Abstract
Background
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) is intended to provide clinicians an overview of “Behavior, Motivational Interviewing, Eating Disorders, and Obesity Management Technologies.“
Methods
The scientific information for this CPS is based upon published scientific citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
Results
This CPS outlines important components of behavior, motivational interviewing, eating disorders, and obesity management technologies as they relate to pre-obesity and obesity. Topics include eating behavior disorder evaluation, the motivations behind eating and physical activity behaviors (including underlying neurophysiology, eating disorders, environmental factors, and personal prioritization), motivational interviewing techniques, and technologies that may assist with pre-obesity/obesity management.
Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on “Behavior, Motivational Interviewing, Eating Disorders, and Obesity Management Technologies” is one of a series of OMA CPSs designed to assist clinicians in the care of patients with the disease of pre-obesity/obesity. Implementation of appropriate clinical practices in these areas may improve the health of patients, especially those with adverse fat mass and adiposopathic metabolic consequences.
Keywords: Behavior, Eating disorder, Motivational interviewing, Obesity, Obesity management technologies
1. Introduction
Beginning in 2013, the Obesity Medicine Association (OMA) created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees [1]. This was followed by a similar Pediatric “Obesity Algorithm” with updates approximately every two years by OMA authors. This OMA CPS on “Behavior, Motivational Interviewing, Eating Disorders, and Obesity Management Technologies was derived from the adult OMA Obesity Algorithm and is one of a series of OMA CPSs designed to assist clinicians in the care of patients with the disease of obesity.
2. Behavior
Along with nutrition, physical activity, and medication, behavior is a major pillar of weight evaluation and management, and is influenced by physiological, psychological, and environmental factors. Genetic predisposition contributes to behavior related to eating [2] and physical activity [3]. Table 1 outlines 10 takeaway messages regarding obesity and behavior.
Table 1.
Ten Takeaway Messages: Obesity and Behavior. Healthful behavior is a key pillar in management of pre-obesity/obesity, and is influenced by a wide variety of factors, including genetics, education, physiology, psychology, environment, record keeping, and support systems.
| Top 10 Takeaway Messages: Obesity and Behavior | |
|---|---|
| 1. | Eating behavior regarding patients with increased body fat often reflects the imbalance of genetic, physiologic, psychologic, and environmental forces that strongly promote weight gain and resist weight reduction, versus forces that weakly resist weight gain. This is analogous to the imbalance in physiologic responses regarding hypoglycemia (marked symptoms and strong signals to immediately consume food) and hyperglycemia (often with limited to no symptoms and/or no signal to change eating behavior). |
| 2. | Eating behavior is affected by all five senses: sight, smell, hearing, taste, and feel. |
| 3. | Eating behavior can be affected by genetic predisposition, mental stress, emotions, habitual time cues, environment, information gap, reward factors, and psychiatric disease. |
| 4. | Eating behavior can be affected by eating disorders (e.g., binge-eating disorder, bulimia nervosa, night-eating syndrome, and sleep-related eating disorder). |
| 5. | Physical inactivity behavior may be due to musculoskeletal, neurologic, pulmonary, cardiac, and other health disorders, as well as fatigue, disinterest, and unhealthful environment (e.g., availability and excessive utilization of conveniences). |
| 6. | Behavior contributing to weight gain and/or regain often reflects personal and physiologic priority imbalances (i.e., “lack of time”) that affect nutritional intake and energy expenditure. |
| 7. | Eating habits and physical activity behavior may be substantially due to genetic predisposition. |
| 8. | Optimal behavior therapies are ones that are: (a) feasible, (b) efficacious, (c) measurable, and (d) those that promote self-ownership. Behavior therapy efficacy is enhanced via frequent encounters with qualified medical professionals, education, stimulus control, cognitive restructuring, goal setting, self- monitoring, behavioral contracting, problem solving, social support, and other contingencies. |
| 9. | For patients ready for change, healthful nutrition and physical activity may be aided by weight management technologies, access to healthful nutrition and physical activity resources (i.e., grocery stores or restaurants with healthful food options, as well as gym memberships, work gyms, or home physical exercise equipment), and/or knowing the existence of social media resources applicable to healthful nutrition and physical activity. In individuals not ready for change, being provided or having access to such resources alone may not facilitate healthful behavior changes. |
| 10. | Healthful behavior change may be more effective and more consistent when accompanied by self-monitoring, record-keeping, education, routine monitoring and assessment by clinicians, and social support/motivation. |
2.1. Why do people eat like they do?
2.1.1. Physiology
Eating is necessary for survival. Body weight and body composition reflect the balance between energy (food) intake and energy expenditure [4]. Physiologically, biological forces that resist weight reduction are often strong while biological forces that resist weight gain are often weak. Hypothalamic dysfunction due to trauma or inflammation is illustrative of a neurophysiological process that can substantially affect hunger and body weight [5]. Eating behavior can be motivated by hunger before meals, a lack of satiety during and after meals, eating to facilitate sleep, and other factors described below [[6], [7], [8]]. In susceptible individuals, food may be an addiction [[10], [11], [9]]. Central nervous system signaling and that impacts eating behavior can be influenced by all five senses [12,13]:
-
•
Sight or images of food
-
•
Smell of food
-
•
Sounds of food (e.g., cooking, wrapper opening) or hearing talk of food
-
•
Taste of food
-
•
Feel of food (texture, quantity, esophageal passage, stomach fullness) and feelings of a lack of food (e.g., peristaltic rumbling of an “empty stomach” and intestine — borborygmi)
2.1.2. Mental stress
Mental stress can lead to unhealthful eating patterns that may contribute to chronic stress-induced limbic (e.g., hypothalamic) and cerebral endocrinopathies and immunopathies [10]. Many individuals who experience chronic mental stress find their personal, work, or emotional priorities overtake healthful nutritional and physical activity priorities. Chronic stress may impair self-regulation and promote unhealthful (i.e., immediately rewarding, ultra-processed) foods over more healthful (i.e., delayed-gratification, unprocessed) foods [10].
2.1.3. Timing and emotions
Timing plays a major role in eating behaviors. People often eat because it's mealtime, independent of neurophysiologic hunger signaling. Special occasions can also lead to modified eating patterns (e.g., holiday celebrations, birthdays) [14]. Emotions can also be tied to eating patterns. Eating and/or offering of food is often a surrogate for love and/or affection for the self or others (i.e., family and friends). Food intake may accompany celebrations of happiness or used to soothe sadness. Eating patterns may reflect time spent avoiding more undesirable situations, with cooking and/or eating being perceived as a necessary accomplishment (“you've got to eat”) that is preferable to more challenging, less desirable, and potentially less successful activities. Finally, eating may also be used to self-treat boredom, fatigue, or stress [5].
2.1.4. Environment
The environment also plays a major role in eating behaviors. People may eat because others around them are eating or simply because food is available [14]. Offers of free food can cue eating, independent of hunger. Ubiquitous and highly researched advertisements can change eating patterns towards ultra-processed, energy-dense foods. People may also eat due to environmental obligations to eat, such as when meals are prepared by family members or friends, when families or friends are gathered together for reasons other than meals, and when at business meetings away from home [15,16]. In such environments, patients may have a perceived obligation to eat beyond the point of satiety (i.e., clean-plate syndrome) or to eat foods in a way inconsistent with a healthful eating plan [14]. Virtual video or audio business meetings while at home might also affect eating, if the home environment has readily available energy dense foods, and if meeting participants eat in response to meeting anxiety or boredom. Finally, individuals may be distracted from mindful eating when food is consumed while watching a screen (e.g., television, smartphone, computer).
2.1.5. Information gap
Many people eat the way they do due to a lack of information and/or education about proper nutrition [17]. Access to nutritional information can be challenging when eating out. While major restaurant chains offer nutritional content of their menu items, many smaller restaurants do not. Thus, it can often be difficult to know the caloric and nutritional content of restaurant foods. Marketing messages can be misleading, such as “low-fat” (which may be high in ultra-processed carbohydrates and calories), “multigrain” (often with relatively less fiber compared to whole grain), “no added sugar” and “natural sugar” (both that may be ultra-processed and calorie dense), and “cholesterol-free,” (which mostly means it is not derived from animals, but still may be high in plant saturated fats, ultra-processed carbohydrates, and be energy dense). “Heart-healthy,” “organic,” “gluten-free,” and “fortified/enriched” are marketing terms that may not always identify foods that are healthful [5,6,12,14] Whether eating out or preparing food at home, it is often challenging to know or guess the healthfulness of foods, without knowing the food's nutritional content.
2.1.6. Reward
For many individuals, eating is a remuneration or reward for an accomplishment or a “good day.” Eating can also be used as reciprocal compensation for a “bad day.” Reward eating is an illustrative example of how eating can be in response to desired pleasure, and not because of hunger [7]. Over-consumption of ultra-refined, hyperpalatable foods high in fat, sugar, and salt may affect the brain's reward system involving dopaminergic, glutamatergic, and dopaminergic pathways in the nucleus accumbens part of the brain [9,10,18,19], which is part of the mesolimbic/reward pathway [20]. Factors that contribute to consumption of unhealthful, ultra-processed, hyperpalatable foods are environmental influences, genetic and familial predispositions, neurobiologic responses, and psychosocial influences – all potentially contributing to cravings and potential loss of eating control, irrespective of adverse health consequences (i.e., obesity) [19].
Additionally, during times of chronic mental stress, emotional eaters often learn that tastier and potentially less healthful foods can provide short-term improvement in anxiety and depression (i.e., stress eating of “comfort food”) [21]. Patients may learn that consumption of ultra-processed hyperpalatable foods may therapeutically dampen hypothalamic pituitary axis stress responses [18]. Finally, intake of ultra-processed hyperpalatable foods, especially sugar, may stimulate opioid release, which may ultimately simulate addiction-like reward deficits between time of eating (i.e., cravings and withdrawal), which in turn may promote compulsive eating [22].
2.1.7. Psychiatric disease
A detailed discussion of the role of psychiatric disease is beyond the scope of this CPS, which is focused on behavior, motivational interviewing, eating disorders, and technologies. However, in short, obesity and depression are bidirectional: obesity can promote depression and depression can promote obesity [23,24]. Both the symptoms and treatment of psychiatric disease may impact eating and their management are integral to obesity management. Among the more common psychiatric diseases contributing to obesity in clinical practice include depression, anxiety, and mood disorders. Numerous depression questionnaire instruments exist. Among the more common general depression tools (i.e., not specific for youth, perinatal period, geriatric population, dementia, or suicidal ideation assessment) include:
2.2. Eating disorders
Eating disorders influence food intake and energy balance. Table 2, Table 3, Table 4, Table 5 summarize the diagnosis and treatment of binge-eating disorder (BED), bulimia nervosa (BN), night-eating syndrome (NES), and sleep-related eating disorder (SRED). While numerous tools/instruments exist, among the more common screening questionnaires generally used for eating disorders include:
-
•
“Sick, Control, One, Fat, and Food” (SCOFF) [30].
-
•
Eating Disorder Examination Questionnaire (EDE-Q) [30].
-
•
Eating Attitudes Test (EAT-26) [31].
-
•
Eating Disorder Screen for Primary Care (EDS-PC) [32].
-
•
Questionnaire for Eating Disorder Diagnosis (Q-EDD) [33].
Table 2.
Binge-eating disorder.
| Binge-eating disorder | |||
|---|---|---|---|
| Diagnosis | Screeninga | Treatment | References |
| Binge-Eating Disorder (BED) is diagnosed when the patient episodically consumes large amounts of food more than once per week for at least three months, without self-induced vomiting (purging), without laxative use, and without extra physical exercise. Patients often feel a lack of self-control, shame, and guilt. Binge eating disorder occurs in approximately 3% of U.S. adults and may occur in up to 50% of patients with severe obesity. | Binge-Eating Scale Binge Eating Disorder Screener-7 (BED7) |
Treatment may include cognitive behavior therapy (i.e., alteration in thinking to facilitate more healthful outcomes). Although not FDA-indicated for this use, pharmacotherapies that may be efficacious in treating binge-eating disorder include some selective serotonin reuptake inhibitors (e.g., fluoxetine, paroxetine, and sertraline) and topiramate. Paroxetine should be used with caution due to potential obesogenic effect [34]. In addition to an indication to treat attention deficit hyperactivity disorder, lisdexamfetamine dimesylate has an FDA indication to treat binge-eating disorder.b | [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44]] |
Listed screening tests are beyond common general eating disorder screening tests described in the first paragraph of this section.
See “Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022” for a detailed discussion of lisdexamfetamine dimesylate.
Table 3.
Bulimia nervosa.
| Bulimia Nervosa | |||
|---|---|---|---|
| Bulimia Nervosa (BN) is an eating disorder characterized as a cycle of recurrent binge eating and compensatory purging, laxative abuse, diuretic abuse, extra exercising, fasting, or strict dieting. Bulimia nervosa may occur in approximately 3% of adults (mostly female), and reportedly has a higher occurrence rate (as high as 10%) among college-aged females. Bulimia nervosa may be indicated by Russell's sign: calluses and abrasions on the dorsum of the hands caused by repeated contact with the teeth during self-induced vomiting; it is also associated with enamel erosion of the teeth (usually lingual surfaces) and sialadenosis (i.e., enlargement of the salivary glands, commonly the parotid glands). Laboratory abnormalities may include hypokalemia (promoted by hypomagnesemia), hypochloremia, or metabolic alkalosis. Elevated amylase suggests possible vomiting and salivary gland irritation. | Screen for Disordered Eating (SDE) SCOFF: Sick (vomiting), Control (loss of control), One stone (loss of approximately 15 pounds in 3 months), Fat (disturbance in body fat image), and Food (obsession with eating behavior) Eating Disorder Inventory (EDI) Eating Attitudes Test (EAT) Eating Disorders Screen for Primary Care (EDS-PC) |
Treatment for bulimia nervosa includes cognitive behavior therapy, possibly in combination with drug treatment. Fluoxetine is an FDA-approved pharmacotherapy for bulimia nervosa; although not FDA-indicated for this use, topiramate and naltrexone may also be efficacious in treating bulimia nervosa. | [40,[45], [46], [47]] [39,41,42,48,49] |
Table 4.
Night-eating syndrome.
| Night-Eating Syndrome | |||
|---|---|---|---|
| Eating Disorder Diagnosis | Screening | Treatment | References |
| Night-eating syndrome (NES) may be present if at least 25% of daily food consumption (often greater than 50%) is consumed after the evening meal. Patients may have recurrent awakenings from sleep that require eating to go back to sleep, often involving carbohydrate-rich snacks. They tend to have little interest in breakfast (morning anorexia). NES may occur in as much as 5% of the U.S. population. | Night Eating Questionnaire (NEQ) |
Treatment for NES involves behavioral therapy regarding nutritional timing and content. While not an FDA-indicated use, selective serotonin re-uptake inhibitors (e.g., sertraline) or topiramate may be useful for NES. | [[50], [51], [52], [53], [54], [55]] |
Table 5.
Sleep-related eating disorder.
| Sleep-related eating disorder | |||
|---|---|---|---|
| Eating Disorder Diagnosis | Screening | Treatment | References |
| Sleep-related eating disorder (SRED) is a parasomnia (i.e., undesired event accompanying sleep) with “sleep-walking,” resulting in repeated episodes of compulsive binge eating and drinking after waking up at night. Non rapid eye movement (NREM) SRED may be more frequent in patients with narcolepsy, sleepwalking, restless legs syndrome, and obstructive sleep apnea, and usually occurs while patients are partially awake Patients with SRED often have no memory of the event afterward. Individuals with SRED may have accidental injuries. An awareness of evening or nocturnal ingestions helps differentiate NES from SRED. | Patients with SRED may not score differently than NES on sleep questionnaires (i.e., Sleep Disturbance Questionnaire). Thus, diagnosis is mainly a clinical one. | The treatment of SRED is dependent on the cause. For example, drug-induced sleep-related eating disorder is best managed by stopping or altering the dose of the offending drug (e.g., benzodiazepines, psychotropic mediations (e.g., olanzapine, risperidone, and quetiapine), mirtazapine, and zolpidem). Patients with SRED should avoid drugs that may worsen the condition. Other treatments include: (1) stress management, (2) “sleep hygiene” (e.g., allowing sufficient sleep time while avoiding screen time, caffeine, and alcohol before bedtime), (3) locks or alarms on ovens, cabinets, and refrigerators, and (4) moving hazards (i.e., furniture) from likely paths to the kitchen to avoid falling. Drug treatment includes selective serotonin reuptake inhibitors, topiramate, and (perhaps paradoxically) clonazepam. |
[53,56,57] [[58], [59], [60], [61]] |
2.3. Why don't people engage in routine physical activity?
2.3.1. Physiology
Pathophysiologic reasons for decreased physical activity include musculoskeletal, neurologic, pulmonary, cardiac, and other health disorders that limit patient ability to engage in routine physical activity. Patients may also experience pain, soreness, or fatigue that likewise limits physical activity [62].
2.3.2. Lack of time prioritization
Many people perceive they do not have time to engage in routine physical activity due to work commitments or family responsibilities. Time may also be preferentially allotted for other entertainments with minimal energy expenditure (i.e., involving screen time), such as television, movies, video games, internet surfing, email, texting, apps, or watching sports [62,63].
2.3.3. Disinterest
Some people have a lack of personal interest in physical activity that limits engagement in regular physical exercise. They may express that “exercise is boring.” They may also experience disinterest due to past failures in achieving physical exercise goals, or past failures in observing body changes with prior alterations in physical activity. Other people may be concerned about their appearance in workout clothes or have a sense of inadequacy when surrounded by others at the gym who are more fit. They may be concerned about garnering unwanted attention or experiencing sexual harassment when they are exercising in a gym or outdoors. Others may fear being bullied about their weight. Finally, some people avoid physical activity that generates perspiration due to the consequences of sweating and effects on hair or odor [62,63].
2.3.4. Environment
Many people do not engage in routine physical activity due to a lack of others engaged in similar physical activity (family and friends), access to a safe environment, access to parks or other areas for leisure activity, access to a gym, or access to workplace exercise equipment. Once started, a common challenge is maintaining routine physical activity. Insufficient education on the importance of physical activity can contribute to a lack of routine physical exercise maintenance. Insufficient knowledge of the risks and techniques of physical exercise (i.e., proper use of exercise equipment) may lead to injury and subsequent inability to maintain routine physical exercise [62,63]. Surrounding availability of conveniences that may limit physical activity include automated transportation (e.g., cars, buses, taxis, shared rides), elevators, escalators, online shopping, and automated equipment that lessen manual labor [62].
2.4. Why do people plateau with weight reduction or regain body weight?
2.4.1. Physiologic priority imbalance
The human body resists weight reduction. Neuro-biologic processes strongly resist under-nutrition (starvation) but weakly resist over-nutrition [64,65]. An analogous example is hypoglycemia and hyperglycemia. Hypoglycemia can be profoundly symptomatic and may promote physiologic and behavioral priority for immediate caloric intake. Conversely, hyperglycemia is often asymptomatic and rarely promotes physiologic and behavioral priority for immediate reduced caloric intake.
2.4.2. Neurobiology
Weight reduction may decrease the secretion of neuroendocrine factors that reduce hunger [e.g., glucagon-like peptide, leptin, insulin, peptide tyrosine-tyrosine (YY), and cholecystokinin]. Concomitantly, weight reduction may increase ghrelin, which may increase hunger. To the extent insulin and leptin “resistance” in the central nervous system impairs satiation, then an increase in physical activity may increase the brain's sensitivity to insulin and leptin, and presumably facilitate a reduction in hunger. This is one possible mechanism why physical activity after weight reduction may help limit body fat regain [[64], [65], [66], [67]].
2.4.3. Energy expenditure (dynamic energy balance)
Patients who undergo weight reduction have reduced resting energy expenditure in part due to decreased energy requirements because of the lost body tissue. However, adaptive thermogenesis may also occur wherein the reduction in resting metabolic rate exceeds that predicted by loss of body tissue [66]. Furthermore, greater muscle efficiency also occurs after weight reduction, resulting in less energy expenditure with the same physical activity before weight reduction [67].
2.4.4. Behavior leading to plateaus or weight regain
Some people experience commitment amnesia, or a forgetfulness of the degree of change and effort required to achieve initial weight reduction success. This supports the benefits of maintaining nutrition and physical activity monitoring logs, even after achievement of desired body weight. Intervening stress, changing life circumstances, or a changing health status may alter priorities, which can lead back to less healthful behavior. Some patients have a setpoint fallacy wherein they have the mistaken belief that once achieved, maintenance of weight reduction will persist, irrespective of subsequent behavior, nutrition, and physical activity. Some patients feel: “I know if I could just get the weight off, I could keep it off.” However, it is the reality of many obesity medicine clinicians, and patients with pre-obesity/obesity, that long-term weight reduction maintenance is often more challenging to achieve, compared to short term weight reduction. Another common challenge is the lack of achieving anticipated weight reduction goals. The “failure” of achieving (sometimes unrealistic) weight reduction goals may be discouraging and diminish motivation to continue behaviors necessary for weight reduction maintenance (“I only lost 30 pounds; but I wanted to lose 100 pounds”).
Priority fatigue can lead to a lack of maintaining healthy body weight priorities or resorting back to previous nutritional and/or physical activity habits after achieving initial weight reduction success. Similar decision fatigue can occur, wherein mental stress or multiple higher priority decisions may impair self-regulation regarding health and may facilitate choosing unhealthful, immediately rewarding and immediately available ultra-processed foods over more healthful, delayed-gratification, unprocessed foods [68].
2.5. Behavior therapy techniques
2.5.1. Elements for optimal success
The most successful behavior therapy techniques have four elements for optimal success. The most successful behavior therapy techniques are [69,70]:
-
1.
Feasible: Practical and accessible (in terms of frequency and consistency)
-
2.
Efficacious: Evidence-based
-
3.
Measurable/Accountable: Trackable and verifiable, often with record keeping and supervised feedback (positive and negative reinforcement) and social support
-
4.
Self-owned: Autonomous, with the patient being the primary stakeholder
2.5.2. Encounters
While autonomous self-ownership is a key component to behavior change success, the success of behavior therapy is enhanced with frequent encounters with medical professionals or other resources free from clinician bias [71]. These encounters may include clinicians (e.g., physicians, nurse practitioners, and physician assistants), nurses, nurse educators, dietitians, physical activity professional trainers (i.e., trainers, physiologists, etc.), mental health professionals, certified health coaches, web-based programs, or mobile access (i.e., test messages, applications, etc.) [72,73]. Many patients may especially benefit from working with clinicians having specific expertise in obesity management.
2.5.3. Education
Education is a key to successful behavior therapy. Pre-obesity/obesity should be understood and treated as a disease. Explaining the complexities of the disease of obesity can be made simpler by conveying how fat cell and fat organ enlargement can result in “sick fat diseases” (e.g., leading to diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, and cancer) and “fat mass” diseases (e.g., leading to arthritis and sleep apnea) [74]. In addition, patients benefit from education on medical health, mental health/stress management, nutrition [17], physical activity [17], and adoption of healthful sleep habits. Healthful eating habits may include reducing the speed of eating, drinking water between meals, choosing and having available healthful snacks, and recognizing and anticipating inevitable weight-reduction plateau [72,73], and even periodic (and preferably transient) bouts of weight regain.
2.5.4. Stimulus control
Stimulus control is a major component of behavior therapy [75]. Examples of eating technique approaches that may maximize satiety include meal timing, nutrient composition (i.e., preferably high fiber, moderately high protein, moderately low glycemic load), and appetite awareness training [8,76]. Patients should avoid eating for reasons other than hunger, avoid frequent snacking, avoid binge-eating, and utilize portion control. Patients should identify and limit access to favorite energy dense foods especially tempting. Ready availability of energy dense foods may “stimulate” the patient to eat such unhealthful foods. Being mindful of eating stimuli may prompt patient actions to limit stimuli-mediated eating and help enhance stimulus control [72].
2.5.5. Cognitive restructuring
As part of a behavior therapeutic plan, cognitive restructuring is a process intended to replace unhealthful and unproductive thoughts and perceptions with more healthful and more productive thoughts and perceptions. Establishing plans to counteract unhelpful or dysfunctional thinking may help lead to more healthful behaviors and actions.
Some patients perceive weight gain and weight reduction only within the context of body image. One objective of cognitive restructuring is to characterize the rationale of realistic weight-reduction in terms of medical and mental health, and not simply body appearance. Overall, cognitive restructuring encourages patients to acknowledge that they are capable of positive thoughts and behaviors, are able to replace unhelpful thoughts and behaviors with more productive ones, and stresses the importance of practicing behavior therapy skills between clinician encounters [75].
2.5.6. Goal setting
As part of behavior therapy, patients are often given step-by-step instructions to accomplish certain nutrition and physical activity goals. A common acronym of goalsetting is “SMART”: Specific, Measurable, Assignable, Realistic, and Time-related. Goal attainment is often best not limited to weight goals. Patients may benefit from receiving credit (if not celebration) for improvement in “non-weight scale” victory goal attainments, such as [77]:
-
•
A focus on things other than food
-
•
Engaging in physical activity (e.g., improved mobility, walking up stairs)
-
•
Improved sleep quality
-
•
Fitting into clothes previously worn before weight gain
-
•
Reducing medications for other chronic diseases
2.5.7. Self-monitoring
Among patients with pre-obesity/obesity desiring weight reduction, the frequency of self-monitoring highly correlates to weight reduction [72]. While some may feel that excessive monitoring of body weight may be counterproductive, most data support the advantages of daily or weekly body weight monitoring. Additionally, body composition analyses can be both diagnostic and prognostic, as well as motivating [4]. Routine self-anthropometric measurements may also be useful (i.e., body fat calipers, home bioelectrical impedance devices, tape measure for waist circumference, and tape measure for muscle mass). Food diaries (e.g., paper records, online services, or mobile applications) can be especially effective for both patient and clinician. Physical activity logs and pedometer/accelerometer measures can help document physical activity. Sleep monitoring and photo journaling may also be useful for weight reduction and record keeping [78].
2.5.8. Behavioral contracting
Behavioral contracting in behavior therapy involves using (non-food-related) rewards as motivation. Possible rewards include tokens or financial incentives for achieving desired metrics [72,79].
2.5.9. Problem solving, social support, and other reinforcement contingencies
Possible strategies related to problem solving, social support and other reinforcement contingencies include stress management, health care team support, mental-health professional support, faith-based interventions, and other group or social support [72,80]. Commercial weight reduction/maintenance programs can be helpful. Optimally, interactions with others will provide positive recognition for successes and establish alternative/back-up procedures to engage during times that challenge adherence to agreed-upon plans (e.g., stressful periods, life changes) [81].
3. Motivational interviewing
Motivational interviewing is a collaborative, yet patient-centered, goal-directed counseling approach intended to guide people toward positive behavior change conducted within an environment of acceptance and compassion [82]. Within the context of obesity medicine, the intent is to promote a healthier body weight and a healthier body composition among patients with pre-obesity/obesity [83]. Table 6 outlines 10 takeaway messages from the OMA regarding obesity and motivational interviewing.
Table 6.
Ten Takeaway Messages: Obesity and Motivational Interviewing. This table lists 10 takeaway messages regarding obesity and motivational interviewing [83].
| Top 10 Takeaway Messages: Obesity and Motivational Interviewing |
|---|
|
|
|
|
|
|
|
|
|
|
3.1. Motivational interviewing and stages of change
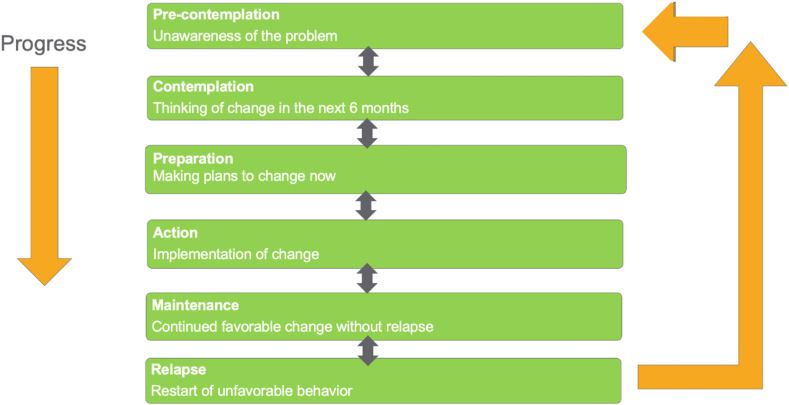
Within the context of obesity medicine, motivational interviewing is a collaborative, goal-oriented discussion between clinician and patient intended to promote healthful change in body weight and body composition [82]. Techniques to facilitate healthful behavior change have largely been derived from decades of learnings from research into smoking cessation and alcohol cessation [84]. Often characterized as consistent with the transtheoretical model of change, human behavior is a process characterized by stages of change (SOC). While the benefits of utilizing this model and SOC for dietary and physical exercise management of obesity may benefit from a more robust evidenced-base dataset [85], having knowledge of the patient's SOC can influence and tailor the direction and emphasis of motivational interviewing [86]. For example, questionnaires regarding SOC may reveal higher action SOC among patients seeking bariatric surgery [87]. Fig. 1 outlines SOC that may be useful to identify during the motivational interviewing process.
Fig. 1.
Stages of Change. Stages of change that may be used to select appropriate motivational interviewing strategies include pre-contemplation, contemplation, preparation, action, maintenance, and relapse [88,89].
3.2. Spirit of motivational interviewing
The spirit of motivational interviewing is centered on partnership, acceptance, compassion, and evocation [82]. Partnership involves working together to find and implement pragmatic solutions without focusing on who is right and who is wrong [90]. Acceptance means meeting the patient where they are without judgment and affirming their ability to solve the problem. Compassion prioritizes the patient's best interests over the authoritarian power of the clinician to direct the patient change [82]. Evocation involves drawing out the patient's thoughts and ideas regarding solutions, without telling the patient what to do [91]. Motivational interviewing may be especially useful when ambivalence to behavior change is high, and confidence, desire, and perceived importance of behavior change are low [82].
3.3. Four processes of a motivational interviewing interview
Motivational interviewing conversations can be described as four processes: engaging, focusing, evoking, and planning.
-
•
Engaging is the establishment of trust and rapport between the patient and clinician for the purpose of affirming patient strengths that may support autonomy towards positive personal change [82].
-
•
Focusing facilitates a targeted conversation regarding directional changes that are needed to achieve specific agreed upon goals [82].
-
•
Evoking involves exploring the patient's ambivalence, motivation, and reasons for change [82].
-
•
Planning occurs when the clinician perceives the patient is sufficiently motivated for healthful change, which then prompts a transition in the discussion towards how to best implement change [82].
3.4. Principles of motivational interviewing
Five major principles of motivational interviewing are shown in Fig. 2.
Fig. 2.
Motivational Interviewing: Principles. The five principles of motivational interviewing are to express empathy, avoid argumentation, develop discrepancies, resolve ambivalence, and support self-efficacy [[92], [93], [94]].
3.4.1. Express empathy
Expressing empathy enhances the potential effectiveness of clinician counseling by enhancing patient satisfaction with the motivational interview process [92,95]. Priorities in motivational interviewing include:
-
•
Engage patients with a warm and genuine manner, demonstrate interest, and express appreciation for patient willingness to discuss their personal challenges of pre-obesity/obesity
-
•
Use active listening to better understand and acknowledge the patient's experience without judgment; active listening is often perceived by patients as the highest form of empathy
-
•
Make reflective statements to verbalize understanding and acceptance when patients share difficult feelings and life situations that may affect their ability to change, including external weight bias, uncomfortable social situations, and a potentially non-supportive partner, family, and friends, as well as identifying internal weight bias, negative self-body image, and other negative thoughts that may benefit from patient cognitive restructuring
-
•
Create a trusting environment where patients feel psychologically safe to experiment with behavior change, in which the clinician supports their efforts and successes, even when the degree of weight reduction does not meet expectations
3.4.2. Avoid arguments
Therapeutic management of body fat for those with pre-obesity or obesity is a sensitive topic to discuss with many patients. While achievement of a healthy body weight is medically advantageous, discussions of body weight, behavior, nutrition, and physical activity can be perceived by the patient as judgmental, and possibly confrontational, potentially resulting in unproductive arguments. This interaction can be made worse when clinicians instinctually respond with the righting reflex, intended to persuade patients to change via a directive counseling style. Such an approach may lead to resistance, where the patient views the problem or solution differently than the clinician, or feels the clinician is being too judgmental and/or authoritative [94]. Discord in the counseling relationship may occur when the patient expresses “sustain talk” (i.e., statements supporting the status quo), which may lead to the patient arguing with, denying, ignoring, or interrupting the clinician who is proposing change. Such discord can be mitigated by counseling style and use of several motivational interviewing techniques that side-step arguments.
-
•
“Rolling with resistance” avoids arguments and confrontations by not challenging patients when they engage in sustain talk or otherwise seem resistant to change [94]. Rolling with resistance may be especially useful during initial interactions with the patient.
-
•Reflective listening expresses empathy, conveys acceptance, and may evoke “change talk” [94,96,97]:
-
oSimple reflection: “You don't think you can lose weight right now.”
-
oAmplified reflection: “You think that people worry too much about your weight; your current body weight is not really a problem for you.”
-
oDouble-sided reflection: “You previously suggested being committed to weight reduction was important for your health; but you now seem to believe that commitment is no longer necessary.”
-
o
-
•Shifting focus can help move the conversation away from an argument or unproductive conversation [94]:
-
o“Your conflict with your contractor is obviously stressful to you; but for now, perhaps we should focus on other issues that led to the entries in your food journal.”
-
o
-
•Reframing the issue may help the patient view their challenges in a different way [94,98]:
-
o“You say you get angry when your family and friends express concern about your body weight. To what degree do you believe they intend to frustrate you, and to what degree do you believe their concern reflects how much they care for you and your health?”
-
o
-
•Normalizing can avoid arguments for patients who have the belief their situation is unique, unexplainable, and thus potentially unresolvable [70,71].
-
o“Many people feel like you: they want to lose weight but find it difficult.”
-
o“While no situation is the same, in general, many people also have problems losing weight and get frustrated when they don't.”
-
o
-
•“Siding with the negative” involves the clinician making a statement siding with the patient's resistance to change or sustain talk. Highlighting the negative side of ambivalence may invite the patient to verbalize an argument for change [94,96,99]
-
o“It sounds like now is not the best time for you to make changes.”
-
oYou're right. You have too much going on right now to make any major changes.”
-
o
-
•“Therapeutic paradox” is a technique that, as opposed to avoidance or finding alternatives, the patient is instructed to engage and/or increase in engagement of an undesirable or feared behavior that might otherwise represent healthful change. The purpose of implementing the therapeutic paradox technique is to demonstrate that the patient has voluntary control.
-
oYou say one of your main challenges is that while you have ready access to a gym, you are intimidated by others around you at the gym. Perhaps you could consider going to the gym at whatever time and in whatever clothes you feel most comfortable. You could start the first few weeks with the plan that you would only stay a few minutes and only use the gym equipment you know best. As you gain more confidence, you may find you can more easily increase your time and expand your exercise gym activities.”
-
o
3.4.3. Discrepancy and ambivalence
Ambivalence is a normal part of the process of change, wherein patients are either uncertain for the need for change, or simultaneously have reasons to change and reasons not to change [82]. Developing discrepancy refers to the process of exploring the mismatch between where the patient is today and where the patient wants to be in the future. Identifying inconsistency between the current behavior and the patient's own life goals and values can help develop discrepancy. Other techniques to develop discrepancy include acknowledging positive and negative aspects of current behavior and providing tailored risk information and feedback [94]. After identifying discrepancy, clinicians can help patients resolve ambivalence by discussing the benefits and risks of change, and risks of no change, and then planning next steps [100,101].
3.4.4. Self-efficacy
Self-efficacy is an important mediator of behavior change in obesity treatment [102]. Motivational interviewing assumes the patient is capable of making change [94]. Change is promoted by focusing on past patient successes and highlighting existing patient skills and strengths [103,104]. Examples of supporting self-efficacy include:
Evoking questions:
-
•
“How might you go about taking the first steps in making a change?”
-
•
“What obstacles might you encounter and how would you overcome them?”
Affirmation statements: [94].
-
•
“I see you have a real commitment toward improving your health.”
-
•
“Your spirituality and family are two strengths that seem to be helping you stick with your plan.”
-
•
“It seems that despite a lot of things happening, you have managed to stay on course, and that is really impressive.”
-
•
“Although you have not seen the results you were hoping for on the scale, the fact you have returned reflects how serious you are about reducing your weight.”
Self-efficacy requires knowledge and skill. Advice and feedback promote patient self-efficacy [104,105]. Motivational interviewers may ask patients with pre-obesity/obesity about their understanding about how weight reduction may affect their health (e.g., blood sugar, blood pressure, blood lipids, heart, breathing, joints, sleep, possible pregnancy, and quality of life).
Overall, summarizing the patient's knowledge, motivations, progress, and positive actions promotes self-efficacy [104,105]:
-
•
“From what you've said, you want to lose weight mainly because you are concerned about your health and because your family is concerned.”
-
•
“It seems that with your commitment to the weight-management plan, and with support from your family, most everyone agrees that overall, you are making great progress.”
-
•
“Although you had made progress in the past, your weight went up a bit this time. But it is good you did not get so discouraged as to cancel your appointment.”
3.5. Evoking change-talk examples
As per the examples above, an integral objective throughout the motivational interview process is directing the conversation in a manner that explores ambivalence and motivation, amplifies discrepancy, and prompts the patient to express reasons for change [94,96,106]: Basic questions relevant to this objective might be:
-
•
“Why do you want to change?”
-
•
“How important is it that you change?”
-
•
“What values are most important to you?”
-
•
“How do your actions fit your values?”
-
•
“How do you plan to change?”
-
•
“How confident are you that you can change?”
Questions that may help explore the influence of the past and future on readiness for change may include [94,96,106]:
-
•
“How were things better in the past?”
-
•
“What may happen if things stay the same?”
-
•
“How would you like for things to change within the next year?”
-
•
“What are the best ways for you to change in the next year?”
Query extremes regarding patient stance and mindset might include [94,96,106]:
-
•
“If you were completely successful in making this change, how would things be better in the future?”
-
•
“What is the worst-case scenario if you do not change?”
-
•
“What is the best-case scenario if you do change?”
3.6. Change metric examples
Another useful technique in motivational interviewing involves assessing the importance of change [94,96]:
-
•
“On a scale of 1–10, where 1 is not important and 10 is most important, how important is it for you to make this change?”
-
•
“Why did you choose this number and not a lower/higher number?”
Assessments of readiness to change [94,96]:
-
•
“On a scale of 1–10, where 1 is not ready to change and 10 is absolutely ready to change, how ready are you to change?”
-
•
“Why did you choose this number and not a lower/higher number?”
Assessments of confidence in the ability to change [94,96]:
-
•
“On a scale of 1–10, where 1 is not at all confident and 10 is absolutely confident, how confident are you in your ability to change?”
-
•
“Why did you choose this number and not a lower/higher number?”
3.7. Decision-balancing examples
Patients often must balance multiple motivations and aspects of change. Decision-balancing can be a helpful tool to explore ambivalence by looking at the pros and cons of change. Examples in motivational interviewing include [94,96]:
-
•
“Write down some of the positive things and negative things about your current eating and physical activity levels.”
-
•
“It sounds like you are frustrated by your current behavior but feel change would be too difficult. It might be helpful to make a chart of the pros and cons of each option – keeping things the same and changing.”
3.8. Motivational interviewing techniques
3.8.1. Micro-counseling (OARS)
Micro-counseling is a motivational interviewing technique that often uses the OARS model: Open-ended questions, Affirmations, Reflections, and Summaries [107].
-
•
Open-ended questions avoid binary answers such as “yes” or “no” and instead invite patients to elaborate on the reasons for and possibility of change.
-
•
Affirmations are expressed recognitions of the patient's strengths and how these strengths can be applied to implement change. Affirmations to the patient by the clinician should be both relevant and genuine.
-
•
Reflections are a central skill in motivational interviewing. Reflective listening is an effective way to express empathy. The clinician can use reflections to reinforce and invite elaboration on change talk, develop discrepancy, amplify/resolve ambivalence, and support self-efficacy.
-
•
Summaries are extended reflections that pull together key points and can help transition into planning next steps. Summaries can steer attention from sustain talk, such as negative past failures, and toward positive but realistic future goals. Summaries should establish metrics to measure success of future goals and outline follow-up plans [107].
3.8.2. The five A's of obesity management and FRAMES
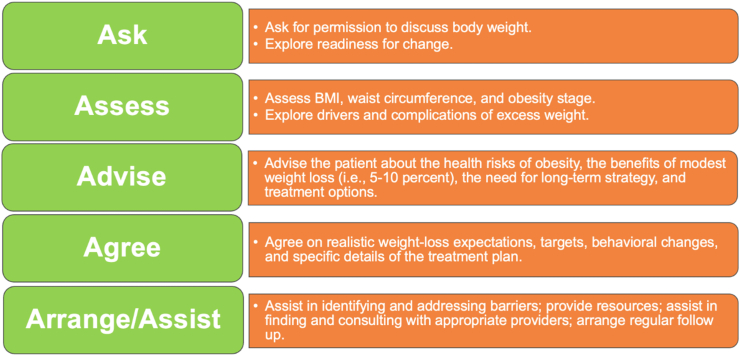
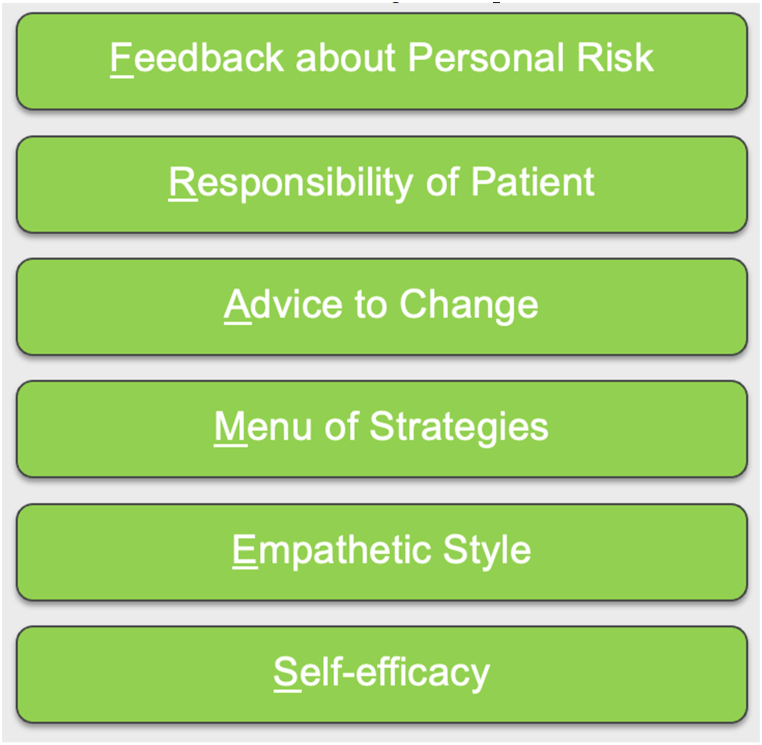
Brief motivational interventions are short, structured applications of motivational interviewing that can be used in busy clinical settings. The five “A” framework was adapted to obesity counseling in primary care: ask, assess, advise, agree, and arrange/assist [108,109]. Fig. 3 describes the five A's of obesity management. Fig. 4 describes the FRAMES technique in motivational interviewing.
Fig. 3.
The Five A's of Obesity Management. The 5 “A's is a helpful brief motivational intervention that incorporates elements of motivational interviewing [108,109].
Fig. 4.
Motivational Interviewing Techniques: FRAMES. FRAMES is a brief intervention that includes feedback, responsibility, advice, a menu of strategies, an empathetic style, and self-efficacy [110,111].
4. Obesity management technologies
Many useful technologies exist for weight management. These technologies can help with motivation, record keeping, and awareness of personal metrics applicable to obesity management.
4.1. Technologies and social media
4.1.1. Applications
Many applications, both free and paid, are available to help patients record and assess nutritional and physical activity metrics [[112], [113], [114]]. The information recorded in apps may be assessed and reviewed by clinicians during face-to-face evaluations, as well as during telehealth visits [74].
4.1.2. Interactive technology
Body-weight scales that provide interactive feedback via email or text messaging are a useful form of interactive technology for weight management [112]. Interactive technology for weight management also includes wearable technologies such as watches [[112], [113], [114], [115], [116], [117], [118], [119]]. These devices can track active minutes, steps, floors climbed, distance, and caloric consumption. They often monitor heart rate, track sleep patterns, and provide daily exercise statistics. They wirelessly sync with smartphones and computers, providing interactive information to the user. Potential benefits of wearable technology beyond standard behavioral intervention depends on the individual, and thus recommending wearable technologies is best based upon a patient-centered approach.
4.1.3. Websites and social media
Websites can provide educational information regarding nutrition, caloric content of foods, physical activity, expected energy expenditure with certain physical activities, meal plans, and recipes. Social media may also be useful for weight management [120]. Posting daily meals and snacks to followers can enhance accountability. Posting physical activity progress to social network group can also be motivating and enhance accountability. Patients can obtain nutritional and physical activity advice from others, including social network support groups specific to weight management. Competition or “wagers” regarding fitness metrics and goals can also increase motivation.
4.2. The National Weight Control Registry
The National Weight Control Registry is a research study of those 18 years or older who have lost at least 30 pounds of weight and maintained weight loss for at least 1 year [121]. The membership includes over 10,000 people, with 80% female and 20% males. Interventions reported by members include [121]:
-
•
Over 90% modified their food intake, modified their physical activity, and exercise about 60 minutes per day
-
•
Over 70% eat breakfast daily and weigh themselves at least once a week
-
•
The most frequently reported physical activity was walking
-
•
Registry participants reported 45% lost weight on their own
-
•
55% lost weight with the help of some type of program
5. Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on Behavior, Motivational Interviewing, Eating Disorders, and Obesity Management Technologies is designed to assist clinicians in the care of patients with the disease of pre-obesity/obesity. Understanding patient motivators and behaviors is essential for obesity medicine practitioners. Additionally, implementation of appropriate techniques in motivational interviewing is helpful in treating patients, and obesity management technologies can play an important role in ongoing treatment. An understanding of behavior therapy, motivational interviewing, and potentially helpful technologies may help clinicians improve the health of their patients, especially those with adverse fat mass and adiposopathic metabolic consequences.
5.1. Transparency [122]
This manuscript was largely derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. This was followed by a similar Pediatric “Obesity Algorithm,” with updates approximately every two years by OMA authors. Authors of prior years’ version of the Obesity Algorithm are included in Supplement #1.
5.2. Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians (Supplement #1). The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
6. Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Author contributions
MF, SC, LO, and HEB reviewed, edited, and approved the document.
Managing disclosures and dualities of interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual disclosures
MF, SC, LO, and HEB have no applicable disclosures.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Disclosures
MS, SC, LO, and HEB report no relevant disclosures.
Acknowledgements and Funding
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2022.100014.
Contributor Information
Michelle Freshwater, Email: mfreshwater@idahoweightloss.com.
Sandra Christensen, Email: sam.chris@im-wm.com.
Lauren Oshman, Email: laoshman@med.umich.edu.
Harold Edward Bays, Email: hbaysmd@outlook.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Bays H.E., McCarthy W., Burridge K., Tondt J., Karjoo S., Christensen S., Ng J., Golden A., Davisson L., Richardson L. Obesity Medicine Association; 2021. Obesity Algorithm eBook.www.obesityalgorithm.orghttps://obesitymedicine.org/obesity-algorithm/ presented by the. [Google Scholar]
- 2.Silventoinen K., Konttinen H. Obesity and eating behavior from the perspective of twin and genetic research. Neurosci Biobehav Rev. 2020;109:150–165. doi: 10.1016/j.neubiorev.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Lightfoot J.T., EJC D.E.G., Booth F.W., Bray M.S., DENH M., Kaprio J., et al. Biological/genetic regulation of physical activity level: consensus from GenBioPAC. Med Sci Sports Exerc. 2018;50:863–873. doi: 10.1249/MSS.0000000000001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneeberger M., Gomis R., Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J Endocrinol. 2014;220:T25–46. doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- 6.Neymotin F., Nemzer L.R. Locus of control and obesity. Front Endocrinol. 2014;5:159. doi: 10.3389/fendo.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteleone P., Piscitelli F., Scognamiglio P., Monteleone A.M., Canestrelli B., Di Marzo V., et al. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metabol. 2012;97:E917–E924. doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- 8.Batra P., Das S.K., Salinardi T., Robinson L., Saltzman E., Scott T., et al. Eating behaviors as predictors of weight loss in a 6 month weight loss intervention. Obesity. 2013;21:2256–2263. doi: 10.1002/oby.20404. [DOI] [PubMed] [Google Scholar]
- 9.Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam T.C., Epel E.S. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Gordon E.L., Ariel-Donges A.H., Bauman V., Merlo L.J. What is the evidence for "food addiction?" A systematic review. Nutrients. 2018;10 doi: 10.3390/nu10040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemps E., Tiggemann M. Approach bias for food cues in obese individuals. Psychol Health. 2015;30:370–380. doi: 10.1080/08870446.2014.974605. [DOI] [PubMed] [Google Scholar]
- 13.Miller A.C., Polgreen L.A., Segre E.M., Polgreen P.M. Variations in marginal Taste perception by body mass index classification: a randomized controlled trial. J Acad Nutr Diet. 2019 doi: 10.1016/j.jand.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Cruwys T., Bevelander K.E., Hermans R.C. Social modeling of eating: a review of when and why social influence affects food intake and choice. Appetite. 2015;86:3–18. doi: 10.1016/j.appet.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Bays H.E., Shrestha A., Niranjan V., Khanna M., Kambhamettu L. Obesity pillars roundtable: obesity and South Asians. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bays H.E., Muñoz-Mantilla D.X., Morgan R., Nwizu C., Garcia T.T. Obesity pillars roundtable: obesity and diversity. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander L., Christensen S.M., Richardson L., Ingersoll A.B., Burridge K., Golden A., et al. Nutrition and physical activity: an obesity medicine association (OMA) clinical practice statement 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yau Y.H., Potenza M.N. Stress and eating behaviors. Minerva Endocrinol. 2013;38:255–267. [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar S., Kochhar K.P., Khan N.A. Fat addiction: psychological and physiological trajectory. Nutrients. 2019;11 doi: 10.3390/nu11112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzone C.M., Liang-Guallpa J., Li C., Wolcott N.S., Boone M.H., Southern M., et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci. 2020;23:1253–1266. doi: 10.1038/s41593-020-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Strien T., Gibson E.L., Baños R., Cebolla A., Winkens L.H.H. Is comfort food actually comforting for emotional eaters? A (moderated) mediation analysis. Physiol Behav. 2019;211 doi: 10.1016/j.physbeh.2019.112671. [DOI] [PubMed] [Google Scholar]
- 22.Lerma-Cabrera J.M., Carvajal F., Lopez-Legarrea P. Food addiction as a new piece of the obesity framework. Nutr J. 2016;15:5. doi: 10.1186/s12937-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milaneschi Y., Simmons W.K., van Rossum E.F.C., Penninx B.W. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatr. 2019;24:18–33. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 24.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W., et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatr. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 25.Fuller N.R., Burns J., Sainsbury A., Horsfield S., da Luz F., Zhang S., et al. Examining the association between depression and obesity during a weight management programme. Clin Obes. 2017;7:354–359. doi: 10.1111/cob.12208. [DOI] [PubMed] [Google Scholar]
- 26.Ma J., Rosas L.G., Lv N., Xiao L., Snowden M.B., Venditti E.M., et al. Effect of integrated behavioral weight loss treatment and problem-solving therapy on body mass index and depressive symptoms among patients with obesity and depression: the RAINBOW randomized clinical trial. JAMA : J Am Med Assoc. 2019;321:869–879. doi: 10.1001/jama.2019.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y.T., Huang W.Y., Kor C.T., Liu K.H., Chen T.Y., Lin P.T., et al. Relationships between depression and anxiety symptoms and adipocyte-derived proteins in postmenopausal women. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Gower B.A., Shelton R.C., Wu X. Gender-specific relationship between obesity and major depression. Front Endocrinol. 2017;8:292. doi: 10.3389/fendo.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paiva-Medeiros P.F., Duarte-Guerra L.S., Santo M.A., Lotufo-Neto F., Wang Y.P. Psychometric properties of the montgomery-Åsberg depression rating scale in severely obese patients. Spanish J Psychol. 2015;18 doi: 10.1017/sjp.2015.072. [DOI] [PubMed] [Google Scholar]
- 30.Seferovic A., Dianes G.N., Juan B., Larsen D., Oyler V., Ragoza Y. What is the best screening tool for eating disorders in the primary care setting? Evidence-Based Pract. 2019;22:12. [Google Scholar]
- 31.Larsson I., Hulthén L., Landén M., Pålsson E., Janson P., Stener-Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35:213–218. doi: 10.1016/j.clnu.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Tromp M.D., Donners A.A., Garssen J., Verster J.C. Sleep, eating disorder symptoms, and daytime functioning. Nat Sci Sleep. 2016;8:35–40. doi: 10.2147/NSS.S97574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green M.A., Scott N.A., Riopel C.M., Skaggs A.K. Feminist identity as a predictor of eating disorder diagnostic status. J Clin Psychol. 2008;64:777–788. doi: 10.1002/jclp.20459. [DOI] [PubMed] [Google Scholar]
- 34.Uguz F., Sahingoz M., Gungor B., Aksoy F., Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatr. 2015;37:46–48. doi: 10.1016/j.genhosppsych.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Smith K.E., Ellison J.M., Crosby R.D., Engel S.G., Mitchell J.E., Crow S.J., et al. The validity of DSM-5 severity specifiers for anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int J Eat Disord. 2017;50:1109–1113. doi: 10.1002/eat.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amianto F., Ottone L., Abbate Daga G., Fassino S. Binge-eating disorder diagnosis and treatment: a recap in front of DSM-5. BMC Psychiatr. 2015;15:70. doi: 10.1186/s12888-015-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler R.C., Berglund P.A., Chiu W.T., Deitz A.C., Hudson J.I., Shahly V., et al. The prevalence and correlates of binge eating disorder in the world health organization world mental health surveys. Biol Psychiatr. 2013;73:904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grupski A.E., Hood M.M., Hall B.J., Azarbad L., Fitzpatrick S.L., Corsica J.A. Examining the Binge Eating Scale in screening for binge eating disorder in bariatric surgery candidates. Obes Surg. 2013;23:1–6. doi: 10.1007/s11695-011-0537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brauhardt A., de Zwaan M., Hilbert A. The therapeutic process in psychological treatments for eating disorders: a systematic review. Int J Eat Disord. 2014;47:565–584. doi: 10.1002/eat.22287. [DOI] [PubMed] [Google Scholar]
- 40.Reas D.L., Grilo C.M. Current and emerging drug treatments for binge eating disorder. Expet Opin Emerg Drugs. 2014;19:99–142. doi: 10.1517/14728214.2014.879291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aigner M., Treasure J., Kaye W., Kasper S., Disorders W.T.F.O.E. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. World J Biol Psychiatr : Off J World Fed Soc Biol Psychiatr. 2011;12:400–443. doi: 10.3109/15622975.2011.602720. [DOI] [PubMed] [Google Scholar]
- 42.Flament M.F., Bissada H., Spettigue W. Evidence-based pharmacotherapy of eating disorders. Int J Neuropsychopharmacol/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012;15:189–207. doi: 10.1017/S1461145711000381. [DOI] [PubMed] [Google Scholar]
- 43.Lisdexamfetamine dimesylate (VYVANSE) prescribing information http://pi.shirecontent.com/PI/PDFs/Vyvanse_USA_ENG.pdf (Accessed August 20, 2016).
- 44.Herman B.K., Deal L.S., DiBenedetti D.B., Nelson L., Fehnel S.E., Brown T.M. Development of the 7-item binge-eating disorder screener (BEDS-7) Prim Care Companion CNS Disord. 2016;18 doi: 10.4088/PCC.15m01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rushing J.M., Jones L.E., Carney C.P. Bulimia nervosa: a primary care review. Prim Care Companion J Clin Psychiatry. 2003;5:217–224. doi: 10.4088/pcc.v05n0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maguen S., Hebenstreit C., Li Y., Dinh J.V., Donalson R., Dalton S., et al. Screen for Disordered Eating: improving the accuracy of eating disorder screening in primary care. General hospital psychiatry. 2018;50:20–25. doi: 10.1016/j.genhosppsych.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Cotton M.A., Ball C., Robinson P. Four simple questions can help screen for eating disorders. Journal of general internal medicine. 2003;18:53–56. doi: 10.1046/j.1525-1497.2003.20374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandberg K., Erford B.T. Choosing assessment instruments for bulimia practice and outcome research. Journal of Counseling & Development. 2013;91:367–379. [Google Scholar]
- 49.U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality . October 2021. Screening for eating disorders in adolescents and adults: an evidence review for the U.S. Preventive services task force. AHRQ Publication No. 21-05284-EF-1. [PubMed] [Google Scholar]
- 50.Allison K.C., Lundgren J.D., O'Reardon J.P., Geliebter A., Gluck M.E., Vinai P., et al. Proposed diagnostic criteria for night eating syndrome. The International journal of eating disorders. 2010;43:241–247. doi: 10.1002/eat.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallant A.R., Lundgren J., Drapeau V. The night-eating syndrome and obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13:528–536. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 52.Milano W., De Rosa M., Milano L., Capasso A. Night eating syndrome: an overview. The Journal of pharmacy and pharmacology. 2012;64:2–10. doi: 10.1111/j.2042-7158.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 53.Stunkard A.J., Allison K.C., Geliebter A., Lundgren J.D., Gluck M.E., O'Reardon J.P. Development of criteria for a diagnosis: lessons from the night eating syndrome. Comprehensive psychiatry. 2009;50:391–399. doi: 10.1016/j.comppsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allison K.C., Tarves E.P. Treatment of night eating syndrome. The Psychiatric clinics of North America. 2011;34:785–796. doi: 10.1016/j.psc.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allison K.C., Lundgren J.D., O'Reardon J.P., Martino N.S., Sarwer D.B., Wadden T.A., et al. The night eating questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Behav. 2008;9:62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Komada Y., Takaesu Y., Matsui K., Nakamura M., Nishida S., Kanno M., et al. Comparison of clinical features between primary and drug-induced sleep-related eating disorder. Neuropsychiatr Dis Treat. 2016;12:1275–1280. doi: 10.2147/NDT.S107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh D., Petrecca A.M., Khuhro A.L. Sleep-related eating disorder (SRED): paradoxical effect of clonazepam. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018;14:1261–1263. doi: 10.5664/jcsm.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leu-Semenescu S., Maranci J.B., Lopez R., Drouot X., Dodet P., Gales A., et al. Comorbid parasomnias in narcolepsy and idiopathic hypersomnia: more REM than NREM parasomnias. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 doi: 10.5664/jcsm.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winkelman J.W., Wipper B., Purks J., Mei L., Schoerning L. Topiramate reduces nocturnal eating in sleep-related eating disorder. Sleep. 2020;43 doi: 10.1093/sleep/zsaa060. [DOI] [PubMed] [Google Scholar]
- 60.Vinai P., Ferri R., Ferini-Strambi L., Cardetti S., Anelli M., Vallauri P., et al. Defining the borders between sleep-related eating disorder and night eating syndrome. Sleep Med. 2012;13:686–690. doi: 10.1016/j.sleep.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Chiaro G., Caletti M.T., Provini F. Treatment of sleep-related eating disorder. Curr Treat Options Neurol. 2015;17:361. doi: 10.1007/s11940-015-0361-6. [DOI] [PubMed] [Google Scholar]
- 62.Peterson J.A. Get moving! Physical activity counseling in primary care. Journal of the American Academy of Nurse Practitioners. 2007;19:349–357. doi: 10.1111/j.1745-7599.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 63.Gupta H. Barriers to and facilitators of long term weight loss maintenance in adult UK people: a thematic analysis. International journal of preventive medicine. 2014;5:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 64.Cornier M.A. Is your brain to blame for weight regain? Physiology & behavior. 2011;104:608–612. doi: 10.1016/j.physbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sainsbury A., Zhang L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13:234–257. doi: 10.1111/j.1467-789X.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- 66.Rosenbaum M., Leibel R.L. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maclean P.S., Bergouignan A., Cornier M.A., Jackman M.R. Biology's response to dieting: the impetus for weight regain. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo S. Dynamic energy balance and obesity prevention. J Obes Metab Syndr. 2018;27:203–212. doi: 10.7570/jomes.2018.27.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howlett N., Trivedi D., Troop N.A., Chater A.M. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Translational behavioral medicine. 2019;9:147–157. doi: 10.1093/tbm/iby010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemstra M., Bird Y., Nwankwo C., Rogers M., Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547–1559. doi: 10.2147/PPA.S103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richardson L.A. Bariatric society is here to help. The Journal of family practice. 2010;59:488. [PubMed] [Google Scholar]
- 72.Jacob J.J., Isaac R. Behavioral therapy for management of obesity. Indian journal of endocrinology and metabolism. 2012;16:28–32. doi: 10.4103/2230-8210.91180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Dorsten B., Lindley E.M. Cognitive and behavioral approaches in the treatment of obesity. The Medical clinics of North America. 2011;95:971–988. doi: 10.1016/j.mcna.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karasu S.R. Psychotherapy-lite: obesity and the role of the mental health practitioner. American journal of psychotherapy. 2013;67:3–22. doi: 10.1176/appi.psychotherapy.2013.67.1.3. [DOI] [PubMed] [Google Scholar]
- 76.Hansen T.T., Andersen S.V., Astrup A., Blundell J., Sjodin A. Is reducing appetite beneficial for body weight management in the context of overweight and obesity? A systematic review and meta-analysis from clinical trials assessing body weight management after exposure to satiety enhancing and/or hunger reducing products. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2019;20:983–997. doi: 10.1111/obr.12854. [DOI] [PubMed] [Google Scholar]
- 77.Rutledge T., Groesz L.M., Linke S.E., Woods G., Herbst K.L. Behavioural weight management for the primary careprovider. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:e290–e297. doi: 10.1111/j.1467-789X.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 78.Harvey J., Krukowski R., Priest J., West D. Log often, lose more: electronic dietary self-monitoring for weight loss. Obesity. 2019;27:380–384. doi: 10.1002/oby.22382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeffery R.W., Bjornson-Benson W.M., Rosenthal B.S., Lindquist R.A., Johnson S.L. Behavioral treatment of obesity with monetary contracting: two-year follow-up. Addictive behaviors. 1984;9:311–313. doi: 10.1016/0306-4603(84)90027-3. [DOI] [PubMed] [Google Scholar]
- 80.Lynch E., Emery-Tiburcio E., Dugan S., White F.S., Thomason C., Jenkins L., et al. Results of alive: a faith-based pilot intervention to improve diet among African American church members. Prog Community Health Partnersh. 2019;13:19–30. doi: 10.1353/cpr.2019.0005. [DOI] [PubMed] [Google Scholar]
- 81.Brambila-Macias J., Shankar B., Capacci S., Mazzocchi M., Perez-Cueto F.J., Verbeke W., et al. Policy interventions to promote healthy eating: a review of what works, what does not, and what is promising. Food and nutrition bulletin. 2011;32:365–375. doi: 10.1177/156482651103200408. [DOI] [PubMed] [Google Scholar]
- 82.Miller W.R., Rollnick S. third ed. Guilford Press; 2013. Motivational interviewing: helping people to change. [Google Scholar]
- 83.Barnes R.D., Ivezaj V. A systematic review of motivational interviewing for weight loss among adults in primary care. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16:304–318. doi: 10.1111/obr.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zimmerman G.L., Olsen C.G., Bosworth M.F. A 'stages of change' approach to helping patients change behavior. American family physician. 2000;61:1409–1416. [PubMed] [Google Scholar]
- 85.Mastellos N., Gunn L.H., Felix L.M., Car J., Majeed A. Transtheoretical model stages of change for dietary and physical exercise modification in weight loss management for overweight and obese adults. The Cochrane database of systematic reviews. 2014 doi: 10.1002/14651858.CD008066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Omer T. Behaviour modification in weight management: the transtheoretical mode of change and the motivational interviewing. J Obes Weight-Loss Medic. 2020;6:38. [Google Scholar]
- 87.Lecube A., Sanchez E., Andres A., Saldana C., Morales M.J., Calanas A., et al. Assessing motivational stages and processes of change for weight management around bariatric surgery: a multicenter study. Obesity surgery. 2019;29:3348–3356. doi: 10.1007/s11695-019-04001-4. [DOI] [PubMed] [Google Scholar]
- 88.Ries A.V., Blackman L.T., Page R.A., Gizlice Z., Benedict S., Barnes K., et al. Goal setting for health behavior change: evidence from an obesity intervention for rural low-income women. Rural and remote health. 2014;14:2682. [PubMed] [Google Scholar]
- 89.Giannisi F., Pervanidou P., Michalaki E., Papanikolaou K., Chrousos G., Yannakoulia M. Parental readiness to implement life-style behaviour changes in relation to children's excess weight. Journal of paediatrics and child health. 2014;50:476–481. doi: 10.1111/jpc.12500. [DOI] [PubMed] [Google Scholar]
- 90.Tyler D.O., Horner S.D. Family-centered collaborative negotiation: a model for facilitating behavior change in primary care. Journal of the American Academy of Nurse Practitioners. 2008;20:194–203. doi: 10.1111/j.1745-7599.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 91.Miller W.R., Rose G.S. Toward a theory of motivational interviewing. The American psychologist. 2009;64:527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pollak K.I., Alexander S.C., Tulsky J.A., Lyna P., Coffman C.J., Dolor R.J., et al. Physician empathy and listening: associations with patient satisfaction and autonomy. Journal of the American Board of Family Medicine : JABFM. 2011;24:665–672. doi: 10.3122/jabfm.2011.06.110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams D.M., Rhodes R.E. The confounded self-efficacy construct: conceptual analysis and recommendations for future research. Health psychology review. 2016;10:113–128. doi: 10.1080/17437199.2014.941998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westra H.A., Aviram A. Core skills in motivational interviewing. Psychotherapy. 2013;50:273–278. doi: 10.1037/a0032409. [DOI] [PubMed] [Google Scholar]
- 95.Pollak K.I., Coffman C.J., Alexander S.C., Ostbye T., Lyna P., Tulsky J.A., et al. Weight's up? Predictors of weight-related communication during primary care visits with overweight adolescents. Patient education and counseling. 2014;96:327–332. doi: 10.1016/j.pec.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Resnicow K., McMaster F. Motivational Interviewing: moving from why to how with autonomy support. The international journal of behavioral nutrition and physical activity. 2012;9:19. doi: 10.1186/1479-5868-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Codern-Bove N., Pujol-Ribera E., Pla M., Gonzalez-Bonilla J., Granollers S., Ballve J.L., et al. Motivational interviewing interactions and the primary health care challenges presented by smokers with low motivation to stop smoking: a conversation analysis. BMC public health. 2014;14:1225. doi: 10.1186/1471-2458-14-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams A.A., Wright K.S. Engaging families through motivational interviewing. Pediatric clinics of North America. 2014;61:907–921. doi: 10.1016/j.pcl.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Pantalon M.V., Sledge W.H., Bauer S.F., Brodsky B., Giannandrea S., Kay J., et al. Important medical decisions: using brief motivational interviewing to enhance patients' autonomous decision-making. Journal of psychiatric practice. 2013;19:98–108. doi: 10.1097/01.pra.0000428556.48588.22. [DOI] [PubMed] [Google Scholar]
- 100.Miller S.T., Oates V.J., Brooks M.A., Shintani A., Gebretsadik T., Jenkins D.M. Preliminary efficacy of group medical nutrition therapy and motivational interviewing among obese African American women with type 2 diabetes: a pilot study. Journal of obesity 2014. 2014 doi: 10.1155/2014/345941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elwyn G., Dehlendorf C., Epstein R.M., Marrin K., White J., Frosch D.L. Shared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Annals of family medicine. 2014;12:270–275. doi: 10.1370/afm.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teixeira P.J., Carraca E.V., Marques M.M., Rutter H., Oppert J.M., De Bourdeaudhuij I., et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC medicine. 2015;13:84. doi: 10.1186/s12916-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Windham M.E., Hastings E.S., Anding R., Hergenroeder A.C., Escobar-Chaves S.L., Wiemann C.M. Teens Talk Healthy Weight": the impact of a motivational digital video disc on parental knowledge of obesity-related diseases in an adolescent clinic. Journal of the Academy of Nutrition and Dietetics. 2014;114:1611–1618. doi: 10.1016/j.jand.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 104.Saelens B.E., Lozano P., Scholz K. A randomized clinical trial comparing delivery of behavioral pediatric obesity treatment using standard and enhanced motivational approaches. Journal of pediatric psychology. 2013;38:954–964. doi: 10.1093/jpepsy/jst054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kushner R.F., Ryan D.H. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA : the journal of the American Medical Association. 2014;312:943–952. doi: 10.1001/jama.2014.10432. [DOI] [PubMed] [Google Scholar]
- 106.Carcone A.I., Naar-King S., Brogan K.E., Albrecht T., Barton E., Foster T., et al. Provider communication behaviors that predict motivation to change in black adolescents with obesity. Journal of developmental and behavioral pediatrics : JDBP. 2013;34:599–608. doi: 10.1097/DBP.0b013e3182a67daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kisely S., Ligate L., Roy M.A., Lavery T. Applying Motivational Interviewing to the initiation of long-acting injectable atypical antipsychotics. Australasian psychiatry : bulletin of Royal Australian and New Zealand College of Psychiatrists. 2012;20:138–142. doi: 10.1177/1039856212437257. [DOI] [PubMed] [Google Scholar]
- 108.Vallis M., Piccinini-Vallis H., Sharma A.M., Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Canadian family physician Medecin de famille canadien. 2013;59:27–31. [PMC free article] [PubMed] [Google Scholar]
- 109.Alexander S.C., Cox M.E., Boling Turer C.L., Lyna P., Ostbye T., Tulsky J.A., et al. Do the five A's work when physicians counsel about weight loss? Family medicine. 2011;43:179–184. [PMC free article] [PubMed] [Google Scholar]
- 110.Searight R. Realistic approaches to counseling in the office setting. American family physician. 2009;79:277–284. [PubMed] [Google Scholar]
- 111.Foote J., DeLuca A., Magura S., Warner A., Grand A., Rosenblum A., et al. A group motivational treatment for chemical dependency. Journal of substance abuse treatment. 1999;17:181–192. doi: 10.1016/s0740-5472(99)00003-3. [DOI] [PubMed] [Google Scholar]
- 112.Dobkin B.H. Wearable motion sensors to continuously measure real-world physical activities. Current opinion in neurology. 2013;26:602–608. doi: 10.1097/WCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evenson K.R., Goto M.M., Furberg R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. The international journal of behavioral nutrition and physical activity. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Allen L.N., Christie G.P. The emergence of personalized health technology. J Med Internet Res. 2016;18 doi: 10.2196/jmir.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mercer K., Li M., Giangregorio L., Burns C., Grindrod K. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR Mhealth Uhealth. 2016;4 doi: 10.2196/mhealth.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jakicic J.M., Davis K.K., Rogers R.J., King W.C., Marcus M.D., Helsel D., et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA : the journal of the American Medical Association. 2016;316:1161–1171. doi: 10.1001/jama.2016.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheatham S.W., Stull K.R., Fantigrassi M., Motel I. The efficacy of wearable activity tracking technology as part of a weight loss program: a systematic review. The Journal of sports medicine and physical fitness. 2018;58:534–548. doi: 10.23736/S0022-4707.17.07437-0. [DOI] [PubMed] [Google Scholar]
- 118.Brickwood K.J., Watson G., O'Brien J., Williams A.D. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kononova A., Li L., Kamp K., Bowen M., Rikard R.V., Cotten S., et al. The use of wearable activity trackers among older adults: focus group study of tracker perceptions, motivators, and barriers in the maintenance stage of behavior change. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/mhealth.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chou W.Y., Prestin A., Kunath S. Obesity in social media: a mixed methods analysis. Translational behavioral medicine. 2014;4:314–323. doi: 10.1007/s13142-014-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.National Weight Control Registry Team. National weight control Registry. http://www.nwcr.ws/(Accessed May 8 2020).
- 122.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.