Abstract
Background
The emergence of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) in early 2020 and subsequent implementation of public health and social measures (PHSM) disrupted the epidemiology of respiratory viruses. This work describes the epidemiology of respiratory syncytial virus (RSV) observed during two winter seasons (weeks 40–20) and inter‐seasonal periods (weeks 21–39) during the pandemic between October 2020 and September 2022.
Methods
Using data submitted to The European Surveillance System (TESSy) by countries or territories in the World Health Organization (WHO) European Region between weeks 40/2020 and 39/2022, we aggregated country‐specific weekly RSV counts of sentinel, non‐sentinel and Severe Acute Respiratory Infection (SARI) surveillance specimens and calculated percentage positivity. Results for both 2020/21 and 2021/22 seasons and inter‐seasons were compared with pre‐pandemic 2016/17 to 2019/20 seasons and inter‐seasons.
Results
Although more specimens were tested than in pre‐COVID‐19 pandemic seasons, very few RSV detections were reported during the 2020/21 season in all surveillance systems. During the 2021 inter‐season, a gradual increase in detections was observed in all systems. In 2021/22, all systems saw early peaks of RSV infection, and during the 2022 inter‐seasonal period, patterns of detections were closer to those seen before the COVID‐19 pandemic.
Conclusion
RSV surveillance continued throughout the COVID‐19 pandemic, with an initial reduction in transmission, followed by very high and out‐of‐season RSV circulation (summer 2021) and then an early start of the 2021/22 season. As of the 2022/23 season, RSV circulation had not yet normalised.
Keywords: COVID‐19 pandemic, epidemiology, Europe, respiratory syncytial virus, severity, surveillance
1. INTRODUCTION
In temperate regions, respiratory syncytial virus (RSV) typically circulates in the winter months, causing more severe illness particularly in infants and older adults that often results in hospitalisation, including admission to intensive care units. 1 , 2 There are often periods of co‐circulation with influenza and other seasonal respiratory viruses. RSV is monitored through sentinel and/or non‐sentinel surveillance in many countries, territories and areas (henceforth referred to as countries) in the World Health Organization (WHO) European Region, the European Union (EU) and European Economic Area (EEA) countries (hereafter referred to as Europe). These sentinel surveillance systems were originally established for influenza and previously described. 3 A new vaccine has been approved in September 2023 by the European Medicines Agency for use in older adults and infants under 6 months of age. Monoclonal immunoglobulins are available as prophylaxis during the typical period of RSV circulation to protect young infants during their first RSV season, especially those with chronic underlying heart and lung disease at higher risk of severe disease. 4
The emergence and spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) in early 2020 and the subsequent public health and social measures (PHSM) implemented by Member states to reduce its transmission and anticipated morbidity and mortality have disrupted the spread of other respiratory viruses, including RSV, in both the southern 5 , 6 , 7 and northern hemispheres. 8 , 9 , 10
This work aims to describe the epidemiology of RSV in Europe during the 2020/21 and 2021/22 winter seasons [weeks 40/2020 (late September) to 20/2021 (mid‐May) and 40/2021 to 20/2022] and two inter‐seasonal periods (weeks 21 to 39/2021 and 21 to 39/2022) through three surveillance systems [primary care sentinel, secondary care Severe Acute Respiratory Infection (SARI) and non‐sentinel surveillance] in comparison with historical pre‐COVID‐19 pandemic data.
2. METHODS
This retrospective epidemiological analysis of RSV used weekly data submitted to The European Surveillance System (TESSy) by the European Regional surveillance network jointly coordinated by ECDC and the WHO Regional Office for Europe. As RSV is not notifiable, reporting is voluntary. Weekly counts of detections and specimens tested in 48 reporting countries between week 40/2020 and week 39/2022 were downloaded on 14 October 2022. Countries were included in this analysis if at least one sample tested was reported.
Using specimens taken from selected cases of influenza‐like illness (ILI) and acute respiratory infection (ARI) presenting to primary care sentinel surveillance system (these systems have been described previously 3 ), we calculated the aggregated weekly counts of RSV detections and tests and the percentage positivity when at least 10 tests were performed. These counts and percentages were compared with the four previous seasons (2016/17 to 2019/20; hereafter referred to as pre‐COVID‐19 pandemic seasons). Using weekly RSV percentage positivity from four pre‐COVID‐19 pandemic seasons of sentinel data, we used the Moving Epidemic Method (MEM) as described to calculate RSV‐specific epidemic thresholds for each country (Table S2) and identify potential changes in seasonal patterns, as previously shown. 11 It is important to note that these thresholds may differ from those used in the country for surveillance purposes. Epidemic activity was determined to have started when percentage positivity was above the country‐specific epidemic threshold for the first of at least two consecutive weeks. The duration of the epidemic period was defined as the total number of weeks above the threshold (including non‐consecutive weeks).
Similarly, aggregated weekly counts of detections and specimens tested were calculated, along with percentage positivity, for SARI sentinel surveillance (hospital inpatients meeting the case definition). Only two countries reported data in 2020/21 and 16 in 2021/22 periods; historical data regarding number of tests performed were unavailable for this surveillance system. Data included aggregated SARI cases by age group (≤4 years, 5–14 years, 15–64 years and ≥65 years).
For the non‐sentinel surveillance system, only the weekly counts of detections were aggregated but percentage positivity could not always be calculated given the unavailability and/or uncertainty around some of the denominators. Specimens from this system were taken from patients that originated from hospitals, schools, primary care facilities not involved in sentinel surveillance, or nursing homes and other institutions.
For all three systems, data in the study period were compared with historical data. Some countries stopped surveillance or reporting data to TESSy over the summer months for some surveillance systems considered. All analyses were conducted in R version 4.0.5. 12
3. RESULTS
3.1. Primary care sentinel surveillance
When compared with pre‐COVID‐19 pandemic seasons, there was an increase in the number of specimens tested for RSV in both the 2020/21 and the 2021/22 seasons: 21,803 and 36,040, respectively, compared with an annual average of 15,796 in pre‐COVID‐19 pandemic seasons (from 21 countries on average), representing a 38% and 128% increase, respectively. More countries reported during the 2021/22 season than during the 2020/21 and pre‐COVID‐19 pandemic seasons (Figure 1A,B and Table 1).
FIGURE 1.
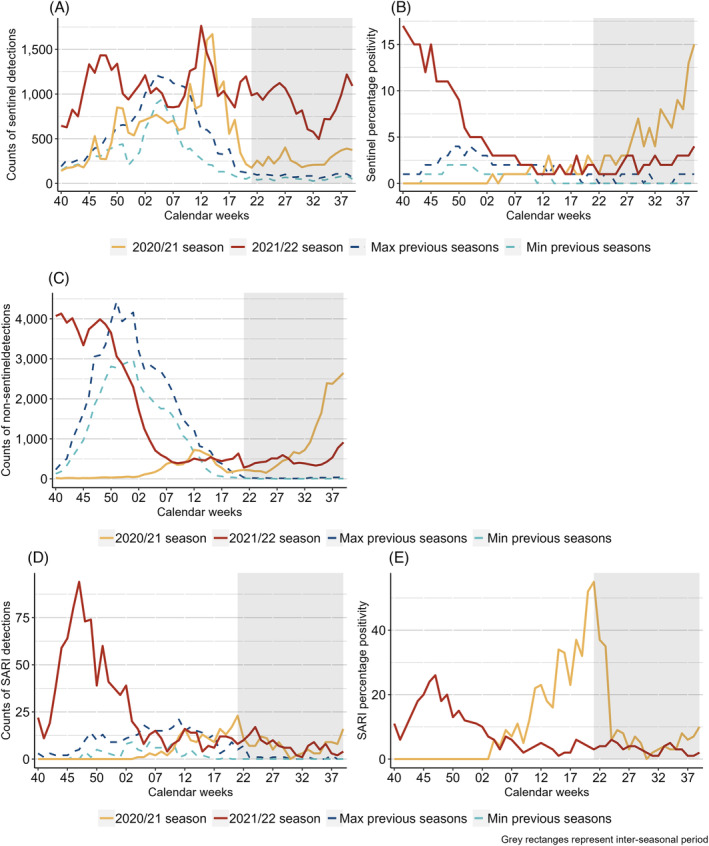
Top row: Count of specimens tested (A) and percentage positivity (B) of RSV specimens per week from sentinel sources compared with the minimum (Min) and maximum (Max) from pre‐COVID‐19 pandemic seasons; middle row: count of non‐sentinel RSV detections compared to the minimum (Min) and maximum (Max) from pre‐COVID‐19 pandemic seasons (C); bottom row: count of specimens tested for RSV (D) and percentage positivity (E) from SARI sites across the selection of countries included, over several seasons. Note: percentage positivity was calculated when at least 10 specimens were tested; grey rectangles represent inter‐seasonal periods; seasons represent the time period ranging from week 40 to 39.
TABLE 1.
Summary breakdown of RSV data by surveillance system for the 2020/21 (weeks 40/2020 to 39/2021) and 2021/22 (weeks 40/2021 to 39/2022) seasons and corresponding inter‐seasonal periods, with percentage positivity in brackets, and previous seasons (seasonal and inter‐seasonal periods), in the selection of countries included.
| 2021/22 season | 2020/21 season | 2016/17–2019/20 seasons | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Seasonal period | Inter‐seasonal period | Seasonal period | Inter‐seasonal period | Mean during seasonal period | Range during seasonal period | Mean during inter‐seasonal period | Range during inter‐seasonal period | ||
| Sentinel surveillance | |||||||||
| Number of countries a | 34 | 27 | 19 | 17 | ‐ | 18–23 | ‐ | 7–12 | |
| Specimens tested | Regional totals | 36,040 | 19,197 | 21,803 | 5076 | 15,796 | 13,853–18,026 | 1338 | 1121–1532 |
| Country ranges | 0–5688 | 3–8031 | 1–4723 | 1–2477 | 2–4502 | 2–5654 | 2–542 | 1–731 | |
| Detections | Regional totals | 2018 (6%) | 375 (2%) | 195 (1%) | 316 (6%) | 1669 (11%) | 1461–1905 | 15 (1%) | 4–26 |
| Country ranges | 0–635 (0%–15%) | 0–103 (0%–15%) | 0–78 (0%–7%) | 0–175 (0%–22%) | 0–312 (0%–50%) b | 0–430 | 0–11 (0%–2%) | 0–20 | |
| Non‐sentinel surveillance | |||||||||
| Number of countriesa | 34 | 24 | 18 | 20 | ‐ | 30–33 | ‐ | 9–15 | |
| Detections | Regional totals | 62,426 | 8876 | 6966 | 18,325 | 49,738 | 44,081–55,614 | 220 | 118–426 |
| Country ranges | 0–14,071 | 0–1955 | 0–5454 | 0–6983 | 0–11,888 | 0–16,456 | 0–61 | 0–164 | |
| SARI surveillance | |||||||||
| Number of countries a | 16 | 11 | 2 | 7 | ‐ | 5–10 | ‐ | 1–3 | |
| Specimens tested | Regional totals | 9478 | 4238 | 682 | 1983 | ‐ | ‐ | ‐ | ‐ |
| Country ranges | 28–2370 | 2–1630 | 94–588 | 27–736 | ‐ | ‐ | ‐ | ‐ | |
| Detections | Regional totals | 948 (10%) | 136 (3%) | 144 (21%) | 148 (7%) | 200 | 178–227 | 7 | 3–15 |
| Country ranges | 0–355 (0%–60%) | 0–98 (0%–18%) | 0–144 (0%–24%) | 0–55 (0%–25%) | 0–93 | 0–105 | 0–3 | 0–9 | |
Reporting at least one specimen tested.
Note that the 50% was based on 10 specimens tested in one country.
A total of 19 countries reported only 195 RSV detections from a total of 21,803 primary care sentinel surveillance specimens tested during the 2020/21 seasonal period. This represents an overall positivity of 1%, ranging up to 7% in France, which was lower than in the four pre‐COVID‐19 pandemic seasons (mean of 1669 positive specimens, 11% positivity from an average of 21 countries) (Figure 1A,B and Table 1). Only seven countries reported at least five detections during the 2020/21 season (Table S1). Only France and Switzerland saw clear waves of activity. Between weeks 5/2020 and 19/2021 in France, percentage positivity was above 10% for all but 2 weeks, whereas Switzerland saw percentage positivity between 4% and 17% between weeks 16 and 20/2021 (Table S1).
During the 2020/21 season, of the nine countries where the epidemic thresholds could be calculated, only France reported peaks of RSV positivity above its threshold. France, Germany and Slovenia experienced early starts to their 2021/22 seasonal period compared with the average starting week of pre‐COVID‐19 pandemic seasons (Figure 2).
FIGURE 2.
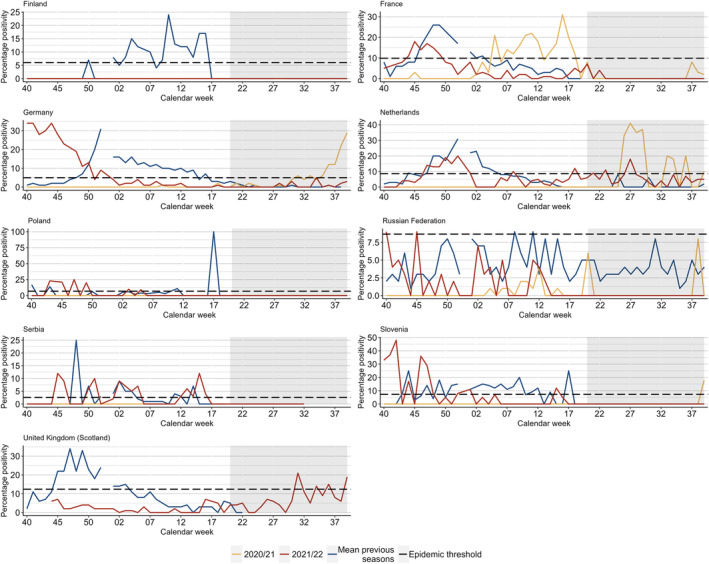
Summary of Moving Epidemic Method (MEM) calculations (calculated by authors) comparing percentage positivity in pre‐COVID‐19 pandemic seasons (2016/17 to 2019/20) and 2020/21 (weeks 40/2020 to 39/2021) and 2021/22 (weeks 40/2021 to 39/2022) seasons, where possible in the selection of countries included. Horizontal lines represent the epidemic threshold for each season where it could be calculated. Note: grey rectangles represent inter‐seasonal periods; the same epidemic threshold calculations were used for the 2021/22 and 2020/21 season. More detailed results can be found in Table S2.
During the 2021 inter‐seasonal period, a total of 316 positive specimens from 5076 specimens tested (6% positivity) were reported from 17 countries (Figure 1A,B and Table 1). A total of nine countries [Estonia (n = 8, 5% positivity), France (n = 5, 4%), Georgia (n = 21, 3%), Germany (n = 175, 7%), Ireland (n = 7, 2%), the Netherlands (n = 39, 22%), Slovenia (n = 16, 16%), Switzerland (n = 35, 9%) and Ukraine (n = 5, 4%)] saw higher than usual activity (Figure S1). Of the countries with inter‐seasonal activity, three (Germany, the Netherlands and Slovenia) exceeded their MEM epidemic threshold, with only the Netherlands having experienced activity during this inter‐seasonal time period in pre‐COVID‐19 pandemic seasons. This represents only sporadic detections, as numbers of collected samples are generally low in the inter‐seasonal time period (Figure 2 and Figure S1).
During the 2021/22 season, 34 countries reported 2018 detections from 36,040 tests performed (6% positivity). This was higher than during the 2020/21 season, but lower than during the pre‐COVID‐19 pandemic seasons (Figure 1A,B and Table 1). A peak of 17% positivity was observed in week 40/2021 which then fell to 3% (week 2/2022) and remained below this level until the end of the season. Regionally, this peak was higher than in the pre‐COVID‐19 pandemic season when regional positivity did not rise above 5% for any given week (Figure 1B and Table 1). In six countries [Denmark, Slovenia, Sweden, United Kingdom (Northern Ireland, Scotland and Wales)], the start of the 2021/22 season was already discernible in the latter part of the 2021 inter‐seasonal period with detections peaking on or around week 40/2021. Where possible to compare with pre‐COVID‐19 pandemic seasons (n = 23 countries), 14 countries experienced similar or higher positivity. Positive specimens continued to be detected in several countries during the second half of the season leading into the 2022 inter‐seasonal period, most notably in Georgia, the Netherlands, Spain and Switzerland (Figure S1B).
During the 2022 inter‐seasonal period, 375 detections were reported from 19,197 samples tested (2% positivity) from 27 countries. Both the number of tests performed and positivity for this period were higher than seen in pre‐COVID‐19 pandemic inter‐seasons: up to 1336 tests and up to 1% positivity (Table 1). Eight countries reported RSV activity outside of the characteristic winter season with at least five detections: Denmark (n = 59, 3% positivity), Georgia (n = 33, 5%), Germany (n = 15, 1%), the Netherlands (n = 40, 6%), Spain (n = 103, 1%), Switzerland (n = 9, 1%), United Kingdom (Scotland) (n = 86, 9%) and Slovenia (n = 12, 15%) (Figure S1 and Table S1). During this period, only the Netherlands and the United Kingdom (Scotland) experienced RSV positivity reaching the epidemic levels previously calculated (Figure 2).
3.2. Non‐sentinel surveillance
A total of 18 countries reported at least one non‐sentinel RSV detection in the 2020/21 season, with a total of 6966 detections. This compares with an average of 49,738 detections from 32 countries in pre‐COVID‐19 pandemic seasons (Figure 1C and Table 1). Between weeks 40/2020 and 1/2021, fewer than 60 weekly detections were recorded, much less than the weekly average during the pre‐COVID‐19 pandemic seasons for the same weeks: 180 (week 40) to 3437 (week 1). Detections peaked in week 12/2021 (Iceland and Ukraine) with 722 RSV detections and fell thereafter until week 18/2021. Between weeks 12 and 18/2021, the number of detections was comparable with pre‐COVID‐19 pandemic seasons (Figure 1C). For the countries with at least one historical season for comparison (n = 17), only Iceland and Ukraine experienced more detections (Table S2) and Finland, Greece, Hungary, Poland, Portugal, Republic of Moldova, Serbia and the United Kingdom saw an earlier peak in 2020/21 than in pre‐COVID‐19 pandemic seasons.
The 2021 inter‐season saw 18,325 RSV detections reported from 20 countries, all of which could be compared with pre‐COVID‐19 pandemic seasons and mostly had higher numbers in 2021 in comparable weeks (average of 220 detections from 13 countries), with the exceptions of Estonia and Latvia (Figure 1C and Tables 1 and S2). This period was characterised by a gradual rise in detections (peaking in week 39/2021 with 2646 detections), which had previously not been reported.
During the 2021/22 season, 62,426 detections were reported from 34 countries, of which 32 could be compared with pre‐COVID‐19 pandemic seasons (Figure 1 and Table 1). The start of the 2021/22 season was a continuation of the high levels of detections already seen during the 2021 inter‐seasonal period, with two peaks of detections: weeks 40/2021 [observed in Denmark, Slovenia, United Kingdom (Northern Ireland, Scotland and Wales)] and 46/2021 (observed in France and Spain). From week 48/2021 until week 7/2022, the numbers of positive detections then gradually fell to levels lower than in pre‐COVID‐19 pandemic seasons. Between weeks 17 and 20/2022, the weekly average of detections was 514, higher than any pre‐COVID‐19 pandemic season (Figure 1C). Of the countries with historical comparisons, nine recorded fewer, 10 recorded more and 13 recorded a similar number of detections than in pre‐COVID‐19 pandemic seasons (Table S2).
Most countries recorded unusually high and/or early peaks of activity during the 2021/22 season. Some countries (Iceland and the Netherlands) saw a biphasic seasonal activity. Others [Denmark, Portugal, Russian Federation, Slovenia, United Kingdom (England, Scotland and Wales)] saw their number of detections decline after an early peak but then rise into the 2022 inter‐seasonal period (Table S2).
The 2022 inter‐season saw 8876 RSV detections from 24 countries, of which 18 had historical data for comparison (Figure 1C and Tables 1 and Table S2). Bulgaria, Iceland, Portugal, Russian Federation and the United Kingdom (Northern Ireland and Scotland) observed more detections than during pre‐COVID‐19 pandemic inter‐seasons but without a distinct peak of activity (Figure S2). In contrast, the Netherlands and the United Kingdom (Wales) did experience a peak of detections in weeks 23/2022 and 28/2022, respectively. Denmark, France, Slovenia and Spain reported a rise in activity late in the period and leading into the 2022/23 season (Figure S2).
3.3. SARI surveillance
During the 2020/21 season, only Belgium and Malta reported SARI data, but only Belgium reported 144 cases identified among 682 patients tested (21% positivity). This compares with an average of 200 detections from seven countries in pre‐COVID‐19 pandemic seasons (Table 1). Detections peaked in week 20/2021 with 55% positivity (n = 17) (Figure 1D,E). Of the 480 patients with known age (all from Belgium), 136 tested positive (28% positivity), with children ≤4 years representing 90% of patients (41% positivity). Children aged 5–14 years saw 26% positivity, which was higher than in the oldest age group (7%) (Table 2).
TABLE 2.
Number of SARI detections (and percentage positivity when at least 10 specimens were tested per age group) per country by age group (in years).
| Country | 2022 inter‐season | 2021/22 season | 2021 inter‐season | 2020/21 season | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 | 5–14 | 15–64 | ≥65 | Total | 0–4 | 5–14 | 15–64 | ≥65 | Total | 0–4 | 5–14 | 15–64 | ≥65 | Total | 0–4 | 5–14 | 15–64 | ≥65 | Total | |
| Armenia | 2 (5%) | 0 (0%) | 0 (0%) | 0 (−%) | 2 (2%) | 9 (6%) | 2 (3%) | 0 (0%) | 0 (−%) | 11 (3%) | 11 (12%) | 0 (0%) | 0 (0%) | 0 (−%) | 11 (5%) | |||||
| Belarus | 0 (0%) | 0 (0%) | 0 (−%) | 0 (−%) | 0 (0%) | 5 (9%) | 1 (3%) | 0 (−%) | 0 (−%) | 6 (7%) | ||||||||||
| Belgium a | 14 (35%) | 0 (−%) | 0 (−%) | 0 (0%) | 14 (16%) | 70 (20%) | 4 (8%) | 5 (2%) | 4 (1%) | 83 (9%) | 41 (39%) | 5 (45%) | 0 (0%) | 7 (14%) | 53 (28%) | 122 (41%) | 5 (26%) | 0 (0%) | 9 (7%) | 136 (28%) |
| Bosnia and Herzegovina | 6 (24%) | 0 (0%) | 0 (−%) | 0 (−%) | 6 (17%) | |||||||||||||||
| Croatia a | 4 (7%) | 0 (0%) | 0 (−%) | 0 (0%) | 4 (1%) | 6 (9%) | 0 (0%) | 1 (−%) | 1 (0%) | 8 (2%) | ||||||||||
| Malta | 0 (−%) | 0 (−%) | 0 (−%) | 1 (1%) | 1 (1%) | 0 (−%) | 0 (−%) | 0 (−%) | 8 (3%) | 8 (3%) | 0 (−%) | 0 (−%) | 0 (−%) | 1 (1%) | 1 (1%) | 0 (−%) | 0 (−%) | 0 (−%) | 1 (0%) | 0 (0%) |
| Montenegro | 24 (67%) | 2 (−%) | 1 (−%) | 0 (−%) | 27 (60%) | |||||||||||||||
| Republic of Moldova | 0 (−%) | 0 (−%) | 0 (−%) | 0 (−%) | 0 (−%) | 21 (26%) | 2 (8%) | 4 (6%) | 0 (0%) | 27 (12%) | ||||||||||
| Russian Federation | 5 (2%) | 1 (1%) | 0 (−%) | 0 (−%) | 6 (2%) | 40 (9%) | 6 (3%) | 1 (1%) | 0 (−%) | 47 (6%) | 18 (11%) | 2 (5%) | 1 (2%) | 0 (−%) | 21 (8%) | |||||
| Türkiye | 4 (1%) | 0 (0%) | 1 (1%) | 0 (−%) | 5 (1%) | 258 (22%) | 8 (4%) | 8 (3%) | 0 (−%) | 274 (17%) | ||||||||||
| Ukraine | 3 (17%) | 2 (−%) | 0 (0%) | 0 (−%) | 5 (7%) | 2 (4%) | 1 (8%) | 0 (0%) | 0 (−%) | 3 (2%) | ||||||||||
| Uzbekistan | 0 (0%) | 0 (0%) | 0 (−%) | 0 (−%) | 0 (0%) | 64 (29%) | 1 (3%) | 2 (73%) | 0 (−%) | 67 (24%) | ||||||||||
| Total | 29 (4%) | 1 (0%) | 1 (1%) | 1 (0%) | 32 (2%) | 506 (20%) | 28 (4%) | 22 (2%) | 13 (1%) | 569 (11%) | 72 (18%) | 8 (10%) | 1 (0%) | 8 (6%) | 89 (10%) | 122 (41%) | 5 (26%) | 0 (0%) | 9 (5%) | 136 (25%) |
Country data have been updated upon request after data collection date.
During the 2021 inter‐season, seven countries (Armenia, Belgium, Georgia, Ireland, Malta, Russian Federation and Ukraine) reported SARI data with a total of 148 RSV detections from 1983 patients (7% positivity). This compares with up to 15 detections from up to three countries in pre‐COVID‐19 pandemic seasons (Tables 1 and S1). Between weeks 24 and 37/2021, the percentage positivity ranged between 2% and 9%, except for week 30/2021 when no detections were reported. Percentage positivity peaked in week 39/2021 at 10%, leading into the 2021/22 season (Figures 1E and S3). When possible to compare with sentinel systems (Georgia, Ireland, Russian Federation and Ukraine), all countries but Ireland saw a delayed peak in SARI positivity after the one in sentinel detections. Among 898 patients with known age tested in five countries, 89 (10%) were positive. Children ≤4 years accounted for 81% (n = 72) of all cases with known age, and 18% of their samples tested positive for RSV. Only Belgium reported over 10% positivity in another age group (those ≥65 years) (Figure 3 and Table 2).
FIGURE 3.
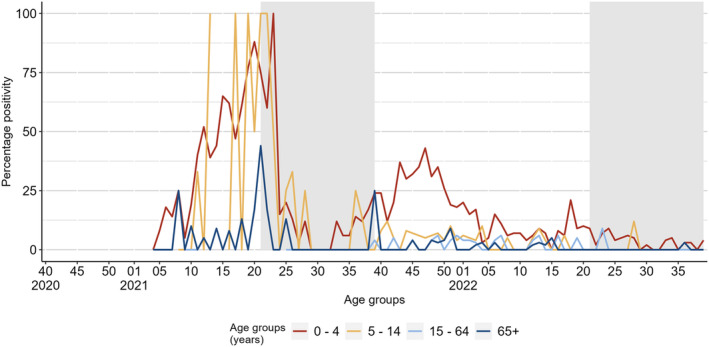
Percentage positivity of positive specimens detected from SARI sites per age group, from the selection of countries included. Grey rectangles represent inter‐seasonal periods.
During the 2021/22 season, 948 SARI cases were reported among 9478 patients tested (10% positivity) in 16 countries (Figure 1D,E and Table 1). A peak of 25% positivity was observed in week 47/2021 in Georgia and Türkiye, after which it fell until week 3/2022 and mostly ranged between 1% and 5% until the end of the season (Figure 1E). Among 5220 patients with known age, 569 detections (11%) were reported by 12 countries. These included 506 (89%) in children ≤4 years and 13 (2%) in persons ≥65 years with a percentage positivity of 20% and 1%, respectively (Table 2). Positive specimens in the youngest age group peaked at the start of the season and in week 18/2022 (Figure 3).
During the 2022 inter‐season, 136 RSV detections were reported from 4238 patients tested (3% positivity) in 11 countries, with positivity ranging between 1% and 6% and peaking in week 24/2022 (Table 1, Figure 1E, Table S1 and Figure S3). Of the 32 cases reported with known age, 29 were ≤4 years from six countries (4% positivity) (Table 2).
4. DISCUSSION
We showed that the COVID‐19 pandemic and its response have impacted the circulation of RSV in Europe since the autumn of 2020. Very little circulation was seen during the 2020/21 season, but unusual inter‐seasonal activity was seen in the summer of 2021, followed by early and high peaks of activity during the 2021/22 season as observed in all surveillance systems. This atypical inter‐seasonal activity continued in many countries in the summer months of 2022. Hospitalised cases of RSV have been predominantly in those aged ≤4 years.
Although countries included in this analysis have tested more specimens from sentinel surveillance systems for RSV during the COVID‐19 pandemic than in prior seasons, fewer viruses were detected during the usual weeks of RSV circulation of the 2020/21 season. The similar findings from three separate surveillance systems (primary care sentinel, non‐sentinel and SARI) and a higher number of countries reporting compared with pre‐COVID‐19 pandemic seasons provide some assurance that this is a true observation of reduced circulation of RSV, likely due to the impact of public health and social measures in the early phase of the pandemic rather than a change in testing practices.
Many countries also observed an unusual increase in RSV activity in the summer months of 2021 and 2022, representing out‐of‐season activity, in all systems, usually translating into an early start of the next epidemic season. In primary care sites in some countries (Denmark, Germany and Slovenia, among others), the 2021/22 season began earlier than during pre‐COVID‐19‐pandemic seasons. In other parts of the Region (such as in France, Ireland and the Netherlands, among others), seasonality returned to similar timing to those observed in pre‐COVID‐19‐pandemic seasons, but with much higher positivity. Both observations may be associated with the lack of RSV circulation observed during the 2020/21 season and hence a widespread lack of exposure to RSV, particularly in younger cohorts, resulting in the build‐up of an increased pool of susceptibles.
When comparing SARI activity to historical data, a higher peak of detections was seen late in the 2020/21 season and early during the 2021/22 season. Because of a lack of historical denominator data, it was not possible to compare the patterns of positivity. Children aged ≤4 years were disproportionately more affected than any other age group, accounting for at least 89% of all SARI cases during all four periods considered whereas those aged ≥65 years accounted for 2% of cases, fewer than would be expected. 13 This may be a result of PHSM against COVID‐19 and continued behavioural changes, especially in older generations, such as continued social distancing or mask wearing even after measures have been lifted. However, it may also be explained by some countries (in Eastern Europe and Central Asia) having a lower threshold for hospitalising young children than other age groups 3 or changing their testing practices during the COVID‐19 pandemic.
Previous studies have looked at the importance of RSV infection causing hospitalisation in the first 2 years of life. 4 , 14 Given that children born during the COVID‐19 pandemic are unlikely to have been exposed to RSV in their first or second year of life, it is possible that when they are older, the severity of a primary RSV infection is reduced. However, it is also possible that the number of children hospitalised during the 2022/23 season will still be greater because of the large number of RSV‐naïve children born since the start of the pandemic, resulting in a high burden of disease and hospitalisation rate. 6 Combined with hospitalisations resulting from other respiratory viruses across all age groups, RSV has the potential to cause high pressure on healthcare systems across the Region. 5
This significant shift in circulation patterns was also observed in other parts of the world where RSV typically also circulates during the winter months such as in Australia and New Zealand 6 , 8 or South Africa. 5 , 6 , 8 , 15 RSV circulation was not uniform across these countries, with some experiencing stronger than expected out‐of‐season activity, 5 whereas others experienced delayed or continued circulation. 6 A delayed circulation of RSV during the summer months had previously been reported after the 2009 A(H1N1) influenza pandemic. 16 The overall reduction in transmission was a very possible result of PHSM implemented (such as mask wearing, stay‐at‐home orders and school orders) to restrict the spread of SARS‐CoV‐2 within and between countries 7 by breaking transmission chains. We know that this implementation was neither uniform between countries nor over time and may be an important factor; similar results were found by other groups. 17
This analysis contains several limitations, including the limited and varying number of countries that report data through various surveillance systems, some of which are not an integral part of routine surveillance but have been part of ECDC projects initiated during the COVID‐19 pandemic. Changes in testing practices and number of reporting sites because of the COVID‐19 pandemic were not considered here. The MEM thresholds were based on only four historical seasons, and very few countries could be included because of the intermittent absence of weekly data or denominators. The interpretation of the calculated epidemic thresholds should therefore be taken with caution and does not necessarily represent the national epidemic thresholds that are set by the individual countries. There were too few countries for a robust investigation into geo‐temporal patterns of increased activity across countries in the Region. The lack of non‐sentinel denominator data did not allow systematic percentage positivity calculations, a potential weakness of this data source when interpreted alone. However, the consistency of non‐sentinel trends with those from other surveillance systems provides some reassurance to the validity of the results. Additionally, the availability of age data for sentinel, non‐sentinel and historical SARI surveillance systems would have allowed a better understanding of which age groups have been most affected by these unusual circulation patterns and whether children were overrepresented. Finally, the limited number of countries reporting SARI age data may not be representative of the Region (also given that these countries are mostly located in its Eastern part) or of the age distribution of patients at large (it is unclear if cases have an a priori equal chance of being tested for RSV regardless of their age). Unfortunately, the absence of historical SARI denominator data and the large age groups into which cases are grouped do not allow for a more detailed interpretation of our results. In addition, during the COVID‐19 pandemic, there were varying degrees of disruption to national sentinel surveillance systems because of changes in health‐seeking behaviours and limitations in the capacity of sites to receive cases and take specimens, impacting the ability of these systems to monitor respiratory viruses, including RSV.
Despite these limitations, this descriptive study illustrates how the emergence of a novel respiratory virus and associated public health measures can significantly disrupt the circulation of established seasonal respiratory viruses. It is likely that the absence of circulation of seasonal respiratory pathogens during a season led to out‐of‐season activity and, potentially, to larger‐than‐expected peaks of activity thereafter, as observed in summer 2021 and the early 2021/22 season. RSV activity during the 2022/23 season continues to follow unusual patterns, and the implementation of integrated surveillance systems monitoring multiple respiratory viruses simultaneously may prove invaluable to both monitor RSV and other seasonal viral respiratory infections and also in planning for the future emergence of a novel pandemic respiratory virus.
5. CONCLUSION
The emergence and spread of SARS‐CoV‐2 and associated control measures in the EU/EEA and in the WHO European Region have significantly impacted the spread and timing of seasonal respiratory viruses such as RSV. For the last two winter seasons, and for the summer periods in between, much higher proportions of RSV detections or a temporal shift in transmissions were detected compared with pre‐COVID‐19 pandemic seasons. Further work is required to determine the possible explanatory factors including the implementation and relaxation in PHSM and factors such as possible SARS‐CoV‐2 viral interference to this shift in timing, activity, and potential difference in age distribution of RSV cases. These results highlight the importance of being aware of and preparing for continued potential unusual patterns in the epidemiology of RSV (and other respiratory viruses). It is also a lesson learned with regard to the impact of future emergence and spread of respiratory viruses with pandemic potential on the epidemiology of seasonal respiratory viruses.
AUTHOR CONTRIBUTIONS
Margaux Marie Isabelle Mesle: Conceptualization; formal analysis; investigation; methodology; validation; visualization; writing—original draft; writing—review and editing. Mary Sinnathamby: Formal analysis; investigation; methodology; writing—original draft; writing—review and editing. Piers Mook: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; validation; visualization; writing—review and editing. WHO European Region respiratory network WHO European Region respiratory network: Writing—review and editing. Richard Pebody: Conceptualization; funding acquisition; methodology; project administration; supervision; validation; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The following authors declare having received funding from the Innovative Medicines Initiative (IMI): Adam Meijer (The Netherlands) and Anne Teirlinck (The Netherlands). All other authors have no conflicts of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/irv.13219.
DISCLAIMER
The authors affiliated with the World Health Organization (WHO) are solely responsible for the views expressed in this publication and do not necessarily represent the decisions or policies of the WHO.
Supporting information
Figure S1: Counts (A) and percentage positivity (B) of RSV‐positive specimens detected from sentinel sources by country and season, from the selection of countries included. Previous seasons range from 2016/17 to 2019/20.
Supplementary figure 2. Counts of RSV detections from non‐sentinel sources by country in the selection of countries included, compared to the minimum (Min) and maximum (Max) detections from pre‐COVID‐19 pandemic seasons.
Supplementary figure 3. Counts (A) and percentage positivity (B) of positive specimens detected from Severe Acute Respiratory Infection (SARI) sites per country, from the selection of countries included.
Supplementary table 1: Summary of specimens tested, detections (and positivity) per country or area in the selection of countries included, surveillance system, and time period. Note: ‘No. previous seasons/inter‐seasons’ refers to the number of seasons between 2016/17 and 2019/20 that have been included in the historical data shown here. NS: ‘Not Statistically significant’.
Supplementary table 2: Summary of Moving Epidemic Method (MEM) threshold values calculated per country where possible. ‘Number of seasons included’ refers to the number of seasons included in the model run for each respective season and country combination.
ACKNOWLEDGEMENTS
We gratefully acknowledge the input and feedback from colleagues at the European Center for Disease Prevention and Control (ECDC), namely, Eeva K Broberg, Edoardo Colzani and Phillip Zucs. We express our gratitude to all ECDC staff involved in the RSV data collection and the TESSy data management team, especially Marius Valentin Valcu, for the technical support. We would also like to acknowledge the physicians and nurses of sentinel network sites and hospitals for their contribution in collecting specimens for RSV testing. We also gratefully acknowledge the input of national clinical, laboratory and public health staff involved in surveillance activities and data submission to TESSy. The authors would also like to thank all countries for the provision of data, including the following: Belarus: Inna Karaban (Head of Department of Hygiene, Epidemiology and Prevention, Ministry of Health, Minsk, Belarus). Belgium: BELSARI‐NET group consisting of Department of Infectiology, UZ Brussel, Brussels (Lucie Seyler), Department of Infectious Diseases, Centre hospitalier Universitaire Saint‐Pierre, Brussels (Nicolas Dauby, Benedicte Delaere, Marc Bourgeois), Department of Laboratory Medicine, Medical Microbiology, Algemeen Ziekenhuis Sint‐Jan, Brugge‐Oostende AV (Evelyn Petit, Marijke Reynders), Department of Microbiology and Infection control, UZ Brussel, Brussels (Els Van Nedervelde, Thomas Demuyser, Arne Witdouck, Siel Daelemans, Virgini Van Buggenhout, Caroline Wylock), Department of Microbiology, Jessa Ziekenhuis, Hasselt (Door Jouck, Koen Magerman, Marieke Bleyen), Epidemiology of Infectious Diseases, Sciensano, Brussels (Melissa Vermeulen, Sebastien Fierens), National Influenza Centre, Sciensano, Brussels (François Dufrasne, Sarah Denayer, Cyril Barbezange), Pediatrics Department, CHU Saint‐Pierre, Université Libre de Bruxelles (ULB), Brussels (Marc Hainaut), Service of Clinical Biology, Grand Hôpital de Charleroi, Charleroi (Benédicte Lissoir) and Service of Infectiology, Grand Hôpital de Charleroi, Charleroi (Xavier Holemans, Catherine Sion). Czechia: Jan Kyncl, Alexander Nagy, Pavel Slezak, Jaromira Vecerova, David Vostrak (all from the National Institute of Public Health, Prague, Czech Republic). Denmark: Members of the Danish National Influenza Team: Hanne‐Dorthe Emborg, Jens Nielsen, Lisbet Krause Knudsen, Lasse Skafte Vestergaard, Amanda Bolt Botnen, Jesper Schak Krog. Estonia: Irina Eero (Laboratory of Infectious Diseases), Liisa Lilje (Laboratory of Infectious Diseases) and Eliisa Metsoja (Estonian Health Board Influenza Centre, chief specialist). France: Bruno Lina, Martine Valette, Sylvie van der Werf, Thierry Blanchon, Nael Zemali, Sylvie Behillil, Delphine Viriot. Ireland: Joan O'Donnell, Jeff Connell, Irish sentinel GP network, ICGP, NVRL, SARI surveillance team and HPSC influenza team. The Netherlands: We thank the Dutch Working Group on Clinical Virology (NWKV) from the Dutch Society for Clinical Microbiology (NVMM) and all participating laboratories for providing the virological data from the weekly laboratory virological report (non‐sentinel data). We thank Anne‐Marie van den Brandt, Bas van der Veer, Lynn Aarts and Hai Xi Wiegmans, representing the wider team of technicians participating in RSV molecular diagnostics. We thank Rianne van Gageldonk‐Lafeber, Marit de Lange, Daphne Reukers and Liz Jenniskens, from the RIVM EPI respiratory surveillance team. We thank Mariette Hooiveld from Nivel, the GPs participating in the Nivel sentinel surveillance network and their patients for participating in the respiratory surveillance. Portugal: Portuguese GP Sentinel Network, Portuguese Laboratory Network for Influenza and other Respiratory Viruses Diagnosis; Ana Rita Torres (Department of Epidemiology, National Institute of Health Doctor Ricardo Jorge); Aryse Melo, Camila Henriques, Inês Costa, Licínia Gomes, Miguel Lança, Nuno Verdasca (National Reference Laboratory for Influenza and Other Respiratory Viruses, Infectious Diseases Department, National Institute of Health Doctor Ricardo Jorge). Spain: Concepción Delgado‐Sanz, Clara Mazagatos, Susana Monge (National Center of Epidemiology, CIBERESP; Carlos III Health Institute); Inmaculada Casas (National Center of Microbiology, CIBERESP; Carlos III Health Institute); Tomás Vega (Servicio de Información de Salud Pública, DGSP, Consejería de Sanidad, Castilla y León). Sweden: AnnaSara Carnahan (Folkhälsomyndigheten, Solna). Switzerland: Swiss Sentinel Network and the Federal Office of Public Health. United Kingdom (Wales): Jana Zitha, Panoraia Kalapotharakou, Anastasia Couzens, Kathleen Pheasant and Laura Gifford (Public Health Wales Virology and ARI surveillance team). Uzbekistan: Dr Bakhodir Yusupaliyev, (Head of the Sanitary and Epidemiological Welfare and Public Health Service of the Republic of Uzbekistan) and Dr Sultan Djemileva, (Head of the Virological Laboratory of the Sanitary and Epidemiological Welfare and Public Health Service of the Republic of Uzbekistan). Kosovo: Zana Deva (National Institute of Public Health of Kosovo) and Pranvera Kacaniku (National Institute of Public Health of Kosovo).
Meslé MMI, Sinnathamby M, Mook P, The WHO European Region Respiratory Network Group , Pebody R. Seasonal and inter‐seasonal RSV activity in the European Region during the COVID‐19 pandemic from autumn 2020 to summer 2022. Influenza Other Respi Viruses. 2023;17(11):e13219. doi: 10.1111/irv.13219
Funding information The WHO Regional Office for Europe was supported for work on influenza and other respiratory viruses by a cooperative agreement from the United States Centers for Disease Control and Prevention (NU51IP000936); the funder had no role in the analysis or interpretation of the data.
Arijana Kalaveshi and Xhevat Jakupi: This designation is without prejudice to positions on status and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo Declaration of Independence. All references to Kosovo in this document should be understood to be in the context of the United Nations Security Council resolution 1244 (1999).
The WHO European Region Respiratory Network Group authorship consists of Anissa Lakhani, Maria Zambon, Odette Popovici, Mihaela Lazăr, Amela Dedeić Ljubović, Nina Rodić Vukmir, Ayşe Başak Altaş, Emine Avci, Katarzyna Łuniewska, Karol Szymański, Greta Gargasiene, Svajune Muralyte, Ausra Dziugyte, Graziella Zahra, Ana Rita Gonçalves, Tania Spedaliero, Guillaume Fournier, Daniel Alvarez‐Vaca, Goranka Petrović, Irena Tabain, Katarina Prosenc, Maja Socan, Jelena Protic, Dragana Dimitrijevic, Alina Druc, Mariana Apostol, Kate Karolina Kalasnikova, Sergejs Nikisins, Janine Reiche, Wei Cai, Adam Meijer, Anne Teirlinck, Amparo Larrauri, Inmaculada Casas, Vincent Enouf, Sophie Vaux, Frederikke Kristensen Lomholt, Ramona Trebbien, Helena Jirincova, Helena Sebestova, Mónika Rózsa, Zsuzsanna Molnár, Gudrun Aspelund, Gudrun Erna Baldvinsdottir, Simon Cottrell, Catherine Moore, Athanasios Kossyvakis, Kassiani Mellou, Olga Sadikova, Johanna Kristina Tamm, Nathalie Bossuyt, Isabelle Thomas, Edita Staroňová, Lyudmila Kudasheva, Boris Pleshkov, Niina Ikonen, Otto Helve, Emma Dickson, Tanya Curran, Kseniya Komissarova, Kirill Stolyarov, Veronika Vysotskaya, Natallia Shmialiova, Božidarka Rakočević, Danijela Vujošević, Romella Abovyan, Shushan Sargsyan, Khatuna Zakhashvili, Ann Machablishvili, Oksana Koshalko, Iryna Demchyshyna, Michal Mandelboim, Aharona Glatman‐Freedman, Rory Gunson, Shivani Karanwal, Raquel Guiomar, Ana Paula Rodrigues, Charlene Bennett, Lisa Domegan, Arijana Kalaveshi, Xhevat Jakupi, Gurbangul Ovliyakulova, Neli Korsun, Nadezhda Vladimirova.
Footnotes
This designation is without prejudice to positions on status and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo Declaration of Independence. All references to Kosovo in this document should be understood to be in the context of the United Nations Security Council resolution 1244 (1999).
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Li Y, Wang X, Broberg EK, Campbell H, Nair H, European RSV Surveillance Network . Seasonality of respiratory syncytial virus and its association with meteorological factors in 13 European countries, week 40 2010 to week 39 2019. Eurosurveillance. 2022;27(16):2100619. doi: 10.2807/1560-7917.ES.2022.27.16.2100619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broberg EK, Waris M, Johansen K, Snacken R, Penttinen P, European Influenza Surveillance Network . Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Eurosurveillance. 2018;23(5):17‐00284. doi: 10.2807/1560-7917.ES.2018.23.5.17-00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mook P, Meerhoff T, Olsen SJ, et al. Alternating patterns of seasonal influenza activity in the WHO European Region following the 2009 pandemic, 2010‐2018. Influenza Resp Viruses. 2020;14:150‐161, 2. doi: 10.1111/irv.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatterjee A, Mavunda K, Krilov LR. Current state of respiratory syncytial virus disease and management. Infect Dis Ther. 2021;10(S1):5‐16. doi: 10.1007/s40121-020-00387-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eden J‐S, Sikazwe C, Xie R, et al. Off‐season RSV epidemics in Australia after easing of COVID‐19 restrictions. Nat Commun. 2022;13(1):2884. doi: 10.1038/s41467-022-30485-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odumade OA, van Haren SD, Angelidou A. Implications of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) pandemic on the epidemiology of pediatric respiratory syncytial virus infection. Clin Infect Dis. 2022;75(Supplement_1):S130‐S135. doi: 10.1093/cid/ciac373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang JW, Bialasiewicz S, Dwyer DE, et al. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID‐19 pandemic. J Med Virol. 2021;93(7):4099‐4101. doi: 10.1002/jmv.26964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binns E, Koenraads M, Hristeva L, et al. Influenza and respiratory syncytial virus during the COVID‐19 pandemic: time for a new paradigm? Pediatr Pulmonol. 2022;57(1):38‐42. doi: 10.1002/ppul.25719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melidou A, Ködmön C, Nahapetyan K, et al. Influenza returns with a season dominated by clade 3C.2a1b.2a.2 A(H3N2) viruses, WHO European Region, 2021/22. Eurosurveillance. 2022;27(15):27. doi: 10.2807/1560-7917.ES.2022.27.15.2200255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Eurosurveillance. 2021;26(29):26. doi: 10.2807/1560-7917.ES.2021.26.29.2100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vos LM, Teirlinck AC, Lozano JE, et al. Use of the moving epidemic method (MEM) to assess national surveillance data for respiratory syncytial virus (RSV) in the Netherlands, 2005 to 2017. Eurosurveillance. 2019;24(20):1800469. doi: 10.2807/1560-7917.ES.2019.24.20.1800469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. https://www.R-project.org/
- 13. Staadegaard L, Caini S, Wangchuk S, et al. The global epidemiology of RSV in community and hospitalized care: findings from 15 countries. Open forum. Open Forum Infect Dis. 2021;8(7):ofab159. doi: 10.1093/ofid/ofab159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert L, Sagfors AM, Openshaw PJM, Culley FJ. Immunity to RSV in early‐life. Front Immunol. 2014;5:5. doi: 10.3389/fimmu.2014.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Mattia G, Nenna R, Mancino E, et al. During the COVID‐19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56(10):3106‐3109. doi: 10.1002/ppul.25582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Wang X, Msosa T, de Wit F, Murdock J, Nair H. The impact of the 2009 influenza pandemic on the seasonality of human respiratory syncytial virus: a systematic analysis. Influenza Resp Viruses. 2021;15(6):804‐812. doi: 10.1111/irv.12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bardsley M, Morbey RA, Hughes HE, et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID‐19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis. 2023;23(1):56‐66. doi: 10.1016/S1473-3099(22)00525-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Counts (A) and percentage positivity (B) of RSV‐positive specimens detected from sentinel sources by country and season, from the selection of countries included. Previous seasons range from 2016/17 to 2019/20.
Supplementary figure 2. Counts of RSV detections from non‐sentinel sources by country in the selection of countries included, compared to the minimum (Min) and maximum (Max) detections from pre‐COVID‐19 pandemic seasons.
Supplementary figure 3. Counts (A) and percentage positivity (B) of positive specimens detected from Severe Acute Respiratory Infection (SARI) sites per country, from the selection of countries included.
Supplementary table 1: Summary of specimens tested, detections (and positivity) per country or area in the selection of countries included, surveillance system, and time period. Note: ‘No. previous seasons/inter‐seasons’ refers to the number of seasons between 2016/17 and 2019/20 that have been included in the historical data shown here. NS: ‘Not Statistically significant’.
Supplementary table 2: Summary of Moving Epidemic Method (MEM) threshold values calculated per country where possible. ‘Number of seasons included’ refers to the number of seasons included in the model run for each respective season and country combination.
Data Availability Statement
Data are available on request from the authors.


