Abstract
Background
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) details nutritional and activity recommendations for the child with normal weight, overweight, and obesity (Appendix A) with consideration of food insecurity. This CPS is intended to provide clinicians with an overview of clinical practices applicable to children and adolescents with body mass indices in the normal range and body mass indices greater than or equal to the 85th percentile for their ages, particularly those with adverse consequences resulting from increased body mass. The information in this CPS is based on scientific evidence, supported by the medical literature, and derived from the clinical experiences of members of the OMA.
Methods
The scientific information and clinical guidance in this CPS is based upon referenced evidence and derived from the clinical perspectives of the authors.
Results
This OMA Clinical Practice Statement details nutritional and activity recommendations for the child with normal weight, overweight, and obesity with consideration of food insecurity. In addition, this CPS addresses nutritional recommendations for complications related to the disease of obesity as well as providing guidance on food insecurity as it impacts children with obesity and their families.
Conclusions
This OMA Clinical Practice Statement on nutritional and activity recommendations for the child with normal weight, overweight, and obesity with consideration of food insecurity is an overview of current recommendations. These recommendations provide a roadmap to the improvement of the health of children and adolescents with obesity, especially those with metabolic, physiological, and psychological complications.
Keywords: Activity recommendation, Assessment, Food insecurity, Nutritional evaluation, Obesity, Pediatric
1. Introduction
The purpose of this CPS regarding nutritional and activity recommendations for the child with normal weight, overweight, and obesity with consideration of food insecurity is to provide clinicians with tools to clinically assess and manage children with obesity. The OMA is an organization of providers in the field of obesity medicine dedicated to the comprehensive care of patients with obesity. OMA members are physicians, nurse practitioners, physician assistants, and other healthcare providers who take a comprehensive, evidence-based approach to treating obesity. This approach is comprised of the four pillars of nutrition, physical activity, behavior, and medication. While it is hoped many clinicians may find the recommendations in this CPS helpful, the final decision regarding the optimal care of the patient with overweight or obesity is dependent upon the individual clinical presentation and the judgment of the clinician who is tasked with directing a treatment plan that is in the best interest of the patient.
2. Nutritional recommendations for children of normal weight
General nutritional recommendations for children and adolescents fall into age categories that are related to overall growth and development stages. For infants from 0 to 12 months of age, guidelines are further divided into birth to 4 months, 4–6 months, 6–8 months, 8–10 months, and 10–12 months due to the rapid physiologic and metabolic changes during the child's first year of life [1]. Recommendations from 0 to 12 months emphasize breastfeeding whenever possible. Fortified formula may be used when breastmilk is not available. Cereals, breads, starches, fruits, vegetables, and proteins (meat or legumes) are gradually introduced from 6 to 12 months of age [2].
From 1 to 4 years of age, children gradually transition to milk/milk products from breastmilk or formula. Intake of breads/starches, fruits, vegetables, and proteins gradually increases with age. Of note, low-fat products are not recommended in the first two years of life, with 3–4 teaspoons per day of fats and oil recommended from ages 3–5 years. Desserts, sweets, soft drinks, candy, jams, jelly and added sugar to foods are not advised during the first 5 years of life [1,2].
During ages 5–18 years, volume in all food categories increases as the child/adolescent grows and develops. Vegetables continue to be encouraged, particularly non-starchy vegetables such as broccoli, spinach, green beans, and tomatoes. Sweets and added sugars (including in all fluids) are not recommended [1,2]. Table 1, Table 2, Table 3 offer more specific guidance, and Table 4 shows the top takeaways from the OMA regarding nutritional principles for children of normal weight.
Table 1.
General intake guidelines (normal weight): 0 to 12 months. Infants from 0 to 12 years of age have unique nutritional considerations; nutritional recommendations are divided into categories for 0–4 months, 4–6 months, 6–8 months, 8–10 months, and 10–12 months [1,2].
| Birth–4 months | 4–6 months | 6–8 months | 8–10 months | 10–12 months | |
|---|---|---|---|---|---|
| Breast milk and/or fortified infant formula | 8 to 12 feedings ∗2 to 6 oz per feeding (18–32 oz per day) |
4 to 6 feedings ∗4 to 6 oz per feeding (27–45 oz per day) |
3 to 5 feedings ∗6 to 8 oz per feeding (24–32 oz per day) |
3 to 4 feedings ∗7 to 8 oz per feeding (24–32 oz per day) |
3 to 4 feedings ∗24–32 oz per day |
| Cereal, breads, starches | None | None | 2–3 servings of iron-fortified baby cereal (serving = 1–2 tbsp) | 2–3 servings of iron-fortified baby cereal (serving = 1–2 tbsp) | 4 servings of iron-fortified bread or other soft starches or baby cereal (serving = 1–2 tbsp) |
| Fruits and vegetables | None | None | Offer plain, cooked, mashed, or strained baby foods vegetables and fruits. Avoid combination foods. No juice. | 2–3 servings (1–2 tbsp) of soft, cut-up, and mashed vegetables and fruits daily. No juice. | 4 servings (2–3 tbsp) daily of fruits and vegetables. No juice. |
| Meats and other protein sources | None | None | Begin to offer plain-cooked meats. Avoid combination dinners. | Begin to offer well-cooked, soft, finely chopped meats. | 1–2 oz daily of soft, finely cut or chopped meats or other protein foods |
While there is no comprehensive research indication of which complimentary foods are best to introduce first, the focus should be on first foods that are higher in iron and zinc, such as pureed meats and fortified-iron rich foods.
Table 2.
General intake guidelines (normal weight): 1 to 4 years. Children from 1 to 4 years of age gradually transition to milk and milk products and begin to incorporate more proteins, breads, grains, starches, fruits, and vegetables [1,2].
| 12–23 months | 2–3 years | 3–4 years | |
|---|---|---|---|
| Milk and Milk Products | 2 cups/day (whole milk or milk products) | 2–2.5 cups/day | 2.5–3 cups/day |
| Serving: 1 cup of milk or cheese, 1½ oz of natural cheese, 1/3 cup shredded cheese | |||
| Meat and Other Protein Foods | 1½ oz/day | 2 oz/day | 2–3 oz/day |
| Serving: (1 oz equivalent) = 1 oz beef, poultry, fish, ¼ cup cooked beans, 1 egg, 1 tbsp peanut butter∗, ½ oz of nuts∗ ∗peanut butter and nuts may be a choking hazard under the age of three | |||
| Breads, Cereal, and Starches | 2 oz/day | 2 oz/day | 2–3 oz/day |
| 1 oz = 1 slice whole grain bread, ½ cup cooked cereal, rice, pasta or 1 cup dry cereal | |||
| Fruits | 1 cup/day | 1 cup/day | 1–1½ cups/day |
| Serving: 1 cup of fruit or ½ cup dried fruit; NO JUICE | |||
| Vegetables (non-starchy vegetables to include sources of vitamin C and A) | 3/4 cup/day | 1 cup/day | 1–1½ cups/day |
| Serving: (1 cup equivalent) = 1 cup of raw or cooked vegetables; 2 cups of raw leafy green greens | |||
| Fats and Oil | Do not limit∗ ∗Low-fat products are not recommended under the age of 2 |
3 teaspoons | 3–4 teaspoons/day |
| Miscellaneous desserts, sweets, soft drinks, candy, jams, jelly | none | none | None |
Table 3.
General intake guidelines (normal weight): 5 to 18 years. From 5 to 18 years of age, the recommendations for children in all food categories increase in volume as the children grow and develop. There is a continued emphasis on the inclusion of non-starchy vegetables; desserts, sweets, and other added-sugar foods are best avoided [1,2].
| 12–23 months | 2–3 years | 3–4 years | |
|---|---|---|---|
| Milk and Milk Products | 2 cups/day (whole milk or milk products) | 2–2.5 cups/day | 2.5–3 cups/day |
| Serving: 1 cup of milk or cheese, 1½ oz of natural cheese, 1/3 cup shredded cheese | |||
| Meat and Other Protein Foods | 1½ oz/day | 2 oz/day | 2–3 oz/day |
| Serving: (1 oz equivalent) = 1 oz beef, poultry, fish, ¼ cup cooked beans, 1 egg, 1 tbsp peanut butter∗, ½ oz of nuts∗ ∗peanut butter and nuts may be a choking hazard under the age of three | |||
| Breads, Cereal, and Starches | 2 oz/day | 2 oz/day | 2–3 oz/day |
| 1 oz = 1 slice whole grain bread, ½ cup cooked cereal, rice, pasta or 1 cup dry cereal | |||
| Fruits | 1 cup/day | 1 cup/day | 1–1½ cups/day |
| Serving: 1 cup of fruit or ½ cup dried fruit; NO JUICE | |||
| Vegetables (non-starchy vegetables to include sources of vitamin C and A) | 3/4 cup/day | 1 cup/day | 1–1½ cups/day |
| Serving: (1 cup equivalent) = 1 cup of raw or cooked vegetables; 2 cups of raw leafy green greens | |||
| Fats and Oil | Do not limit∗ ∗Low-fat products are not recommended under the age of 2 |
3 teaspoons | 3–4 teaspoons/day |
| Miscellaneous desserts, sweets, soft drinks, candy, jams, jelly | None | none | None |
Table 4.
Top takeaways: nutrition recommendations for children of normal weight. Shown are the top takeaways from the OMA regarding nutrition for children of normal weight, including increases in food quantities, encouragement of vegetables, and avoidance of sweets and added sugars.
| 1. | Dietary recommendations for infants and children reflect their stages of growth and development. |
| 2. | Breastfeeding is encouraged during the first 12 months of life, with the gradual introduction of solids after 6 months of age. |
| 3. | Low-fat products are not recommended under the age of 2 years. Fats and oils are gradually increased from 3 to 6 teaspoons per day from ages 2–18 years. |
| 4. | Desserts, sweets, and added sugar, especially in fluids, are not recommended at any age. |
| 5. | Vegetables, especially non-starchy vegetables, are encouraged in various forms starting at 6 months of age and continuing through adulthood, but juices should be avoided. |
3. Activity recommendations for children of normal weight, overweight, and obesity
Activity recommendations for children and adolescents of normal weight and for those with overweight/obesity also fall into age categories that correspond to growth and development stages. Caregivers should follow recommendations from pediatric providers skilled in growth and development principles.
For infants from 0 to 12 months of age, movement and active play with caregivers is encouraged for short periods several times a day. The setting should encourage and stimulate movement experiences, and the environment should meet or exceed recommended safety standards for performing large-muscle activities. These activities should promote skill development appropriate to the specific developmental skill of the infant. The key focus is exploring movement through the infant's environment through structured and unstructured physical activity [3,4].
For the child 12–36 months of age, activity should include a minimum of 30 minutes of structured and 60 minutes of unstructured physical activity per day. During this growth phase, activities should maximize any opportunity to develop movement skills that will serve as the building blocks for future motor skillfulness and physical activity. Children benefit from access to indoor and outdoor areas for performing large-muscle activities. Caretakers should promote movement skills by providing activity and physical movement experiences [3,4].
Recommendations for the child ages 3–5 years are similar, with structured physical activity increasing to 60 minutes daily. For children ages 5 through adolescence, a minimum of 60 minutes, with a preference of several hours, is recommended for age-appropriate physical activity and play. These activities should include several bouts of physical activity lasting 15 minutes or more each day designed to achieve optimal health, wellness, fitness, and performance benefits. Extended periods of inactivity are discouraged, especially during the daytime hours [3,4]. Table 5 shows the top takeaways from the OMA regarding activity recommendations for children for normal weight.
Table 5.
Top takeaways: activity recommendations for children of normal weight, overweight, and obesity. Shown are the OMA's top takeaway messages regarding activity recommendations for children of normal weight [5].
| 1. | Activity recommendations exist for children and adolescents of normal weight and overweight/obesity at each developmental stage. |
| 2. | Structured activity and unstructured play are equally key components of an optimal activity plan. |
| 3. | Extended periods of inactivity are discouraged at any age. |
4. Nutrition management options for children with overweight and obesity
Evidence suggests that weight loss in children with obesity is possible regardless of the macronutrient distribution in an energy-reduced diet [6]. Similar results have been found in adult studies [7]. Tailoring the macronutrient content to address specific obesity-related complications may be helpful, but more research is needed. The pediatric provider uses the principles of precision medicine to consider which nutritional option best addresses a particular child and family's needs. Examples of macronutrient options and comparisons of common recommendations follow in Fig. 1, Fig. 2. Table 6 shows the top takeaways from the OMA regarding nutritional interventions for the child with obesity. All nutritional interventions are more effective in combination with activity and behavioral counseling, which includes addressing conditions detrimental to the child and family's social determinants of health.
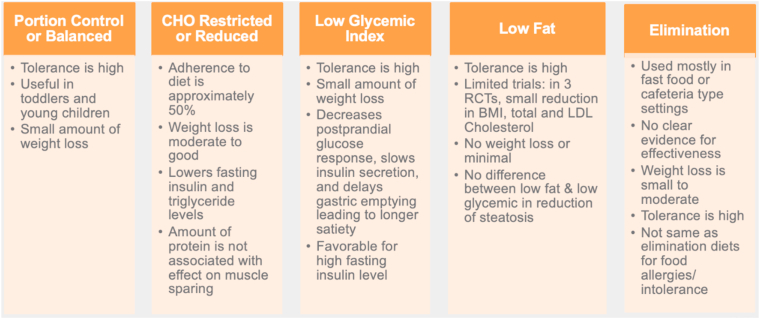
Fig. 1.
Nutritional Therapy: Comparison of Common Recommendations I. Nutritional management for pediatric patients can take several forms. These include portion control, CHO-restricted or reduced diets, low glycemic index foods, low-fat diets, and elimination diets; information, pros, and cons for each are shown [6,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]].
CHO: carbohydrate; RCT: randomized controlled trial; P: protein; KD: Ketogenic diet; BMI: body mass index; LDL: low-density lipoprotein.
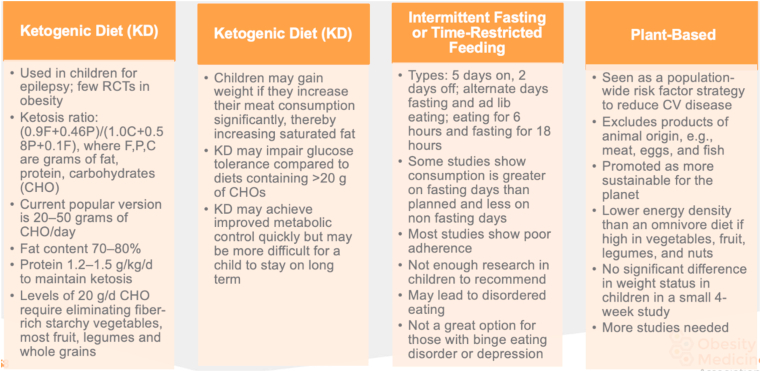
Fig. 2.
Nutritional Therapy: Comparison of Common Recommendations II. Nutritional management for pediatric patients can take several forms, including diets that are popular with adults. They may include the ketogenic diet, intermittent fasting/time-restricted feeding, and plant-based diets; information, pros, and cons are shown [[20], [21], [22]].
CHO: carbohydrate; RCT: randomized controlled trial.
Table 6.
Top takeaways: nutritional interventions for the child with overweight and obesity. Shown are the top takeaways from the OMA regarding nutritional interventions for the child with obesity.
| 1. | Improvement in weight status in children with overweight and obesity is possible regardless of the macronutrient distribution in an energy-reduced diet. |
| 2. | The choice of which macronutrient option to implement is based on a thorough assessment with the family along with shared decision making. |
| 3. | Frequent evaluation of the macronutrient choice allows for adjustment or change to a better option, reducing wasted time and frustration. |
| 4. | All nutritional interventions are more effective in combination with activity and behavioral counseling, which includes addressing conditions detrimental to the child/family's social determinants of health. |
5. Nutritional recommendations for complications associated with obesity in children
Precision medicine uses traditional principles of dietary management of obesity-related complications, tailoring them to specific children, complications, and circumstances. General dietary management for children with obesity-related complications (type 2 diabetes, hypertension, metabolic syndrome, familial hypercholesterolemia, non-alcoholic fatty liver disease, and attention deficit hyperactivity disorder) follow; these can be adapted for the specific developmental age, disease progression, and individual response of the patient.
5.1. Type 2 diabetes
Conventional management of type 2 diabetes (T2D) suggests a nutrient-dense diet consisting of high fiber and low sugar, with approximately 30 grams of carbohydrate (CHO) per meal. If the baseline fasting insulin level is elevated, an alternative meal plan might include low CHO content with a well-balanced diet and nonrestricted caloric intake. The source of CHO is important and varies with individuals in maintaining glucose control. Frequent monitoring is recommended. Guidance from nutritional professionals is critical for long-term success [23,24].
5.2. Hypertension
The Dietary Approaches to Stop Hypertension (DASH) diet is a nutritional plan based on an intake of 2300 mg of sodium per day or less (1500 mg/day or less for individuals identifying as non-Hispanic Black). Adherence to the plan is associated with a lowering of blood pressure. The plan concentrates on vegetables, fruits, whole grains, low-fat meats/proteins, fish, and low-fat dairy with limitations on sugar, sweets, and sodium [[25], [26], [27]].
5.3. Metabolic syndrome
The Framingham Study is a long-term, population-based study that began in 1948 to investigate cardiovascular disease; in this study, whole grain intake was inversely associated with metabolic disease prevalence while glycemic index was positively associated with prevalence [28].
The source and quality of dietary carbohydrates may differentially optimize insulin action. Fasting insulin concentrations are lower among individuals reporting higher dietary fiber or whole grain intake [29].
5.4. Familial hypercholesterolemia (FH)
The National Heart, Lung, and Blood Institute (NHLBI) Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents has created recommendations to help clinicians promote cardiovascular (CV) health and manage CV risk factors in children and adolescents [30]. Once the diagnosis of FH is made, these recommendations suggest initiating the NHLBI Child 2 diet plan based on less than 7% saturated fat and less than 200 mg/day of cholesterol per day. Since most children with obesity have LDL <140 mg/dl, if higher levels are found, the clinician should suspect heterozygous FH [31]. It is important for both children and parents to know that while reducing dietary saturated fat and dietary cholesterol may have lifelong health benefits, the cholesterol blood levels of children and adults is mainly dependent upon the clinical impact of the genetic abnormality causing FH. No child with FH or parent of a child with FH should have expectations that cholesterol blood levels will substantially improve with diet alone [32]. Failure to convey this message may lead to unwarranted blame and guilt, as well as unnecessary delay in starting pharmacological therapy (e.g., statins) [33,34].
5.5. Non-alcoholic fatty liver disease (NAFLD)
The spectrum of liver disease related to obesity varies considerably in the degree of pathology and genetic predisposition. The dietary intervention for known or presumed NAFLD is a nutrient-dense diet rich in fiber (legumes, whole grains), protein, and unsaturated fats. A significant reduction in foods and beverages with added sugar, salt, saturated fat, and refined carbohydrates (high glycemic index) is encouraged [35].
5.6. Attention deficit hyperactivity disorder (ADHD)
Overall, children with ADHD should follow a healthy diet with well-balanced nutrients that is low in added sugar, low in sodium, and high in fiber. While no specific diet has been shown to improve symptoms of ADHD, some research suggests that ADHD is associated with disordered eating that my contribute to weight gain [36]. Some children with ADHD demonstrate difficulty with mundane and repetitive tasks such as following a dietary plan. Strategies such as encouraging choices in diet selection offers a needed sense of control for the child [37]. Table 7 shows the top takeaways from the OMA regarding nutritional recommendations for complications associated with obesity in children.
Table 7.
Top Takeaways: Nutritional Recommendations for Complications Associated with Obesity in Children. Shown are the top takeaways from the OMA regarding nutritional recommendations for complications associated with obesity in children.
| 1. | Precision medicine uses traditional principles of dietary management of obesity-related complications, tailoring them to specific children, complications, and circumstances. |
| 2. | The source of CHO is important in managing T2D and varies with individuals in maintaining glucose control. |
| 3. | The DASH diet is a nutritional plan based on a 2300 mg of sodium/day (1500 mg/day for individuals identifying as non-Hispanic Black). |
| 4. | The NHBLI Expert Panel recommends the Child 2 Diet Plan to help clinicians promote cardiovascular (CV) health and manage children and adolescents at risk for CV disease. |
| 5. | Dietary intervention for known or presumed NAFLD is a nutrient-dense diet rich in fiber (legumes, whole grains), protein, and unsaturated fats. |
| 6. | Children with ADHD should follow a healthy diet with well-balanced nutrients that is low in added sugar, low in sodium, and high in fiber, as no specific diet has been shown to improve symptoms of ADHD. |
T2D: type 2 diabetes; CHO: carbohydrates; NHBLI: National Heart, Lung, and Blood Institute; NAFLD: non-alcoholic fatty liver disease; ADHD: attention deficit hyperactivity disorder.
6. Activity management for the pediatric patient with overweight and obesity
There is ongoing evidence that youth with obesity may experience a range of neuromuscular injuries and physical complications including increased pain, reduced muscle strength, postural malalignment, increased fatigue, reduced flexibility, and impaired balance, motor skills, and gait [38]. A review of 21 systematic reviews found a substantial impact on the physical health of children with overweight and obesity, predominantly represented by impairments in body structures and function [39]. Children with overweight and obesity are often compromised during weight-bearing aerobic exercise; specifically, they are metabolically compromised due to impaired fat oxidation and insulin sensitivity, biomechanically disadvantaged during walking and running, and emotionally compromised due to teasing and bullying [40,41]. Neuromuscular impairments may limit engagement in effective obesity treatment and require a redesign of obesity interventions specific to the needs and capabilities of the child and family.
The impact of physical activity on metabolism is dependent on the type, intensity and volume of activity pursued. Impact is also dependent on the genetic potential, gender and age, health, and current training status of the individual child. The benefits of long-term exercise training, particularly for youth with obesity, include reduction in fat mass, increases in fat free mass and fat oxidation, improvement in metabolic function and insulin sensitivity, and control of low-grade systemic inflammation [42].
Tools are available to support health professionals in assessing, adapting, and evaluating their treatment plan related to neuromusculoskeletal health in children with overweight and obesity [38,42]. Collaborative goal setting and use of the FITT-VP principles (frequency, intensity, time, type, volume, and progression) is recommended [38,43].
Strategies for youth with any degree of physical inactivity include using an incremental approach to reach the recommended 60 minutes per day by increasing activity by 10% per week [42,44]. Progressing too quickly is counterproductive and may lead to injury. Realistic and obtainable physical activity goals comparable to the child's own baseline abilities, not normal weight peers, allow for individualized management [44]. Regular assessment of the child's physical activity level allows the health care professional to provide positive feedback and adjust recommendations as needed. Other characteristics of successful activities include:
-
•
Fun and entertaining
-
•
Developmentally appropriate
-
•
Promote aerobic fitness, muscular strength and endurance, and flexibility
-
•
Consider the physical and emotional limits of the child
Table 8 shows the top takeaways from the OMA regarding activity management options for the pediatric patient with obesity.
Table 8.
Top Takeaways for Activity Management Options for the Pediatric Patient with Overweight and Obesity. Shown are the top takeaways from the OMA regarding activity management options for the pediatric patient with overweight and obesity.
| 1. | Appropriate and reliable physical activity assessment tools are available for pediatric patients with overweight and obesity. |
| 2. | Physiologic, metabolic, and psychologic factors may limit physical activity in pediatric patients with overweight and obesity. |
| 3. | Evidence-based recommendations and/or protocols exist for physical activity in the treatment of the pediatric patient with overweight and obesity. |
| 4. | Physical activity health professionals are available for appropriate referrals for the child with overweight and obesity. |
7. Summary of recommendations for the child above the 85th percentile (overweight & obesity)
The short-term goals in treating children with overweight and obesity include interrupting the trajectory of abnormal weight gain and assessing and managing any obesity-related complications. The long-term goal in treating children with overweight and obesity is slow, steady reduction of BMI percentile until obesity-related complications are resolved followed by weight maintenance [45]. Multidisciplinary interventions include nutritional strategies, activity modifications, behavioral strategies, and improved sleep hygiene. Current evidence suggests that improved weight status can be achieved in children and adolescents with overweight or obesity irrespective of the macronutrient distribution of a reduced-energy diet. Tailoring the macronutrient content to target specific cardio-metabolic risk factors, such as a low-carbohydrate diet to treat insulin resistance, may be possible [6]. Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7 show management strategies for children at different stages of development, and Table 9 shows a summary of OMA recommendations for children above the 85th percentile (overweight & obesity).
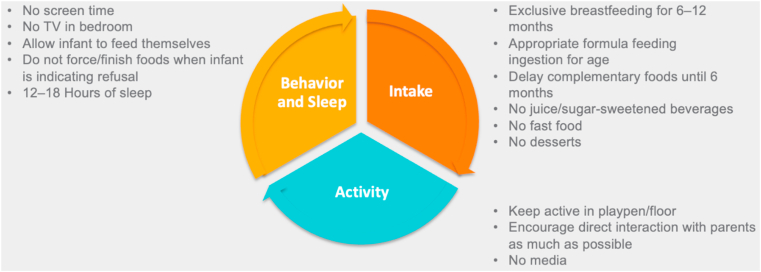
Fig. 3.
Management of the Infant with Overweight and Obesity: 0 to 24 Months. Recommendations for the infant with overweight and obesity (0–24 months) address optimal behavior and sleep patterns, food intake, and activity levels [1,2,46,47].
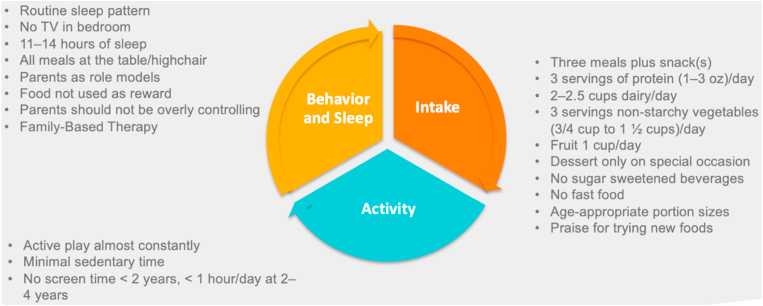
Fig. 4.
Management of the Toddler with Overweight and Obesity: 2 to 4 Years. Recommendations for the toddler with overweight and obesity (2–4 years) address optimal behavior and sleep patterns, food intake, and activity levels [1,2,46,47].
Fig. 5.
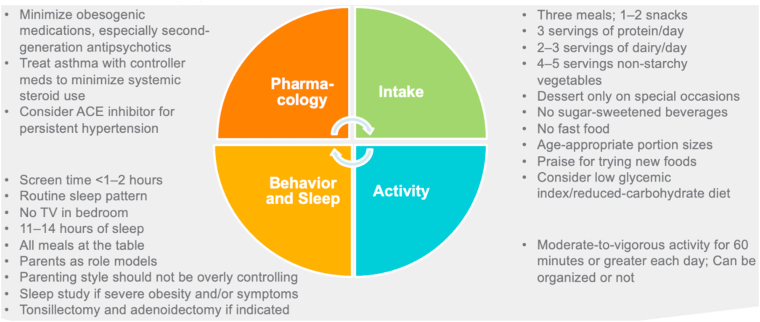
Management of the Young Child with Overweight and Obesity: 5 to 9 Years. Recommendations for the young child with overweight and obesity (5–9 years) address optimal behavior and sleep patterns, pharmacology, food intake, and activity levels [1,2,46,47].
ACE: Angiotensin-converting enzyme.
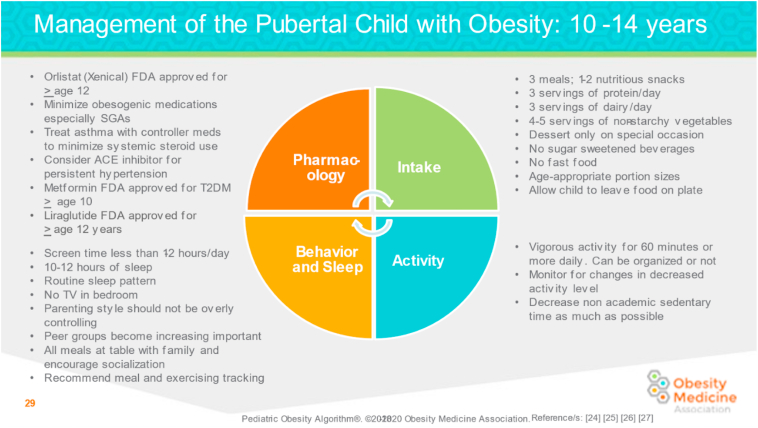
Fig. 6.
Management of the Pubertal Child with Overweight and Obesity: 10 to 14 Years. Recommendations for the pubertal child with obesity (10–14 years) address optimal behavior and sleep patterns, pharmacology, food intake, and activity levels [1,2,46,47].
ACE: Angiotensin-converting enzyme; SGA: second-generation antipsychotics; T2D: type 2 diabetes.
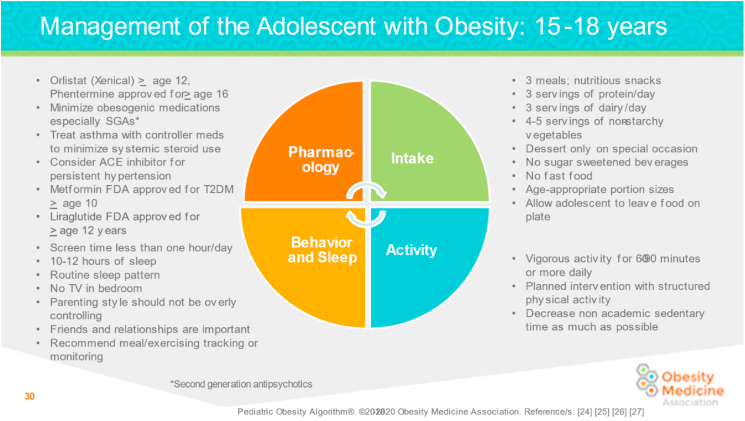
Fig. 7.
Management of the Adolescent Child with Overweight and Obesity: 15 to 18 Years. Recommendations for the adolescent child with overweight and obesity (15–18 years) address optimal behavior and sleep patterns, pharmacology, food intake, and activity levels [1,2,46,47].
ACE: Angiotensin-converting enzyme; SGA: second-generation antipsychotics; T2D: type 2 diabetes.
Table 9.
Top Takeaways: Summary Recommendations for the Child Above the 85thPercentile (Overweight and Obesity). Shown are the top takeaways from the OMA regarding recommendations for the child above the 85th percentile, including goals and interventions.
| 1. | The short-term goals in treating children with overweight and obesity include: |
|
|
|
|
| 2. | The long-term goal in treating children with obesity is slow, steady weight loss until obesity-related complications are resolved and then weight maintenance. |
| 3. | Interventions include nutritional strategies, activity modifications, behavioral strategies, and improved sleep hygiene. |
8. Food insecurity: impact and guidance
Food insecurity is defined as being uncertain of having, or unable to acquire, enough food to meet the needs of all family members because of insufficient money or other resources for food [48]. As of 2020, the US Department of Agriculture reports that 10.5% of Americans are food insecure, with 6.6% reporting low food security and 3.9% reporting very low food security; in households with children, 14.8% reported food insecurity, which represents 6.1 million children [49]. A 2017 study showed that the odds of being obese were 5 times higher for children from food insecure households when compared to children from food-secure households [50]. Those with greater risk of food insecurity include children in immigrant families, families headed by single women, increased members in household, and parental separation/divorce [51]. While food insecurity is prevalent in these groups, families with greater resources are also periodically at risk. 16% of low-income families do not receive federal support to supplement household food [52]. Participation in a Food Assistance Program (such as NSLP/WIC/SNAP) shows a reduction in food insecurity [53]. Intermittent access to adequate food can result in unhealthy eating patterns and increased stress, anxiety, and depression [[54], [55], [56], [57], [58], [59]]. Other sequelae from the chronic stress of FI include dysregulated behavior, emotional distress, and suicidal ideation (particularly in adolescents) [52,60,61].
The American Academy of Pediatrics (AAP) recommends that all children seen by their health professional are screened for food insecurity using a validated tool [51]. A positive screen for FI includes a positive response to either or both of the following 2 questions [62]:
-
1.
Within the past 12 months, we worried whether our food would run out before we got money to buy more. (Yes or No)
-
2.
Within the past 12 months, the food we bought just didn't last and we didn't have money to get more. (Yes or No)
The AAP recommendations for a positive screening include being familiar with community, state, and federal resources; having an awareness of nutritional content of these resources; and having an awareness of factors that increase vulnerability for FI. The AAP also encourages pediatric providers to advocate for their patients with FI at the local, state, and national levels [51]. Table 10 shows the top takeaways from the OMA regarding food insecurity.
Table 10.
Top Takeaways: Food Insecurity: Impact and Guidance. Shown are the top takeaways from the OMA regarding food insecurity as it relates to pediatric patients with overweight and obesity.
| 1. | Food insecurity is defined as being uncertain of having, or unable to acquire, enough food to meet the needs of all family members because of insufficient money or other resources for food [48]. |
| 2. | As of 2020, 10.5% of Americans are food insecure, 6.6% report low food security, 3.9% report very low food security [49]. |
| 3. | In households with children, 14.8% reported food insecurity representing 6.1 million children [49]. |
| 4. | The odds of children having obesity are 5 times higher in food insecure households compared to food-secure households [50]. |
| 5. | Children from immigrant families, families headed by single women, families with increased members in household, and parental separation/divorce have greater risk of FI [51]. |
| 6. | Families with greater resources are also periodically at risk for FI [52]. |
| 7. | Participation in a food assistance program (such as NSLP/WIC/SNAP) shows a reduction in FI [53]. |
| 8. | Intermittent access to adequate food can result in unhealthy eating patterns and increased stress, anxiety, and depression [[54], [55], [56], [57], [58], [59]]. |
| 9. | The American Academy of Pediatrics (AAP) recommends that all children seen by their health professional are screened for food insecurity using a validated tool [51]. |
| 10. | A positive screen for FI includes a positive response to either or both of the following 2 questions [62]: |
| 1. Within the past 12 months, we worried whether our food would run out before we got money to buy more. (Yes or No) | |
| 2. Within the past 12 months, the food we bought just didn't last and we didn't have money to get more. (Yes or No) |
9. Conclusions
This Clinical Practice Statement on nutritional and activity recommendations for the child with normal weight, overweight, and obesity with consideration of food insecurity provides clinicians with recommendations regarding their pediatric patients. The nutritional and activity interventions presented may lead to improvements in the health and wellbeing of children and adolescents with overweight and obesity, especially those with metabolic, physiological, and psychological complications.
Transparency [63]
This manuscript was largely derived and edited from the 2020–2022 Obesity Medicine Association (OMA) Pediatric Obesity Algorithm. Beginning in 2016, the OMA created and maintained an online Pediatric “Obesity Algorithm” (i.e., educational slides and eBook) that underwent updates approximately every two years by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. Authors of prior years’ versions are included in Supplement #1. This manuscript is the first published version of the applicable chapter/s of the 2020–2022 OMA Pediatric Obesity Algorithm.
Group composition
Over the years, the authors of the OMA Pediatric Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. (Supplement #1) The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
NTB transcribed the first draft of this CPS from the 2021 OMA Pediatric Obesity Algorithm. SEC then reviewed, edited, and approved the document for pre-peer review submission and post-peer review publication.
Disclosures (declaration of potential competing interest)
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMAOMA Pediatric Algorithms nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Pediatric Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual Disclosures
SEC declares a relationship with Novo Nordisk as a member of an Advisory Board and a relationship with Rhythm Pharmaceuticals as a member of their Gold Panel. NTB reports no disclosures pertaining to this project.
Evidence
The content of the OMA Pediatric Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
After approval by the authors, a draft manuscript was peer-reviewed and approved by the OMA Board of Trustees prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with overweight and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Suzanne Elizabeth Cuda reports a relationship with Rhythm Pharmaceuticals that includes: speaking and lecture fees and travel reimbursement. Suzanne Elizabeth Cuda reports a relationship with Novo Nordisk Inc that includes: consulting or advisory and travel reimbursement.
Acknowledgements
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2022.100012.
Contributor Information
Nancy T. Browne, Email: nancytkacz@sbcglobal.net.
Suzanne E. Cuda, Email: suzanne@alamocityhealthykid.com.
Appendix B.
| Weight Category (2–19 years) | BMI Percentile Range for Age & Gender (2–19 years) |
|---|---|
| Underweight | Less than 5th percentile |
| Healthy Weight | 5th to ≤ 85th percentile |
| Overweight | 85th to ≤ 95th percentile |
| Obesity | 95th to ≤ 99th percentile |
| Severe Obesity | Above 99th percentile |
Glossary: Definition of terms in pediatric obesity.
Appendix B. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davis M.M., Gance-Cleveland B., Hassink S., Johnson R., Paradis G., Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007;120(Suppl 4):S229–S253. doi: 10.1542/peds.2007-2329E. [DOI] [PubMed] [Google Scholar]
- 2.Krebs N.F., Himes J.H., Jacobson D., Nicklas T.A., Guilday P., Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 3.SHAPE America . Human Kinetics Publishing; IL: 2009. Active start: a statement of physical activity guidelines for children from birth to age 5 nEC. [Google Scholar]
- 4.Pediatrics AAo Active healthy living: prevention of childhood obesity through increased physical activity. Pediatrics. 2006;117:1834–1842. doi: 10.1542/peds.2006-0472. [DOI] [PubMed] [Google Scholar]
- 5.Yogman ea Physical activity guidelines for Americans, 2nd edition. Committee on psychosocial aspects of child and family health and council on communications and media. Pediatrics. 2018;142(3) [Google Scholar]
- 6.Gow M.L., Ho M., Burrows T.L., Baur L.A., Stewart L., Hutchesson M.J., et al. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutr Rev. 2014;72:453–470. doi: 10.1111/nure.12111. [DOI] [PubMed] [Google Scholar]
- 7.Ge L., Sadeghirad B., Ball G.D.C., da Costa B.R., Hitchcock C.L., Svendrovski A., et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369:m696. doi: 10.1136/bmj.m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gundersen C.Z.J. Childhood food insecurity in the U.S.: trends, causes, and policy options. 2014. http://www.futureofchildren.org/publications/docs/ResearchReport-July.2018.pdf Retrieved from.
- 9.Ramon-Krauel M., Salsberg S.L., Ebbeling C.B., Voss S.D., Mulkern R.V., Apura M.M., et al. A low-glycemic-load versus low-fat diet in the treatment of fatty liver in obese children. Child Obes. 2013;9:252–260. doi: 10.1089/chi.2013.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein L.H., Paluch R.A., Beecher M.D., Roemmich J.N. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity. 2008;16:318–326. doi: 10.1038/oby.2007.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krebs N.F., Gao D., Gralla J., Collins J.S., Johnson S.L. Efficacy and safety of a high protein, low carbohydrate diet for weight loss in severely obese adolescents. J Pediatr. 2010;157:252–258. doi: 10.1016/j.jpeds.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher J.O., Goran M.I., Rowe S., Hetherington M.M. Forefronts in portion size. An overview and synthesis of a roundtable discussion. Appetite. 2015;88:1–4. doi: 10.1016/j.appet.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Kirk S., Woo J.G., Brehm B., Daniels S.R., Saelens B.E. Changes in eating behaviors of children with obesity in response to carbohydrate-modified and portion-controlled diets. Child Obes. 2017;13:377–383. doi: 10.1089/chi.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kral T.V., Hetherington M.M. Variability in children's eating response to portion size. A biobehavioral perspective. Appetite. 2015;88:5–10. doi: 10.1016/j.appet.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Watowicz R.P.T.R., Hummel J.C. The protein-sparing modified fast for adolescents with severe obesity. Infant Child Adolesc Nutr. 2015;7:233–241. 2015. [Google Scholar]
- 16.Visuthranukul C., Sirimongkol P., Prachansuwan A., Pruksananonda C., Chomtho S. Low-glycemic index diet may improve insulin sensitivity in obese children. Pediatr Res. 2015;78:567–573. doi: 10.1038/pr.2015.142. [DOI] [PubMed] [Google Scholar]
- 17.Schwingshackl L., Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins D.J., Kendall C.W., Augustin L.S., Franceschi S., Hamidi M., Marchie A., et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76 doi: 10.1093/ajcn/76/1.266S. 266s-73s. [DOI] [PubMed] [Google Scholar]
- 19.Dodds P., Wolfenden L., Chapman K., Wellard L., Hughes C., Wiggers J. The effect of energy and traffic light labelling on parent and child fast food selection: a randomised controlled trial. Appetite. 2014;73:23–30. doi: 10.1016/j.appet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Grandl G., Straub L., Rudigier C., Arnold M., Wueest S., Konrad D., et al. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J Physiol. 2018;596:4597–4609. doi: 10.1113/JP275173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockman M.C., Thomas D., Burke J., Apovian C.M. Intermittent fasting: is the wait worth the weight? Curr Obes Rep. 2018;7:172–185. doi: 10.1007/s13679-018-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmond M.A., Sobiecki J., Fewtrell M., Wells J.C.K. Plant-based diets for children as a means of improving adult cardiometabolic health. Nutr Rev. 2018;76:260–273. doi: 10.1093/nutrit/nux079. [DOI] [PubMed] [Google Scholar]
- 23.Gardner C.D., Trepanowski J.F., Del Gobbo L.C., Hauser M.E., Rigdon J., Ioannidis J.P.A., et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight Adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319:667–679. doi: 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebbeling C.B., Feldman H.A., Klein G.L., Wong J.M.W., Bielak L., Steltz S.K., et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. doi: 10.1136/bmj.k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J.F.W., Lehnerd M.E., Houser R.F., Rimm E.B. Dietary Approaches to Stop hypertension diet, weight status, and blood pressure among children and adolescents: national health and nutrition examination surveys 2003-2012. J Acad Nutr Diet. 2017;117 doi: 10.1016/j.jand.2017.03.026. 1437-44.e2. [DOI] [PubMed] [Google Scholar]
- 26.Carey R.M., Calhoun D.A., Bakris G.L., Brook R.D., Daugherty S.L., Dennison-Himmelfarb C.R., et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander L., Christensen S.M., Richardson L., Ingersoll A.B., Burridge K., Golden A., et al. Nutrition and physical activity: an obesity medicine association (OMA) clinical practice statement 2022. Obesity Pillars. 2022:1. doi: 10.1016/j.obpill.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKeown N.M., Meigs J.B., Liu S., Saltzman E., Wilson P.W., Jacques P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–546. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 29.Pereira M.A., Jacobs D.R., Jr., Pins J.J., Raatz S.K., Gross M.D., Slavin J.L., et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 30.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada-Shiba M., Ohta T., Ohtake A., Ogura M., Dobashi K., Nohara A., et al. Guidance for pediatric familial hypercholesterolemia 2017. J Atheroscler Thromb. 2018;25:539–553. doi: 10.5551/jat.CR002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkas F., Nomikos T., Liberopoulos E., Panagiotakos D. Diet and cardiovascular disease risk among individuals with familial hypercholesterolemia: systematic review and meta-analysis. Nutrients. 2020;12 doi: 10.3390/nu12082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuorio A., Kuoppala J., Kovanen P.T., Humphries S.E., Tonstad S., Wiegman A., et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev. 2014:CD006401. doi: 10.1002/14651858.CD006401.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Maliachova O., Stabouli S. Familial hypercholesterolemia in children and adolescents: diagnosis and treatment. Curr Pharm Des. 2018;24:3672–3677. doi: 10.2174/1381612824666181010145807. [DOI] [PubMed] [Google Scholar]
- 35.Ullah R., Rauf N., Nabi G., Ullah H., Shen Y., Zhou Y.D., et al. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci. 2019;15:265–276. doi: 10.7150/ijbs.30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy L.D., Fleming J.P., Klar D. Treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int J Obes. 2009;33:326–334. doi: 10.1038/ijo.2009.5. [DOI] [PubMed] [Google Scholar]
- 37.Bowling A., Davison K., Haneuse S., Beardslee W., Miller D.P. ADHD medication, dietary patterns, physical activity, and BMI in children: a longitudinal analysis of the ECLS-K study. Obesity. 2017;25:1802–1808. doi: 10.1002/oby.21949. [DOI] [PubMed] [Google Scholar]
- 38.O'Malley G.C., Shultz S.P., Thivel D., Tsiros M.D. Neuromusculoskeletal health in pediatric obesity: incorporating evidence into clinical examination. Curr Obes Rep. 2021;10:467–477. doi: 10.1007/s13679-021-00463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsiros M.D., Tian E.J., Shultz S.P., Olds T., Hills A.P., Duff J., et al. Obesity, the new childhood disability? An umbrella review on the association between adiposity and physical function. Obes Rev. 2020;21 doi: 10.1111/obr.13121. [DOI] [PubMed] [Google Scholar]
- 40.Chung S.T., Onuzuruike A.U., Magge S.N. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411:166–183. doi: 10.1111/nyas.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puhl R.M., Lessard L.M. Weight stigma in youth: prevalence, consequences, and considerations for clinical practice. Curr Obes Rep. 2020;9:402–411. doi: 10.1007/s13679-020-00408-8. [DOI] [PubMed] [Google Scholar]
- 42.Sothern M. Safe and effective exercise for overweight youth. CRC Press; 2014. Profile of the overweight child. [Google Scholar]
- 43.Bushman B. Developing the P (for progression) in a FITTVP exercise prescription. ACSM's Health & Fit J. 2018;22(3):6–9. doi: 10.1249/fit.00000.00000.000378.2018. [DOI] [Google Scholar]
- 44.Han A., Fu A., Cobley S., Sanders R.H. Effectiveness of exercise intervention on improving fundamental movement skills and motor coordination in overweight/obese children and adolescents: a systematic review. J Sci Med Sport. 2018;21:89–102. doi: 10.1016/j.jsams.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Institute for Healthy Childhood Weight Algorithm for the assessment and management of childhood obesity in patients 2 years and older. 2015. https://www.paaap.org/uploads/1/2/4/3/124369935/551b74_5a52cf9033cb48b09aba3c0280a15402.pdf Available at:
- 46.Barlow S.E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 47.Spear B.A., Barlow S.E., Ervin C., Ludwig D.S., Saelens B.E., Schetzina K.E., et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 48.Browne N.T. Food insecurity: assessment and intervention. J Pediatr Surg Nurs. 2017;6:7–10. [Google Scholar]
- 49.Coleman-Jensen A.R.M., Gregory C.A., Singh A. U.S. Department of Agriculture, Economic Research Service; 2021. Household food security in the United States in 2020, ERR-298. [Google Scholar]
- 50.Kral T.V.E., Chittams J., Moore R.H. Relationship between food insecurity, child weight status, and parent-reported child eating and snacking behaviors. J Spec Pediatr Nurs. 2017;22 doi: 10.1111/jspn.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnidge E., LaBarge G., Krupsky K., Arthur J. Screening for food insecurity in pediatric clinical settings: opportunities and barriers. J Community Health. 2017;42:51–57. doi: 10.1007/s10900-016-0229-z. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics Promoting food security for all children. Pediatrics. 2015;136:e1431–e1438. doi: 10.1542/peds.2015-3301. [DOI] [PubMed] [Google Scholar]
- 53.Food assistance programs (NSLP/WIC/SNAP) participation shows reduction in FI. Healthy people. 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-health/interventions-resources/food-insecurity
- 54.Cook J.T., Black M., Chilton M., Cutts D., Ettinger de Cuba S., Heeren T.C., et al. Are food insecurity's health impacts underestimated in the U.S. population? Marginal food security also predicts adverse health outcomes in young U.S. children and mothers. Adv Nutr. 2013;4:51–61. doi: 10.3945/an.112.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eicher-Miller H.A., Mason A.C., Weaver C.M., McCabe G.P., Boushey C.J. Food insecurity is associated with iron deficiency anemia in US adolescents. Am J Clin Nutr. 2009;90:1358–1371. doi: 10.3945/ajcn.2009.27886. [DOI] [PubMed] [Google Scholar]
- 56.Eisenmann J.C., Gundersen C., Lohman B.J., Garasky S., Stewart S.D. Is food insecurity related to overweight and obesity in children and adolescents? A summary of studies, 1995-2009. Obes Rev. 2011;12:e73–83. doi: 10.1111/j.1467-789X.2010.00820.x. [DOI] [PubMed] [Google Scholar]
- 57.Fox C.K., Cairns N., Sunni M., Turnberg G.L., Gross A.C. Addressing food insecurity in a pediatric weight management clinic: a pilot intervention. J Pediatr Health Care. 2016;30:e11–e15. doi: 10.1016/j.pedhc.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Gundersen C., Kreider B. Bounding the effects of food insecurity on children's health outcomes. J Health Econ. 2009;28:971–983. doi: 10.1016/j.jhealeco.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Laraia B.A. Food insecurity and chronic disease. Adv Nutr. 2013;4:203–212. doi: 10.3945/an.112.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howard L.L. Transitions between food insecurity and food security predict children's social skill development during elementary school. Br J Nutr. 2011;105:1852–1860. doi: 10.1017/S0007114510005623. [DOI] [PubMed] [Google Scholar]
- 61.Gross R.S., Mendelsohn A.L. Food insecurity during early childhood: marker for disparities in healthy growth and development. Pediatrics. 2019;144 doi: 10.1542/peds.2019-2430. [DOI] [PubMed] [Google Scholar]
- 62.Hager E.R., Quigg A.M., Black M.M., Coleman S.M., Heeren T., Rose-Jacobs R., et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26–32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- 63.Institute of Medicine (U.S . National Academies Press; Washington, DC: 2011. Committee on standards for developing trustworthy clinical practice guidelines., graham R. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.