Abstract
Human cognitive performance is a key function whose biological foundations have been partially revealed by genetic and brain imaging studies. The sleep electroencephalogram (EEG) is tightly linked to structural and functional features of the central nervous system and serves as another promising biomarker. We used data from MrOS, a large cohort of older men and cross-validated regularized regression to link sleep EEG features to cognitive performance in cross-sectional analyses. In independent validation samples 2.5–10% of variance in cognitive performance can be accounted for by sleep EEG features, depending on the covariates used. Demographic characteristics account for more covariance between sleep EEG and cognition than health variables, and consequently reduce this association by a greater degree, but even with the strictest covariate sets a statistically significant association is present. Sigma power in NREM and beta power in REM sleep were associated with better cognitive performance, while theta power in REM sleep was associated with worse performance, with no substantial effect of coherence and other sleep EEG metrics. Our findings show that cognitive performance is associated with the sleep EEG (), with the strongest effect ascribed to spindle-frequency activity. This association becomes weaker after adjusting for demographic () and health variables (), but its resilience to covariate inclusion suggest that it also partially reflects trait-like differences in cognitive ability.
Keywords: Sleep EEG, Cognition, Health, Sleep spindle, Intelligence
1. Introduction
Cognitive performance in humans is a fundamental neuropsychological function which predicts both sociological outcomes (Gottfredson, 1997; Kuncel and Hezlett, 2010; Strenze, 2007) and the development or progression of disease (Deary et al., 2021). Human cognitive performance varies, among others, as a consequence of genetic factors (Plomin and von Stumm, 2018), long-acting environmental influences like schooling or toxin exposure (Protzko, 2017; Ritchie and Tucker‑Drob, 2018), proximal environmental factors such as stress or sleep deprivation (Alhola and Polo‑Kantola, 2007; Lim and Dinges, 2010; Wickens et al., 2015), as well as various somatic and psychiatric illnesses (Karlamangla et al., 2014; Kendler et al., 2018; Wraw et al., 2018).
Finding the biological foundations of individual differences in cognitive performance has been a mainstay of neuroscience research for the past decades (Haier, 2016; Karlamangla et al., 2014). Early studies of human cognitive biomarkers have typically been conducted in small samples analyzed with non-standardized methods, a problem generally present in the psychological (Giner‑Sorolla, 2012; Lilienfeld, 2017), and neuroscience literature (Cohen, 2017; Hong et al., 2019). When biomarkers of human cognition have low effect size – that is, individual differences in cognitive performance arise due to the summed effects of many small biological differences – then the signal-noise ratio of the detected associations is poor in small samples, leading to many false positive findings which do not replicate while the true associations may remain undetected (Button et al., 2013; Szucs and Ioannidis, 2017). Recently, however, large-scale studies have explored some biomarkers of human cognition, especially genetic (Davies et al., 2018; Savage et al., 2018) and magnetic resonance imaging (MRI) related (Deary et al., 2022; Kharabian Masouleh et al., 2019; Marek et al., 2022; Pohl et al., 2019; Ritchie et al., 2015) features. These studies have revealed some biological characteristics linked to various aspects of human cognitive performance, however, as they account for a small fraction of the variance, they currently do not provide a full mechanistic description about the origin of individual differences in cognitive performance.
The sleep electroencephalogram (EEG) is another important biomarker of cognitive performance. This is for two main reasons. The first reason that while the sleep EEG changes substantially over the course of the human lifespan (Carrier et al., 2001; Feinberg and Campbell, 2013, 2010; Sun et al., 2019), sleep EEG measures obtained from the same individual on different nights within a reasonably short time period are highly similar (Finelli et al., 2001; Reynolds et al., 2019; Tan et al., 2001, 2000) even if the night or the preceding day is perturbed (De Gennaro et al., 2005), while they exhibit substantial inter-individual variability. In other words, sleep EEG features are trait-like, which renders them strong potential candidate biomarkers of other temporally stable traits. The second reason relates to the biological significance of the EEG signal. The sleep EEG, when recorded from the scalp, reflects the joint activity of relatively large neuronal assemblies in the underlying brain tissue with excellent temporal (although limited spatial) precision. Thus, the sleep EEG can provide information about both the structural (Buchmann et al., 2011; Mander et al., 2017b; Saletin et al., 2013; Vien et al., 2019) and functional (Fernandez and Lüthi, 2020; Mander et al., 2017a) features of the central nervous system which are potentially not available for other imaging modalities. Notably, certain oscillations detectable from the sleep EEG – most importantly, slow waves and sleep spindles – have physiologically clearly described generating mechanisms and, in part, functions (Fernandez and Lüthi, 2020; Tononi and Cirelli, 2014). If these oscillations are associated with cognitive performance or another human characteristic, then this provides mechanistic information about the biological foundations of this trait and may highlight intervention targets if the trait is clinically relevant. The trait-like nature and intimate link to both structural and functional features of the central nervous system render the sleep EEG a highly promising biomarker of other individually stable human characteristics linked to the central nervous system, such as cognitive performance.
Despite its potential, the sleep EEG is somewhat underutilized in the search for cognitive biomarkers, although this is changing with the advent of large, freely available sleep EEG cohorts (Redline and Purcell, 2021). Some literature, however, has clearly linked the sleep EEG to cognitive performance. Notably, the sleep EEG can be linked to cognitive performance for at least two different reasons, both of which are potentially significant, although more so for two different fields of scientific inquiry and with different applications.
First, both sleep and cognitive performance changes as a function of age (Mander et al., 2017a; Salthouse, 2004), as well as in association with various common health conditions (García‑Marín et al., 2021; Wraw et al., 2018). Thus, any association between sleep features and cognitive performance can arise due to age or poor health serving as either a common cause or a mediating factor in both. For example, aging may lead to both a reduction of slow wave sleep and worse cognitive performance (common cause), or obesity may lead to poor sleep and this in turn may lead to poor cognitive performance (mediation). These associations are likely to arise in geriatric sleep cohorts where mean participant age is high, its variability is considerable and participants frequently suffer from age-related ailments. A recent landmark study (Djonlagic et al., 2021) has explored the association between the sleep EEG and cognitive performance considering these issues. The study found that numerous features of the sleep EEG, such as features of sleep spindles and slow waves, were associated with cognitive performance, even after correcting for chronological age and health-related covariates. It also reported that sleep EEG features associated with higher age are also generally associated with worse cognitive performance, even after correcting for age.
Second, a line of research has linked sleep in general and the sleep EEG in particular to psychometric intelligence (Bódizs et al., 2005; Schabus et al., 2006; Ujma et al., 2016, 2015, 2014), generally in healthy young participants where comorbidities were not likely an issue. A meta-analysis linked the amplitude of sleep spindles to higher scores on IQ tests (Ujma, 2018). Individual studies found that spectral features were (Geiger et al., 2011; Ujma et al., 2017), but coherence (Ujma et al., 2019) was not associated with IQ test performance in healthy participants. These studies also highlighted the role of spindle-frequency oscillations in cognitive functioning.
Our goal in the current study was to extend previous knowledge about the relationship between the sleep EEG and cognition by unifying the most advantageous aspects of previous studies. Our study used a large sample of over 3000 participants with full-night PSG recordings. It was designed to be multivariate and hypothesis-free, using a data-driven, cross validated approach to identify sleep features which can be discovered and replicated as correlates of cognition. Finally, it used a stepwise application of demographic and health-related covariates to identify links between sleep and cognition which are underlain by these factors.
2. Methods
2.1. Electroencephalography recordings
For our principal exploratory analyses, we used data from the MrOS Sleep Study. MrOS Sleep is an ancillary study of the parent Osteoporotic Fractures in Men Study (MrOS). Details of the protocol of the study have been documented in previous publications (Blank et al., 2005; Orwoll et al., 2005). Briefly, between 2000 and 2002, 5994 community-dwelling men 65 years or older were enrolled at 6 clinical centers in a baseline examination (mean age in current sample: 73.06 years, SD=5.55 years). Between December 2003 and March 2005, 3135 of these participants were recruited to the Sleep Study when they underwent full unattended polysomnography and 3 to 5-day actigraphy studies (Blackwell et al., 2011; Zhang et al., 2018). In these studies, EEG was recorded from C3 and C4 with a sampling frequency of 256 Hz and a high-pass hardware filter of 0.15 Hz. Both channels were recorded with gold cup electrodes, originally referenced to Fpz, and re-referenced to the contralateral mastoids. All recordings were visually scored by experts (see Djonlagic et al. (2021) for further recording details). Artifacts were automatically rejected. Artifact rejection was performed on a 4-second basis using Hjorth parameters. The three Hjorth parameters were calculated for all 4-second epochs and those deviating from the within-participant mean of the given vigilance state (NREM or REM) by at least 2 standard deviations were rejected as artifactual (Purcell et al., 2017). The selection of participants for the current study is illustrated on Fig. 1.
Fig. 1.
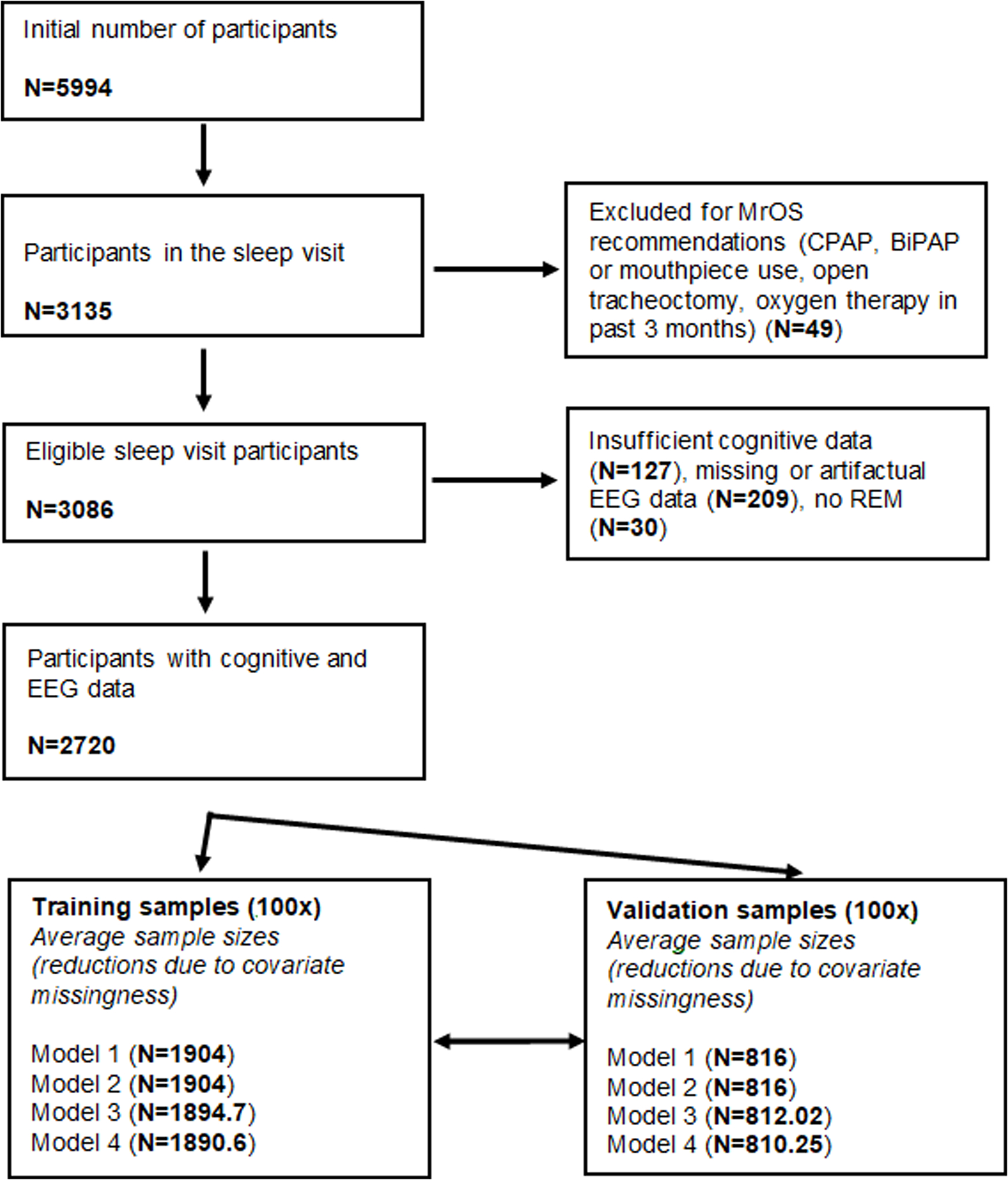
Sample size flowchart. Fractional sample sizes in the training and validation sample sets reflect the fact that across the 100 random splits some variation in sample size was observed.
2.2. EEG feature extraction
From the EEG data, we extracted a set of features as intended predictors of general mental functioning. This set of predictors was selected as plausible correlates of cognitive performance based on previous literature (see Introduction). Some additional EEG features were calculated for exploratory analyses designed to discover if simply calculable global EEG features are associated with cognition.
Power spectral density (PSD), 0–48 Hz with 0.25 Hz bin resolution, separately for C3 and C4 and in REM and NREM sleep. All PSD estimates were log10 transformed (to normalize variances) and relativized (to eliminate inter-individual PSD differences due to voltage differences due to e.g. skull thickness). PSD was relativized by subtracting the mean of all PSD values (across bins, within each participant, channel and sleep state) from all individual PSD values. PSD was selected as a candidate measure based on previous studies (Djonlagic et al., 2021; Geiger et al., 2011; Ujma et al., 2017) which linked PSD including, but not limited to, the sleep spindle frequency range to cognitive outcomes.
PSD laterality: C3-C4 PSD difference in both NREM and REM, calculated from the averaged, relativized data. Laterality was selected as an experimental candidate measure, based on previous studies showing considerable hemispheric lateralization and topographically specific correlates of neurocognitive performance (Bódizs et al., 2017; Doucette et al., 2015).
NREM-REM PSD difference on the mean of both channels, calculated from the averaged, relativized data. NREM-REM PSD differences were chosen as experimental measures in order to investigate whether vigilance state specificity of frequency components is associated with cognition.
Weighted phase lag index (wPLI), 0–48 Hz with 0.25 Hz bin resolution, calculated between C3 and C4 in REM and NREM sleep. wPLI (Vinck et al., 2011) is a measure of signal synchronization in two sources which is designed to penalize zero phase lags to reduce the effects of spurious signal similarity due to volume conduction. Although wPLI was found not to be associated with cognition in a previous smaller study (Ujma et al., 2019), this finding needed replication in a better powered sample.
Hjorth parameters (Hjorth, 1970) activity, mobility and complexity on both channels, in REM and NREM separately. Hjorth parameters are simple measures describing the stationarity of a signal, and estimate the total power, the mean frequency and the bandwidth of the signal, respectively. Hjorth parameters are selected for inclusion because as global descriptors of the EEG waveform they might capture relevant inter-individual differences, and because they were already calculated for artifact detection (see also Section 2.1).
The Modulation Index (Tort et al., 2010) between delta (0.5–4 Hz) phase and sigma (10–16 Hz) power on both channels, in REM and NREM separately. Modulation Index quantifies the degree to which a higher-frequency signal is modulated by a lower-frequency signal. In this case, this measure was intended to estimate the degree to which sleep spindles are coupled by slow waves (Gonzalez et al., 2018) a feature which a set of previous studies found to be associated with cognition (Bódizs et al., 2005; Djonlagic et al., 2021; Hahn et al., 2020).
Linear and quadratic overnight trends for all previous predictors. These were included as experimental predictors in order to investigate whether the rhythm and strength of homeostatic and circadian processes, as indexed by sleep EEG features (Bódizs et al., 2022; G Horváth et al., 2022), are associated with cognitive performance. These were estimated by regressing time since recording start at the start of each epoch on the predictor values calculated from each epoch. For this we estimated magnitude-squared coherence for each epoch, using the mscohere() MATLAB function and splitting each epoch into eight overlapping windows to gain a within-epoch estimate of coherency. In order to simplify analyses, for PSD and wPLI we calculated the average delta (0.1–4 Hz), theta (4–8 Hz), alpha (8–10 Hz), low sigma (10–13 Hz), high sigma (13–16 Hz), beta (16–25 Hz) and gamma (25–48 Hz) power in each epoch instead of regressing time on power in each individual frequency bin. Frequency bins on the borders between frequency bands were included in the calculation of the higher frequency band.
All features (except wPLI) were calculated for each 4-second epochs in the signal with 50% overlap and then averaged across windows to yield a single value in each participant. For wPLI, the imaginary part of the cross-spectrum was calculated for each window in this manner, and then averaged by using real components as weights according to the formula provided by Vinck et al. For PSD and wPLI, Hamming windowing was used.
For a less fine-grained analysis of EEG spectral components, we averaged PSD estimates within the following ranges to obtain band power: delta (0.1–4 Hz), theta (4–8 Hz), alpha (8–10 Hz), low sigma (10–13 Hz), high sigma (13–16 Hz), beta (16–25 Hz), gamma (25–48 Hz). Power at band boundaries was always assigned to the higher-frequency band.
2.3. EEG spectral parametrization
Spectral components of the sleep EEG do not necessarily reflect actual oscillations (Bódizs et al., 2021; Donoghue et al., 2020). Much of the variance in spectrum of the sleep EEG can be modelled with just two parameters, a spectral intercept and a slope coefficient describing the exponent of the 1/f power law function (aperiodic components). Oscillations cause a deviation from this deterministic pattern (periodic components). We used FOOOF (Donoghue et al., 2020) (“Fitting Oscillations & One Over f”, available at https://github.com/fooof-tools/fooof) to decompose absolute spectra into periodic and aperiodic components, estimating the power law function in the full (0.25–48 Hz) range. We allowed periodic components (spectral peaks) with a width of 0.5–6 Hz and a minimum peak height of 2 standard deviations above the aperiodic spectrum. We discarded participants for whom periodic and aperiodic components accounted for less than 95% of the variance in the power spectrum (). Spectral parametrization was performed separately on both EEG channels and in NREM and REM sleep. Based on the zero-order correlations between cognitive performance and EEG power (see Results) we searched for peaks in the REM theta, REM beta and NREM sigma ranges. The bandwidth, frequency and power (height above the aperiodic spectrum) of these peaks was saved. If a participant did not have a detected peak in these ranges, the value of the spectral peak was set to 0 and the value of the bandwidth and frequency were set to the sample average. If a participant had multiple peaks in these frequency ranges, we retained the one closest in frequency to the maximum of the zero-order correlation between power spectral density and cognitive performance (6.5 Hz in REM theta, 23 Hz in REM beta and 14 Hz in NREM sigma).
2.4. Cognitive data
Cognitive testing typically occurred before visits to the sleep laboratory, with some flexibility in the protocol. Participants completed cognitive tests on average 6.9 days (SD=15.8 days) before the sleep visit. Concurrent with the sleep study, participants filled out three cognitive tests: the Modified Mini-Mental State Test (3MS), Trails B, and Digit Vigilance (DV). 3MS (Teng and Chui, 1987) is a global test of global functioning and orientation. Trails B (Reitan, 1958) is a timed trail-making test which measures attention, visual scanning and executive functions. The Digit Vigilance test (Lewis and Rennick, 1979) requires participant to cross out as quickly as possible each ‘6′ in a large matrix of numbers, if they are followed by a larger number. It is a test of vigilance and visual tracking ability. From these tests, we considered the following variables: 3MS total score, Trails B completion time, DV completion time, and DV omission errors (false negatives). 3MS total scores were square root-transformed to improve normality and their inverse was taken to ensure that in all tests higher scores mean worse performance. The other scores were used without transformation.
In this sample, raw cognitive test scores may have been strongly affected by factors other than general mental functioning, most notably age and health. Consequently, we regressed out a set of covariates from the raw scores. Because (with the exception of age) the route of causation between the confounding variables and test scores is unclear, we explored four models with four, increasingly extensive sets of covariates:
Model 1: no covariates
Model 2: regressing out technical/demographic variables (recording site, age including quadratic, cubic and fourth-order effects, and race/ethnicity)
Model 3: regressing out technical/demographic variables, plus health (medication use, systolic and diastolic blood pressure, caffeine, alcohol and cigarette consumption before sleeping, comorbidities listed at the baseline visit [arthritis/gout, cancer, cataracts, congestive heart failure, diabetes, glaucoma, kidney stones, osteoporosis, Parkinson’s, prostatitis, stroke], comorbidities listed at the Sleep Study [angina pectoris, peripheral, cerebral or coronary disease, arterial fibrillation, heart rate problems, sleep disorders]). At both the baseline and sleep visits, participants answered a questionnaire about comorbidities with the following formula: “Have you ever had (disease name)?”, except angina pectoris, which was measured with the Rose Angina Questionnaire. In order to reduce missing data, we considered participants not providing information about a comorbidity to not have that condition. We also used the use of 49 common medications (based on a physician’s review of the participant’s common medications presented during a personal visit) as covariates (see Supplementary text for a detailed list).
Model 4: regressing out demographic/technical variables, physical health and quality of life, including mental health and sleep symptoms (SF12 Modified Physical/Mental Summary Scale score, Geriatric Depression Scale score, Goldberg Anxiety and Depression Scale scores, Epworth Sleepiness Scale scores, Pittsburgh Sleep Quality Inventory total score, Functional Outcomes of Sleep Questionnaire total score). For simplicity, we refer to the covariates only included in Model 4 as ‘quality of life’.
In all models, we also regressed out the effect of confounders from the EEG predictors.
2.5. Statistical analysis
In our main analyses, the principal question was: to what extent can cognitive performance be predicted from sleep EEG biomarkers? In order to answer this question, we used sleep EEG features as independent variables and cognitive performance as the dependent variable in regularized regression models (Tibshirani, 1996; Zou and Hastie, 2005). Regularized regression is an iterative learning algorithm which minimizes the following function:
In plain words, regularized regression performs ordinary linear regression, but it also assigns a penalty to the prediction error, which increases as a function of 1) more predictors with non-zero regression coefficients in the model 2) an iteratively changing penalty parameter. Regularized regression can use L1 (LASSO regression) or L2 (ridge regression) regularization, or a combination of the two (elastic net regression. The combination of L1 and L2 regularization can be tuned with a parameter yields ridge regression and yields LASSO regression, while interim values yield elastic net regularization. Due to regularization, all of these procedures are able to handle more predictors than there are cases, while robust results are ensured and overtraining is protected against by cross-validation.
We randomly split all MrOS participants into a training (70%) and a validation (30%) sample. The size of training samples averaged and validation samples (depending on covariate availability, see also Fig. 1). Regularized regression models were fitted with 10-fold cross-validation in the training sample. We split the training sample into 10 random subsamples and iteratively fitted the regression model using a range of values (penalty parameters) after pooling nine of them, with the tenth serving as a holdout sample to assess performance. This was repeated in all combinations of subsamples. Finally, the model fitted in this way was carried forward to the validation sample which was not used during training. The effect size of interest (henceforth referred to as validity) was the correlation between predicted and actual cognitive functioning in the validation sample. We performed this analysis 100 times to explore the effect of randomly assorting participants into training and validation samples. We repeated this procedure for dependent and independent variables after regressing out the effects of each covariate set (Model 1–4), and across a range of values (0–1 with increments of 0.1) that switch between ridge (), elastic net () and LASSO () regressions, yielding 44 model specifications (covariate sets and ) and 100 models with random subsamples for each specification.
Regularized regression was performed using the cvglmnet() MATLAB function, based on the glmnet() package (Friedman et al., 2010). Due to missingness of data, the number of participants slightly varied in the validation samples, but it was on average in Model 1 and 2, in Model 3 and in Model 4 (minima: , respectively).
2.6. Data and code availability
All PSG data are freely available via the National Sleep Research Resource (http://sleepdata.org). Model results and code used for analyses are available on Zenodo at 10.5281/zenodo.7684266.
3. Results
3.1. Covariate effects on cognition and sleep
Potential covariates accounted for up to 20% of the variance in cognitive scores, with the least in Digit Vigilance errors and the most in Trails B completion time (Fig. 2). About two thirds of this variance in 3MS, Digit Vigilance completion time and Trails B completion time and virtually all of this variance in Digit Vigilance errors was accounted for by demographic covariates alone. Supplementary Figs. 1–4 provide detailed data about the association between covariates and power spectral density. Supplementary Fig. 5 illustrates the relationship between covariates and cognitive test scores.
Fig. 2.
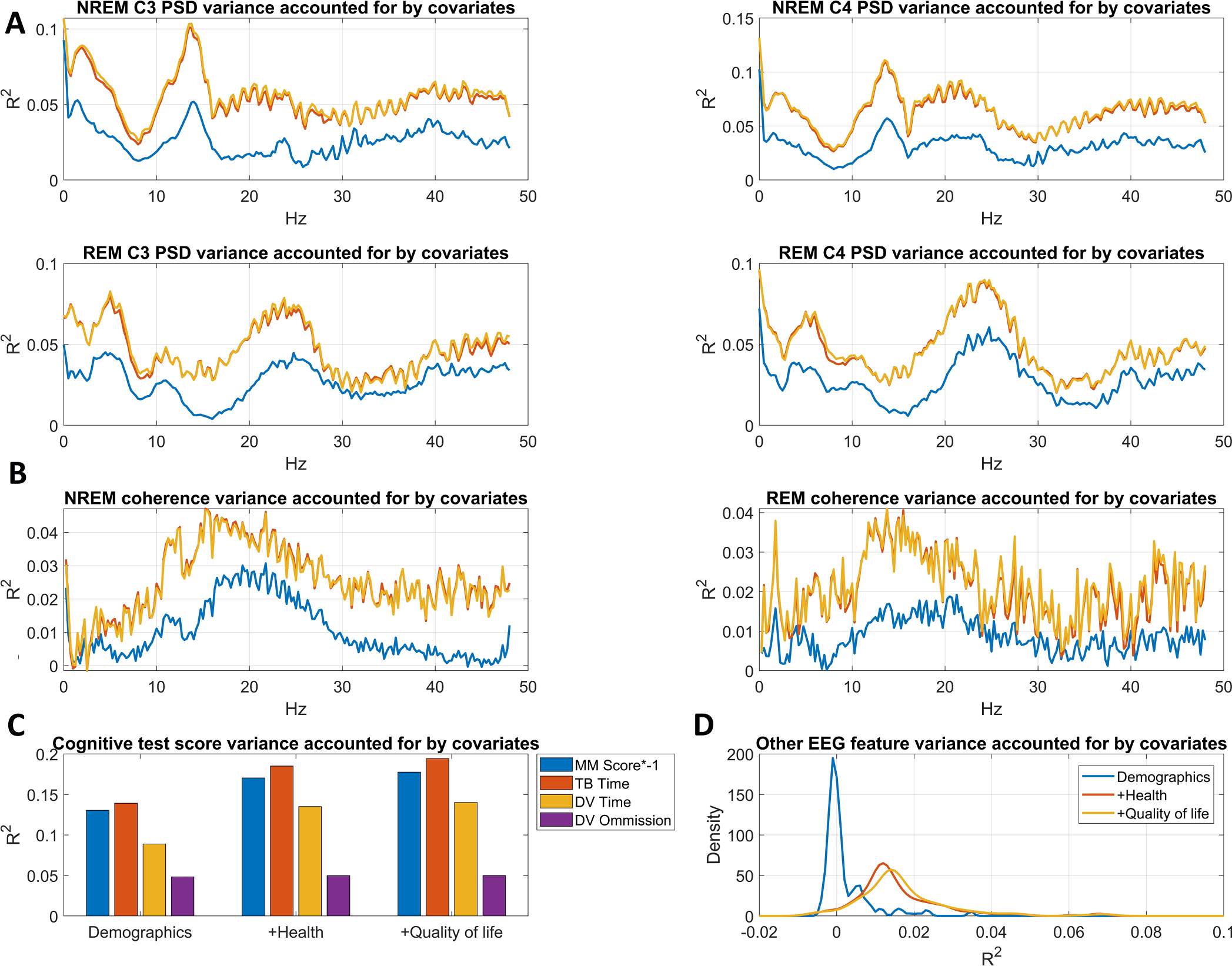
Variance accounted for by the three covariate sets (Model 2: demographic covariates added, Model 3: health covariates added, Model 4: quality of life covariates added). Panel A shows power spectral density variance accounted for as a function of frequency. Panel B shows wPLI variance accounted for as a function of frequency. Panel C shows variance accounted for in raw cognitive test scores. Panel D shows the distribution of R2 values across the 190 other EEG features. Note that the color codes used for the three models on this panel also apply for panels A and B.
In sleep measures, potential covariates accounted for up to 10% of the total variance, with about half attributable to demographics. Covariates accounted for the most variance in NREM delta, NREM sigma, REM theta and REM beta power and in wPLI values from a broad frequency range encompassing the sigma and beta bands. Notably, health-related covariates affected sleep measures in a very similar manner to age, and quality of life accounted for very little additional variance, although it encompassed explicit questionnaire-based measures of sleep.
3.2. Principal component analysis of test scores
Cognitive test scores were all positively correlated, with or without regressing out covariates (Supplementary Table S1). In all models, we performed principal component analysis on the unstandardized residuals of cognitive scores. In all models, a single principal component with eigenvalue>1 emerged. This first principal component accounted for 42.5–46.3% of the variance, with values decreasing somewhat with the inclusion of more confounders. In each model, we extracted principal component scores on this first unrotated principal component as the measure of general cognitive performance. Measures from the 4 models were highly correlated (Supplementary Table S2, ), in line with the observation that the confounders only accounted for a modest amount of variance in test scores.
3.3. Correlations between the sleep EEG and cognition
In our initial analysis, we calculated zero-order correlations between general cognitive functioning and sleep measures in the entire MrOS sample. Results were in line with previous reports (Djonlagic et al., 2021; Ujma et al., 2017). In NREM sleep, higher relative power in the alpha, sigma and beta range (7.75 Hz-22.5 Hz) range was associated with better cognitive performance, with a clear peak in the fast spindle range around 14 Hz. High-frequency power (>26 Hz) was associated with lower cognitive performance. In REM sleep, power in the beta range (19.5–32 Hz) was associated with better cognitive performance, while higher power in the theta (~3.25–8.25 Hz) range was associated with lower performance. (Frequencies reported for Model 3 by the broadest possible definition including associations from any channel.) This pattern of results was consistent across the four models with different covariate sets, although effect sizes were reduced in more heavily corrected models and only reached r>0.1 for the NREM sigma association in Model 4 (demographic, health and quality of life covariates).
We found no consistent correlations between cognitive function and wPLI values or other EEG features. Fig. 3 illustrates bivariate correlations between cognitive performance and sleep EEG measures.
Fig. 3.

Correlations between general cognitive performance, power spectral density (Panel A), wPLI-based functional connectivity (Panel B) and other EEG features (Panel C). Correlations are shown after increasingly strict covariate sets: no covariates (Model 1), demographic covariates added (Model 2), health covariates added (Model 3), quality of life covariates added (Model 4). For PSD and wPLI, correlations are shown as a function of frequency. For other EEG features, only the distribution of correlation is shown for simplicity, as no correlation is significant after adjusting for multiple comparisons. On all plots, black lines indicate the critical correlation coefficient.
3.4. The sleep EEG predicts cognitive performance
In our initial models, we used ridge regression () to predict general cognitive functioning. We used 70% of MrOS participants as training and 30% as validation, repeating this process 100 times to get an estimate of the variation in model performance due to random sampling of the training and validation samples. Fig. 4 provides a detailed illustration of prediction performance. Across the 100 random samples, the mean validity (out-of-sample correlation between predicted and actual cognitive performance) amounted to 0.283 (SD=0.026, range 0.219–0.359) in Model 1 (no covariates), 0.186 (SD=0.027, range 0.084–0.246) in Model 2 (demographic covariates added), 0.155 (SD=0.026, range 0.077–0.211) for Model 3 (health covariates added), and 0.152 (SD=0.026, range 0.067–0.212) for Model 4 (quality of life covariates added) (Fig. 4, Panel A). Using Spearman correlations to estimate validities made minimal difference to the findings (mean validity for Model was 0.276, for Model 0.171, for Model 3 0.146, and for Model 4 0.147).
Fig. 4.
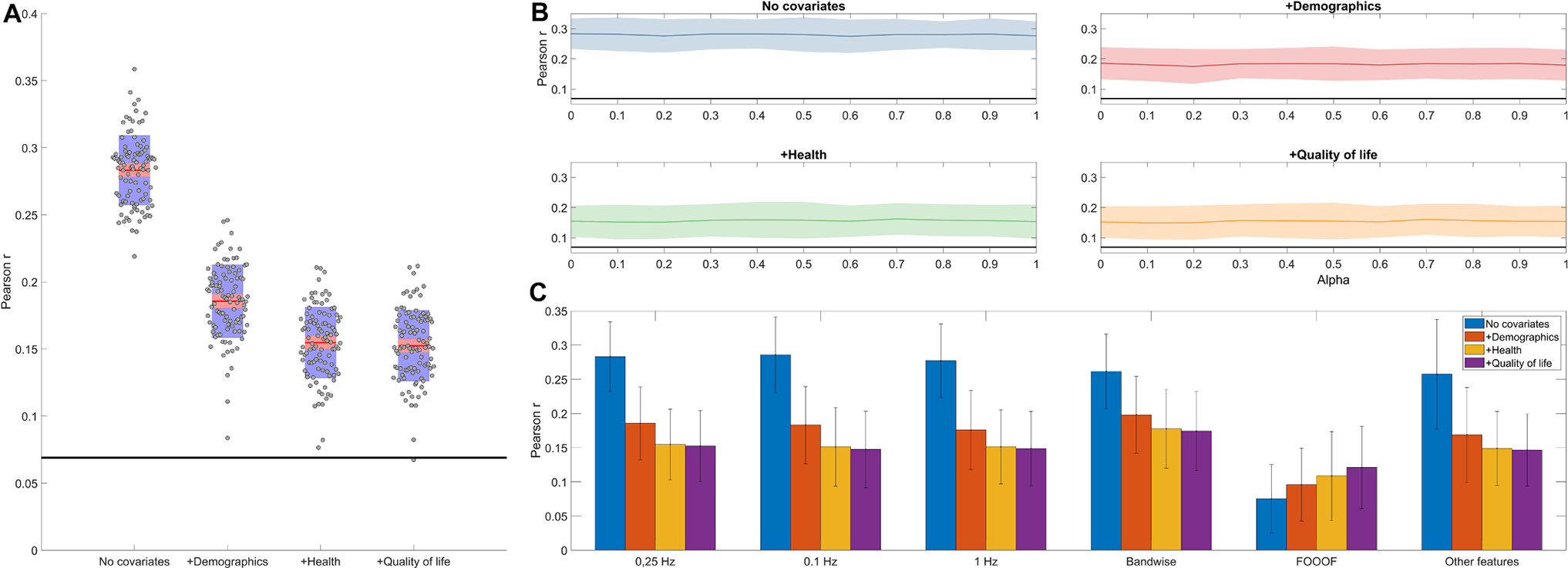
Panel A. EEG-based predictive validity (out-of-sample correlation between predicted and actual cognitive performance) for four increasingly strict covariate sets. Model 1 contains no covariates, Model 2 controls for demographic covariates, Model 3 adds controls for health covariates, while Model 4 adds controls for quality of life covariates. Red lines show the average performance across 100 model runs with random training-validation splits. gray dots show individual model runs. Red shading illustrates the standard error of the mean, while blue shading illustrates the standard deviation across model runs. A thick black line illustrates the critical correlation coefficient at the mean sample size of validation samples. Panel B: out-of-sample correlations between predicted and actual cognitive functioning as a function of regularization type and covariate choice. Regularization type is iteratively changed between alpha=0 (ridge regression), 0<alpha<1 (elastic net) or alpha=1 (LASSO). A thick black line indicates the critical correlation coefficient at the mean sample size of the validation samples. Shadings indicate empirical confidence intervals (1.96 standard deviations of the out-of-sample correlations calculated from 100 model runs). Panel C: model validity with different predictors sets: binwise PSD with three different spectral resolutions (0.1, 0.25 and 1 Hz), bandwise PSD, spectral parameters (intercept, slope, bandwidth, frequency and power of REM theta/beta and NREM sigma peaks) derived by FOOOF, and 0.25 Hz binwise PSD with the other EEG features (see Methods) added.
Empirical p-values can be considered to be 0 as no model had non-positive validity, but with 100 model runs we had a limited resolution of possible empirical p-values. A more conservative, semi-parametric p-value was calculated by considering the standard deviation of validity across models to be an empirical standard error. Dividing the mean validity by this number to obtain a z-statistic and converting it into a p-value yields p-values of <10−13, 8 × 10−12, 5 × 10−9, and 7 × 10−9 for Models 1–4, respectively. We note that individual models were usually all highly statistically significant, as across 400 model runs only a single non-significant p-value () was observed and only 16 p-values exceeded 0.001.
3.5. Alpha tuning has no effect on model performance
We found no evidence that models using (elastic net or LASSO) performed better than (ridge regression). Models using such values produced validities significantly different from ridge regression in only three cases out of the 40 comparisons: for Model 1 produced significantly lower validity than ); for Model 2 produced significantly lower validity than ); while for Model 4, produced significantly higher validity than ). As these deviations were rare, small in magnitude and did not fit into a theoretically expected or empirically observed pattern, we considered them to be likely spurious and proceeded with the computationally simpler ridge regression as our preferred model. Fig. 4, Panel B summarizes the performance of various regularized regression models.
3.6. EEG features other than PSD lack predictive value
In further steps, we explored whether the predictive performance of the sleep EEG changes by adding further features or changing their resolution. First, we compared models based only on PSD data with models that also incorporated wPLI, phase-amplitude coupling, Hjorth parameters as well as linear and quadratic overnight trends as predictors. We found that these more complex models actually statistically significantly underperformed relative to PSD-only models in Model 1 () and Model 2 (), while there was a statistically non-significant trend for better performance in PSD-only models in Model 3 () and Model 4 () (Fig. 4, Panel C). That is, PSD remained the best predictor of cognitive performance with no meaningful additional variance accounted for by other predictors.
3.7. Spectral resolution does not affect predictive validity
In the next step, we run models based on PSD data using two additional levels of PSD resolution (1 Hz and 0.1 Hz using zero-padding). Models based on the more sparse PSD (1 Hz resolution) tended to yield slightly lower out-of-sample correlations (), but this difference only reached significance in Model 2 (). Models with the fine-grained PSD (0.1 Hz resolution) did not produce even a consistent trend for higher validity values ( for the four models, ) (Fig. 4, Panel C). Thus, 0.25 Hz remained our preferred resolution for binwise analysis.
3.8. Band power has comparable predictive validity to binwise power
We attempted using band power in seven frequency bands (see Methods for details) as predictors of general cognitive functioning. It is of note that from these models we dropped not only the fine-grained power estimates, but also PSD laterality and REM-NREM PSD differences, using just 28 predictors (power in seven frequency bands over two channels in NREM and REM) in the regularized regression model. We found that these models significantly underperformed in Model 1 (), but actually outperformed binwise models in Model 2 (), Model 3 () and Model 4 (). The mean out-of-sample correlations across the 100 runs yielded by band power models were 0.262 () for Model 1, 0.198 () for Model 2, 0.178 () for Model 3 and 0.174 () for Model 4 (Fig. 4, Panel C).
3.9. Spectral parametrization
Spectral components of the sleep EEG do not only reflect oscillatory components, but also background activity and sinusoidal components introduced by Fourier analysis to approximate non-sinusoidal oscillations in the actual signal (Bódizs et al., 2021; Donoghue et al., 2020). Therefore, as an alternative analytical strategy, we attempted to decompose spectra into aperiodic (an intercept and an exponent to describe non-oscillatory activity in a simple power law function) and periodic (oscillations exceeding the trend of the power law function) components and use these as predictors of cognitive functioning. Spectral parametrization was performed using FOOOF (Donoghue et al., 2020). In each participant, on each channel and in NREM and REM separately we calculated spectral intercepts, spectral slopes, as well as the bandwidth, frequency and power of REM theta, REM beta and NREM sigma peaks.
Results confirmed the findings related to PSD analyses. In univariate analyses, across all four covariate sets, better cognitive performance was significantly associated (after correction for multiple comparisons) with a higher spectral intercept, a steeper spectral slope, higher power in the NREM sigma and lower power in the REM theta ranges, and higher REM beta power on C3. The correlation between REM beta power on C4 was only found in Model 1 (no covariates) and Model 2 (demographic covariates). No other spectral parameter was consistently associated with cognitive performance, but a trend emerged between a higher-frequency REM beta peak and better cognitive performance (Fig. 5, Panel A). These findings were replicated for peak missingness. Lacking NREM sigma or REM beta peaks was significantly associated with lower cognitive performance, while lacking REM theta peaks showed a trend level association with higher cognitive performance (Fig. 5, Panel B), mirroring both PSD-based analyses and bivariate correlations with spectral parameters.
Fig. 5.
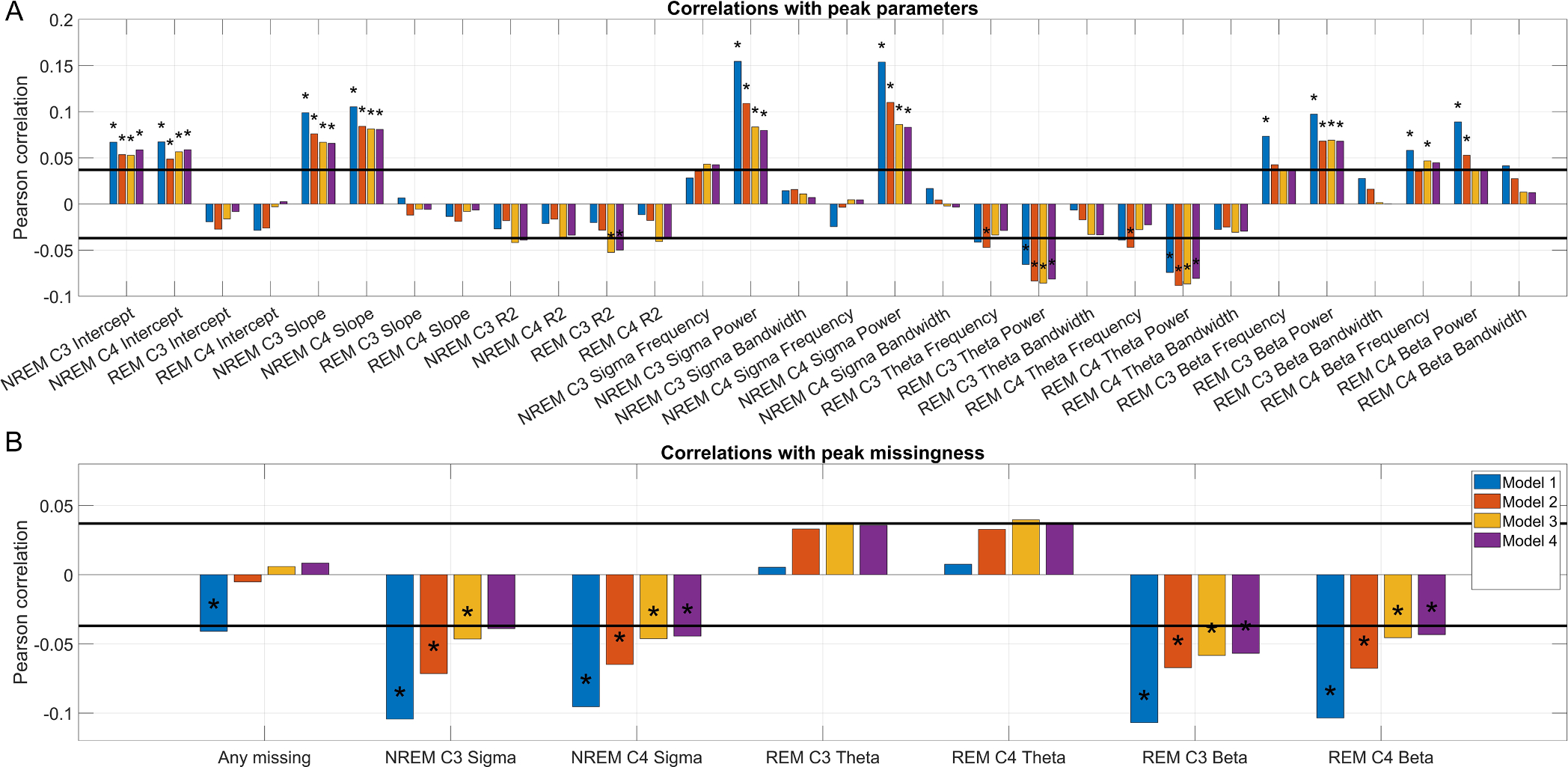
Panel A: Bivariate correlations between spectral parameters (intercept, slope, variance accounted for, and the frequency, power and bandwidth of NREM sigma, REM theta and REM beta peaks) and cognitive performance, residualized for four covariate sets. Dual horizontal lines illustrate the critical significance level assuming zero missingness. Asterisks mark correlations which remain significant after controlling for multiple comparisons. Panel B: Point-biserial correlations between the missingness of spectral peaks (NREM sigma, REM theta and REM beta peaks) and cognitive performance, residualized for four covariate sets. Dual horizontal lines illustrate the critical significance level assuming zero missingness. Asterisks mark correlations which remain significant after controlling for multiple comparisons.
As before, we trained regularized regression models to predict cognitive performance from EEG spectrum parameters, while controlling for the potential confounders specified in Models 1–4. Multivariate models based on these spectral parameters underperformed relative to models based on the 0.25 Hz PSD (Fig. 4, Panel D). Out-of-sample correlations [empirical standard errors] with cognitive performance were: 0.075 [0.026], 0.096 [0.027], 0.109 [0.033] and 0.121 [0.031] for Models 1–4, respectively, all differences from the 0.25 Hz PSD model are significant at p<0.001.
4. Discussion
Our study used a hypothesis-free, multivariate, cross-validated method to identify markers of cognitive functioning from the sleep electroencephalogram. We found that such markers exist, they mostly consist of power spectral density in the NREM sigma and REM theta and beta power, a part of their effect is mediated by observed demographic and health-related covariates and quality of life, but another part is independent from these.
Cognitive functioning is a significant human trait which predicts sociological outcomes (Strenze, 2007), the development and progression and disease (Deary et al., 2021), but a decline in which is also the symptom of various pathological conditions (Karlamangla et al., 2014). A search for biomarkers of cognitive function has been ongoing for decades. Early studies searching for cognitive function biomarkers were often underpowered and univariate, with the choice of the putative biomarker motivated by the intuition of researchers. As a result, many failed to yield replicable results, even if the original findings seemed biologically plausible (Chabris et al., 2013, 2012). The response to the failure of these studies has generally been to launch hypothesis-free, cross-validated association studies which rely on very large statistical power to precisely identify even small biological effects on the target phenotype, and sum of many small biological effects to yield a predictive score whose power is assessed in an independent sample. The most prominent hypothesis-free, cross validated studies have been genome-wide association studies (GWAS) using genetic data (Tam et al., 2019) and whole-brain regression using magnetic resonance imaging data (Marek et al., 2022). Several such studies were concerned with cognitive functioning (Deary et al., 2022; Pohl et al., 2019). EEG-based machine learning studies have also been published (Al Zoubi et al., 2018; Gemein et al., 2020; Sun et al., 2019), but to date, ours is the first to apply a hypothesis-free, cross-validated association method to sleep EEG data to identify biomarkers of cognitive functioning.
In our models, NREM sigma, REM theta and REM beta activity clearly emerged as correlates of cognitive functioning, with out-of-sample multiple correlations of . These validities compare favorably to previously published predictive models based on brain imaging (Hilger et al., 2022; Vieira et al., 2022) or genetic information (Krapohl et al., 2018; Okbay et al., 2022). For example, a recent genome-wide association study using data over 3 million people (Okbay et al., 2022) found that a multiple correlation of can be achieved between genetically predicted and actual cognitive performance. The ABCD Neurocognitive Prediction Challenge, a competition in 2019 which prompted contestants to design multivariate models predicting cognition from whole-brain imaging data, was won by a submission (Mihalik et al., 2019) which showed a correlation of 0.05–0.15 between predicted and actual cognition (although with data residualized for whole brain volume and social characteristics). Whole brain volume correlates about 0.3 with intelligence (Pietschnig et al., 2015), usually with significant but modest information added by other morphological features (Cox et al., 2019). Notably, these studies rely on a richer set of independent variables and much larger samples. This underscores the importance of sleep oscillations as trait-level biomarkers of cognition. While our focus was on trait-level associations, further studies may find an even stronger correlation between sleep features and cognition measured immediately on the following day, where the direct effects of sleep play a role.
Regarding NREM sigma activity, our works replicates a larger body of literature (Djonlagic et al., 2021; Reynolds et al., 2018; Ujma, 2018) which found associations between various aspects of cognitive performance and sleep spindles. As sleep spindles arise from thalamocortical networks (Fernandez and Lüthi, 2020), our results point to the importance of the integrity of this system as a biological prerequisite of cognitive functioning. Although both sleep spindles and cognition are affected by aging, our findings are robust to statistical corrections for age, indicating that this is not the causal mechanism connecting sigma power and cognition. We observed the highest correlation () between sigma power and cognition at 14 Hz, which is in the fast spindle frequency range, supporting a link between fast spindles and cognition (Ujma, 2018). Although only central derivations with a predominance of fast rather than slow spindles were available in the current study, previous research with smaller samples but more topographically representative channel sets confirmed that cognition is predominantly associated with fast spindles (Ujma, 2018; Ujma et al., 2017).
We previously reported (Ujma et al., 2017) that REM beta oscillations had a positive, while REM delta-theta oscillations (albeit at a lower frequency with a maximum at 3.5 Hz) had a negative association with cognitive performance. A previous analysis of the current sample (Djonlagic et al., 2021) also found that REM beta power was correlated with Digit Vigilance scores, although it did not consider a composite cognitive score as the dependent variable and it failed to find a similar association in another sleep cohort. While an invasive EEG study of humans (Vijayan et al., 2017) identified a REM theta-beta network in the anterior cingulate and the dorsolateral prefrontal cortex, likely underlying the oscillations identified in our current study, more research is needed to understand the functional properties of this system. Given the power and replication issues plaguing human neuroscience (Button et al., 2013; Szucs and Ioannidis, 2017), it is significant that we could replicate the observation that NREM sigma, REM theta and REM beta oscillations are correlated to human cognitive functioning, which should facilitate research into the biological origins of these oscillations.
We observed that while approximately half of the association between sleep EEG features and cognitive functioning was accounted for by measured covariates, the other half persisted despite statistical controls for a very large number of potential moderators. The largest drop in this association was seen between the first two models, by adding demographic covariates, of which we hypothesized age to be the most significant. As age is associated with both changes in cognitive performance (Salthouse, 2004) and in changes in the sleep EEG (Carrier et al., 2001; Landolt et al., 1996; Sun et al., 2019), an algorithm may find age-related sleep biomarkers which are, in turn, also related to worse cognitive performance. This expectation was confirmed by the fact that in the second step (Model 2, demographic covariates including linear and nonlinear effects of age added) validity dropped substantially to from 0.283 to 0.186. In the third step (Model 3, health-related covariates added, including comorbidities and medication) validity dropped further, but only slightly, to 0.155. Thus, while a small amount of the sleep EEG-cognition covariance was due to some participants’ comorbidities and/or medications being related to both sleep EEG patterns and cognitive performance, this was comparatively a small effect and even among participants of the same medical history we would expect these EEG markers to be related to cognitive performance. In the fourth step (Model 4, quality of life covariates added), we did not observe a substantial drop validity, which was on average 0.152. Interestingly, the covariates added at this step not only included geriatric functioning scales (SF12 and GDS) the scores of which could be strongly related to well-preserved cognitive functioning at higher ages, but also sleep quality rating scales (ESS, PSQI and FOSQ). Results from Model 4 disconfirm the hypothesis that EEG biomarkers of cognition index poor sleep which impairs next-day cognition, or age-related cognitive and physical decline which is also reflected in sleep alterations. Controlling for the previously added covariates, self-reported sleep quality and geriatric functioning hardly mediates any of the association between sleep EEG markers and cognition. We also note that only a small number of participants () completed cognitive tests on the day after their sleep laboratory visit.
We did not observe substantial zero-order correlations between sleep EEG biomarkers other than power spectral density, and consequently we only included this measure as a predictor in our base model. Furthermore, based on experiences from brain imaging (Marek et al., 2022) and genetics (Chabris et al., 2013), we expected that predictive validity will be driven by a relatively large number of sleep EEG features, each having only a weak zero-order association with cognition. Therefore, our initial models only included PSD as a predictor, but with a relatively fine (0.25 Hz) resolution.
Relating to the first expectation, in exploratory analyses we indeed found that adding wPLI, Hjorth parameters, delta-sigma coupling and overnight effects of all predictors to our models did not improve predictive accuracy. This confirms our finding that sleep EEG functional connectivity is not significantly associated with cognitive performance (Ujma et al., 2019), but it is in contrast with some studies, generally performed in small samples which found that delta-sigma coupling (or a more explicitly measured grouping of sleep spindles by slow waves) is associated with cognitive outcomes (Hahn et al., 2020; Ladenbauer et al., 2017; Latchoumane et al., 2017; Muehlroth et al., 2019). Notably, it is also in contrast with a similar analysis of the present sample (Djonlagic et al., 2021), which found that the coupling of individually detected sleep spindles and slow oscillations was associated with better performance on some cognitive tests (Trails B and 3MS). In the current analyses, the NREM Modulation Index of the delta and sigma frequency ranges was only weakly and non-significantly associated with better cognitive composite scores, but with the correct sign on both C3 and C4 (). Our current study deliberately used measures of spectral power instead of individually detected oscillations due to the methodological issues of sleep oscillation detection (Muehlroth and Werkle‑Bergner, 2020; Warby et al., 2014), especially in older samples, in particular the issues of various sleep spindle detection algorithms in capturing the spindle-cognition association (Ujma, 2018). It is possible that adequately parametrized oscillation detectors yield better estimates of slow oscillation-spindle coupling than delta-sigma Modulation Index, which may be associated with cognition. This finding highlights that while in the case of some biomarkers alternative measures yield highly comparable results (for example, sleep spindle amplitude, sigma power and sigma peak height in the parametrized spectrum all correlate with cognition), in other cases it may be necessary to measure biomarker in a precisely defined way to make associations detectable.
Relating to the second expectation, however, we found that 1) even zero-order correlations between PSD and cognitive performance are substantial, often excluding ) increasing spectral resolution does not improve and reducing it does not impair predictive accuracy, and 3) very similar validities can be achieved by just retaining a coarse estimate of PSD in seven frequency bands as predictors. On the other hand, we also observed that 1) using parametrized spectra (slope, intercept and three spectral peaks) as predictors did reduce validity, and 2) the use of LASSO (which assumes sparsity, forcing regression coefficients to zero for all except a few predictors from correlated sets) was not preferred to ridge regression (which distributes regression weights among correlated predictors). These observations, taken together, suggest that while the associations between sleep EEG features (especially NREM sigma and REM beta power) and cognition are orders of magnitude stronger than what is usually observed in genetics and brain imaging, the set of associated features cannot be reduced to a handful of readily observable spectral peaks or one or two frequency ranges. Power in spectral components of the sleep EEG which are assigned to the ‘aperiodic’ part of the spectrum is substantially associated with cognitive performance.
Our work suffers from a number of limitations. First, as we use a cross-sectional design, we cannot clearly ascertain routes of causation, which also pertains to covariate selection. We emphasize that although Model 1–4 uses an increasingly strict set of covariates, stricter models are not necessarily theoretically preferred. This is because various comorbidities (Calvin et al., 2017; Wraw et al., 2015), general well-being at a high age (Deary et al., 2021), and even less pronounced age-related changes in the sleep EEG (Pótári et al., 2017) have been associated with premorbid cognitive functioning. Therefore, comorbidities may not be true confounders but simply the consequences of pre-existing cognitive abilities which are subsequently reflected in both cognitive test scores and sleep EEG patterns. The theoretical case is stronger for preferring Model 2 (demographic covariates) over Model 1 (no covariates), as both age (Carrier et al., 2001; Sun et al., 2019), and self-reported ethnicity (Profant et al., 2002; Purcell et al., 2017; Rao et al., 2009) has a likely spurious association with sleep EEG patterns, and the same can be assumed for recording site. In any case, it is clear that even with a potentially overcontrolling strict covariate set cognitive functioning is related to features of the sleep EEG. Second, although our findings are robust to a large set of health-related covariates and replicate in an independent sample, it is not fully elucidated to what extend we found sleep EEG correlates of age-related cognitive decline or those of pre-existing cognitive abilities which persisted into an old age. For this limitation to be overcome, similar investigations in healthy, younger samples are necessary. Third, the scope of our investigation was limited by the low spatial resolution of our EEG instruments (two central channels). It is possible that other EEG features with different, specific topographies are also associated with cognition. Finally, we emphasize that the associations we find between EEG patterns and cognitive performance are modest and most of the variance in cognitive performance is not accounted for by patterns of the sleep EEG.
In sum, our work showed using a large dataset and a data-driven, cross validated approach that features of the sleep electroencephalogram are related to cognitive functioning in elderly participants, even after controlling for a broad set of covariates. Power in the NREM sigma, REM theta and REM beta bands is especially strongly implicated. These features of the sleep EEG exhibit zero-order correlations often exceeding , and similar multiple correlations to brain imaging-based or genetic predictors (Deary et al., 2022), usually established in discovery samples orders of magnitude larger than ours.
Supplementary Material
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR002369. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The National Heart, Lung, and Blood Institute provided funding for the ancillary MrOS Sleep Study, “Outcomes of Sleep Disorders in Older Men,” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The National Sleep Research Resource was supported by the National Heart, Lung, and Blood Institute (R24 HL114473, 75N92019R002).
This research has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the PD-21 (project number: 138935) and TKP2021-EGA-25 funding schemes.
Footnotes
Declaration of Competing Interest
None.
CRediT authorship contribution statement
Péter P. Ujma: Conceptualization, Methodology, Software, Validation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Róbert Bódizs: Writing – original draft, Writing – review & editing. Martin Dresler: Writing – original draft, Writing – review & editing. Péter Simor: Writing – original draft, Writing – review & editing. Shaun Purcell: Investigation, Resources, Writing – original draft, Writing – review & editing. Katie L. Stone: Investigation, Resources, Writing – original draft, Writing – review & editing. Kristine Yaffe: Investigation, Resources, Writing – original draft, Writing – review & editing. Susan Redline: Investigation, Resources, Writing – original draft, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2023.120319.
Data availability
Links to data and code are shared in the manuscript.
References
- Alhola P, Polo-Kantola P, 2007. Sleep deprivation: impact on cognitive performance. Neuropsychiatr. Dis. Treat. 3, 553–567. [PMC free article] [PubMed] [Google Scholar]
- Al Zoubi O, Ki Wong C, Kuplicki RT, Yeh H-W, Mayeli A, Refai H, Paulus M, Bodurka J, 2018. Predicting age from brain EEG signals-A machine learning approach. Front. Aging Neurosci. 10, 184. 10.3389/fnagi.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL, Osteoporotic Fractures in Men (MrOS) Study Group, 2011. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 34, 1347–1356. 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR, 2005. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp. Clin. Trials 26, 557–568. 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bódizs R, Gombos F, Ujma PP, Szakadát S, Sándor P, Simor P, Pótári A, Konrad BN, Genzel L, Steiger A, Dresler M, Kovács I, 2017. The hemispheric lateralization of sleep spindles in humans. Sleep Spind. Cort. Up State. 1, 42–54. 10.1556/2053.01.2017.002. [DOI] [Google Scholar]
- Bodizs R, Horváth CG, Szalárdy O, Ujma PP, Simor P, Gombos F, Kovács I, Genzel L, Dresler M, 2022. Sleep-spindle frequency: overnight dynamics, afternoon nap effects, and possible circadian modulation. J. Sleep Res. 31, e13514. 10.1111/jsr.13514. [DOI] [PubMed] [Google Scholar]
- Bódizs R, Kis T, Lázár AS, Havrán L, Rigó P, Clemens Z, Halász P, 2005. Prediction of general mental ability based on neural oscillation measures of sleep. J. Sleep Res. 14, 285–292. 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- Bódizs R, Szalárdy O, Horváth C, Ujma PP, Gombos F, Simor P, Pótári A, Zeising M, Steiger A, Dresler M, 2021. A set of composite, non-redundant EEG measures of NREM sleep based on the power law scaling of the Fourier spectrum. Sci. Rep. 11, 2041. 10.1038/s41598-021-81230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Kurth S, Ringli M, Geiger A, Jenni OG, Huber R, 2011. Anatomical markers of sleep slow wave activity derived from structural magnetic resonance images. J. Sleep Res. 20, 506–513. 10.1111/j.1365-2869.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Calvin CM, Batty GD, Der G, Brett CE, Taylor A, Pattie A, Čukić I, Deary IJ, 2017. Childhood intelligence in relation to major causes of death in 68 year follow-up: prospective population study. BMJ 357, j2708. 10.1136/bmj.j2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH, 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38, 232–242. 10.1111/1469-8986.3820232. [DOI] [PubMed] [Google Scholar]
- Chabris CF, Hebert BM, Benjamin DJ, Beauchamp J, Cesarini D, van der Loos M, Johannesson M, Magnusson PKE, Lichtenstein P, Atwood CS, Freese J, Hauser TS, Hauser RM, Christakis N, Laibson D, 2012. Most reported genetic associations with general intelligence are probably false positives. Psychol. Sci. 23, 1314–1323. 10.1177/0956797611435528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris CF, Lee JJ, Benjamin DJ, Beauchamp JP, Glaeser EL, Borst G, Pinker S, Laibson DI, 2013. Why it is hard to find genes associated with social science traits: theoretical and empirical considerations. Am. J. Public Health 103 (Suppl 1), S152–S166. 10.2105/AJPH.2013.301327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, 2017. Rigor and replication in time-frequency analyses of cognitive electrophysiology data. Int. J. Psychophysiol. 111, 80–87. 10.1016/j.ijpsycho.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Fawns-Ritchie C, Tucker-Drob EM, Deary IJ, 2019. Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76, 101376. 10.1016/j.intell.2019.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, Hagenaars SP, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Liewald DCM, Okely JA, Ahola-Olli AV, Barnes CLK, Bertram L, Bis JC, Burdick KE, Christoforou A, DeRosse P, Djurovic S, Espeseth T, Giakoumaki S, Giddaluru S, Gustavson DE, Hayward C, Hofer E, Ikram MA, Karlsson R, Knowles E, Lahti J, Leber M, Li S, Mather KA, Melle I, Morris D, Oldmeadow C, Palviainen T, Payton A, Pazoki R, Petrovic K, Reynolds CA, Sargurupremraj M, Scholz M, Smith JA, Smith AV, Terzikhan N, Thalamuthu A, Trompet S, van der Lee SJ, Ware EB, Windham BG, Wright MJ, Yang J, Yu J, Ames D, Amin N, Amouyel P, Andreassen OA, Armstrong NJ, Assareh AA, Attia JR, Attix D, Avramopoulos D, Bennett DA, Böhmer AC, Boyle PA, Brodaty H, Campbell H, Cannon TD, Cirulli ET, Congdon E, Conley ED, Corley J, Cox SR, Dale AM, Dehghan A, Dick D, Dickinson D, Eriksson JG, Evangelou E, Faul JD, Ford I, Freimer NA, Gao H, Giegling I, Gillespie NA, Gordon SD, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Hartmann AM, Hatzimanolis A, Heiss G, Holliday EG, Joshi PK, Kähönen M, Kardia SLR, et al. , 2018. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9, 2098. 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Cox SR, Hill WD, 2022. Genetic variation, brain, and intelligence differences. Mol. Psychiatry 27, 335–353. 10.1038/s41380-021-01027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Hill WD, Gale CR, 2021. Intelligence, health and death. Nat. Hum. Behav. 5, 416–430. 10.1038/s41562-021-01078-9. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M, 2005. An electroencephalographic fingerprint of human sleep. Neuroimage 26, 114–122. 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Djonlagic I, Mariani S, Fitzpatrick AL, Van Der Klei VMGTH, Johnson DA, Wood AC, Seeman T, Nguyen HT, Prerau MJ, Luchsinger JA, Dzierzewski JM, Rapp SR, Tranah GJ, Yaffe K, Burdick KE, Stone KL, Redline S, Purcell SM, 2021. Macro and micro sleep architecture and cognitive performance in older adults. Nat. Hum. Behav. 5, 123–145. 10.1038/s41562-020-00964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, Noto T, Lara AH, Wallis JD, Knight RT, Shestyuk A, Voytek B, 2020. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665. 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette MR, Kurth S, Chevalier N, Munakata Y, LeBourgeois MK, 2015. Topography of slow sigma power during sleep is associated with processing speed in preschool children. Brain Sci. 5, 494–508. 10.3390/brainsci5040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG, 2013. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R296. 10.1152/ajpregu.00422.2012, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG, 2010. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn 72, 56–65. 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Fernandez LMJ, Lüthi A, 2020. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868. 10.1152/physrev.00042.2018. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Achermann P, Borbély AA, 2001. Individual “fingerprints” in human sleep EEG topography. Neuropsychopharmacology 25, S57–S62. 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R, 2010. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22. 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G Horváth C, Szalárdy O, Ujma PP, Simor P, Gombos F, Kovács I, Dresler M, Bódizs R, 2022. Overnight dynamics in scale-free and oscillatory spectral parameters of NREM sleep EEG. Sci. Rep. 12, 18409. 10.1038/s41598-022-23033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marín LM, Campos AI, Martin NG, Cuéllar-Partida G, Rentería ME, 2021. Inference of causal relationships between sleep-related traits and 1,527 phenotypes using genetic data. Sleep 44. 10.1093/sleep/zsaa154. [DOI] [PubMed] [Google Scholar]
- Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P, 2011. The sleep EEG as a marker of intellectual ability in school age children. Sleep 34, 181–189. 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemein LAW, Schirrmeister RT, Chrabąszcz P, Wilson D, Boedecker J, Schulze-Bonhage A, Hutter F, Ball T, 2020. Machine-learning-based diagnostics of EEG pathology. Neuroimage 220, 117021. 10.1016/j.neuroimage.2020.117021. [DOI] [PubMed] [Google Scholar]
- Giner-Sorolla R, 2012. Science or Art? How aesthetic standards grease the way through the publication bottleneck but undermine science. Perspect. Psychol. Sci. 7, 562–571. 10.1177/1745691612457576. [DOI] [PubMed] [Google Scholar]
- Gonzalez CE, Mak-McCully RA, Rosen BQ, Cash SS, Chauvel PY, Bastuji H, Rey M, Halgren E, 2018. Theta bursts precede, and spindles follow, cortical and thalamic downstates in human NREM sleep. J. Neurosci. 38, 9989–10001. 10.1523/JNEUROSCI.0476-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson LS, 1997. Why g matters: the complexity of everyday life. Intelligence 24, 79–132. 10.1016/S0160-2896(97)90014-3. [DOI] [Google Scholar]
- Hahn MA, Heib D, Schabus M, Hoedlmoser K, Helfrich RF, 2020. Slow oscillation-spindle coupling predicts enhanced memory formation from childhood to adolescence. Elife 9. 10.7554/eLife.53730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, 2016. The Neuroscience of Intelligence. Cambridge University Press. [Google Scholar]
- Hilger K, Spinath FM, Troche S, Schubert A-L, 2022. The biological basis of intelligence: benchmark findings. Intelligence 93, 101665. 10.1016/j.intell.2022.101665. [DOI] [Google Scholar]
- Hjorth B, 1970. EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 29, 306–310. 10.1016/0013-4694(70)90143-4. [DOI] [PubMed] [Google Scholar]
- Hong Y-W, Yoo Y, Han J, Wager TD, Woo C-W, 2019. False-positive neuroimaging: undisclosed flexibility in testing spatial hypotheses allows presenting anything as a replicated finding. Neuroimage 195, 384–395. 10.1016/j.neuroimage.2019.03.070. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE, 2014. Biological correlates of adult cognition: midlife in the United States (MIDUS). Neurobiol. Aging 35, 387–394. 10.1016/j.neurobiolaging.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Keefe RSE, Sundquist K, Sundquist J, 2018. The joint impact of cognitive performance in adolescence and familial cognitive aptitude on risk for major psychiatric disorders: a delineation of four potential pathways to illness. Mol. Psychiatry 23, 1076–1083. 10.1038/mp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, Genon S, Alzheimer’s Disease Neuroimaging Initiative, 2019. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife 8. 10.7554/eLife.43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapohl E, Patel H, Newhouse S, Curtis CJ, von Stumm S, Dale PS, Zabaneh D, Breen G, O’Reilly PF, Plomin R, 2018. Multi-polygenic score approach to trait prediction. Mol. Psychiatry 23, 1368–1374. 10.1038/mp.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncel NR, Hezlett SA, 2010. Fact and fiction in cognitive ability testing for admissions and hiring decisions. Curr. Dir. Psychol. Sci. 19, 339–345. 10.1177/0963721410389459. [DOI] [Google Scholar]
- Ladenbauer Julia, Ladenbauer Josef, Külzow N, de Boor R, Avramova E, Grittner U, Flöel A, 2017. Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. J. Neurosci. 37, 7111–7124. 10.1523/JNEUROSCI.0260-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA, 1996. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 738, 205–212. 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S, 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435. 10.1016/j.neuron.2017.06.025 e6. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Rennick PM, 1979. Manual For the Repeatable Cognitive-Perceptual-Motor Battery. Axon, Clinton Township, MI. [Google Scholar]
- Lilienfeld SO, 2017. Psychology’s replication crisis and the grant culture: righting the ship. Perspect. Psychol. Sci. 12, 660–664. 10.1177/1745691616687745. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF, 2010. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 136, 375–389. 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Walker MP, 2017a. Sleep and human aging. Neuron 94, 19–36. 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Zhu AH, Lindquist JR, Villeneuve S, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP, 2017b. White matter structure in older adults moderates the benefit of sleep spindles on motor memory consolidation. J. Neurosci. 37, 11675–11687. 10.1523/JNEUROSCI.3033-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, Malone SM, Kandala S, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, Moore LA, Conan GM, Uriarte J, Snider K, Lynch BJ, Wilgenbusch JC, Pengo T, Tam A, Chen J, Newbold DJ, Zheng A, Seider NA, Van AN, Metoki A, Chauvin RJ, Laumann TO, Greene DJ, Petersen SE, Garavan H, Thompson WK, Nichols TE, Yeo BTT, Barch DM, Luna B, Fair DA, Dosenbach NUF, 2022. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660. 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik A, Brudfors M, Robu M, Ferreira FS, Lin H, Rau A, Wu T, Blumberg SB, Kanber B, Tariq M, Garcia MDME, Zor C, Nikitichev DI, Mourao-Miranda J, Oxtoby NP, 2019. ABCD Neurocognitive Prediction Challenge 2019: predicting individual fluid intelligence scores from structural MRI using probabilistic segmentation and kernel ridge regression. arXiv. 10.48550/arxiv.1905.10831. [DOI] [Google Scholar]
- Muehlroth BE, Sander MC, Fandakova Y, Grandy TH, Rasch B, Shing YL, Werkle-Bergner M, 2019. Precise slow oscillation-spindle coupling promotes memory consolidation in younger and older adults. Sci. Rep. 9, 1940. 10.1038/s41598-018-36557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlroth BE, Werkle-Bergner M, 2020. Understanding the interplay of sleep and aging: methodological challenges. Psychophysiology 57, e13523. 10.1111/psyp.13523. [DOI] [PubMed] [Google Scholar]
- Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, Sidorenko J, Kweon H, Goldman G, Gjorgjieva T, Jiang Y, Hicks B, Tian C, Hinds DA, Ahlskog R, Magnusson PKE, Oskarsson S, Hayward C, Campbell A, Porteous DJ, Freese J, Herd P, 23andMe Research Team, Social Science Genetic Association Consortium, Watson C, Jala J, Conley D, Koellinger PD, Johannesson M, Laibson D, Meyer MN, Lee JJ, Kong A, Yengo L, Cesarini D, Turley P, Visscher PM, Beauchamp JP, Benjamin DJ, Young AI, 2022. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat. Genet 54, 437–449. 10.1038/s41588-022-01016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K, 2005. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp. Clin. Trials 26, 569–585. 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M, 2015. Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci. Biobehav. Rev. 57, 411–432. 10.1016/j.neubiorev.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Plomin R, von Stumm S, 2018. The new genetics of intelligence. Nat. Rev. Genet. 19, 148–159. 10.1038/nrg.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolescent brain cognitive development neurocognitive prediction: first challenge. In: Pohl KM, Thompson WK, Adeli E, Linguraru MG. (Eds.), 2019. ABCD-NP 2019, Held in Conjunction with MICCAI 2019, Shenzhen, China, October 13, 2019, Proceedings, Lecture notes in computer science. Springer International Publishing, Cham. 10.1007/978-3-030-31901-4. [DOI] [Google Scholar]
- Pótári A, Ujma PP, Konrad BN, Genzel L, Simor P, Körmendi J, Gombos F, Steiger A, Dresler M, Bódizs R, 2017. Age-related changes in sleep EEG are attenuated in highly intelligent individuals. Neuroimage 146, 554–560. 10.1016/j.neuroimage.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Profant J, Ancoli-Israel S, Dimsdale JE, 2002. Are there ethnic differences in sleep architecture? Am. J. Hum. Biol. 14, 321–326. 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- Protzko J, 2017. Raising IQ among school-aged children: five meta-analyses and a review of randomized controlled trials. Developm. Rev. 10.1016/j.dr.2017.05.001. [DOI] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, Panagiotaropoulou G, Saxena R, Pan JQ, Smoller JW, Redline S, Stickgold R, 2017. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun. 8, 15930. 10.1038/ncomms15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE, 2009. Ethnic differences in electroencephalographic sleep patterns in adolescents. Asian J. Psychiatr. 2, 17–24. 10.1016/j.ajp.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Purcell SM, 2021. Sleep and Big Data: harnessing data, technology, and analytics for monitoring sleep and improving diagnostics, prediction, and interventions-an era for Sleep-Omics? Sleep 44. 10.1093/sleep/zsab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, 1958. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills 8, 271–276. 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Reynolds CM, Gradisar M, Short MA, 2019. Reliability of sleep spindle measurements in adolescents: how many nights are necessary? J. Sleep Res. 28, e12698. 10.1111/jsr.12698. [DOI] [PubMed] [Google Scholar]
- Reynolds CM, Short MA, Gradisar M, 2018. Sleep spindles and cognitive performance across adolescence: a meta-analytic review. J. Adolesc. 66, 55–70. 10.1016/j.adolescence.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Booth T, Valdés Hernández MDC, Corley J, Maniega SM, Gow AJ, Royle NA, Pattie A, Karama S, Starr JM, Bastin ME, Wardlaw JM, Deary IJ, 2015. Beyond a bigger brain: multivariable structural brain imaging and intelligence. Intelligence 51, 47–56. 10.1016/j.intell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Tucker-Drob EM, 2018. How much does education improve intelligence? A meta-analysis. Psychol. Sci. 29, 1358–1369. 10.1177/0956797618774253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletin JM, van der Helm E, Walker MP, 2013. Structural brain correlates of human sleep oscillations. Neuroimage 83, 658–668. 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, 2004. Localizing age-related individual differences in a hierarchical structure. Intelligence 32. 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski JA, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hägg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Muñoz-Manchado AB, Quinlan EB, Schumann G, Skene NG, Webb BT, White T, Arking DE, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, DeRosse P, Dickinson D, Djurovic S, Donohoe G, Conley ED, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Koltai D, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Räikkönen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Tiemeier H, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Posthuma D, 2018. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919. 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Hödlmoser K, Gruber G, Sauter C, Anderer P, Klösch G, Parapatics S, Saletu B, Klimesch W, Zeitlhofer J, 2006. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur. J. Neurosci. 23, 1738–1746. 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- Strenze T, 2007. Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence 35, 401–426. 10.1016/j.intell.2006.09.004. [DOI] [Google Scholar]
- Sun H, Paixao L, Oliva JT, Goparaju B, Carvalho DZ, van Leeuwen KG, Akeju O, Thomas RJ, Cash SS, Bianchi MT, Westover MB, 2019. Brain age from the electroencephalogram of sleep. Neurobiol. Aging 74, 112–120. 10.1016/j.neurobiolaging.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D, Ioannidis JPA, 2017. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol 15, e2000797. 10.1371/journal.pbio.2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D, 2019. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 20, 467–484. 10.1038/s41576-019-0127-1. [DOI] [PubMed] [Google Scholar]
- Tan X, Campbell IG, Feinberg I, 2001. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clin. Neurophysiol. 112, 1540–1552. [DOI] [PubMed] [Google Scholar]
- Tan X, Campbell IG, Palagini L, Feinberg I, 2000. High internight reliability of computer-measured NREM delta, sigma, and beta: biological implications. Biol. Psychiatry 48, 1010–1019. 10.1016/s0006-3223(00)00873-8. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC, 1987. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 48, 314–318. [PubMed] [Google Scholar]
- Tibshirani R, 1996. Regression shrinkage and selection via the lasso. J. Roy. Statist. Soc.: Ser. B (Methodolog.) 58, 267–288. 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Tononi G, Cirelli C, 2014. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Komorowski R, Eichenbaum H, Kopell N, 2010. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 104, 1195–1210. 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujma PP, Bódizs R, Gombos F, Stintzing J, Konrad BN, Genzel L, Steiger A, Dresler M, 2015. Nap sleep spindle correlates of intelligence. Sci. Rep. 5, 17159. 10.1038/srep17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujma PP, Konrad BN, Genzel L, Bleifuss A, Simor P, Pótári A, Körmendi J, Gombos F, Steiger A, Bódizs R, Dresler M, 2014. Sleep spindles and intelligence: evidence for a sexual dimorphism. J. Neurosci. 34, 16358–16368. 10.1523/JNEUROSCI.1857-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujma PP, Konrad BN, Gombos F, Simor P, Pótári A, Genzel L, Pawlowski M, Steiger A, Bódizs R, Dresler M, 2017. The sleep EEG spectrum is a sexually dimorphic marker of general intelligence. Sci. Rep. 7, 18070. 10.1038/s41598-017-18124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujma PP, Konrad BN, Simor P, Gombos F, Körmendi J, Steiger A, Dresler M, Bódizs R, 2019. Sleep EEG functional connectivity varies with age and sex, but not general intelligence. Neurobiol. Aging 78, 87–97. 10.1016/j.neurobiolaging.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Ujma PP, Sándor P, Szakadát S, Gombos F, Bódizs R, 2016. Sleep spindles and intelligence in early childhood-developmental and trait-dependent aspects. Dev. Psychol. 52, 2118–2129. 10.1037/dev0000233. [DOI] [PubMed] [Google Scholar]
- Ujma PP, 2018. Sleep spindles and general cognitive ability – a meta-analysis. Sleep Spindles & Cortical Up States 1–17. 10.1556/2053.2.2018.01. [DOI] [Google Scholar]
- Vieira BH, Pamplona GSP, Fachinello K, Silva AK, Foss MP, Salmon CEG, 2022. On the prediction of human intelligence from neuroimaging: a systematic review of methods and reporting. Intelligence 93, 101654. 10.1016/j.intell.2022.101654. [DOI] [Google Scholar]
- Vien C, Boré A, Boutin A, Pinsard B, Carrier J, Doyon J, Fogel S, 2019. Thalamo-cortical white matter underlies motor memory consolidation via modulation of sleep spindles in young and older adults. Neuroscience 402, 104–115. 10.1016/j.neuroscience.2018.12.049. [DOI] [PubMed] [Google Scholar]
- Vijayan S, Lepage KQ, Kopell NJ, Cash SS, 2017. Frontal beta-theta network during REM sleep. Elife 6. 10.7554/eLife.18894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA, 2011. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 55, 1548–1565. 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Warby SC, Wendt SL, Welinder P, Munk EGS, Carrillo O, Sorensen HBD, Jennum P, Peppard PE, Perona P, Mignot E, 2014. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat. Meth. 11, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens CD, Hutchins SD, Laux L, Sebok A, 2015. The impact of sleep disruption on complex cognitive tasks: a meta-analysis. Hum. Fact. 57, 930–946. 10.1177/0018720815571935. [DOI] [PubMed] [Google Scholar]
- Wraw C, Deary IJ, Gale CR, Der G, 2015. Intelligence in youth and health at age 50. Intelligence 53, 23–32. 10.1016/j.intell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraw C, Der G, Gale CR, Deary IJ, 2018. Intelligence in youth and health behaviours in middle age. Intelligence 69, 71–86. 10.1016/j.intell.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G-Q, Cui L, Mueller R, Tao S, Kim M, Rueschman M, Mariani S, Mobley D, Redline S, 2018. The National Sleep Research Resource: towards a sleep data commons. J. Am. Med. Inform. Assoc. 25, 1351–1358. 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T, 2005. Regularization and variable selection via the elastic net. J. Royal Statist. Soc. B 67, 301–320. 10.1111/j.1467-9868.2005.00503.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All PSG data are freely available via the National Sleep Research Resource (http://sleepdata.org). Model results and code used for analyses are available on Zenodo at 10.5281/zenodo.7684266.
Links to data and code are shared in the manuscript.


