited 2022 Nov 30];46:D1062–7. 10.1093/nar/gkx1153 [DOI] [PMC free article] [PubMed]
- 84.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The Human Genome Browser at UCSC. Genome Res [Internet]. 2002 [cited 2022 Nov 30];12:996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed]
- 85.Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, et al. Ensembl 2022. Nucleic Acids Res [Internet]. 2022 [cited 2022 Nov 30];50:D988–95. 10.1093/nar/gkab1049 [DOI] [PMC free article] [PubMed]
- 86.The UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res [Internet]. 2021 [cited 2022 Nov 30];49:D480–9. 10.1093/nar/gkaa1100 [DOI] [PMC free article] [PubMed]
- 87.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat Protoc [Internet]. 2016 [cited 2022 Nov 30];11:1–9. 10.1038/nprot.2015.123 [DOI] [PubMed]
- 88.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet [Internet]. 2013 [cited 2022 Nov 30];0 7:Unit7.20. 10.1002/0471142905.hg0720s76 [DOI] [PMC free article] [PubMed]
- 89.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res [Internet] 2019;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res [Internet]. 2003;31:3568–71. Available from: 10.1093/nar/gkg616 Accessed 30 Nov 2022 [DOI] [PMC free article] [PubMed]
- 91.Desmet FO, Hamroun D, Lalande M, Collod-Bëroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res [Internet] 2009;37:e67. doi: 10.1093/NAR/GKP215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. https://home.liebertpub.com/cmb [Internet]. 2004;11:377–94. 10.1089/1066527041410418 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the Supplement; more detailed information is available from the corresponding author on reasonable request.