Abstract
In this study we investigated whether the enterohemorrhagic Escherichia coli (EHEC) hemolysin gene ehxA could be used as an indicator of pathogenicity in Shiga-like-toxin-producing Escherichia coli (SLTEC) isolates. The isolates in a collection of 770 SLTEC strains of human and bovine origins were assigned to group 1 (230 human and 138 bovine SLTEC isolates belonging to serotypes frequently implicated in human disease), group 2 (85 human and 183 bovine isolates belonging to serotypes less frequently implicated in disease), and group 3 (134 bovine isolates belonging to serotypes not implicated in disease). PCR amplification was used to examine all of the SLTEC isolates for the presence of ehxA and the virulence-associated genes eae, slt-I, and slt-II. The percentages of human isolates in groups 1 and 2 that were positive for ehxA were 89 and 46%, respectively, and the percentages of bovine isolates in groups 1 to 3 that were positive for ehxA were 89, 51, and 52%, respectively. The percentages of human isolates in groups 1 and 2 that were positive for eae were 92 and 27%, respectively, and the percentages of bovine isolates in groups 1 to 3 that were positive for eae were 78, 15, and 19%, respectively. The frequencies of both ehxA and eae were significantly higher for group 1 isolates than for group 2 isolates. The presence of the ehxA gene was associated with serotype, as was the presence of the eae gene. Some serotypes, such as O117:H4, lacked both eae and ehxA and have been associated with severe disease, but only infrequently. The slt-I genes were more frequent in group 1 isolates than in group 2 isolates, and the slt-II genes were more frequent in group 2 isolates than in group 1 isolates. In a second experiment we determined the occurrence of the ehxA and slt genes in E. coli isolated from bovine feces. Fecal samples from 175 animals were streaked onto washed sheep erythrocyte agar plates. Eight E. coli-like colonies representing all of the morphological types were transferred to MacConkey agar. A total of 1,080 E. coli isolates were examined, and the ehxA gene was detected in 12 independent strains, only 3 of which were positive for slt. We concluded that the ehxA gene was less correlated with virulence than the eae gene was and that EHEC hemolysin alone has limited value for screening bovine feces for pathogenic SLTEC because of presence of the ehxA gene in bovine isolates that are not SLTEC.
Shiga-like-toxin-producing Escherichia coli (SLTEC) strains are major food-borne bacterial pathogens that have been implicated in diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) (2, 12, 19, 22, 24). One serotype, O157:H7, is the dominant serotype associated with disease worldwide, but other serotypes, notably O26:H11, O103:H2, O111:H-, and O113:H21, are also frequently implicated in disease (2, 4, 9, 12, 16, 17, 21, 22, 24, 32, 37, 47, 52, 54, 60). Numerous other serotypes of SLTEC are either associated with disease at a low frequency or have not been implicated in disease. To date, more than 160 serotypes of SLTEC have been identified among SLTEC strains isolated from human sources, and more than 200 serotypes have been isolated from cattle (1, 5, 22, 51, 58, 59, 61). SLTEC strains are detectable at high frequencies in the feces of normal cattle, and contaminated ground beef is the most common source of human infection. Other foods, such as milk, lettuce, apple cider, and radish sprouts, contaminated with SLTEC have also been shown to be sources of human infection (2, 31).
The occurrence of several large outbreaks of O157:H7 SLTEC disease in recent years (2) has led to intensive efforts to monitor contamination of ground beef with this organism. However, because numerous other serotypes of SLTEC also cause disease in humans, workers are attempting to determine which serotypes of SLTEC may cause disease in humans. Detection of virulence factors is the classical method used to identify classes of pathogenic E. coli, and if such an approach is to be applied to SLTEC, then the virulence factors of this class of pathogenic E. coli need to be identified.
A number of virulence factors have been identified in SLTEC. Shiga-like toxins (SLTs) may contribute to diarrhea (26, 33, 50) and appear to be necessary for HUS and hemorrhagic colitis (50). Genes encoded by a chromosomal region called the locus for enterocyte effacement (28) are necessary for development of attaching and effacing lesions, which are characteristic of SLTEC belonging to serotype O157:H7 and most other common SLTEC serotypes (30). One of these genes, designated eae (E. coli attaching and effacing), encodes a surface protein (intimin) which mediates close adherence of SLTEC to enterocytes (15, 29, 46, 56). Other genes in the locus for enterocyte effacement are responsible for signal transduction between the bacteria and the intestinal epithelial cells, and still other genes encode a type III secretory system. Production of a heat-stable enterotoxin has been observed in some SLTEC strains, and this enterotoxin may contribute to virulence (41).
A plasmid that is approximately 90 kb long is present in O157:H7 SLTEC, as well as in certain other SLTEC serotypes (23, 25, 44, 55, 57). SLTEC strains which resemble prototype serotype O157:H7 strains in having the ability to cause hemorrhagic colitis and HUS, possessing a related approximately 90-kb plasmid, and harboring the eae gene are called enterohemorrhagic E. coli (EHEC) (25). One of the operons on the O157:H7 plasmid codes for synthesis and secretion of a hemolysin called EHEC hemolysin (42, 45). There is increasing evidence that EHEC hemolysin may be a marker or determinant of SLTEC virulence (4, 6, 8, 25, 43, 48), but other plasmid-encoded products have also been suggested as possible virulence factors (11, 23).
Because O157:H7 SLTEC strains account for 70 to 80% of recognized cases of SLTEC diseases, methods have been developed to screen for this serotype in ground beef and in humans with diarrheal illness. However, E. coli O157:H7 is far outnumbered in cattle and beef by other SLTEC, some of which also cause serious disease (22). Consequently, detection of virulent SLTEC requires methods which distinguish E. coli O157:H7 and other virulent SLTEC from the numerous, less virulent SLTEC. Unfortunately, there is no suitable animal model which can be used to determine the importance of putative virulence factors in relation to the virulence of specific SLTEC. An alternative approach, which was used in this study, is to examine the association of proposed virulence factors, such as EHEC hemolysin, with serotypes that differ in importance in human disease.
One purpose of this study was to assess whether the occurrence of the EHEC hemolysin is correlated with the frequency of association of serotypes of SLTEC with human disease. Bovine SLTEC were also examined, since human disease typically is initiated by bovine isolates. A second purpose was to assess whether selection of colonies with a EHEC hemolysin phenotype could be used as a simple and rapid method to screen for the presence of pathogenic SLTEC in cattle feces.
MATERIALS AND METHODS
SLTEC from human and bovine sources.
A total of 770 unique SLTEC strains were collected from researchers. Not more than one isolate belonging to a particular serotype was obtained from the same animal or person or from the same outbreak of disease in humans. The bovine isolates were obtained from healthy cattle in Canada or the United States and were not associated with any of the human isolates in the collection, whereas the human isolates were obtained from Australia, Belgium, Canada, Denmark, Germany, The Netherlands, Sri Lanka, Switzerland, and the United States and most of these isolates were from persons with disease. Information concerning the kind of illness of the patients from whom the isolates were recovered was available for 194 of the 315 human isolates. Although the isolates were received with serotype designations, they were all serotyped at the Health Canada Laboratory in Guelph, Ontario, Canada. The isolates were divided into three groups. Group 1 consisted of 230 human isolates and 138 bovine isolates belonging to 11 serotypes which occur at moderate to high frequencies in human disease; we studied a minimum of 15 group 1 isolates per serotype (Table 1). Group 2 consisted of 85 human isolates and 183 bovine isolates belonging to serotypes that are less frequently implicated in human disease (Table 2), and group 3 consisted of bovine SLTEC strains belonging to serotypes that have not been implicated in human disease (Table 3).
TABLE 1.
Frequencies of occurrence of ehxA, eae, and slt in isolates belonging to 11 serotypes of SLTEC commonly associated with disease in humans
| Serotype | No. of isolates
|
% of isolates with ehxA
|
% of isolates with eae
|
% of isolates with slt-I
|
% of isolates with slt-II
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Bovine | Human | Bovine | Human | Bovine | Human | Bovine | Human | Bovine | |
| O5:H-a | 5 | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 20 |
| O26:H- | 14 | 3 | 71 | 100 | 100 | 100 | 71 | 100 | 29 | 0 |
| O26:H11 | 31 | 15 | 81 | 93 | 97 | 100 | 84 | 100 | 16 | 7 |
| O103:H2 | 24 | 20 | 96 | 95 | 100 | 100 | 100 | 100 | 4 | 0 |
| O111:H- | 26 | 10 | 85 | 80 | 96 | 100 | 100 | 100 | 42 | 10 |
| O111:H8 | 16 | 7 | 69 | 100 | 100 | 100 | 100 | 100 | 50 | 29 |
| O113:H21 | 12 | 16 | 83 | 44 | 0 | 0 | 8 | 31 | 92 | 94 |
| O145:H- | 11 | 11 | 100 | 100 | 100 | 100 | 36 | 36 | 64 | 64 |
| O153:H25 | 3 | 14 | 67 | 86 | 0 | 0 | 67 | 57 | 67 | 71 |
| O157:H7 | 60 | 30 | 97 | 100 | 98 | 100 | 38 | 63 | 85 | 50 |
| O157:H-b | 28 | 2 | 96 | 100 | 100 | 100 | 57 | 50 | 100 | 100 |
| %c | 89 | 89 | 92 | 78 | 67 | 73 | 56 | 51 | ||
H-, no H antigen present.
Two of the human isolates which had the gene failed to express the phenotype.
Percentage of the total number of isolates (230 human isolates and 138 bovine isolates).
TABLE 2.
Frequencies of occurrence of ehxA, eae, and slt in isolates belonging to serotypes of SLTEC less commonly associated with disease in humans
| Serotype | No. of isolates
|
% of isolates with ehxA
|
% of isolates with eae
|
% of isolates with slt-I
|
% of isolates with slt-II
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Bovine | Human | Bovine | Human | Bovine | Human | Bovine | Human | Bovine | |
| O2:H6 | 2 | 0 | 0 | 0 | 0 | 100 | ||||
| O2:H29 | 0 | 4 | 0 | 0 | 0 | 100 | ||||
| O7:H4 | 0 | 2 | 0 | 0 | 0 | 100 | ||||
| O15:H-a | 6 | 0 | 50 | 50 | 33 | 83 | ||||
| O22:H8 | 2 | 12 | 100 | 100 | 0 | 0 | 100 | 92 | 100 | 83 |
| O38:H21 | 2 | 4 | 100 | 100 | 0 | 0 | 50 | 50 | 100 | 100 |
| O45:H2 | 3 | 1 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 |
| O48:H21 | 4 | 0 | 100 | 0 | 50 | 100 | ||||
| O55:H7 | 3 | 0 | 0 | 100 | 33 | 67 | ||||
| O80:H- | 0 | 3 | 100 | 100 | 33 | 100 | ||||
| O91:H- | 3 | 1 | 0 | 100 | 0 | 0 | 100 | 100 | 100 | 0 |
| O91:H10 | 2 | 0 | 0 | 0 | 0 | 100 | ||||
| O91:H21 | 6 | 3 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 100 |
| O98:H- | 0 | 2 | 100 | 0 | 50 | 50 | ||||
| O113:H4 | 1 | 15 | 0 | 0 | 0 | 0 | 0 | 93 | 100 | 53 |
| O115:H18 | 0 | 2 | 100 | 0 | 100 | 100 | ||||
| O117:H4 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 75 | 100 | 100 |
| O118:H12 | 2 | 0 | 0 | 0 | 0 | 100 | ||||
| O118:H16 | 0 | 2 | 100 | 100 | 100 | 0 | ||||
| O119:H- | 0 | 8 | 25 | 13 | 100 | 0 | ||||
| O121:H19 | 8 | 0 | 38 | 88 | 0 | 100 | ||||
| O128:H- | 2 | 0 | 50 | 0 | 50 | 50 | ||||
| O128:H2 | 5 | 0 | 80 | 0 | 80 | 100 | ||||
| O132:H- | 0 | 11 | 0 | 0 | 9 | 100 | ||||
| O163:H19 | 0 | 5 | 100 | 0 | 40 | 80 | ||||
| O165:H- | 0 | 2 | 50 | 100 | 0 | 100 | ||||
| O165:H25 | 2 | 0 | 0 | 100 | 0 | 100 | ||||
| OX3:H21 | 1 | 35 | 0 | 17 | 0 | 0 | 0 | 14 | 100 | 97 |
| O?:H2b | 1 | 9 | 0 | 44 | 0 | 0 | 0 | 56 | 100 | 89 |
| O?:H4 | 0 | 3 | 0 | 0 | 67 | 100 | ||||
| O?:H8 | 0 | 3 | 100 | 0 | 67 | 67 | ||||
| O?:H19 | 0 | 10 | 90 | 0 | 50 | 90 | ||||
| O?:H25 | 0 | 13 | 100 | 8 | 62 | 67 | ||||
| O?:H- | 1 | 21 | 0 | 71 | 0 | 71 | 0 | 19 | 100 | 81 |
| Othersc | 24 | 8 | 46 | 63 | 21 | 13 | 79 | 50 | 46 | 63 |
| %d | 46 | 51 | 27 | 14 | 45 | 47 | 79 | 79 | ||
H-, no H antigen present.
?, antigen present but not designated.
Serotype for which there was only one isolate in the collection.
Percentage of the total number of isolates (85 human isolates and 183 bovine isolates).
TABLE 3.
Frequencies of occurrence of ehxA, eae, and slt in bovine isolates belonging to serotypes of SLTEC not reported to be associated with disease in humans
| Serotype | No. of isolates | % of isolates with ehxA | % of isolates with eae | % of isolates with slt-I | % of isolates with slt-II |
|---|---|---|---|---|---|
| O2:H-a | 2 | 100 | 0 | 50 | 50 |
| O6:H34 | 5 | 80 | 0 | 20 | 80 |
| O8:H19 | 5 | 100 | 0 | 0 | 100 |
| O39:H49 | 2 | 100 | 0 | 100 | 0 |
| O46:H38 | 6 | 100 | 0 | 100 | 67 |
| O69:H11 | 3 | 100 | 100 | 67 | 33 |
| O76:H?b | 2 | 0 | 0 | 100 | 0 |
| O84:H- | 7 | 100 | 100 | 100 | 0 |
| O88:H25 | 5 | 100 | 0 | 80 | 100 |
| O98:H25 | 4 | 100 | 100 | 100 | 0 |
| O113:H- | 5 | 20 | 0 | 80 | 100 |
| O116:H21 | 3 | 100 | 0 | 67 | 67 |
| O119:H25 | 3 | 100 | 100 | 100 | 0 |
| O121:H7 | 6 | 0 | 0 | 100 | 0 |
| O136:H12 | 5 | 0 | 0 | 100 | 0 |
| O145:H8 | 2 | 100 | 50 | 100 | 50 |
| O153:H31 | 9 | 0 | 0 | 100 | 0 |
| O156:H- | 30 | 10 | 0 | 7 | 100 |
| O156:H25 | 3 | 100 | 100 | 100 | 0 |
| O?:H32b | 2 | 0 | 0 | 100 | 0 |
| OR:H16 | 2 | 100 | 0 | 0 | 100 |
| Othersc | 23 | 65 | 17 | 74 | 48 |
| %d | 52 | 19 | 63 | 53 |
H-, no H antigen present.
?, antigen present but not designated.
Serotype for which there was only one isolate in the collection.
Percentage of the total number of isolates (134 isolates).
Detection of genes for SLT-I, SLT-II, Eae, and EHEC hemolysin.
PCR amplification was used to detect the slt-I, slt-II, eae, and ehxA genes in all 770 SLTEC isolates. The PCR amplification protocols used were those described by Pollard et al. (35) for slt-I and slt-II, by Sandhu et al. (40) for eae, and by Sandhu et al. (39) for ehxA. Positive and negative controls for each of these genes were included. The PCR amplification protocols were also applied to a collection of 116 bacterial isolates which consisted of 50 bovine fecal E. coli isolates, 6 human enterotoxigenic E. coli isolates, 10 human enteropathogenic E. coli isolates, 7 Salmonella typhimurium isolates, 9 Citrobacter freundii isolates, 4 Hafnia alvei isolates, 8 Pseudomonas aeruginosa isolates, 4 Aeromonas hydrophila isolates, 8 Klebsiella pneumoniae isolates, and 10 Yersinia enterocolitica isolates.
Detection of expression of EHEC hemolysin.
All isolates that were positive for the ehxA gene as determined by PCR were tested for the ability to cause lysis of washed sheep erythrocytes in an agar medium and for the inability to cause lysis of unwashed erythrocytes in the same medium (7). Each isolate was streaked along an approximately 1-cm line on the agar, and the plates were incubated at 37°C. The plates were examined for hemolysis around the bacterial streaks after 3 and 16 h. Hemolysis on the washed-erythrocyte-containing agar plates after 16 h but not after 3 h, combined with no hemolysis on the unwashed-erythrocyte-containing agar plates, was considered to be due to EHEC hemolysin. When there was hemolysis on both types of plates after 3 h of incubation, the isolate was considered an isolate which expressed the alpha-hemolysin phenotype (7). E. coli O157:H7 strain E32511 served as a positive control for EHEC hemolysin, E. coli 412 served as a positive control for alpha-hemolysin, and EHEC hemolysin-negative strain KK7/1 was used as a negative control.
Comparison of EHEC hemolysin-positive and -negative isolates within serotypes.
EHEC hemolysin-negative isolates belonging to eight serotypes that were typically EHEC hemolysin positive were selected for further investigation. EHEC hemolysin-negative isolates and an equal number of randomly selected EHEC hemolysin-positive isolates belonging to the same serotype were subjected to a biotyping and plasmid profile analysis. A total of 58 isolates, including 2, 14, 10, 12, 12, 4, 2, and 2 serotype O26:H-, O26:H11, O111:H-, O111:H8, O113:H21, O121:H19, O153:H25, and O157:H7 isolates, respectively, were examined. Biotypes were determined with the Vitek gram-negative identification system (bioMerieux Canada, St. Laurent, Quebec, Canada). Small-scale plasmid preparations were obtained by alkaline lysis (38) with one phenol-chloroform extraction. The plasmids were separated by agarose gel electrophoresis, and the plasmid profiles of EHEC hemolysin-positive and -negative isolates belonging to the same serotype were compared. The plasmid DNA was transferred to a nylon membrane and probed with an EHEC hemolysin-specific digoxigenin-labelled DNA probe which included the entire ehxA gene except for the amino-terminal 70 bp (10). The washes were done under high-stringency conditions (0.1× SSC at 65°C [1× SSC is 15 mM sodium chloride plus 1.5 mM sodium citrate, pH 7.0]). The plasmid extracts were also probed by similar methods under high-stringency conditions with DNA probes for the espP gene and with probe PB16, a probe for a region flanking the ehx operon (10).
Occurrence of EHEC hemolysin, SLT, and alpha-hemolysin in E. coli isolates from the feces of normal cattle.
We isolated five to eight fecal E. coli strains from each of 175 cattle; a total of 1,080 isolates were obtained. Approximately one-half of the samples examined were from beef cattle from one farm, and one-half were from dairy cattle from another farm. All of the samples were collected over a 3-week period in the summer. The fecal samples were streaked onto washed-erythrocyte-containing agar, which was incubated overnight at 37°C. Eight colonies were selected to represent the various morphological types of colonies that resembled E. coli. When there were hemolytic E. coli-like colonies, at least one of each morphological type of hemolytic colony was selected. All of the selected isolates were subcultured on MacConkey agar, and lactose-fermenting colonies were then used for the study. The isolates which contained genes for SLT, EHEC hemolysin, or alpha-hemolysin were characterized biochemically with the Vitek gram-negative identification system (bioMerieux Canada) to confirm that they were E. coli strains. The remaining isolates were considered to be E. coli strains on the basis of positive indole tests and negative hydrogen sulfide, urease, and citrate tests. All 1,080 isolates were scored for the presence of the EHEC hemolysin and alpha-hemolysin phenotypes, the ehxA gene, and a conserved region of the slt genes. A digoxigenin-labelled PCR amplification product from the ehxA gene of E. coli E32511 was used as a specific probe to detect the ehxA gene by colony hybridization (39). A similarly produced probe derived from the conserved region of slt in an O157:H7 E. coli strain (36) was used to identify isolates with slt genes. Positive and negative control strains were used for both genes. All probe-positive isolates were retested, and uncertainties in the colony hybridization results were resolved by PCR amplification with primers specific for EHEC hemolysin, alpha-hemolysin (39), slt-I (35), and slt-II (35). Thirty isolates which were positive for ehxA and/or slt were serotyped and tested by PCR for the presence of the eae gene. An equal number of the remaining isolates, selected at random, were tested for the eae gene, and all of the isolates that were positive for ehxA, slt, or eae were serotyped at the Health Canada Laboratory in Guelph, Ontario, Canada.
Statistical methods.
The frequencies of ehxA, eae, slt-I, and slt-II were compared by using a z test for comparisons of proportions in overall comparisons and a two-tailed Fisher exact test for separate comparisons within each serotype. To avoid the confounding effects of serotype in group 2, analyses were also carried out by using only the serotypes for which both human and bovine isolates were available.
RESULTS
Serotyping.
The serotyping results generally confirmed the information provided by the donors. However, there were 15 isolates that were designated by the donors as H- that were shown to possess H antigens. Eight of these were isolates received as O111:H- that were shown to be O111:H8.
Association of EHEC hemolysin with pathogenic SLTEC.
The frequencies of occurrence of the ehxA gene in human group 1 and 2 isolates were 89 and 46%, respectively, the frequencies of occurrence of the ehxA gene in bovine isolates belonging to groups 1 and 2 were 89 and 51%, respectively (Tables 1 and 2). The frequency of occurrence of the ehxA gene in the group 3 bovine isolates was 52% (Table 3), a value that was almost identical to the value obtained for the group 2 bovine isolates (51%). For both human and bovine isolates, the ehxA gene was significantly more frequent in group 1 isolates than in group 2 isolates (P < 0.0001 for all comparisons), and the frequencies of ehxA in group 2 and 3 bovine isolates did not differ significantly (P = 0.80). There was no significant difference in the frequencies of ehxA when human and bovine isolates belonging to groups 1 and 2 isolates were compared either overall or within serotypes (P > 0.05).
There was an almost 100% correlation between presence of the ehxA gene and expression of the EHEC hemolysin phenotype with washed sheep erythrocytes; only two isolates (both O157:H-) had the genes and did not express them (Table 1). However, 22 of the ehxA-positive isolates produced hemolysis on washed sheep erythrocytes after 3 h of incubation. Two of the four O48:H21 isolates were hemolytic with unwashed sheep erythrocytes after 16 h of incubation. All of the O132:H- isolates produced alpha-hemolysin. One isolate (O6:H-) was positive as determined by PCR for both EHEC and alpha-hemolysins and had an alpha-hemolysin phenotype. The presence or absence of the ehxA gene was highly related to serotype (Tables 1 through 3).
Association of the eae gene with pathogenic SLTEC.
The eae gene was present in 92 and 27% of the human isolates belonging to groups 1 and 2, respectively, and in 78, 14, and 19% of the bovine isolates belonging to groups 1 to 3, respectively (Tables 1 through 3). Thus, the eae gene occurred four to five times more frequently in group 1 isolates than in group 2 or 3 isolates. For both human and bovine isolates the prevalence of eae in group 1 was significantly higher than the prevalence of this gene in the other groups (P < 0.0001 for all comparisons). The frequencies of eae were significantly higher in human group 1 and 2 isolates than in bovine group 1 and 2 isolates (P < 0.001 and P = 0.011 for groups 1 and 2, respectively). However, the presence of the eae gene was highly correlated with serotype, and there was no significant difference between human and bovine isolates when comparisons were made within serotypes. For the group 1 isolates, 9 of the 11 serotypes were eae positive (Table 1). The frequency of eae in a serotype tended to be 0 or 100% more consistently than the frequency of ehxA (Tables 1 through 3).
Association of the slt genes with pathogenic SLTEC.
For both human and bovine isolates, the slt-I gene was more frequent (P = 0.0004 and P < 0.0001, respectively) and the slt-II gene was less frequent (P = 0.0001 and P < 0.0001, respectively) in group 1 isolates than in group 2 isolates (Tables 1 and 2). We observed no significant difference in the frequency of either slt gene when group 1 bovine isolates and group 3 isolates were compared (Tables 1 and 3). In group 1, isolates belonging to certain serotypes (O5:H-, O26:H-, O26:H11, and O103:H2) were predominantly positive only for slt-I, whereas all of the O111:H- and O111:H8 isolates were positive for slt-I and more than one-third of them were positive for slt-II as well. Most O113:H21 isolates were positive only for slt-II; most O153:H25, O157:H7, and O157:H- isolates were positive for slt-II, and many were also positive for slt-I. The O145:H- isolates possessed either the slt-I gene or the slt-II gene but not both.
Biotype and plasmid profiles of isolates belonging to serotypes that included ehxA-positive and ehxA-negative isolates.
Biotyping of 29 ehxA-negative isolates and 29 serotype-matched ehxA-positive isolates showed that positive and negative isolates belonging to the same serotype generally had identical or similar biotypes (data not shown). For example, 11 of the 12 serotype O113:H21 isolates had the same biotype, and the other isolate differed in one sugar fermentation reaction.
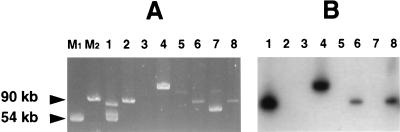
The results of probing plasmid DNA extracted from the 58 isolates are summarized in Table 4. All 29 isolates that were negative for ehxA as determined by the PCR did not hybridize with the probe for EHEC hemolysin, whereas all 29 ehxA-positive isolates had a plasmid that was 70 kb long or longer and was probe positive. In six of the ehxA-negative isolates (one O121:H19 isolate, one O153:H25 isolate, one O157:H7 isolate, one O26:H- isolate, and two O26:H11 isolates), there were no plasmids whose sizes were comparable to the size of the probe-positive plasmid (Fig. 1, lane 3). In the remaining 23 ehxA-negative isolates, there were plasmids whose sizes were similar to the size of the ehxA-positive plasmid (Fig. 1, lanes 2, 5, and 7). The plasmid extracts were also probed for the espP gene, which is found on some EHEC hemolysin plasmids (11), and all 29 ehxA-positive isolates were positive with the probe used. However, among the ehxA-negative isolates, three of five O111:H- isolates and two of six O111:H8 isolates were positive, as were all six O113:H21 isolates. Hybridization with the PB16 probe was observed with all but one of the ehxA-positive isolates belonging to the eae-positive serotypes but, as expected, with no isolates belonging to eae-negative serotypes O113:H21 and O153:H25.
TABLE 4.
Patterns of hybridization of ehxA-positive and ehxA-negative isolates with three probes for DNA sequences associated with the EHEC plasmida
| Serotype | Phenotype | No. of isolates | No. of isolates positive with all three probesb | No. of isolates negative with all three probesc | No. of isolates positive only with probes for ehxA and espPb | No. of isolates positive only with probe for espPb |
|---|---|---|---|---|---|---|
| O26:H11 | Ehx+ | 7 | 7 | 0 | 0 | 0 |
| Ehx− | 7 | 0 | 7 | 0 | 0 | |
| O121:H19 | Ehx+ | 2 | 2 | 0 | 0 | 0 |
| Ehx− | 2 | 0 | 2 | 0 | 0 | |
| O157:H7 | Ehx+ | 1 | 1 | 0 | 0 | 0 |
| Ehx− | 1 | 1 | 1 | 0 | 0 | |
| O113:H21d | Ehx+ | 6 | 0 | 0 | 6 | 0 |
| Ehx− | 6 | 0 | 0 | 0 | 6 | |
| O153:H25d | Ehx+ | 1 | 0 | 0 | 1 | 0 |
| Ehx− | 1 | 0 | 1 | 0 | 0 | |
| O26:H- | Ehx+ | 1 | 0 | 0 | 1 | 0 |
| Ehx− | 1 | 0 | 1 | 0 | 0 | |
| O111:H- | Ehx+ | 5 | 5 | 0 | 0 | 0 |
| Ehx− | 5 | 0 | 2 | 0 | 3 | |
| O111:H8 | Ehx+ | 6 | 6 | 0 | 0 | 0 |
| Ehx− | 6 | 0 | 4 | 0 | 2 |
The three probes, ehxA, espP, and PB16, targeted the EHEC hemolysin A gene, an extracellular serine protease (11), and a sequence specific for the EHEC plasmid of eae-positive SLTEC (10), respectively.
Indicates that the EHEC plasmid is present.
Indicates that the EHEC plasmid has been lost.
eae-negative serotypes which do not hybridize with the PB16 probe.
FIG. 1.
Large plasmids (A) and Southern blot (B) of plasmid DNA hybridized with a probe for ehxA. Lanes M1 and M2 contained molecular weight markers. Lanes 1 through 3, lanes 4 and 5, and lanes 6 and 7 contained plasmid extracts of SLTEC isolates belonging to serotypes O121:H19, O113:H21, and O26:H11, respectively. Lane 8 contained plasmid DNA from a positive control O157:H7 SLTEC isolate known to contain a 90-kb EHEC plasmid. The isolates in lanes 1, 4, and 6 had an EHEC hemolysin-positive phenotype and were probe positive. The isolates in lanes 2, 3, 5, and 7 had an EHEC hemolysin-negative phenotype and were probe negative. Note that the EHEC plasmid in the eae-negative O113:H21 isolate was considerably larger than the approximately 90-kb plasmid in the SLTEC isolates which were eae positive.
Detection of genes for EhxA, Eae, Slt-I, and Slt-II in a collection of bovine fecal E. coli and selected gram-negative bacteria.
PCR amplification which targeted the ehxA gene showed that all of the isolates in a collection consisting of 50 E. coli isolates from normal bovine feces and 66 other gram-negative bacteria lacked the EHEC hemolysin A gene and were also negative for the eae, slt-I, and slt-II genes.
Occurrence of EHEC hemolysin, SLT, and alpha-hemolysin in E. coli isolates from the feces of normal cattle.
The results of testing an E. coli population from the feces of normal cattle are summarized in Table 5. Both hybridization and PCR amplification showed that the EHEC hemolysin A gene sequences were present in 27 of the isolates, but hemolysis of washed erythrocytes after 16 h of incubation was detected with only 23 of these isolates. Three of six isolates which had the genes for SLT and expressed characteristic Vero cell cytotoxicity also produced EHEC hemolysin. The remaining three isolates produced alpha-hemolysin. A total of 140 of the isolates were positive for alpha-hemolysin A gene sequences, and all except one of these isolates produced alpha-hemolysin.
TABLE 5.
Occurrence and expression of genes for EHEC hemolysin, SLT, and alpha-hemolysin in fecal E. coli isolates from normal cattle
| No. of isolatesa | EHEC hemolysin phenotype | EHEC hemolysin A gene | SLTb | Alpha-hemolysinc |
|---|---|---|---|---|
| 20 (5) | + | + | − | − |
| 3 (3) | + | + | + | − |
| 4 (4) | − | + | − | − |
| 3 (2) | − | − | + | + |
| 137 | − | − | − | + |
| 913 | − | − | − | − |
| All isolatesd | 23 (2.1) | 27 (2.5) | 6 (0.6) | 140 (13.0) |
| All strainse | 8 | 12 | 5 | NDf |
The numbers in parentheses are the numbers of independent strains derived from different animals.
+, isolates were positive as determined by hybridization, PCR amplification, and the Vero cell assay.
+, isolates were positive as determined by hybridization and PCR amplification (most of the isolates that were positive expressed the alpha-hemolysin phenotype; the only exception was one of the isolates that belonged to the group containing 137 isolates).
Number of isolates. The numbers in parentheses are percentages.
Number of strains.
ND, not determined (isolates were not serotyped and therefore were not identified as members of strains).
One of the 30 isolates chosen at random from the isolates which were negative for ehxA, slt, or alpha-hemolysin was positive for eae. The results of a serotyping analysis of 31 isolates that were positive for ehxA, slt, or eae are shown in Table 6. Most isolates that were positive for the ehxA gene were eae negative, but most eae-positive isolates were positive for the ehxA gene. The three alpha-hemolysin-positive O121:H7 isolates that were PCR positive for the conserved region of the slt genes were PCR negative when the primers for slt-I and slt-II were used.
TABLE 6.
Serotypes of normal bovine fecal isolates that were positive for ehxA, slt, eae, and/or alpha-hemolysin (hlyA) genes
| Serotype | No. of isolates | ehxA | slt | eae | hlyA |
|---|---|---|---|---|---|
| O46:H38 | 1 | + | − | − | − |
| O64:H7 | 1 | + | − | − | − |
| O136:H12 | 5 | + | − | − | − |
| OR:H12a | 1 | + | − | − | − |
| O?:H2b | 8 | + | − | − | − |
| O?:H16 | 5 | + | − | − | − |
| O22:H8 | 1 | + | + | − | − |
| O22:H? | 1 | + | + | − | − |
| O113:H-c | 1 | + | + | − | − |
| O121:H7 | 3 | − | + | − | + |
| O?:H25 | 1 | + | − | + | − |
| O119:H27 | 1 | + | − | + | − |
| O156:H25 | 1 | + | − | + | − |
| O156:H8 | 1 | − | − | + | − |
OR, rough lipopolysaccharide.
?, not identifiable.
H-, no H antigen present.
Several of the 31 isolates (Table 6) which were positive for at least one of the properties being investigated were from the same animal. All five O136:H12 isolates and the OR:H12 isolate were recovered from the same animal and had the same biotype. All eight O?:H2 isolates were from the same animal; four of the five O?:H16 isolates were from the same animal; and two of the three O121:H7 isolates were from the same animal. Thus, the 20 isolates which had and expressed the ehxA gene and also had the slt gene (Table 5) represented five independent strains. Similarly, the three slt-positive, alpha-hemolysin-positive isolates (Table 5) represented two independent strains.
If we considered independent strains, there were eight strains that had the EHEC hemolysin phenotype, and three of these also had slt genes (Table 5). There were 12 strains that had the ehxA gene, and 3 of these had slt genes. Two of five slt-positive independent strains lacked the EHEC hemolysin phenotype.
DISCUSSION
Based on studies performed with small numbers of SLTEC isolates or serotypes, researchers have suggested that the presence of the EHEC hemolysin genes or plasmid may be an indicator of virulence of SLTEC for humans (4, 7, 34, 59). It is clear that the plasmid and the associated EHEC hemolysin are present in O157:H7 and O157:H- isolates, as well as O26:H-, O26:H11, O111:H-, and O111:H8 isolates (4, 11, 23, 34, 42, 43). The present study extended these observations for the O157, O26, and O111 organisms (Table 1) and yielded data for several other serotypes that have been implicated in human disease (Tables 1 and 2). Our study showed that 89% of 230 human isolates belonging to 11 serotypes that are frequently implicated in disease but only 46% of 85 human isolates belonging to serotypes that are less frequently involved in disease were positive for the ehxA gene. These findings are consistent with an association between ehxA and disease. However, since ehxA is on a plasmid with other genes, it could be a marker for virulence or a contributor to virulence. It is unlikely, however, that the close association of EHEC hemolysin with the SLTEC serotypes most frequently implicated in human disease and the association of alpha-hemolysin with SLTEC that cause edema disease in pigs (53) are simply chance associations. It is clear that ehxA is not essential for virulence since some serotypes, such as O117:H4, lack the gene but have been reported to cause HUS, albeit infrequently (24).
Our data suggested that the EHEC plasmid had been lost by 18 ehxA-negative isolates that were negative with all three plasmid probes (Table 4) but not by 11 ehxA-negative isolates that were positive with the espP probe (Table 4). For the latter isolates, it is likely that a block of genes, rather than the whole plasmid, had been lost. Although unlikely, an alternative explanation is that the espP gene was present on a plasmid that was different from the EHEC plasmid. Loss of the EHEC plasmid during storage of bacteria has been reported by Wieler et al. (59) for bovine SLTEC isolates belonging to serotypes O26:H- and O111:H- and by Schmidt and Karch (43) for O111:H- strains of human origin. Interestingly, Schmidt and Karch (43) showed that the plasmid was present in 88% of 18 O111:H- isolates from HUS patients, compared with 22% of 18 O111:H- isolates from patients with diarrhea, and these authors suggested that EHEC hemolysin may enhance the ability of SLTEC O111:H- strains to cause extraintestinal complications in humans. The high level of EHEC hemolysin-negative O111:H- isolates in human diarrhea could have been due to infection with plasmid-negative isolates from the bovine reservoir or to plasmid loss in humans. In porcine enterotoxigenic E. coli, colostral antibodies against K88 pili have been shown to promote loss of the K88 plasmid (30) in vitro. Perhaps a similar mechanism operates against the EHEC plasmid in the intestines of some individuals.
Barrett et al. (3) noted a strong association between eae and ehxA in non-O157 SLTEC isolates from cattle and humans; 44 of 45 isolates were either negative or positive for both probes. The study of Barrett et al. (3) involved predominantly the major serotypes implicated in disease, and the results of these authors are similar to those obtained for the same serotypes in the present study. However, in the present study there were several serotypes in which eae and ehxA showed no association (Tables 1 through 3), particularly serotypes less frequently implicated or not implicated in disease (Tables 2 and 3). Since both properties are highly related to serotype, the serotypes in a collection should markedly influence the patterns that are observed. The significant differences in the frequencies of eae in human and bovine group 1 and 2 isolates are related to serotype, and when isolates are compared within a serotype, these differences disappear.
The presence of the putative virulence factors was highly related to serotype and appeared to be independent of the source of the isolates. Thus, bovine and human isolates belonging to the same serotype exhibited similar patterns for ehxA, eae, and slt (Tables 1 through 3). Disease information was available for 62% of the human isolates. When this information was available for sufficient numbers of isolates belonging to the same serotype (e.g., information was available for most O157:H7 and O157:H- isolates), there was no difference in the association of eae and ehxA with asymptomatic carriage, diarrhea, hemorrhagic colitis, or HUS. Similar results were reported previously for seven O128:H2 isolates (60). The findings obtained with the O157 isolates are not surprising, since almost all SLTEC isolates belonging to this O group are positive for eae and ehxA (3, 4, 34; this study). The types of SLT produced by the SLTEC were also related to serotype, as has been shown by other workers (3, 4, 7, 16, 22, 37, 59).
The SLTEC that are detected in ground beef probably originate in cattle feces. Thus, if EHEC hemolysin is to be used as an aid in screening ground beef for SLTEC that produce EHEC hemolysin, it is important to obtain data on the frequency of occurrence of EHEC hemolysin-positive E. coli and the association of this trait with slt genes in E. coli in normal cattle feces. In the strains of E. coli from normal cattle feces, the ehxA gene occurred independent of the slt genes three times as frequently as it occurred in association with them (Table 5). The EHEC hemolysin phenotype was detected independent of the slt genes 1.7 times as frequently as it was detected in association with them. In a study involving E. coli isolates from the feces of 1,305 dairy heifers, Cray et al. (14) found that the ehxA gene was detected twice as frequently as the slt genes and was independent of the slt genes as frequently as it was found in association with them. Therefore, any screening for EHEC hemolysin-positive SLTEC in ground beef should seek to identify colonies that are positive for both properties.
The ehxA-positive and/or slt-positive isolates (Table 6) all belonged to serotypes previously isolated from cattle (1). The eae-positive, slt-negative O156:H8 organism also represents a serotype previously identified as an SLTEC serotype obtained from bovine feces (1, 61). Some O119 isolates are enteropathogenic E. coli (18), and others are SLTEC (9, 59). The slt-negative isolates may be SLTEC that have lost the genes for SLT, or they may be organisms which never received these genes. These isolates were tested within a short time after isolation, and it is unlikely that they would all have lost the genes, especially when there were several isolates of the same strain. These organisms will be interesting organisms for further study.
Beutin et al. (8) have shown that the EHEC hemolysin phenotype may be used for rapid screening for SLTEC in the stools of humans with HUS or hemorrhagic colitis. The presence of hemolysis after 3 h of incubation indicated that alpha-hemolysin was present, and delayed hemolysis (16 h) indicated that EHEC hemolysin was present (7, 8). However, we observed that a number of isolates which exhibited hemolysis on washed-erythrocyte-containing agar after 3 h of incubation carried the ehxA gene. Most of these isolates failed to lyse unwashed erythrocytes, but a small number did lyse unwashed erythrocytes after 16 h of incubation. It was necessary to rely on PCR amplification or hybridization to be certain of the identity of the hemolysin in the isolates which produced atypical patterns.
Beutin et al. (8) detected EHEC hemolysin-positive E. coli in 9 of 10 human stool samples and alpha-hemolytic E. coli in 4 of 10 human stool samples collected from two patients with enteritis, five patients with HUS, and one healthy individual. Studies of alpha-hemolytic E. coli in human feces have shown that the frequencies of occurrence vary from 3 to 30% (20, 27, 49). Similar values were obtained for bovine fecal isolates in the present study (Table 5) and in a previous study of Beutin et al. (5), although a value of 76% was reported by Smith in 1963 (53). Despite the presence of alpha-hemolytic E. coli, Beutin et al. (8) were successful in using detection of EHEC hemolysin to screen stool samples for SLTEC in the population of humans which they studied. However, our data suggest that application of this approach to normal cattle feces or to meat contaminated with cattle feces may not be as effective due to the low correlation between the presence of ehxA and the presence of slt genes in isolates from cattle feces.
The findings of the present study are consistent with the concept that SLTEC have an array of properties that contribute to the ability of the organisms to cause disease (3). Some properties that were not examined in the present study such as acid resistance (13), probably contribute to the virulence of O157 SLTEC and need to be investigated with SLTEC belonging to other serotypes. Host factors are also probably very important in the outcome of exposure to SLTEC. The isolates and serotypes which have many or all of the EHEC virulence factors are likely to induce disease in most individuals after a low dose of bacteria is ingested. Less virulent SLTEC may cause disease only after ingestion of larger doses and/or in individuals who are highly susceptible due to impairment of specific or nonspecific defenses. Thus, there is probably a virulence continuum, and it may not be possible to draw a clear-cut line of distinction between pathogenic and nonpathogenic SLTEC. To date, the most consistent factor associated with virulence is serotype, and it is important to pursue measures that will result in simplified and more rapid identification of serotypes. Each serotype may be associated with a certain probability of causing disease, and the risk of exposure to a certain number of organisms could be estimated, but good animal models which mimic the disease in humans remain an important missing ingredient in attempts to evaluate the virulence of SLTEC. In the absence of a suitable animal model to assess virulence, cumulative frequency data for serotypes associated with human disease, especially bloody diarrhea and/or HUS, should help define SLTEC serotypes that carry the greatest risk.
ACKNOWLEDGMENTS
This work was supported by a grant from the International Life Sciences Institute (North America).
We acknowledge the contributions to this work made by many colleagues. Kris Rahn and Jutta Hammermueller assisted with strain acquisition and plasmid characterization. Karl Bettelheim, Lothar Beutin, Andre Burnens, Wendy Johnson, S. Notermans, James Paton, Kulbir Sandhu, and Nancy Strockbine generously donated strains.
REFERENCES
- 1.Aleksic S. WHO report on Shiga-like toxin producing Escherichia coli (SLTEC), with emphasis on zoonotic aspects. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 2.Armstrong G L, Hollingsworth J, Morris J G., Jr Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol Rev. 1996;18:29–51. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- 3.Barrett T J, Kaper J B, Jerse A E, Wachsmuth I K. Virulence factors in Shiga-like-toxin-producing Escherichia coli isolated from humans and cattle. J Infect Dis. 1992;165:979–980. doi: 10.1093/infdis/165.5.979. [DOI] [PubMed] [Google Scholar]
- 4.Beutin L, Aleksic S, Zimmermann S, Gleier K. Virulence factors and phenotypical traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med Microbiol Immunol. 1994;183:13–21. doi: 10.1007/BF00193627. [DOI] [PubMed] [Google Scholar]
- 5.Beutin L, Geier D, Steinruck H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutin L, Geier D, Zimmermann S, Karch H. Virulence markers of Shiga-like-toxin-producing Escherichia coli strains originating from healthy domestic animals of different species. J Clin Microbiol. 1995;33:631–635. doi: 10.1128/jcm.33.3.631-635.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutin L, Montenegro M A, Orskov I, Orskov F, Prada J, Zimmermann S, Stephan R. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J Clin Microbiol. 1989;27:2559–2564. doi: 10.1128/jcm.27.11.2559-2564.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutin L, Zimmermann S, Gleier K. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J Clin Microbiol. 1996;34:2812–2814. doi: 10.1128/jcm.34.11.2812-2814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitzan M, Karch H, Maas M G, Meyer T, Russmann H, Aleksic S, Bockemuhl J. Clinical and genetic aspects of Shiga-like toxin production in traditional enteropathogenic Escherichia coli. Int J Med Microbiol. 1991;274:496–505. doi: 10.1016/s0934-8840(11)80087-3. [DOI] [PubMed] [Google Scholar]
- 10.Boerlin P B, Chen S, Colbourne J, Johnson R, De Grandis S, Gyles C. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect Immun. 1998;66:2553–2561. doi: 10.1128/iai.66.6.2553-2561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 12.Burnens A P, Boss P, Orskov F, Orskov I, Schaad U B, Muller F, Heinzle R, Nicolet J. Occurrence and phenotypic properties of verotoxin producing Escherichia coli in sporadic cases of gastroenteritis. Eur J Clin Microbiol Infect Dis. 1992;11:631–634. doi: 10.1007/BF01961673. [DOI] [PubMed] [Google Scholar]
- 13.Conner D E, Kotrola J S. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl Environ Microbiol. 1995;61:382–385. doi: 10.1128/aem.61.1.382-385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cray W C, Thomas L A, Schneider R A, Moon H W. Virulence attributes of Escherichia coli isolated from dairy heifer feces. Vet Microbiol. 1996;53:369–374. doi: 10.1016/s0378-1135(96)01261-8. [DOI] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorn C R, Scotland S M, Smith H R, Willshaw G A, Rowe B. Properties of Vero cytotoxin-producing Escherichia coli of human and animal origin belonging to serotypes other than O157:H7. Epidemiol Infect. 1989;103:83–95. doi: 10.1017/s0950268800030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldwater P N, Bettelheim K A. The role of enterohaemorrhagic Escherichia coli serotypes other than O157:H7 as causes of disease in Australia. Communicable Dis Intelligence. 1995;19:2–4. [Google Scholar]
- 18.Goncalves A G, Campos L C, Gomes T A, Rodrigues J, Sperandio V, Whittam T S, Trabulsi L R. Virulence properties and clonal structures of Escherichia coli O119 serotypes. Infect Immun. 1997;65:2034–2040. doi: 10.1128/iai.65.6.2034-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 20.Hacker J, Schroter G, Schrettenbrunner A, Hughes C, Goebel W. Hemolytic Escherichia coli strains in the human fecal flora as potential urinary pathogens. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1983;254:370–378. [PubMed] [Google Scholar]
- 21.Huppertz H, Busch D, Schmidt H, Aleksic S, Karch H. Diarrhea in young children associated with Escherichia coli non-O157 organisms that produce Shiga-like toxin. J Pediatr. 1996;128:341–346. doi: 10.1016/s0022-3476(96)70278-0. [DOI] [PubMed] [Google Scholar]
- 22.Johnson R P, Clarke R C, Wilson J B, Read S C, Rahn K, Renwick S A, Sandhu K A, Alves D A, Karmali M A, Lior H, McEwen S A, Spika J S, Gyles C L. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 23.Karch H, Heesemann J, Laufs R, O’Brien A D, Tacket C O, Levine M M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987;55:455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine M M, Xu J G, Kaper J B, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth I K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 serotypes and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Buret A, Robins-Browne R, Stiel D, Loughlin E. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 27.Marques L R, Abe C N, Griffin P M, Gomes T A. Association between alpha-hemolysin production and HeLa cell-detaching activity in fecal isolates of Escherichia coli. J Clin Microbiol. 1995;33:2707–2709. doi: 10.1128/jcm.33.10.2707-2709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O’Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy L K, MacKenzie T, Pickard D J, Dougan G. Effects of immune colostrum on the expression of a K88 plasmid encoded determinant: role of plasmid stability and influence of phenotypic expression of K88 fimbriae. J Gen Microbiol. 1986;132:2497–2503. doi: 10.1099/00221287-132-9-2497. [DOI] [PubMed] [Google Scholar]
- 31.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai C H, Ahmed N, Lior H, Johnson W M, Sims H V, Woods D E. Epidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective study. J Infect Dis. 1988;157:1054–1057. doi: 10.1093/infdis/157.5.1054. [DOI] [PubMed] [Google Scholar]
- 33.Pai C H, Kelly J K, Meyers G. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierard D, Stevens D, Moriau L, Lior H, Lauwers S. Isolation and virulence factors of verocytotoxin-producing Escherichia coli in human stool samples. Clin Microbiol Infect. 1997;3:531–540. doi: 10.1111/j.1469-0691.1997.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 35.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read S C, Clarke R C, Martin A, DeGrandis S A, Hii J, McEwen S, Gyles C L. Polymerase chain reaction for detection of verocytotoxigenic Escherichia coli isolated from animal and food sources. Mol Cell Probes. 1992;6:153–161. doi: 10.1016/0890-8508(92)90060-b. [DOI] [PubMed] [Google Scholar]
- 37.Russman H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Sandhu K S, Clarke R C, Gyles C L. Hemolysin phenotypes and genotypes of eaeA-positive and eaeA-negative bovine verotoxigenic Escherichia coli. Adv Exp Med Biol. 1997;412:295–302. doi: 10.1007/978-1-4899-1828-4_49. [DOI] [PubMed] [Google Scholar]
- 40.Sandhu K S, Clarke R C, McFadden K, Brouwer A, Louie M, Wilson J, Lior H, Gyles C L. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in southwest Ontario. Epidemiol Infect. 1996;116:1–7. doi: 10.1017/s095026880005888x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savarino S J, McVeigh A, Watson J, Cravioto A, Molina J, Echeverria P, Bahn M K, Levine M M, Fasano A. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J Infect Dis. 1996;173:1019–1022. doi: 10.1093/infdis/173.4.1019. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt H, Karch H. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1996;34:2364–2367. doi: 10.1128/jcm.34.10.2364-2367.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H, Karch H, Beutin L. The large-sized plasmids of enterohemorrhagic Escherichia coli O157 strains encode hemolysins which are presumably members of the E. coli alpha-hemolysin family. FEMS Microbiol Lett. 1994;117:189–196. doi: 10.1111/j.1574-6968.1994.tb06763.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt H, Kernbach C, Karch H. Analysis of EHEC-hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:907–914. doi: 10.1099/00221287-142-4-907. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt H, Plaschke B, Franke S, Russmann H, Schwarzkopf A, Heesemann J, Karch H. Differentiation in virulence patterns of Escherichia coli possessing eae genes. Med Microbiol Immunol. 1994;183:23–31. doi: 10.1007/BF00193628. [DOI] [PubMed] [Google Scholar]
- 47.Scotland S M, Rowe B, Smith H R, Willshaw G A, Gross R J. Vero cytotoxin-producing strains of Escherichia coli from children with haemolytic uraemic syndrome and their detection by specific DNA probes. J Med Microbiol. 1988;25:237–243. doi: 10.1099/00222615-25-4-237. [DOI] [PubMed] [Google Scholar]
- 48.Scotland S M, Willshaw G A, Smith H R, Rowe B. Properties of strains of Escherichia coli O26:H11 in relation to their enteropathogenic or enterohemorrhagic classification. J Infect Dis. 1990;162:1069–1074. doi: 10.1093/infdis/162.5.1069. [DOI] [PubMed] [Google Scholar]
- 49.Siitonen A. Escherichia coli in fecal flora of healthy adults: serotypes, P and type 1C fimbriae, non-P mannose-resistant adhesins, and hemolytic activity. J Infect Dis. 1992;166:1058–1065. doi: 10.1093/infdis/166.5.1058. [DOI] [PubMed] [Google Scholar]
- 50.Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C, Fondacaro J, Gemski P, Boedecker E. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106:306–317. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 51.Smith H R, Willshaw G A, Scotland S M, Thomas A, Rowe B. Properties of Vero cytotoxin-producing Escherichia coli isolated from human and non-human sources. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1987;278:436–444. doi: 10.1016/s0934-8840(11)80860-1. [DOI] [PubMed] [Google Scholar]
- 52.Smith H R, Rowe B, Gross R J, Fry N K, Scotland S M. Haemorrhagic colitis and verocytotoxin-producing Escherichia coli in England and Wales. Lancet. 1987;i:1062–1064. doi: 10.1016/s0140-6736(87)90485-5. [DOI] [PubMed] [Google Scholar]
- 53.Smith H W. The haemolysins of Escherichia coli. J Pathol Bacteriol. 1963;85:197–211. doi: 10.1002/path.1700850119. [DOI] [PubMed] [Google Scholar]
- 54.Tamura K, Sakazaki R, Murase M, Kosaka Y. Serotyping and categorisation of Escherichia coli strains isolated between 1958 and 1992 from diarrhoeal diseases in Asia. J Med Microbiol. 1996;45:353–358. doi: 10.1099/00222615-45-5-353. [DOI] [PubMed] [Google Scholar]
- 55.Toth I, Cohen M L, Rumschlag H S, Riley L W, White E H, Carr J H, Bond W W, Wachsmuth I K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990;58:1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tzipori S, Gunzer F, Donnenberg M S, de Montigny L, Kaper J B, Donohue Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzipori S, Karch H, Wachsmuth K I, Robins Browne R M, O’Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wieler L H, Bauerfeind R, Baljer G. Characterization of Shiga-like toxin producing Escherichia coli (SLTEC) isolated from calves with and without diarrhoea. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:243–253. doi: 10.1016/s0934-8840(11)80011-3. [DOI] [PubMed] [Google Scholar]
- 59.Wieler L H, Wieler E, Erpenstein C, Schlapp T, Steinrück H, Bauerfeind R, Byomi A, Baljer G. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willshaw G A, Scotland S M, Smith H R, Rowe B. Properties of Vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J Infect Dis. 1992;166:797–802. doi: 10.1093/infdis/166.4.797. [DOI] [PubMed] [Google Scholar]
- 61.Wilson J B, McEwen S A, Clarke R C, Leslie K E, Wilson R A, Waltner-Toews D, Gyles C L. Distribution and characteristics of verocytotoxigenic Escherichia coli isolated from Ontario dairy cattle. Epidemiol Infect. 1992;108:423–439. doi: 10.1017/s0950268800049931. [DOI] [PMC free article] [PubMed] [Google Scholar]