Abstract
Background & Aims:
Currently, there is no consistent information on the course of fibrosis-4 (FIB-4) score changes in non-alcoholic fatty liver disease (NAFLD) or their association with subsequent risk of cirrhosis and/or hepatocellular carcinoma (HCC). Thus, we aimed to evaluate the association between longitudinal changes in FIB-4 and subsequent risk of HCC and a composite endpoint of cirrhosis and HCC in patients with NAFLD.
Methods:
We conducted a retrospective cohort study of patients with NAFLD seen in 130 Veterans Administration hospitals between 1/1/2004–12/31/2008, with follow-up through to 12/31/2018. We calculated FIB-4 longitudinally and categorized patients based on risk of advanced fibrosis (low-risk FIB-4 <1.45, indeterminate-risk FIB-4 1.45–2.67, and high-risk FIB-4 >2.67). We used landmark Fine-Gray competing risks models to determine the effects of change in FIB-4 between NAFLD diagnosis date and 3-year landmark time on the subsequent risk of HCC and a composite endpoint.
Results:
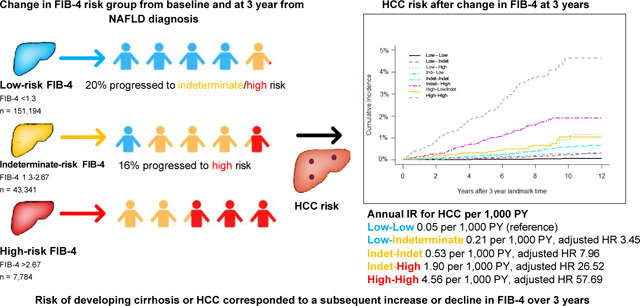
Among the 202,319 patients with NAFLD in the 3-year landmark analysis, 473 progressed to HCC at an incidence rate of 0.28 per 1,000 person years (PY) (95% CI 0.26–0.30). The incidence rate of the composite endpoint was 1.31 per 1,000 PY (95% CI 1.25–1.37). At baseline, 74.7%, 21.4%, and 3.8% of patients had a low, indeterminate, and high FIB-4, respectively. Compared to patients who were at stable low FIB-4 at both time points, the risk of HCC and that of the composite endpoint was higher for all other subgroups with the highest risk in patients with persistently high FIB-4 (HCC adjusted sub-distribution hazard ratio 57.7, 95% CI 40.5–82.2 and composite endpoint hazard ratio 28.6, 95% CI 24.6–33.2).
Conclusion:
Longitudinal changes in FIB-4 were strongly associated with progression to cirrhosis and HCC.
Keywords: Risk stratification, HCC, NAFLD, non-invasive fibrosis markers, fibrosis
Graphical Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now the most common liver disease with a population prevalence among adults estimated to be 20–30% worldwide.1–3 NAFLD is growing at a rate parallel with the obesity epidemic and is predicted to become one of the leading causes of cirrhosis and hepatocellular carcinoma (HCC).4,5 Unfortunately, NAFLD-related HCC (NAFLD-HCC) is associated with a high mortality rate due to being diagnosed at a later stage and in older individuals.6–9 There are several challenges in identifying at-risk individuals with NAFLD who may benefit from HCC surveillance. For example, progression to cirrhosis, the main precursor to HCC, is lower with variable risk in NAFLD compared to other etiologies. Furthermore, up to one-third of HCC cases arise in the setting of NAFLD without cirrhosis; however, current clinical guidelines do not recommend HCC surveillance in individuals with NAFLD without cirrhosis.10,11
The heterogeneous risk of progression to cirrhosis and NAFLD-HCC in patients may come from variations in the extent and evolution of liver fibrosis, which may be captured by non-invasive markers.12,13 In this regard, blood-based biomarkers of liver fibrosis are promising because they can be standardized and easily applied in limited resource settings compared to use of liver biopsy or repeated imaging for fibrosis assessment. Using these markers may be a cost-effective and easily implementable method for risk stratifying patients. Changes in the fibrosis-4 (FIB-4) score over time were strongly associated with risk of HCC in a cohort of patients treated for chronic hepatitis C. Currently, there is no consistent information on the course of FIB-4 changes in NAFLD or its association with subsequent risk of cirrhosis and/or NAFLD-HCC.14 These data could be important for risk stratification of patients. For example, identifying patients who may be at a high enough risk for HCC can alter surveillance decisions, with more aggressive surveillance considered in those more likely to have a high risk. To clarify these important questions, we evaluated changes in FIB-4 over time and the associated risk of progression, including progression to HCC, among a large retrospective cohort of patients with NAFLD who sought care at 130 Veterans Affairs (VA) hospitals and affiliated clinics. VA is the largest integrated healthcare system in the US. It is also a semi-closed system with a stable population with comprehensive clinical, laboratory and outcome data rendering longitudinal studies feasible.
Materials and methods
Data sources
We used the national VA Corporate Data Warehouse (CDW) and VA Central Cancer Registry (CCR). CDW includes all diagnostic codes (ICD-9, ICD-10, and CPT), radiology reports from the Radiology Raw Data domain, laboratory test results, and pharmacy data for each patient encounter.15 CDW also contains information from annual Alcohol Use Disorders Identification Test (AUDIT-C) screen and Vital Status files with date of death from four sources.8 CCR is a centralized repository for over 750,000 VA patients with cancer and includes information on date of diagnosis, primary site, and histology. We also accessed electronic medical records for patients nationwide through the VA Compensation and Pension Records Interchange.
Study population
We defined patients as having NAFLD if they had two or more elevated alanine aminotransferase (ALT) values (>−40 IU/ml for men and >−31 IU/ml for women) in the ambulatory setting and more than 6 months apart, with no positive serologic laboratory testing for HBV (i.e., HBV surface antigen) or HCV (i.e., HCV RNA).8 We excluded patients with any alcohol-related ICD-9 codes or positive AUDIT-C scores (>−4 in men and >−3 in women) any time prior to or during study follow-up. We also excluded patients with evidence of rare chronic liver disorders (hereditary hemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilsons disease, alpha-1 antitrypsin disease, or autoimmune hepatitis) defined based on ICD-9 codes. This combined definition was highly predictive of NAFLD diagnosis based on explicit chart review (positive predictive value 89%, 95% CI 84–94%; negative predictive value 98%, 95% CI 93–99%).8
We used the date of first elevated ALT as the date of NAFLD diagnosis. We included patients with a NAFLD diagnosis date from January 1, 2004 to December 31, 2008 in this analysis because AUDIT-C was implemented in the VA in 2004. Patients had to be 18 years or older and have an evaluable FIB-4 (see below) within 6 months of NAFLD diagnosis to be included in our analysis. For our analysis, we constructed two separate cohorts of patients and followed them to December 31, 2018 to examine our outcomes of interest (see statistical analysis section).
Variable specification
FIB-4
We calculated FIB-4 longitudinally using age and laboratory results from aspartate aminotransferase (AST), ALT and platelet tests performed within 6 months of each other in ambulatory settings as described: FIB-4 = Age (years) × AST (U/L)/[platelets (109/L)×ALT1/2 (U/L)].16 We examined FIB-4 as a continuous variable and also categorized it using established (<1.45, >2.67) cut-offs.17 FIB-4 scores >2.67 (positive predictive value 80%) are predictive of having high risk for advanced fibrosis, whereas values <1.45 (negative predictive value: 90%) are predictive of absence of advanced fibrosis.18
Other covariates
Demographic variables collected included age, sex, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other). We defined diabetes and hypertension by >−2 outpatient or >−1 inpatient ICD-9 code or >−1 filled prescription of diabetes medications (oral hypoglycemic medications or insu-lin) or anti-hypertensives respectively; we used the first evidence of the condition as the date of diagnosis. The key features of metabolic syndrome-associated dyslipidemia included high serum triglycerides and low HDL levels.19 We defined dyslipidemia by examining serial laboratory values for abnormal serum triglycerides (>−200) and/or HDL (<40), starting from the value (within 1 year prior to or after) closest to the NAFLD diagnosis date and then updated over time. We used height and weight values (any time before to 1 year after) nearest to the NAFLD diagnosis to define BMI. Healthcare utilization was measured as the number of clinical visits within the first year from the NAFLD diagnosis date.
Outcome
The study outcomes were incident HCC and a composite endpoint of either cirrhosis or HCC, whichever was diagnosed first. We used a hierarchical approach to define the occurrence of HCC, as described previously.8,15 Briefly, the VA CCR contains variables related to primary site and diagnosis, which we used to determine HCC by keyword searches for “liver” and “hepatocellular carcinoma”, respectively. In addition to using CCR, we also identified HCC cases by the presence of ICD 9/10 codes. We conducted a manual review of the VA electronic medical records for each discordant patient who had an ICD-9/10 code but was not identified as having HCC in the CCR data to determine their true HCC status. This hierarchical approach ensured high validity of all the captured HCC cases. We defined cirrhosis as >−2 outpatient or >−1 inpatient ICD 9/10 code for cirrhosis or its complications (such as ascites, encephalopathy, varices with or without bleeding) and used the date of first instance of the code to define date of cirrhosis diagnosis. We obtained all-cause mortality data from VA Vital Status file.
Statistical analysis
We used landmark Fine and Gray model for competing risks constructed at two representative landmark times (3 and 5 years after the NAFLD diagnosis date)14 to examine the associations between evolution of fibrosis scores between NAFLD diagnosis and these landmark times and the subsequent risk of progression to HCC or the composite outcome through end of follow-up (December 31, 2018). This approach accounts for immortal time bias by defining exposures based on longitudinal history before the landmark, with outcome events that occurred after the landmark.20
Our main outcome was the time from the landmark to HCC or composite outcome of cirrhosis or HCC; we treated death as a competing risk in the Fine and Gray model. At each of the two landmarks for each endpoint (HCC or composite endpoint), the data only included at-risk patients (e.g., those who had NAFLD with at least two elevated ALT tests prior to the landmark time and who were alive without experiencing the outcome of interest) at that time. Patients who had their second elevated ALT after landmark time (immortal time bias), died or had event before landmark time, or had missing baseline FIB-4 values were excluded. Inclusion and exclusion criteria for study entry into the 3-year and 5-year landmark cohort analysis is detailed in Fig. S1. Patients who did not develop events (HCC, cirrhosis, or death) by the end of follow-up were censored. The model at a landmark time was based on the regression model from the specified landmark time. The predictors incorporated longitudinal information of a patient up to the landmark time, including change in FIB-4 as well as changes in age and metabolic traits based on time, updating variables prior to the landmark time. The use of two landmark times allowed us to evaluate whether the relationship between prior exposure and subsequent outcome is consistent over the follow-up period. At each landmark time, data were missing for some patients (for example, 10.6% of race/ ethnicity, 18.3% of ALT, 19.9% of AST, 23.8% of platelet count, and 12.5% of BMI data were missing at the 3-year landmark time). We used multiple imputation (PROC MI and PROC MIANALYZE in SAS) and fit the models to the five imputed datasets. The multiple imputation was done at each landmark time separately. The variables involved in the imputation model included all covariates and time-to-event outcomes corresponding to the landmark time.
In our preliminary analyses, we also evaluated the linear association between baseline and change in FIB-4 score as a continuous variable and subsequent development of HCC. Their effects were expressed as spline functions in the linear predictor of the Fine-Gray model in order to accommodate any non-linear association (an example result at the 3-year landmark time is shown in Fig. S2). The spline functions were approximately linear with a monotone increasing trend, suggesting that both higher baseline FIB-4 and increasing FIB-4 during the follow-up were associated with higher risk of HCC. However, as is often the case in non-parametric curve estimation, the confidence intervals were wide in the two ends of the curve where data are sparse with possible skewness and outliers. Given this, we opted to use a categorical version for FIB-4 in our final analysis to avoid instability that might be caused by outliers, skewness, and data sparsity. The chosen cut-off values for categorization are widely used in clinical research.21–23
Sensitivity and secondary analyses
In our secondary analyses, we examined whether associations between risk factors (including change in FIB-4) and HCC were modified by cirrhosis status using stratified analyses. We classified patients as having cirrhosis if they had a diagnostic code for cirrhosis prior to each landmark time. Some data, including FIB-4 values at landmark time, were missing for our cohort. To examine the validity of our results, we also performed a complete case analysis including only patients with non-missing FIB-4 values.
In clinical practice, NAFLD is often diagnosed by the presence of abnormal liver enzymes in the absence of other causes of liver disease (HBV, HCV, alcohol abuse, rare chronic liver disease), as is employed in the current study. However, patients with NAFLD can have persistently normal enzymes and our cohort construction strategy missed these patients. To examine the robustness of our results in this group, we conducted a secondary analysis using a separate retrospective cohort of patients with hepatic steatosis and persistently normal liver enzymes, as described in detail previously.15 Briefly, in this previous study, patients were classified as participants with hepatic steatosis and normal enzymes based on a validated natural language processing algorithm that identified hepatic steatosis in abdominal imaging reports in electronic medical records) and longitudinal laboratory data, respectively. For this cohort, we extended follow-up time to December 31, 2018 and constructed an analysis at the 3-year landmark time.
We used SAS version 9.4 (Charlotte, North Carolina) for all statistical analyses. p values <0.05 were considered significant. This study was approved by the Institutional Review Boards of Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Hospital, Houston, Texas. Informed consent was waived by the board because the study was register-based. For further details regarding the materials and methods used, please refer to the Supplementary CTAT Table.
Results
Clinical characteristics
Table 1 lists the characteristics of patients included in the 3- and 5-year landmark time cohorts for HCC. Mean follow-up time from 3-year and 5-year landmark time were 8.2 (SD, 2.8) and 6.6 (SD, 2.3) years. Among those included in the 3-year landmark analysis, the mean age at NAFLD diagnosis was 55.2 years (SD,12.9), 93.9% were male, 69.5% were white and 11.4% were African American. At baseline, the mean FIB-4 score was 1.20 (SD, 0.91), nearly three-quarters (74.7%) of patients had a FIB-4 value <1.45, 21.4% had FIB-4 value between 1.45 and 2.67, and 3.9% had FIB-4 >2.67. Medical comorbidities related to metabolic syndrome were frequently present; 57.6% had obesity, 51.9% hypertension, 47.5% had dyslipidemia, and 20.4% had diabetes. These proportions increased over time. For example, by year 3, 82.3% had dyslipidemia, 79.2% had hypertension, and 36.4% had diabetes.
Table 1.
Demographic and clinical characteristics of patients with NAFLD in the 3- and 5-year landmark cohorts.
| 3-year landmark cohort |
5-year landmark cohort |
|||
|---|---|---|---|---|
| Baseline | At 3-year landmark time n = 202,319 | Baseline | At 5-year landmark time n = 208,690 | |
|
| ||||
| n (%) | ||||
| Age in years, mean (SD) | 55.2 (12.9) | 58.2 (12.9) | 54.7 (12.9) | 59.7 (12.9) |
| Sex | ||||
| Female | 12,288 (6.1) | 12,288 (6.1) | 1,2444 (6.0) | 12,444 (6.0) |
| Male | 190,031 (93.9) | 190,031 (93.9) | 196,246 (94.0) | 196,246 (94.0) |
| Ethnicity/race | ||||
| White | 140,585 (69.5) | 140,585 (69.5) | 145,041 (69.5) | 145,041 (69.5) |
| African American | 23,047 (11.4) | 23,047 (11.4) | 24,639 (11.8) | 24,639 (11.8) |
| Hispanic | 11,665 (5.8) | 11,665 (5.8) | 12,073 (5.8) | 12,073 (5.8) |
| Other | 5,492 (2.7) | 5,492 (2.7) | 5,726 (2.7) | 5,726 (2.7) |
| Missing | 21,530 (10.6) | 21,530 (10.6) | 21,211 (10.2) | 21,211 (10.2) |
| Cirrhosis diagnosis | 344 (0.2) | 1,095 (0.5) | 271 (0.1) | 1,309 (0.6) |
| FIB-4 value | ||||
| Mean (SD) | 1.20 (0.91) | 1.45 (1.11) | 1.20 (0.86) | 1.53 (1.27) |
| <1.45 | 151,194 (74.7) | 91,582 (45.3) | 158,089 (75.8) | 86,934 (41.7) |
| 1.45–2.67 | 43,341 (21.4) | 45,096 (22.4) | 43,420 (20.8) | 50,419 (24.2) |
| >2.67 | 7,784 (3.9) | 10,592 (5.2) | 7,181 (3.4) | 12,601 (6.0) |
| Missing | 0 | 55,049 (27.2) | 0 | 58,736 (28.2) |
| Diabetes | 41,266 (20.4) | 73,592 (36.4) | 40,816 (19.6) | 83,112 (39.8) |
| Obesity, BMI >30 | 112,905 (57.6) | 105,657 (59.0) | 116,716 (57.9) | 106,888 (59.0) |
| Hypertension | 105,005 (51.9) | 160,228 (79.2) | 106,157 (50.9) | 171,443 (82.2) |
| Dyslipidemia | 96,084 (47.5) | 166,474 (82.3) | 97,331 (46.6) | 179,490 (86.0) |
FIB-4, fibrosis-4; NAFLD, non-alcoholic fatty liver disease.
Among patients included in the 3-year landmark analysis for HCC, 74.7%, 21.4%, and 3.8% of patients had a low, indeterminate, and high FIB-4, respectively. Most patients remained stable within the same FIB-4 risk group at 3-year landmark time (low-low 79.1%, indeterminate-indeterminate 61.3%, high-high 55.3%) (Table 2). In total, 20.9% of patients who were at low risk at baseline progressed to either indeterminate or high risk after 3 years, and 44.7% of those who were indeterminate at baseline progressed to high risk after 3 years. In those who were indeterminate at baseline, 22.4% declined to low risk after 3 years, and 44.7% declined from high to low/indeterminate risk after 3 years. We observed similar trends in FIB-4 between baseline and 5-year landmark time (Table S1).
Table 2.
Association between fibrosis risk groups, defined based on FIB-4 values at baseline and 3-year landmark time and subsequent risk of developing HCC.
| Baseline | 3-year landmark | n (%)a | HCC events | HCC incidence per 1,000 PY (95% CI)b |
|---|---|---|---|---|
|
| ||||
| Overall cohort | 202,319 | 473 | 0.28 (0.27,0.30) | |
| Low risk n = 151,194 | Low risk | 119,547 (79.1) | 56 | 0.05 (0.04–0.07) |
| Indeterminate risk | 29,542 (19.5) | 50 | 0.21 (0.16–0.28) | |
| High risk | 2,105 (1.4) | 11 | 0.76 (0.38–1.37) | |
| Indeterminate risk n = 43,341 | Low risk | 9,729 (22.4) | 20 | 0.26 (0.16–0.40) |
| Indeterminate risk | 26,581 (61.3) | 109 | 0.53 (0.43–0.64) | |
| High risk | 7,031 (16.2) | 90 | 1.90 (1.52–2.33) | |
| High riskd n = 7,784 | Low/indeterminate risk | 3,483 (44.7) | 21 | 0.86 (0.53–1.31) |
| High risk | 4,301 (55.3) | 116 | 4.56 (3.77–5.47) | |
|
| ||||
| Baselinec | 3-year landmark | n (%)a | Cirrhosis or HCC eventsa | Cirrhosis or HCC incidence per 1,000 PY (95% CI)b |
|
| ||||
| Overall cohort | 201,224 | 2,161 | 1.31 (1.25–1.37) | |
| Low risk n = 150,911 | Low risk | 119,401 (79.1) | 416 | 0.41 (0.37–0.45) |
| Indeterminate risk | 29,435 (19.5) | 317 | 1.34 (1.19–1.49) | |
| High risk | 2,075 (1.4) | 119 | 8.59 (7.11–10.28) | |
| Indeterminate risk n = 42,996 | Low risk | 9,672 (22.5) | 96 | 1.25 (1.01–1.52) |
| Indeterminate risk | 26,456 (61.5) | 453 | 2.22 (2.02–2.44) | |
| High risk | 6,868 (16.0) | 358 | 7.87 (7.07–8.73) | |
| High riskd n = 7,317 | Low/indeterminate risk | 3,407 (46.6) | 73 | 3.06 (2.40–3.85) |
| High risk | 3,910 (53.4) | 329 | 14.25 (12.76–15.89) | |
FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; PY, person years.
Percentage (%) calculated with number within FIB-4 group at baseline as the denominator.
Incidence rate calculated using imputed data.
Excluded patients who developed composite outcome (cirrhosis or HCC) prior to 3-year landmark time.
Data from high to low and indeterminate risk were combined due low number of patients and events in high to low-risk group.
We excluded patients who had already progressed to cirrhosis from the cohorts for 3- and 5-year landmark analyses focusing on the composite endpoint. Otherwise, the demographic and clinical characteristics of these cohorts were similar to those reported in Table 1.
Associations between changes in FIB-4 and subsequent risk of HCC
Among the 202,319 patients included in the 3-year landmark analysis, there were 473 patients diagnosed with HCC following the landmark time at an incidence rate (IR) of 0.28 (95% CI 0.27–0.30) per 1,000 person years (PY). Among 208,690 patients included in the 5-year landmark analysis, 403 developed HCC at an IR of 0.29 (95% CI 0.26–0.32) per 1,000 PY after the 5-year landmark time.
Table 2 describes the longitudinal change in FIB-4 and subsequent risk of developing HCC. The IR of HCC increased with an increase in FIB-4 value from baseline. At the 3-year landmark time, among patients who remained stable at low risk (low FIB-4 at baseline and at 3 years), HCC developed at an IR of 0.05 (95% CI 0.04–0.07) per 1,000 PY but increased to 0.21 (95% CI 0.16–0.28) and 0.76 (95% CI 0.38–1.37) per 1,000 PY in patients who were low risk at baseline but progressed to indeterminate or high risk, respectively. Patients who remained stable at indeterminate risk developed HCC at an IR of 0.53 per 1,000 PY (95% CI 0.43–0.64). In those who progressed from intermediate to high risk or remained stable at high risk after 3 years, HCC developed at an IR of 1.90 (95% CI 1.52–2.33) and 4.56 (95% CI 3.77–5.47), respectively. Cumulative risk of developing HCC stratified from the change of FIB-4 risk groups after 3-year landmark time are depicted in Fig. 1.
Fig. 1.
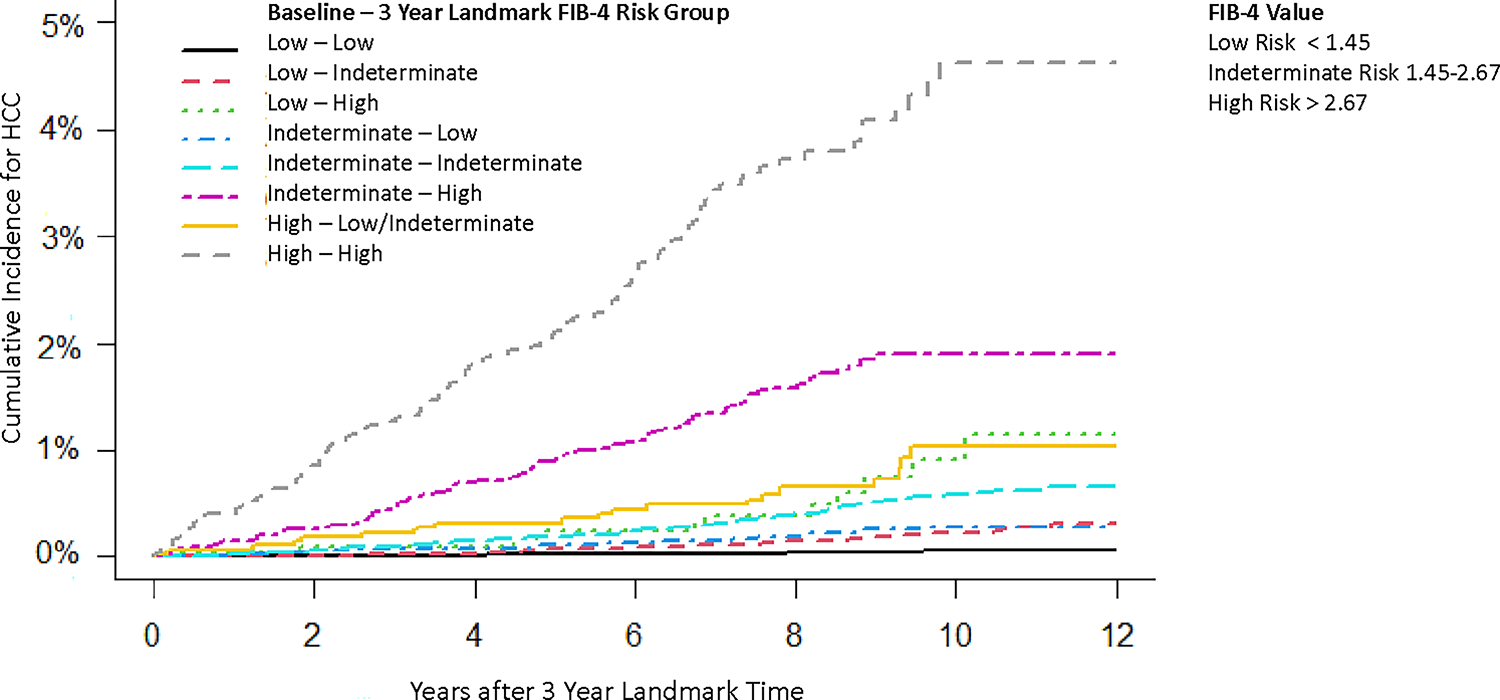
Cumulative risk of developing HCC stratified on the FIB-4 transition groups from the time of the second FIB-4 test at 3 years. FIB-4 value: low risk <1.45; indeterminate risk 1.45–2.67; high risk: >2.67. FIB-4, fibrosis-4; HCC, hepatocellular carcinoma. (This figure appears in color on the web.)
Table 3 displays the association between change in FIB-4 between baseline and at 3 years and subsequent risk of incident HCC adjusted for race/ethnicity and metabolic comorbidities. Compared to patients who remained stable at low risk, the IR of developing HCC was 8-fold (adjusted sub-distribution hazard ratio [adjusted HR] 7.96, 95% CI 5.67–11.19) and 58- fold (adjusted HR 57.69, 95% CI 40.50–82.18) higher in those who remained stable at indeterminate and high risk, respectively. Risk of developing HCC corresponded to a subsequent increase or decline in risk group over 3 years. Other significant predictors for increased IR of HCC irrespective of FIB-4 included diabetes (adjusted HR 2.65, 95% CI 2.12–3.30), hypertension (adjusted HR 1.83, 95% CI 1.18–0.69), and obesity with BMI >−30 (adjusted HR 1.43, 95% CI 1.16–1.76).
Table 3.
Association between changes in FIB-4 and risk of HCC and composite outcome after 3-year landmark in patients with NAFLD.
| HCC |
Cirrhosis or HCCa |
|||
|---|---|---|---|---|
| Unadjusted HR | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|
| ||||
| Change in FIB-4 | ||||
| Low-low | Reference | Reference | Reference | Reference |
| Low-indeterminate | 3.91 (2.60–5.90) | 3.45 (2.28–5.21) | 3.20 (2.72–3.77) | 2.87 (2.43–3.38) |
| Low-high | 14.06 (6.97–28.35) | 11.51 (5.70–23.25) | 20.12 (16.28–24.87) | 17.09 (13.77–21.20) |
| Indeterminate-low | 4.58 (2.63–7.96) | 3.92 (2.25–6.82) | 3.14 (2.48–3.98) | 2.75 (2.43–3.38) |
| Indeterminate-indeterminate | 9.48 (6.78–13.26) | 7.96 (5.67–11.19) | 5.24 (4.55–6.03) | 4.51 (3.90–5.20) |
| Indeterminate-high | 32.94 (23.07–47.01) | 26.52 (18.46–38.12) | 18.75 (16.21–21.69) | 15.67 (13.50–18.20) |
| High-indeterminate/low | 11.89 (6.41–22.05) | 10.35 (5.58–19.21) | 6.84 (5.24–8.83) | 6.12 (4.69–7.99) |
| High-high | 71.44 (50.47–101.13) | 57.69 (40.50–82.18) | 33.59 (29.00–38.91) | 28.64 (24.61–33.32) |
|
| ||||
| Clinical characteristics | ||||
| Race/ethnicity | ||||
| White | Reference | Reference | Reference | Reference |
| African American | 0.44 (0.28–0.68) | 0.53 (0.34–0.82) | 0.52 (0.44–0.62) | 0.62 (0.52–0.73) |
| Hispanic | 1.17 (0.82–1.69) | 1.40 (0.97–2.02) | 1.14 (0.97–1.33) | 1.36 (1.15–1.60) |
| Other | 0.88 (0.48–1.61) | 1.08 (0.58–1.98) | 0.74 (0.56–0.98) | 0.90 (0.68–1.19) |
| Diabetes | 3.99 (1.81–6.17) | 2.65 (2.12–3.30) | 3.54 (1.49–5.59) | 2.46 (2.24–2.71) |
| Obesity, BMI >30 | 1.57 (1.28–1.92) | 1.43 (1.16–1.76) | 1.69 (1.55–1.85) | 1.55 (1.42–1.70) |
| Hypertension | 4.72 (2.29–7.15) | 1.83 (1.18–2.86) | 3.29 (1.17–5.41) | 1.36 (1.15–1.62) |
| Dyslipidemia | 1.19 (0–3.44) | 0.52 (0.40–0.69) | 1.33 (0–3.42) | 0.65 (0.57–0.73) |
FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease.
Composite outcome of cirrhosis or HCC analyzed only in patients with NAFLD without evidence of cirrhosis prior to landmark time.
Associations between changes in FIB-4 and subsequent risk of cirrhosis or HCC (composite outcome)
We examined the risk of developing a liver-related event (cirrhosis or HCC as a composite outcome) with longitudinal change in FIB-4 over time (Table 2). Among 201,224 patients included in this analysis, 2,161 patients developed cirrhosis or HCC after the 3-year landmark time, with an overall IR of the composite endpoint of 1.31 per 1,000 PY (95% CI 1.25–1.37). The IR for the composite endpoint in those who remained stable at low-risk FIB-4 at 3 years was 0.41 per 1,00 PY (95% CI 0.37–0.45) but increased to an IR of 8.59 (95% CI 7.11–10.28) in those who progressed to high risk, with similar findings seen in those who were in the intermediate-risk group at baseline. As expected, those remained at high risk at 3 years had the highest IR of 14.25 (95% CI 12.76–15.89). In multivariable analyses, those who progressed from low risk to high risk had a 17.1-fold higher risk (adjusted HR 17.09, 95% CI 13.77–21.20) of developing cirrhosis/HCC than those who were stable at low risk. Patients who progressed from intermediate to high risk (adjusted HR 15.67, 95% CI 13.50–18.20) or remained stable at high risk (adjusted HR 28.6, 95% CI 24.6–33.2) also had a significantly higher risk of developing cirrhosis/HCC than those who were stable at low risk. Regression to a lower risk group conferred a reduction in the risk of developing cirrhosis/HCC. The association between change in FIB-4 and risk of developing HCC or the composite endpoint after the 5-year landmark time is shown in Table S2, with findings consistent with the 3-year landmark analysis.
Secondary and sensitivity analyses
Cirrhosis
Cirrhosis is the most important precursor lesion for HCC. We examined the association between changes of FIB-4 and HCC IR in patients with a diagnosis of cirrhosis at each landmark time (Table S3). At 3 years, 3,013 patients had a clinical diagnosis of cirrhosis. Of these, 22.9% (n = 690) had stable high FIB-4 (at baseline and 3-year landmark time). HCC IR was the highest in this group (26.62 per 1,000 PY, 95% CI 21.29–32.87). HCC IR was lowest at 3.43 per 1,000 PY (95% CI 1.88, 5.76) in patients with cirrhosis who had stable low FIB-4 over time. HCC IR fell in-between for other subgroups. In patients without cirrhosis, those who had a stable high FIB-4 had an HCC IR of 1.35 per 1,000 PY (95% CI 0.91–1.93).
Hepatic steatosis with normal ALT
We analyzed risk of cirrhosis and/or HCC over time in patients with normal liver enzymes and radiologic evidence of steatosis, shown in Table S4. In this subgroup of 2,377 patients with normal liver enzymes and hepatic steatosis, 23 developed cirrhosis and 3 developed HCC following the 3-year landmark time. The IR for HCC also increased with higher FIB-4 values over time, with the highest IR seen in those who remained stable at high risk at the 3-year landmark (16.21 per 1,000 PY, 95% CI 6.52–33.40).
Analyses in patients with non-missing FIB-4
In the primary analysis, we employed multiple imputation methods for missing FIB-4 values. To examine the validity of our results, we also performed a complete case analysis including only patients with NAFLD with non-missing FIB-4 values at the 3-year landmark time and calculated the subsequent IR for HCC (Table S5). Overall, we found similar HCC IR between FIB-4 groups in all patients with NAFLD and in those with non-missing FIB-4 values at the 3-year landmark time. For example, HCC IR for all patients with stable low FIB-4 was 0.05 per 1,000 PY (95% CI 0.04–0.07) and 0.06 per 1,000 PY (95% CI 0.05–0.09) in patients with only non-missing FIB-4 values. Similarly, in those who were stable at high risk, HCC IR was 4.56 per 1,000 PY (95% CI 3.77–5.47) in all patients and 4.61 per 1,000 PY (95% CI 3.73–5.65) in those with non-missing FIB-4 values.
We also compared clinical characteristics of patients with and without missing FIB-4 values at 3-year landmark time shown in Table S6. Sociodemographic differences were comparable but patients with missing FIB-4 values had a slightly lower prevalence of metabolic comorbidities. However, the number of incident HCC cases among patients with or without FIB-4 data remained similar.
Discussion
Risk stratification lies at the core of medical management for patients with NAFLD. Non-invasive markers of liver fibrosis have the potential to guide risk stratification in patients with NAFLD. In this large cohort of patients with NAFLD, we found longitudinal changes in FIB-4 to be associated with the magnitude of risk of subsequent liver-related endpoints. Progression or regression of fibrosis from baseline was associated with an incremental increase or decrease in the IR of HCC and the composite endpoint, respectively. Specifically, patients with persistently high FIB-4 values had a >50-fold higher risk of developing HCC than those with stable low values over time. Similarly, an increase in FIB-4 value over time was also strongly associated with development of the composite endpoint, cirrhosis and HCC. This study also highlights the need for concordant FIB-4 measurements before clinical decision making. With non-invasive tests for fibrosis readily available in clinical practice, serial measurements of fibrosis markers such as FIB-4 may be a cost-effective and easily implementable method for clinicians to identify patients with NAFLD who are at low risk of progressing to cirrhosis and HCC.
Identifying high-risk NAFLD subgroups in whom HCC surveillance provides a clear survival benefit has remained a significant challenge.24–26 Cirrhosis is the predominant risk factor for HCC in patients with NAFLD. However, we and others have reported that the annual risk of HCC in patients with NAFLD cirrhosis is lower than the 2 to 7% risk reported from cohorts with active viral (HCV or HBV) cirrhosis26 and can be variable depending on age and other factors. Better stratification of HCC risk in patients with NAFLD cirrhosis can allow for tailored HCC surveillance, possibly matching surveillance intensity and type with patients’ underlying risk. This approach can maximize the benefits of surveillance by shifting resource intensive efforts towards high-risk patients while reducing harms of testing (including false positive results) in patients at low risk of HCC. In our study, we found that the annual risk of HCC was as high as 2.5% in patients with cirrhosis and persistently high FIB-4 values, whereas it was below 0.3% in patients who had a cirrhosis diagnosis but persistently low FIB-4 values. These data could have important implications for tailored HCC surveillance in patients with NAFLD cirrhosis.
NAFLD-HCC that develops in the absence of cirrhosis remains a major clinical challenge. High-risk FIB-4, especially if it remained persistently high, was strongly associated with HCC risk in patients without a cirrhosis diagnosis. Furthermore, longitudinal FIB-4 measurements were strongly associated with developing the composite outcome, cirrhosis or HCC. Given the low IR for NAFLD-HCC and our inability to winnow down individuals at-risk of developing HCC, identifying patients at-risk of a liver-related event may be a reasonable approach in clinical practice. In those with NAFLD, our results demonstrate the clinical significance of detecting individuals with high-risk FIB-4 and suggest the potential to use this information to optimize risk stratification and management of this large group of patients. Our data can improve shared decision making between patients and their clinicians by providing a quantifiable risk assessment, including how it changes over time. Our results about longitudinal changes in fibrosis and risk of progression to cirrhosis or HCC can also help future cost-effectiveness studies in determining who among this heterogeneous NAFLD population may benefit from close monitoring. One of the best uses of these data in patients with NAFLD may be to set the stage for developing precision prevention strategies and care pathways tailored to the individual’s risk. We found progression of fibrosis to be a key determinant in progression risk for patients with NAFLD, and serial measurements of fibrosis should be integrated into the NAFLD care pathway. Recent guidelines recommend utilizing non-invasive fibrosis tests, including FIB-4 measurements, to screen for those at high risk of advanced fibrosis among patients with NAFLD.27 Moreover, a proposed joint societal algorithm for risk stratification in patients with NAFLD recommends serial non-invasive testing for fibrosis every 2–3 years and close monitoring in secondary care clinics (i.e. hepatology and endocrinology) in those with advanced fibrosis.24 Our data corroborate this systematic approach to managing NAFLD. We show that risk of developing the composite endpoint, cirrhosis or HCC, is magnified by at least 3- to 28-fold in those with at least one indeterminate FIB-4 value within 3 years compared to those with persistently low FIB-4 values. This risk further increases with duration and progression of fibrosis over time. Progression from low to high-risk FIB-4 values after 3 years was associated with a 14-fold higher risk for the composite endpoint, which demonstrates the time-dependent association of fibrosis and risk of cirrhosis or HCC in NAFLD. Patients with persistently high FIB-4 values after 3 years were found to have a 1.4% annual IR for developing a liver-related event, and represent at-risk individuals that may benefit from repeated FIB-4 measurements and surveillance. The strength of association between change in FIB-4 and HCC in patients with NAFLD suggests that incorporating longitudinal information on non-invasive markers may also strengthen the predictive ability of risk prediction models in future studies.
Our study has a few limitations. Our VA cohort was predominantly male which may limit the generalizability of incidence estimates. Further studies of non-veteran populations would be needed to confirm our results. However, the VA NAFLD cohort represents one of the largest population-based cohorts and the biological processes related to progression risk in this cohort are likely generalizable to cohorts outside the VA. FIB-4 values were missing in a significant subset of patients at different landmark times. However, there were no clinically meaningful differences in patients with and without repeat FIB-4 tests with regard to demographic factors or outcomes. Furthermore, there were no meaningful differences in our primary analyses that relied on multiple imputation and the secondary analysis using complete cases, providing support for the validity of the results. We might have missed early-stage HCCs, which would have biased our findings towards a lower estimate of HCC risk (i.e. actual HCC risk would be higher than observed in this study). Most HCCs progress to advanced and clinically symptomatic stages during a short period of time and would be identified by routine clinical care. Other limitations include potential misclassification of patients with advanced fibrosis or cirrhosis; however, given the FIB-4 has 90% negative predictive value for advanced fibrosis we expect this to have been minimal.18 Although many Veterans receive their care exclusively through VA facilities, some patients may seek their care outside the VA, resulting in incomplete capture of their data.
In conclusion, we found longitudinal changes of non-invasive markers for liver fibrosis were associated with future risk of cirrhosis and HCC in a large US population of patients with NAFLD. Persistently high-risk FIB-4 may be an additional risk stratification tool. Integrating serial measurements of non-invasive tests for fibrosis in the care pathway for patients with NAFLD can help tailor risk prevention. These data also provide valuable information for future cost-effectiveness studies determining which high-risk NAFLD subgroups will benefit from frequent monitoring and HCC surveillance.
Supplementary Material
Highlights.
An increase in FIB-4 value over time was associated with risk of developing cirrhosis and HCC in patients with NAFLD.
High FIB-4 (>2.67) at baseline and 3 years linked to a >50- fold higher risk of HCC than persistently low FIB-4 (<1.45) values.
75% of patients were at low, 21% indeterminate (1.45–2.67) and 4% high risk of advanced fibrosis based on baseline FIB-4 value.
More than 50% of patients with NAFLD remained within the same FIB-4 risk group after 3 years.
Repeating FIB-4 measurements in clinical practice was strongly associated with progression to cirrhosis and HCC.
Impact and implications.
Tools to stratify the risk of HCC development in patients with NAFLD are currently lacking. The fibrosis-4 (FIB-4) score is a widely available non-invasive test for liver fibrosis, a primary determinant of the development of cirrhosis and HCC. In a large retrospective cohort of patients with NAFLD, we found that serial changes in FIB-4 over time were strongly associated with progression to cirrhosis and HCC. Integrating serial measurements of non-invasive tests for fibrosis into the care pathway for patients with NAFLD could help tailor HCC risk prevention.
Acknowledgments
We would like to thank Cancer Prevention & Research Institute of Texas, National Cancer Institute, and The VA Health Services Research and Development, Center for Innovations in Quality, Effectiveness and Safety for their support for this work.
Financial support
This material is based on work supported by the Cancer Prevention & Research Institute of Texas (CPRIT) RP200633 (GC, JRK, XY, FK). This work is also supported by the National Cancer Institute’s (NCI) U01 CA230997, U01 CA271887, R01 CA256977 and P30DK056338 and Department of Veterans Affairs, Health Services Research and Development, Center for Innovations in Quality, Effectiveness and Safety (IQuESt) (CIN 13–413), at Michael E. DeBakey VA Medical Center, Houston (JRK, XY, FK).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUDIT-C
Alcohol Use Disorders Identification Test
- BMI
body mass index
- CCR
Central Cancer Registry
- CDW
Corporate Data Warehouse
- FIB-4
fibrosis-4
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IR
incidence rate
- NAFLD
non-alcoholic fatty liver disease
- PY
person years
- VA
Veterans Affairs
Footnotes
Conflict of interest
Dr. Kanwal and Kramer are investigators at IQuESt. The authors have no other relevant financial disclosures or conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.10.034.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author [FK]. The data are not publicly available due to privacy/ ethical restrictions.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [3].Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- [4].Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national Health and nutrition examination survey data. Am J Gastroenterol 2017;112:581–587. [DOI] [PubMed] [Google Scholar]
- [5].Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol 2016;14:301–308. e301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012;55:1809–1819. [DOI] [PubMed] [Google Scholar]
- [7].Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002;36:1349–1354. [DOI] [PubMed] [Google Scholar]
- [8].Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828–1837 e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2020;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cholankeril G, Perumpail RB, Pham EA, Ahmed A, Harrison SA. Nonalcoholic fatty liver disease: epidemiology, natural history, and diagnostic challenges. Hepatology 2016;64:954. [DOI] [PubMed] [Google Scholar]
- [11].Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol 2020;20:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–1625 e1612. [DOI] [PubMed] [Google Scholar]
- [14].Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020;71:44–55. [DOI] [PubMed] [Google Scholar]
- [15].Natarajan Y, Kramer JR, Yu X, Li L, Thrift AP, El-Serag HB, et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology 2020;72:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- [17].Redman JS, Natarajan Y, Hou JK, Wang J, Hanif M, Feng H, et al. Accurate identification of fatty liver disease in data Warehouse utilizing natural language processing. Dig Dis Sci 2017;62:2713–2718. [DOI] [PubMed] [Google Scholar]
- [18].Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Agarwal P, Moshier E, Ru M, Ohri N, Ennis R, Rosenzweig K, et al. Immortal time bias in observational studies of time-to-event outcomes: assessing effects of postmastectomy radiation therapy using the national cancer database. Cancer Control 2018;25:1073274818789355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McPherson S, Hardy T, Dufour J-F, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Official J Am Coll Gastroenterol ACG 2017;112: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology 2020;158:200–214. [DOI] [PubMed] [Google Scholar]
- [23].Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020;73:1023–1029. [DOI] [PubMed] [Google Scholar]
- [24].Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 2019;157:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158: 1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- [27].European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guideline P, Chair, representative EGB, Panel m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75: 659–689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [FK]. The data are not publicly available due to privacy/ ethical restrictions.


