Abstract
Background
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on Nutrition and Physical Activity provides clinicians an overview of nutrition and physical activity principles applicable to the care of patients with increased body fat, especially those with adverse fat mass and adiposopathic metabolic consequences.
Methods
The scientific information and clinical guidance is based upon referenced evidence and derived from the clinical perspectives of the authors.
Results
This OMA CPS on Nutrition and Physical Activity provides basic clinical information regarding carbohydrates, proteins, fats (including trans fats, saturated fats, polyunsaturated fats, and monounsaturated fats), general principles of healthful nutrition, nutritional factors associated with improved health outcomes, and food labels. Included are the clinical implications of isocaloric substitution of refined carbohydrates with saturated fats and vice-versa, as well as definitions of low-calorie, very low-calorie, carbohydrate-restricted, and fat-restricted dietary intakes. Specific dietary plans discussed include carbohydrate-restricted diets, fat-restricted diets, very low-calorie diets, the Mediterranean diet, Therapeutic Lifestyle diet, Dietary Approaches to Stop Hypertension (DASH), ketogenic (modified Atkins) diet, Ornish diet, Paleo diet, vegetarian or vegan diet (whole food/plant-based), intermittent fasting/time restricted feeding, and commercial diet programs. This clinical practice statement also examines the health benefits of physical activity and provides practical pre-exercise medical evaluation guidance as well as suggestions regarding types and recommended amounts of dynamic (aerobic) training, resistance (anaerobic) training, leisure time physical activity, and non-exercise activity thermogenesis (NEAT). Additional guidance is provided regarding muscle physiology, exercise prescription, metabolic equivalent tasks (METS), and methods to track physical activity progress.
Conclusion
This Obesity Medicine Association Clinical Practice Statement on Nutrition and Physical Activity provides clinicians an overview of nutrition and physical activity. Implementation of appropriate nutrition and physical activity in patients with pre-obesity and/or obesity may improve the health of patients, especially those with adverse fat mass and adiposopathic metabolic consequences.
Keywords: Clinical practice statement, Nutrition, Obesity, Physical activity
1. Introduction
The purpose of the Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on Nutrition and Physical Activity is to provide clinicians an overview of nutrition and physical activity principles applicable to the care of patients with increased body fat, especially those with adverse fat mass and adiposopathic metabolic consequences. The OMA is an organization of providers in the field of obesity medicine dedicated to the comprehensive care of patients with obesity. OMA members are physicians, nurse practitioners, physician assistants, and other allied healthcare providers who engage in a comprehensive, patient-centered, evidence-based approach towards managing obesity. This approach is comprised of the four pillars of nutrition, physical activity, behavior, and medication. “Obesity Pillars” is the journal of the Obesity Medicine Association.
2. Nutrition
Medical nutrition therapy is an essential pillar regarding treatment of patients with obesity. Table 1 outlines 10 takeaway messages regarding nutrition and obesity. Table 2 provides general principles of healthful nutrition. Fig. 1 describes nutrition factors associated with improved health outcomes. The principles outlined pertain to general nutrition and may not apply to the individual patient.
Table 1.
Ten Takeaway Messages: Obesity and Nutrition. This table summarizes ten illustrative healthful dietary intakes as they apply to medical nutrition therapy for obesity [1].
| Ten Takeaway Messages: Obesity and Nutrition |
|---|
| 1. Health outcomes are most improved with medical nutrition therapy when the dietary interventions are evidence-based, quantitative, qualitative, and conducive to patient adherence. |
| 2. A low-calorie diet is ∼1200–1800 kcal/day; a very low-calorie diet is generally <800 kcal/day. |
| 3. Fat-restricted diets are often defined as 10–30% of total calories from fat. |
| 4. Low-carbohydrate diets are generally defined as 50–150 g of carbohydrates per day; very low-carbohydrate diets contain <50 g of carbohydrates per day. |
| 5. The intake of both ultra-processed (refined) carbohydrates and saturated fats increases the risk of cardiovascular disease. The isocaloric substitution of ultra-processed carbohydrates with saturated fats does not improve cardiovascular disease risk; the isocaloric substitution of saturated fats with ultra-processed carbohydrates does not improve cardiovascular disease risk. |
| 6. The Ketogenic Diet is a carbohydrate-restricted intervention that typically discourages unhealthful ultra-processed and refined foods, foods high in glycemic index/load, and foods rich in trans fatty acids. Ketosis may reduce hunger. |
| 7. The Mediterranean Diet is not a defined diet, but rather a generalized meal pattern that encourages olive oil, vegetables, fruits, legumes, whole grains, nuts, seeds, seafood, fermented dairy products, poultry, eggs, and red wine; it discourages high amounts of red meats, meat products, and ultra-processed carbohydrates. |
| 8. The DASH Diet is a dietary pattern that encourages vegetables, fruits, whole grains, fat-free or low-fat dairy products, fish, poultry, lean meats, nuts, seeds, legumes, fiber, foods containing calcium, potassium, and magnesium; it discourages sodium >2300 mg per day, total fat >27% of total daily calories, cholesterol >150 mg per day for a 2100 Calorie eating plan, red and ultra-processed meats, sugar-sweetened beverages, and foods with added sugars. |
| 9. The vegetarian diet encourages vegetables, fruits, whole grains, legumes, seeds, and nuts and discourages meats. A vegan diet encourages vegetables, fruits, whole grains, legumes, seeds, and nuts, and discourages all animal products. |
| 10. Fasting (alternative day, intermittent, or time-restricted feeding) may contribute to overall caloric restriction and weight reduction. |
Table 2.
General Principles of Healthful Nutrition. Listed are general nutritional recommendations [4].
| General Principles of Healthful Nutrition | |
|---|---|
| Limit: | Encourage: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
∗ Some clinical data suggest variance in the potential unhealthful nature of some saturated fats, depending on the clinical scenario. (See discussion below).
∗∗ Patients with obesity who lose weight and become leaner may increase their intestinal absorption of cholesterol, which may help explain why some patients on a ketogenic diet may have marked increases in cholesterol blood levels. (See discussion below) [5].
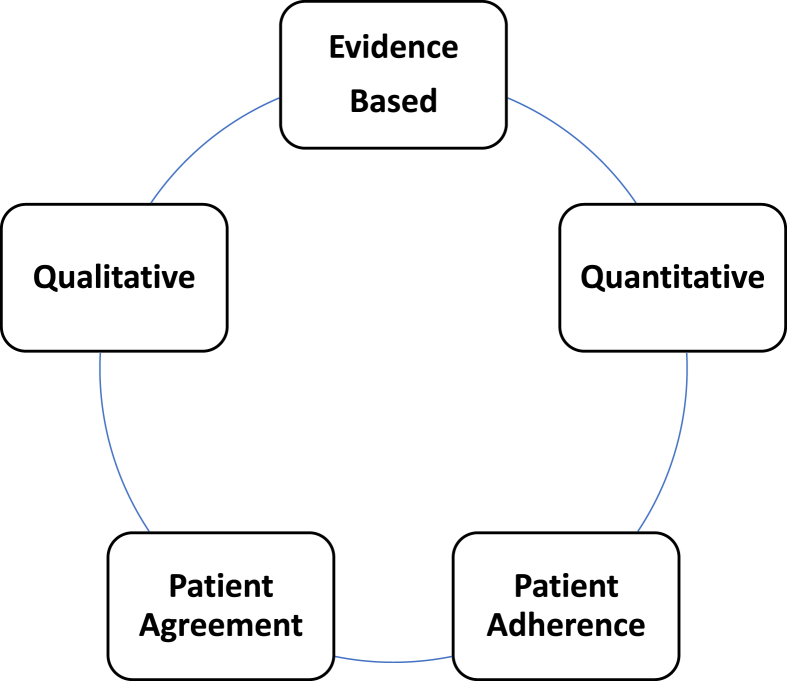
Fig. 1.
Nutrition Factors Associated with Improved Health Outcomes. Regarding medical nutrition therapy for obesity, the most effective approaches are evidence-based, consider both qualitative and qualitative aspects of dietary intake, and promote patient agreement and adherence. While possibly counterintuitive, randomized clinical trials do not necessarily support improved weight reduction when diets are based upon patient food preferences. In fact, meta-analyses suggest that patient choices in weight reduction strategies have no significant effect on duration or attrition, with greater weight reduction often occurring in the control groups. However, the effectiveness of any therapeutic intervention is likely enhanced when patients are engaged and agree to treatment plans [[6], [7], [8]].
2.1. Macronutrients
2.1.1. Carbohydrates
Carbohydrates are a type of macronutrient composed of carbon, hydrogen, and oxygen atoms, often with a hydrogen-oxygen atom ratio of 2:1. Types of carbohydrates commonly found in foods and drinks include sugars, starches, and fiber. Carbohydrates serve as a source of energy (4 kcal/gram) as well as components of cellular structures [9]. Simple sugars include monosaccharides and disaccharides. Examples of monosaccharides include hexose sugars such as glucose (blood sugar), fructose (fruit sugar), and galactose (combines with glucose to form lactose). Examples of disaccharides include maltose (two linked glucose molecules found in grains), sucrose (glucose linked to fructose found in table sugar), and lactose (glucose linked to galactose found in dairy products). Examples of polysaccharides (chain of simple sugars) include glycogen (i.e., branched polysaccharide of glucose found in animals), starch (i.e., branched and chain polysaccharide of glucose found in plants), chitin (i.e., structural carbohydrates for exoskeleton of arthropods and cell walls of fungi), and plant cell wall structural elements such as cellulose, hemicellulose, and pectin. Animals (e.g., humans) have cell membranes and not cell walls. The principal components of animal cell membranes are lipids (e.g., phospholipids and cholesterol), proteins, and carbohydrates. Carbohydrates in cell membranes are mostly attached to proteins (glycoproteins) or lipids (glycolipids).
The digestion of carbohydrates begins in the mouth (e.g., chewing and saliva with amylase). Carbohydrate digestion continues in the intestine via body enzymes (e.g., pancreatic amylase and intestinal maltase and lactase) and bacteria where polysaccharides (i.e., starches from plant foods and glycogen from animal foods) are converted to monosaccharides (i.e., simple sugars), which are then absorbed across intestinal cell membranes. Fructose may be absorbed by facilitated diffusion via intestinal glucose transporter 5 (GLUT-5); glucose and galactose are actively transported by intestinal sodium glucose cotransporter-1 (SGLT-1) [10]. Glucose can be broken down into carbon dioxide and water, which is a glycolytic process that generates biochemical energy in the form of adenosine triphosphate or ATP via cellular respiration (i.e., glycolysis, citric acid cycle, electron transport, oxidative phosphorylation). Conversely, excessive carbohydrates can be converted to and stored as fat via a process called lipogenesis, which is stimulated by insulin.
The satiation from carbohydrates in foods (e.g., fruits) is substantially dependent upon the presence of fiber. Fiber-free juice can be consumed 11 times faster than intact apples, with apples more satiating than puree, and puree more satiating than fruit juice. Especially in susceptible individuals (e.g., those with insulin resistance), fruit juice consumption may result in higher insulin levels compared to whole fruit consumption [11]. In addition, more calories per unit volume are found in apple juice than in intact apples. An intact apple and a glass of apple juice may occupy the same volume in the stomach, but the fiber-containing apple may contain fewer calories, depending on the concentration of the apple juice.
Carbohydrates are generally not considered an essential macronutrient. The liver, kidney, and possibly small intestine can synthesize glucose (i.e., gluconeogenesis). However, genetic defects of glucose metabolism and/or storage (e.g., glycogen storage disease) may cause carbohydrates to be conditionally essential. While calorie deficiency can lead to marasmus (insufficient calories), in patients without impairment in carbohydrate metabolism or storage, no known carbohydrate deficiency exists [12].
2.1.2. Fats
Fats, or lipids, are a diverse group of compounds used to store energy (i.e., 9 kcal/gram of fat) [9], that provide body insulation through body fat, and that facilitate or contribute to immune response (i.e., omega-3 fatty acids), cell membrane structure (phospholipids), brain tissue composition (cerebrosides contain the fatty acid sphingosine attached to galactose or glucose), synthesis of bile acid, cholesterol, absorption of fat-soluble vitamins (A, D, E, and K), and synthesis of steroid hormones. Cholesterol is sterol derived from animal fats, not carbohydrates.
Omega-3 alpha linolenic acid (ALA) and omega-6 linoleic acid (LA) are two fatty acids that cannot be made by the body. These are termed “essential” fatty acids and must be consumed in the diet. Omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and gamma linolenic acid (an omega-6 fatty acid) are sometimes considered “conditionally essential,” meaning they can be endogenously derived on the condition of a lack of intake of essential fatty acids. Given humans are only able to produce small amounts of EPA and DHA, oral intake of EPA and DHA is often recommended from cold water marine fish. The USDA Dietary Reference Intake (DRI) for fat is at least 30 g/day.
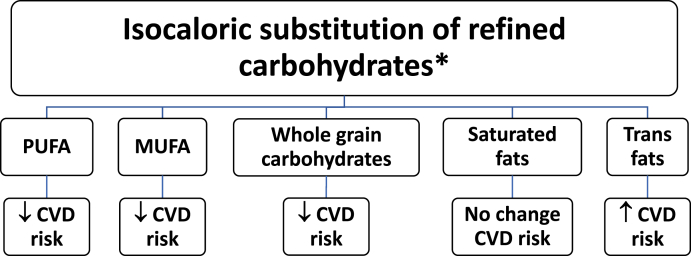
Replacing saturated fats with polyunsaturated or monounsaturated fats may reduce cardiovascular disease risk. Replacing saturated fats with ultra-processed (refined) carbohydrates and sugar is not associated with reduced cardiovascular disease risk, as shown in Fig. 3 [13].
Fig. 3.
Macronutrient Effects on CVD Risk. The health effects of isocaloric substitutions depends on the macronutrient [13,[26], [27], [28], [29]].
Abbreviations: PUFA: Polyunsaturated Fatty Acids; MUFA: Monounsaturated Fatty Acids: CVD: Cardiovascular disease
∗ This figure is focused on isocaloric substitutions and does not necessarily reflect health effects of substitutions that result in changes in weight.
Insulin promotes fatty acid and triglyceride synthesis and storage (lipogenesis) and inhibits fat breakdown (lipolysis). Foods that cause a rise in blood glucose, such as sugars, starches, or, to a smaller degree, amino acids, will stimulate the secretion of insulin from the pancreas. A nutritional therapy plan that limits the prandial rise in insulin levels may decrease ectopic fat deposition (e.g., visceral fat, intrahepatic fat, and intrapericardial fat), and improve components of the metabolic syndrome independent of weight reduction.
2.1.2.1. Trans fats
Trans fats are created through a process of artificially hydrogenating polyunsaturated fats (vegetable oils) into more saturated fats, allowing for higher melting temperatures, which is more desirable for processed foods, cooking, and frying. Partially hydrogenated vegetable oils were developed because they tasted better in foods and were less expensive than saturated fats derived from animals (lard). Some early shortenings (i.e., fats used in cooking) made from partially hydrogenated vegetable oil (cottonseed and soybean oil) originally contained 50% trans fats and were marketed as being a more healthful alternative to animal fat, because they were derived from “vegetables.” Although many contain partially hydrogenated palm and soybean oils, common shortenings now contain minimal trans fats, soybean oil, and fully hydrogenated palm oil (i.e., 3 g saturated fats, 6 g polyunsaturated fats, 2.5 monounsaturated fats) [14].
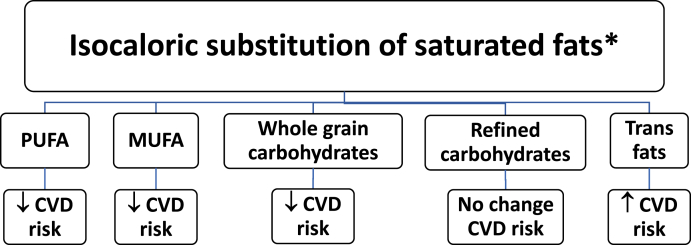
Trans fats may increase low-density lipoprotein cholesterol, reduce high-density lipoprotein cholesterol, and increase the risk of cardiovascular disease (i.e., myocardial infarction and stroke), type 2 diabetes mellitus, and certain cancers [15] (Fig. 2, Fig. 3). While the FDA banned partially hydrogenated oil in 2018, trans fats are sometimes reportedly still found in some cakes, pies, cookies (especially with frosting), biscuits, microwavable breakfasts, stick margarine, crackers, microwave popcorn, cream-filled candies, doughnuts, fried fast foods, and frozen pizza.
Fig. 2.
Macronutrient Effects on CVD Risk. The health effects of isocaloric substitutions depends on the macronutrient [13,[26], [27], [28], [29]].
Abbreviations: PUFA: Polyunsaturated Fatty Acids; MUFA: Monounsaturated Fatty Acids: CVD: Cardiovascular disease
∗ This figure is focused on isocaloric substitutions and does not necessarily reflect health effects of substitutions that result in changes in weight.
Conjugated linoleic acid (CLA) is a naturally occurring trans/cis fat derived from ruminants (i.e., fermentation of plant-based foods via microbes in the stomach prior to digestion). Naturally occurring CLA in foods is generally not thought detrimental to health; conjugated trans linkages are not included as trans fats for nutritional regulations and food labeling [16].
2.1.2.2. Saturated fats
Saturated fats are composed of carbon chain fatty acids with no double bonds. They are solid or semisolid at room temperature. Many natural foods containing saturated fats also contain polyunsaturated and monounsaturated fat. Coconut and palm oils have a high percent of saturated fats (both medium and large chain) and are commonly found in snack foods. Long chain saturated fatty acids (>12 carbons) are found in meats, dairy products, tropical oils (i.e., coconut and palm oil), and hydrogenated vegetable oils (i.e., shortening). Medium chain saturated fatty acids (8–12 carbons) are found in coconut and palm oil. Stearic acid (C18:0) represents about 25% of saturated fats in the U.S. adult diet and has minimal effects upon low density lipoprotein (LDL) cholesterol. Conversely, palmitic acid (C16:0) represents about 50% of saturated fats in the U.S. adult diet and increases LDL cholesterol [13,17].
Saturated fat consumption may impair vascular endothelial function; polyunsaturated fats such as omega-3 fatty acids may improve endothelial function [18,19]. Conversely, saturated fats are less likely than unsaturated fats (e.g., with cooking) to become oxidized or become rancid [17]. Reports are inconsistent regarding the relationship of saturated fat-containing dairy products and cardiovascular disease. Dairy food intake is included in “diets” that are generally considered to be healthful (e.g., Mediterranean Diet). Some reports suggest that fermented dairy products (e.g., cheeses and yogurt) may be more healthful than other high fat dairy products (e.g., butter) [20,21].
Most studies suggesting saturated fats are unhealthful (i.e., especially regarding increased cardiovascular disease risk) evaluated isocaloric substitution for other nutrients. Most studies have not prospectively evaluated the health effects of different types of saturated fats during clinically meaningful weight reduction, and not when accompanied by improvement in adiposopathic obesity-related metabolic diseases [13] (Fig. 2, Fig. 3). Many patients with pre-obesity/obesity who undergo weight reduction via carbohydrate restricted diets may experience improvement in fat mass disease symptoms and/or improvement or remission in diabetes mellitus, hypertension, dyslipidemia (i.e., triglycerides), and thuds reduced CVD risk factors [13]. Having said this, some patients with genetic dyslipidemias may have moderate to marked increases in low-density lipoprotein cholesterol with carbohydrate restriction, which, if excessive or uncontrolled, should prompt replacement of saturated fats with poly or monounsaturated fats and restriction of dietary cholesterol [22] (see discussion below).
2.1.2.3. Polyunsaturated fats
Polyunsaturated fats contain carbon chain fatty acids with multiple double bonds. They are typically liquid at room temperature (e.g., vegetable and fish oils). Nuts (e.g., walnuts, almonds, macadamia nuts, hazelnuts, pecans) contain both polyunsaturated and monounsaturated fats and are thought to reduce the risk of cardiovascular disease (Fig. 2, Fig. 3). Omega-3 fatty acids are polyunsaturated fats, such as those found in oceanic cold-water fish and are often considered cardioprotective. In patients at risk for atherosclerotic cardiovascular disease and elevated triglyceride levels, prescription dose omega-3 fatty acids (4 g per day of eicosapentaenoic acid) can reduce triglyceride levels and reduce the risk of cardiovascular disease [23].
Cooking oils beyond their individual smoking points or repeated use of the same cooking oils may increase oxidation, generate unhealthful free radicals, and have some minor potential to create trans isomers; more likely with unrefined, unbleached, undeodorized, raw, pure, virgin polyunsaturated fats [24,25].
2.1.2.4. Monounsaturated fats
Monounsaturated fats contain carbon chain fatty acids with one double bond. They are typically liquid at room temperature (e.g., olive and canola oils). Isocaloric substitution of monounsaturated fats for saturated fats may reduce cardiovascular disease risk factors and cardiovascular disease risk (Fig. 2, Fig. 3).
Smoking points can vary widely depending on the oil. While some polyunsaturated fats have among the lowest smoking points (e.g., unrefined safflower and sunflower oils are approximately 200–300° F), some sources of monounsaturated oils have among the higher smoking points (e.g., pomace and extra light olive oil and avocado oil ∼ 500° F). Stoves and ovens can be as hot as 500° F. Baking and stir-frying with canola oil does not significantly increase the generation of trans fats [25].
2.1.3. Protein
Protein contains amino acids and serves as the major structural building block of the human body: bone, muscle, skin, brain, and nucleic acids. Proteins can also serve as a source of energy (4 kcal/gram) [9]. Essential amino acids are those that cannot be made by the human body and must be consumed in the diet. These 9 essential amino acids include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Some amino acids can be used as an energy source (i.e., converted to glucose or ketones when needed), and can be used as a substrate to make carbohydrates in a process called gluconeogenesis. Two conditions that are associated with severe protein deficiency include Kwashiorkor and Marasmus.
Kwashiorkor occurs with sufficient calories, but with protein deficiency of such severity as to cause muscle wasting (often with normal or increased body fat), hypoalbuminemia, and movement of potassium and other intracellular ions to the extracellular space. The subsequent osmotic water movement to the extracellular space is clinically manifest by edema. Marasmus is a term typically used to describe malnutrition in infants due to inadequate energy intake of all forms of nutrients.
The biologic value of a protein is the proportion absorbed from a food and incorporated into proteins of the body. For example, an egg has a protein biologic value of 100 (top efficiency). However, a single egg may only have a protein Daily Value of ∼12%, with the Daily Value being the percent of a nutrient recommended per day, based upon a 2000 Calorie a day diet. If a food protein lacks one of the nine essential amino acids, then it is given a biologic value of zero. Gelatin (and collagen) lack tryptophan; hence, gelatin and collagen are often given low biologic values (i.e., sometimes as low as 0). The USDA Dietary Reference Intake (DRI) for protein is 0.8–2.0 g/kg/day depending upon age, sex, and physical activity.
2.1.4. Alcohol
Pure alcohol contains ∼7 kcal/gram. Limiting alcoholic drinks is often an important principle of medical nutrition therapy. Beyond their alcohol content, many alcoholic drinks are energy dense (e.g., two margaritas may be well over 1000 Calories) and should be avoided in patients being treated for pre-obesity or obesity.
2.2. Food labels
The Dietary Reference Intake (DRI) is a set of reference ranges published by the US Institute of Medicine, and includes Recommended Dietary Allowance (RDA), Adequate Intake (AI), Tolerable Upper Intake Level (UL), and Estimated Average Requirement (EAR).
The RDA/AI for trans fatty acids (0%) and saturated fats (<10%) are generally recommended to be as low as possible while consuming a nutritionally adequate diet. Added sugar should be less than 10% of consumed calories. Perhaps most applicable to patients is that the DRI establishes the recommended percent dietary allowance (RDA), which is noted in food labels, and lists the recommended percent intake of a nutrient sufficient to meet the requirements of 98% of healthy individuals. These dietary guidelines are intended to be informational and not specifically intended to be a clinical guideline for treating chronic disease, including obesity [30].
Food labels list the amount of total fat (saturated and trans fat), cholesterol, sodium, total carbohydrates, sugar, fiber, vitamin D, calcium, iron, and potassium in the food, as well as the percent Daily Values (DV) in food based upon a 2,000 Calorie diet. Perhaps most relevant to energy intake and weight management is that food labels also list servings per container, serving size, and Calories. A calorie is the amount of heat energy required to raise the temperature of 1 g of water 1 °C. A Calorie (capital “C”) is the same as a kilocalorie (kcal), which is the heat energy required to raise the temperature of 1 kg of water by 1 °C. One kcal is equal to 4.184 kJ. Kilocalories are used in food labels, usually expressed as Calories. When referring to food or physical exercise, it is common that the term “calorie” actually refers to kilocalorie (kcal) [31].
When available, reading and understanding food labels at grocery stores and restaurants can be an empowering form of education that can affect food choice, form the basis of accountability, and effect positive change. For example, a single serving of a certain popular ice cream can be ∼350 Calories, but the entire 2 cup/1 pint container contains ∼900 Calories or more.
2.3. Caloric intake
Hunger is the physiologic craving or need for food (e.g., increased by ghrelin, neuropeptide Y, asprosin and/or decreased by leptin and many other hormones). Appetite is the desire to eat food, which may be physiologic due to hunger or may be independent of hunger via responses to psychosocial environments and/or cued responses to senses such as touch, sight, hearing, smell, and taste. Satiation is the feeling of fullness within a meal. Satiety is the feeling of fullness between meals. Cravings, or desire to eat food, can be measured by validated scales, often utilizing visual analog scales. Other measures include the Intuitive Eating Scale-2, Dutch Eating Behavior Questionnaire, Power of Food scale, and the Eating Inventory (Three-Factor Eating Questionnaire or Stunkard-Messick Eating Questionnaire).
Multiple factors influence satiety and satiation and thus affect daily ad libitum calorie intake. Such factors include the amount/volume of food (i.e., quantitative), type of food (i.e., qualitative carbohydrates, fats, and proteins), food form and texture, food palatability, and dietary fiber intake. These factors have potential clinical application, such as when recommending fiber, complex carbohydrates and proteins to enhance satiation [32]. Other factors that may influence food intake includes food availability, environmental triggers, sensory specific satiety, sleep deprivation and circadian rhythm alignment/malalignment, physical activity, mental stress, ketosis, and body composition (i.e., muscle and fat mass) [[33], [34], [35], [36]].
Sleep deprivation may increase hunger (i.e., especially for energy dense foods), decrease physical activity, increase partitioning of body energy to body fat, (i.e., particularly abdominal or visceral fat), reduce insulin sensitivity, and preferentially promote fat mass accumulation relative to lean mass accumulation [37]. In addition, sleep deprivation can promote metabolic derangements that lead to worsening hepatic steatosis, development of non-alcoholic fatty liver disease (NAFLD) that progresses to non-alcoholic steatohepatitis (NASH), and worsening metabolic syndrome [[38], [39], [40]].
2.3.1. Caloric organ delivery and storage
Caloric partitioning is the distribution of consumed energy to specific tissues. Carbohydrates are preferentially utilized in muscle for immediate energy needs. During positive caloric balance, carbohydrates are stored in the liver and muscles as glycogen. If not utilized for energy needs, then carbohydrates may ultimately be converted to fat, mainly in adipose tissue. Fats are utilized in muscles (i.e., fatty acids) at lower levels of physical activity, and predominantly stored in adipose tissue during periods of positive caloric balance. Proteins may be preferentially delivered, utilized, and stored in muscle tissue [36].
A greater proportion of consumed energy will partition to muscle during resistance training, and a greater proportion of energy will partition to fat without resistance training. During negative caloric balance, the derivation of energy from muscle tissue is likely to be less during concomitant resistance training, with some mitigation of muscle wasting and the possibility of increase muscle mass during weight reduction when accompanied by high levels of resistance training [32].
2.4. Choice of nutrition plan
The most appropriate nutritional therapy for management of obesity is one that is safe, effective, and one that the patient is most likely to adhere. In patients with obesity, a goal is to encourage food intake that results in a negative caloric balance to achieve and maintain a healthy body weight with consideration of the following:
-
•
Eating behaviors and meal patterns
-
•
Cultural background, traditions, and food availability
-
•
Time constraints and financial limitations
-
•
Nutritional knowledge and cooking skills
-
•
Medical conditions potentially affected by the nutrition plan
-
•
Medical conditions impacting the optimal nutrition plan
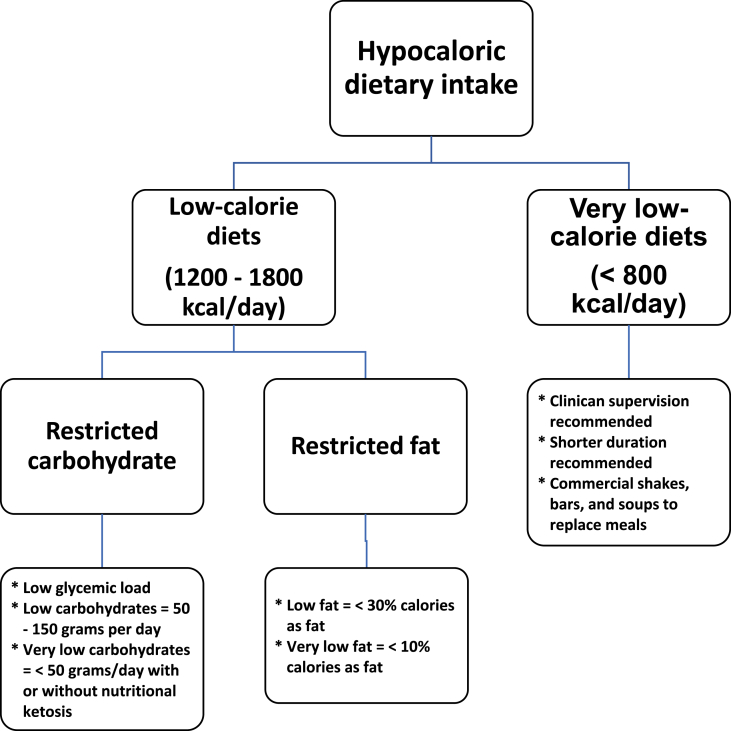
Medical nutrition therapy approaches for weight reduction in patients with pre-obesity or obesity typically focus on the caloric manipulation of the three macronutrients: carbohydrates, fats, or proteins. Very low-calorie diets contain less than 800 kcal/day and require close medical supervision for safety reasons. Low calorie diets range from 1200 to 1800 kcal/day (1200–1500 for women and 1500–1800 for men) [41]. Restricting dietary saturated fat leads to a greater reduction in total and LDL cholesterol, whereas restricting dietary carbohydrates leads to a greater reduction in serum triglycerides and an increase in HDL-cholesterol levels [42]. Reduction of carbohydrates can lead to a greater reduction in serum glucose and hemoglobin A1C. Fig. 4 describes dietary energy consumption intended to cause negative caloric balance and reduction of fat mass. Different dietary approaches are reviewed below.
Fig. 4.
Dietary Energy Consumption Intended to Cause Negative Caloric Balance and Reduction of Fat Mass. This figure summarizes the types and definitions of different hypocaloric diets [[41], [42], [43], [44]].
3. Hypocaloric medical nutrition therapies
3.1. Carbohydrate-restricted hypocaloric diet
A low-carbohydrate diet is often defined as 50–150 g of carbohydrates per day. A very low-carbohydrate diet is often defined as < 50 g of carbohydrates per day. A carbohydrate restricted diet may produce modestly greater weight reduction compared to fat-restricted dietary intake, at least for the first 6 months. After 6 months, the net weight reduction may be similar to other calorie-restricted nutritional interventions [45]. Carbohydrate-restricted hypocaloric diets may reduce fasting glucose, insulin, and triglyceride levels, and modestly increase high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels, as well as modestly reduce blood pressure. Some of these metabolic effects may occur with or without weight reduction.
A low-carbohydrate ketogenic diet (LCKD) may improve diabetes mellitus complications (i.e., nephropathy). In patients with epilepsy, a very low-carbohydrate ketogenic diet (VLCKD) may reduce seizures. Finally, LCKD may help increase energy expenditure during maintenance of weight reduction [46,47].
A carbohydrate-restricted diet is sometimes associated with malaise during early implementation and may produce carbohydrate cravings within the first few days of implementation, which may be mitigated by adding low-glycemic-index carbohydrate foods. A carbohydrate-restricted diet may induce gout flares in patients with history of gout, especially during initial implementation, and may also present challenges in patients where dietary protein restriction may be recommended (i.e., severe kidney disease). Due to the possibility of hypoglycemia and hypotension in patients treated for diabetes mellitus and hypertension respectively, blood sugar and blood pressure should be monitored for potential adjustment in applicable metabolic drug treatments. As with any new aggressive medical nutrition therapy, clinicians should consider whether patients (i.e., especially those having well-controlled blood pressure and blood sugar) might proactively require dose reduction or discontinuation of some antihypertensive drugs or hypoglycemic agents, particularly insulin or sulfonylureas [48].
3.2. Fat-restricted hypocaloric diet
A fat-restricted diet is often defined as 10–30% of total calories from fat. After six months, fat-restrictive, low-calorie nutritional interventions generally produce similar weight reductions as a “low-carb diet.” If accompanied by weight reduction, a fat-restricted diet may reduce fasting glucose and insulin levels and modestly reduce blood pressure. It may also modestly decrease low-density and high-density lipoprotein cholesterol levels [49,50].
Hunger control may present challenges with a fat-restricted hypocaloric diet, which may be mitigated with anti-obesity pharmacotherapy. If fat restriction results in a substantial increase in carbohydrate consumption, and if weight reduction is not achieved, then an increase in dietary carbohydrate intake may contribute to hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and reduced levels of high-density lipoprotein cholesterol.
3.3. Very Low-Calorie Diet (VLCD)
Very low-calorie diets (VLCDs) are defined as diets containing less than 800 kcal/day and are typically implemented utilizing specifically formulated meal-replacement products under the supervision of a trained clinician. VLCDs may also be achievable without meal replacements by a trained clinician. A VLCD produces more rapid weight reduction than low-calorie fat-restricted or carbohydrate-restricted diets due to lower energy intake [51]. VLCDs may reduce fasting glucose, insulin, triglyceride, low-density lipoprotein cholesterol levels, may reduce blood pressure, and may modestly increase high-density lipoprotein cholesterol levels [51,52]. Common adverse effects of VLCD include fatigue, nausea, constipation, diarrhea, hair loss, brittle nails, cold intolerance, dysmenorrhea, and some increased risk for gallstones, kidney stones, and gout flares. Due to the possibility of hypoglycemia and hypotension in patients treated for diabetes mellitus and hypertension, respectively, blood sugar and blood pressure should be monitored for potential adjustment in applicable metabolic drug treatments. In many cases, anti-diabetes mellitus and antihypertensive drugs might best be reduced in dose or discontinued before start of a VLCD, if this is determined to be in the best safety interest of the patient.
Finally, if insufficient mineral intake occurs with a VLCD, then this may predispose patients to palpitations, cardiac dysrhythmias, and muscle cramps. Weight regain will occur if patients are not taught how to maintain healthful eating when transitioning to non-meal-replacement eating patterns or to a higher-calorie diet [51,53].
4. Dietary patterns
4.1. Ketogenic diet (Keto or Modified Atkins Diet)
The Ketogenic Diet is illustrative of a carbohydrate-restricted nutritional intervention that promotes utilization of fat for energy and generates ketosis, which may reduce hunger.
-
•Encouraged [33,54,55]:
-
oThe induction phase allows no more than 20 g of carbohydrates per day from non-starchy vegetables and leafy greens, encourages adequate protein, and includes a higher proportion of dietary fat to reduce insulin levels and generate a state of nutritional ketosis.
-
oThe ongoing weight reduction phase allows a wider variety of vegetables, seeds and nuts, and low-glycemic fruits (i.e., strawberries and blueberries).
-
oThe pre-maintenance phase, after the goal weight is achieved, allows increased carbohydrate intake to be slowly increased if weight gain does not occur.
-
oIn the maintenance phase, 60–90 g of carbohydrates per day is allowed if weight and health benefits are maintained, which may include legumes, whole grains, and fruits.
-
oAll phases encourage a balance of saturated, monounsaturated, and polyunsaturated fatty acids.
-
o
- •
-
•Advantages:
-
oMay contribute to clinically meaningful weight reduction in patients with pre-obesity or obesity [56].
-
oMay reduce hunger [33].
-
oLower carbohydrate food intake will typically result in lower postprandial glucose and insulin levels [57].
-
oIf associated with weight reduction, a ketogenic diet may improve glucose metabolism with improved insulin sensitivity, reduced fasting glucose, and reduced fasting insulin levels [58].
-
oMay lower diastolic blood pressure [54].
-
oMay reduce triglyceride and increase high density lipoprotein cholesterol levels [54].
-
oKetonemia may help treat seizures [59].
-
oEffects upon physical exercise performance are inconsistent [60].
-
oPossible patient-specific adjunct to multifactorial therapy for certain kinds of cancers [61].
-
o
-
•Disadvantages:
-
oMay increase low density lipoprotein (LDL) cholesterol levels, sometimes substantially so in patients with genetic hypercholesterolemia [54] or who increase intestinal cholesterol absorption with weight reduction (see discussion below).
-
oMay not improve insulin sensitivity in patients not experiencing weight reduction [58].
-
oMay cause transient fatigue and mild decrease in mental cognition upon the start of a ketogenic diet [56].
-
oEffects upon physical exercise performance are inconsistent [60].
-
o
4.1.1. Management of the rare patient with moderate/marked increases in LDL cholesterol and/or LDL particle number with ketogenic diet [62]
If clinically meaningful weight reduction is achieved, then the increase in low density lipoprotein cholesterol and/or LDL particle number with ketogenic diet is generally modest [62]. However, the ketogenic diet is sometimes associated with individual, rare cases of moderate to marked increases in LDL cholesterol levels [63]. If this occurs, then a reasonable first step is confirmation and evaluation. Any unexpected or unexplained clinically meaningful change in lipid levels should initially be addressed with repeat lipid testing. If the change in lipid measures is confirmed, then this should prompt evaluation for new onset or worsening of secondary causes of hypercholesterolemia (e.g., diabetes mellitus, hypothyroidism, nephrotic syndrome, liver disease) and recent changes in medications that may worsen cholesterol levels (e.g., some beta-blockers, corticosteroids, amiodarone, cyclosporin, anabolic steroids, protease inhibitors, some diuretics). If it is determined that the weight loss via the ketogenic diet is likely the cause for elevations in low-density lipoprotein cholesterol levels, then this may be because the weight reduction has facilitated an increase in intestinal cholesterol absorption [5]. In severe cases, the clinician might consider evaluation for diet-sensitive genetic dyslipidemias (e.g., sitosterolemia).
If a patient treated with a ketogenic diet is confirmed to have developed moderate/marked increases in LDL cholesterol and/or LDL particle number with ketogenic diet, then management may include:
-
•
Replace dietary saturated fats with polyunsaturated or monounsaturated fats.
-
•
Reduce dietary cholesterol.
-
•
Consider a trial of ezetimibe (i.e., if the patient is suspected to be a hyper-absorber of intestinal cholesterol).
-
•
Consider cholesterol-lowering drug treatment (e.g., statin).
-
•
Consider a trial period off the ketogenic diet to determine if elevated lipid levels resolve.
4.1.2. Sitosterolemia is an illustrative example of a diet-sensitive genetic condition that can result in high cholesterol levels [64]
Beta-sitosterolemia (phytosterolemia) is a rare autosomal recessive disorder that may phenotypically resemble heterozygous familial hypercholesterolemia. Beta-sitosterolemia is due to bi-allele mutations in one or both genes encoding for intestinal sterol co-transporters [i.e., adenosine triphosphate binding cassette transporters (ABC) G5 and/or G8]. Loss of function of ABC G5 and G8 impairs the efflux of absorbed consumed plant sterols and animal cholesterol from intestinal and hepatic cells into the intestinal and biliary lumen.
Clinical findings related to beta-sitosterolemia include tendon xanthomas and increased cardiovascular disease risk out of proportion to the patient's lipid profile. Some patients may also have low platelet counts. The degree of elevation in cholesterol levels can vary, with some patients exhibiting marked hypercholesterolemia, despite no immediate family history of hypercholesterolemia. The diagnosis should be suspected in patients with wide fluctuations of cholesterol levels during nutritional changes.
Diagnosis can be made clinically, or biochemically by measuring plant sterol levels (sitosterol, campesterol, and possibly stigmasterol), or documenting bi-allelic loss-of-function mutations in ABC G5 and/or G8. Patients with beta-sitosterolemia may respond poorly to statins but may respond well to reduced dietary plant sterol and cholesterol consumption as well as treatment with bile acid sequestrants. Beta-sitosterolemia responds to cholesterol/sterol absorption inhibitors such as ezetimibe, which is the only drug approved to treat beta-sitosterolemia (i.e., ezetimibe lowers LDL cholesterol and LDL particle number) [[65], [66], [67]].
4.2. Mediterranean Diet
The Mediterranean Diet is not a defined “diet,” but rather a generalized term describing several meal pattern variants. The Mediterranean Diet has among the most consistent and robust scientific support in reducing atherosclerotic cardiovascular disease risk [[68], [69], [70], [71]].
-
•Encouraged [72]:
-
oOlive oil as main source of fat
-
oVegetables, fruit, legumes, whole grains, nuts, and seeds
-
oIntake of red wine.
-
oModerate consumption of seafood, fermented dairy products (e.g., cheese and yogurt), poultry, and eggs
-
o
-
•Discouraged [72]:
-
oLimit consumption of high amounts of red meat, meat products, and ultra-processed carbohydrates
-
oSaturated fats are often discouraged with the Mediterranean Diet; olive oil is a staple of most definitions of the Mediterranean Diet. However, some Mediterranean cuisine may include lard and butter for cooking, and olive oil for dressing salads and vegetables
-
o
-
•Advantages:
-
oMay reduce the risk of cardiovascular disease
-
oThe diet is high in olive oil and certain nuts. Therefore, it is high in mono-unsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), which are predominantly omega-3 rather than omega-6 fatty acids.
-
oAlthough the Mediterranean Diet has higher fat content than other common diets (40% of total calories), it is low in saturated fats (less than 10% of calories)
-
oPhenol-rich extra virgin olive oil has been shown to exert hepato-protective effects through the induction of cellular antioxidant response and inhibition of inflammatory pathways (e.g., visceral adipocyte inflammatory cytokine expression) and steatosis. This can also contribute to anti-cancer effects [73].
-
oThis diet is high in fiber (average 25–35 g a day), fruits, and vegetables that are rich in dietary active compounds like phytochemicals, antioxidant compounds, vitamins, phenolics, and flavonoids. Dietary fiber helps reduce hunger and serum peak glucose levels.
-
oIt may improve insulin resistance and lipid profiles.
-
oIt is safe to implement in children and adults.
-
o
-
•Disadvantages:
-
oWeight loss may be less than a very low-carbohydrate diet [74].
-
oFood choices may be more expensive than less healthful foods.
-
o
4.3. DASH diet
The “Dietary Approaches to Stop Hypertension” (DASH) is a diet pattern promoted by the U.S. National Heart Lung and Blood Institute, primarily to treat high blood pressure [75,76].
-
•Encouraged [75]:
-
oVegetables, fruits, and whole grains
-
oFat-free or low-fat dairy products
-
oFish, poultry, and lean meats
-
oNuts, seeds, and legumes
-
oFiber and the minerals calcium, potassium, and magnesium
-
o
-
•Discouraged [75]:
-
oLimit sodium: 1,500 - 2,300 mg per day
-
oLimit total fat: ∼27% of total daily calories.
-
oLimit saturated fat: <6% of total daily calories
-
oLimit cholesterol: ≤150 mg per day for a 2,100-Calorie eating plan
-
oAvoid red and processed meats
-
oAvoid sugar-sweetened beverages
-
oAvoid foods with added sugars
-
o
-
•Advantages:
-
oMay reduce cardiovascular disease risk
-
oMay improve blood pressure and dyslipidemia
-
o
-
•Disadvantages:
-
oMay be challenging to maintain
-
oLargely eliminates convenience foods
-
oMay not be ideal for weight loss
-
o
4.4. Vegetarian diet
A vegetarian nutritional intervention includes a meal plan consisting of foods that come mostly from plants. Plant-based nutritional intake is generally associated with weight reduction, reduced risk of heart disease (including heart failure), and beneficial effects on metabolic diseases, some cancers, and possibly all-cause mortality [[77], [78], [79], [80], [81], [82], [83], [84], [85]]. However, these potential benefits may be negated when more healthful plant-based whole foods (i.e., with natural fiber and nutrients) are replaced by ultra-processed foods, fried foods, and refined carbohydrates [86]. Vegetarian diets may also result in deficiencies of micronutrients and minerals such as vitamin B12 and iron, which may require clinical monitoring and nutrient replacement when appropriate [87]. Patients who begin a vegetarian diet may benefit from dietitian counseling regarding healthful macro and micronutrient consumption.
Table 4 shows common variants of the vegetarian diet.
-
•Encouraged:
-
oVegetables
-
oFruits
-
oWhole grains
-
oLegumes
-
oSeeds
-
oNuts
-
oSome varieties may include eggs, milk, seafood, and occasional chicken.
-
o
-
•Discouraged:
-
oAnimal protein from fowl, fish/seafood, beef, pork, and lamb
-
o
-
•Advantages:
-
oPlant-based diets may reduce the risk of cardiovascular disease.
-
oDiets high in fruits, vegetables, and fiber are high in antioxidants, polyphenols, and anti-inflammatory phytochemicals and may reduce oxidative stress.
-
oThe high-fiber and low-calorie content of plants promotes weight loss and maintenance.
-
o
-
•Disadvantages:
-
oHealth benefits of a vegetarian diet may not be realized upon consumption of unhealthful, energy dense, ultra-processed, plant-based foods that are high in glycemic index, low in fiber, low in micronutrients, and high in calories and trans fats.
-
oSome vegetarian diets contain high levels of beans, which, without proper preparation, can be high in phytic acid and can cause vitamin deficiencies and poor absorption of iron, zinc, and calcium [88].
-
o
Table 4.
Vegetarian Diet Variants. Common variants of the vegetarian diet, including veganism, lacto-vegetarianism, lacto-ovo vegetarianism, pescatarianism, and flexitarianism [77,78].
| VEGETARIAN DIET VARIANTS | |
|---|---|
| Vegan (“total vegetarian”) | Only plant-based foods (e.g., fruits, vegetables, legumes, grains, seeds, and nuts) with no animal proteins or animal by-products, such as eggs, milk, or honey |
| Lacto-vegetarian | Plant foods plus some or all dairy products (e.g., cheese) |
| Lacto-ovo vegetarian (or ovo-lactovegetarian) | Plant foods, dairy products, and eggs |
| Semi- or Partial Vegetarian | Plant foods and may include chicken or fish, dairy products, and eggs, but not red meat |
| Pescatarian | Plant foods and seafood |
| Flexitarian | Mostly plant-based foods (minimally processed), with occasional fish, meat, and animal products in moderation |
4.5. Therapeutic Lifestyle Change Diet (TLC)
The TLC Diet is a low-fat meal plan variant that was recommended by the National Cholesterol Education Program, Adult Treatment Panel [[89], [90], [91]]. It was historically the “diet” most utilized in the conduct of lipid clinical trials and is still utilized today in many lipid clinical trials.
-
•Encouraged:
-
oTotal fat: 25–35% of daily calories
-
oPolyunsaturated fat: Up to 10% of total daily calories
-
oMonounsaturated fat: Up to 20% of total daily calories
-
oCarbohydrate: 50% to 60% of total calories
-
oSoluble fiber: At least 5–10 g a day, preferably 10–25 g a day
-
o2 g per day of plant stanols or sterols through foods or dietary supplements
-
o
-
•Discouraged:
-
oLimit saturated fat: < 7% of total calories
-
oLimit cholesterol: < 200 mg a day
-
oAvoid foods with trans fatty acids.
-
o
-
•Advantages:
-
oEncourages healthful lifestyle habits
-
oMay improve lipid levels
-
o
-
•Disadvantages:
-
oRequires close tracking of macronutrients that may be difficult for some patients
-
oMay have limited weight loss effects
-
o
4.6. Ornish diet
The Ornish Diet is illustrative of a fat-restricted nutritional intervention [[92], [93], [94]].
-
•Encouraged:
-
oFoods are best eaten in their natural form
-
oVegetables, fruits, whole grains, and legumes
-
oOne serving of a soy product each day
-
oLimited amounts of green tea
-
oFish oil 3–4 g each day
-
oSmall meals eaten frequently throughout the day.
-
o
-
•Discouraged:
-
oLimit dietary fat: < 10% of total daily calories
-
oLimit dietary cholesterol: ≤ 10 mg per day
-
oLimit sugar, sodium, and alcohol
-
oAvoid animal products (red meat, poultry, and fish) and caffeine (except green tea)
-
oAvoid foods with trans fatty acids, including vegetable shortening, stick margarines, and commercially prepared foods, such as frostings; cake, cookie, and biscuit mixes; crackers and microwave popcorn; and deep-fried foods
-
oAvoid refined carbohydrates and oils
-
o
-
•Advantages:
-
oMay reduce the risk of cardiovascular disease
-
o
-
•Disadvantages:
-
oThe Ornish diet is a very restricted diet that may be hard to maintain long term.
-
oMay have limited weight loss effects
-
o
4.7. Paleolithic diet
The Paleolithic nutritional intervention is based upon a dietary pattern presumed to exist during the Paleolithic period (i.e., lasting 3.4 million years and ending 6000–2000 BCE). It differs from some other diets in that it excludes grains, dairy, and ultra-processed foods [[95], [96], [97]].
-
•Encouraged:
-
oFresh vegetables, fruits, and root vegetables
-
oGrass-fed lean red meats
-
oFish/seafood
-
oEggs
-
oNuts and seeds
-
oNaturally produced oils (olive, walnut, flaxseed, macadamia, avocado, and coconut)
-
o
-
•Discouraged:
-
oCereal grains
-
oLegumes, including peanuts
-
oDairy products
-
oPotatoes
-
oUltra-processed foods
-
oRefined sugar, refined vegetable oils, and salt
-
o
-
•Advantages:
-
oRemoval of preservatives, fillers, and non-natural additives in food
-
oAnti-inflammatory
-
oImproved satiety with the type of foods in the diet
-
oMay promote weight loss and improvement in insulin sensitivity and blood pressure in some individuals
-
o
-
•Disadvantages:
-
oMay be more expensive than less healthful food choices
-
oDifficult for vegetarians
-
oMay not promote clinically meaningful weight loss in many patients
-
o
4.8. Fasting (e.g., alternative day, intermittent, time-restrictive feeding)
Fasting is a lack of eating/feeding that may limit overall caloric intake, used therapeutically with the intent to avoid inducing malnutrition [98,99]. Periodic fasting (PF) is defined as limiting food for greater than two consecutive days followed by one week of normal feeding. Intermittent fasting (IF) limits food intake on certain days. IF has three main protocols. In the 5:2 protocol, food is limited for two days, and normal feeding resumes for the other five days of the week. The 2:1 protocol has one day of limited feeding followed by two days of normal meals. The 1:1 protocol alternates between limited and full feeding in equal durations. Another subset of IF is time restricted feeding (TRF), where food is limited to a fixed period during the day [100].
-
•Potential advantages:
-
oReducing “decision fatigue” regarding food selection [101].
-
oQuickly reversible
- o
- o
- o
-
o
-
•Potential disadvantages:
-
oDoes not necessarily emphasize healthful meal quality [108].
-
oMay not be appropriate for patients with eating disorders (e.g., bulimia or binge-eating disorder) [109].
-
oIncreases the risk of hypoglycemia among patients with diabetes mellitus who do not appropriately adjust their hypoglycemic anti-diabetes drug treatments (e.g., insulin, sulfonylurea) [108].
-
oUnclear if sustainable on a lifetime basis for a lifelong disease (i.e., obesity) [99].
-
oMost long-term evidence of efficacy, health benefits and safety are derived from animal studies [104].
-
oProlonged fasting (weeks or more) may promote gout, urate nephrolithiasis, postural hypotension, and cardiac dysrhythmias [102].
-
o
5. Physical activity
Physical activity can be defined as skeletal muscle movements requiring energy expenditure [110,111]. Physical exercise is physical activity that is “planned, structured, repetitive, and aims to improve or maintain one or more components of physical fitness” [110]. Physical activity also includes skeletal muscle use for leisure enjoyment (i.e., gardening), ambulation and transportation, and work-related activities - often termed NEAT (non-exercise activity thermogenesis). Physical inactivity increases the risk of cardiovascular disease (CVD) [111]. Table 5 describes 10 takeaway messages regarding obesity and physical activity.
Table 5.
Top 10 Takeaway Messages: Obesity and Physical Activity. This table lists ten important takeaway messages from the OMA regarding obesity and physical activity.
| Top 10 Takeaway Messages: Obesity and Physical Activity |
|---|
| Routine physical activity may improve body composition. |
| Routine physical activity may improve adiposopathic endocrine and immune body processes. |
| Physical activity may improve metabolic, musculoskeletal, cardiovascular, pulmonary, mental, sexual, and cognitive health. |
| Dynamic training may promote weight reduction and may help prevent weight gain or regain. |
| Resistance training may improve body composition, prevent muscle loss during weight reduction, and increase resting energy expenditure. |
| In addition to physical exercise, increased energy expenditure can be achieved via increased leisure time physical activity and non-exercise activity thermogenesis (NEAT). |
| A common physical exercise prescription (FITTE) includes frequency, intensity, time spent, type of activity, and enjoyment. |
| Metabolic equivalent tasks (METS) are used to assess the intensity of physical exercise, with one MET equal to the amount of energy expended for 1 min while lying down at rest [equal to 3.5 mL of oxygen consumption per kilogram of bodyweight per minute (3.5 mL/kg/min) in a middle-aged male with a normal BMI]. Oxygen consumption per kilogram body weight per minute decreases with increased adiposity and decreased muscle mass [115]. |
| Standing is equal to 2 METS; walking 4 miles per hour is equal to 4 METS; running 10 miles per hour is equal to 16 METS. |
| Tracking physical activity can be done via a variety of activity logs as well as by taking body composition measurements using a reliable technique. |
5.1. Dynamic (Aerobic) training
Moderate physical activity is better than minimal physical activity [112]. At least 150 minutes (2.5 hours) per week of moderate physical activity or at least 75 minutes (1.25 hours) per week of vigorous intensity aerobic exercise has the most health benefits, promotes modest weight reduction, and may help prevent weight gain or regain [113]. Some patients may further benefit from at least 300 minutes (5 hours) per week of moderate physical activity or at least 150 minutes (2.5 hours) per week of vigorous intensity aerobic exercise; this will promote more robust weight reduction and better prevent weight regain after weight reduction [113].
5.2. Resistance (Anaerobic) strength training [112,114]
Resistance training involves the strengthening of major muscle groups two or more times per week. The emphasis is on increasing total muscle mass, which is most efficiently achieved by training large muscle groups which may increase the percentage of lean body mass. Resistance training utilizes appropriate weight-lifting techniques using a variety of free weights, machines, and resistance bands, which may reduce boredom and provide greater flexibility regarding scheduling and location. In resistance training, development of “core” muscles is important for posture and balance stabilization; this includes muscles located at the midsection of the body (i.e., abdomen, back, hips).
During negative caloric balance, resistance training can help mitigate muscle loss and limit reduced resting metabolic rate. Short-term sore muscles may be expected. Sore joints suggest poor technique, with a possible need for medical evaluation and physical activity modification. In resistance training, it is often best to prioritize muscle mass metrics (e.g., muscle tape measurements) versus the amount of weight lifted.
6. Physical activity and thermogenesis
6.1. Exercise Activity Thermogenesis (EAT)
EAT is planned, structured, and repetitive physical activity conducted with the objective of improving health (e.g., sports and gym activities). Similar to the fuel of gasoline for motor vehicles, available energy in muscle (i.e., the “fuel” of adenosine triphosphate or ATP) is used to facilitate motion (mechanical work), with some energy released as heat (thermogenesis). The efficiency in converting ATP to muscle mechanical work is around 30%; dynamic exercise efficiency can be increased with training and weight reduction [116]. Muscle work efficiency may decrease with resistance training [117], resulting in more energy expended as heat.
Whether at steady-state or during physical activity, body temperature is tightly regulated by the autonomic nervous system. At steady state in muscle, adipose tissue, and other body tissues, generation of body heat is largely regulated by the function of mitochondria uncoupling proteins, with increasing mitochondrial inefficiency resulting in more body energy generating heat than energy stored, and with most of the body's stored energy being stored in triglyceride-containing adipose tissue. During physical activity, an increase in body temperature triggers the central nervous system (e.g., hypothalamus) to cool the body via increased dilation of skin smooth muscle blood vessels, increased heart rate, and increased sweat production, all of which help facilitate heat loss during physical exercise [116].
6.2. Non-Exercise Activity Thermogenesis (NEAT)
NEAT is defined as energy expenditure not typically considered physical exercise (e.g., maintaining posture, standing, stair climbing, fidgeting, cleaning, singing, and other activities of daily living). Walking can be considered EAT or NEAT. NEAT often represents the widest variance in total energy expenditure among individuals and can range between 150 and 500 kcal/day, which is often more than bouts of physical exercise. Along with genetic/epigenetic, biological (i.e., increased proportion of brown adipogenesis), and environmental factors, NEAT is an example of a behavioral factor that can help explain perception that some individuals are “naturally lean” and/or maintain a healthier body weight compared to others, even with the same caloric intake and same routine “exercise” activity [[118], [119], [120]].
7. Steps
One of the most common forms of physical activity is steps. Increasing the number of steps taken per day can be achieved by altering daily activity, or by scheduled walking/running. Compared to being seated for hours (such as in the workplace), it is better to walk at least 10 minutes per hour, which might be better achieved by implementing behavior modifications such as preferentially taking stairs instead of elevators and parking further from a destination. The number of steps per day can be monitored via a pedometer or other tracking device. The average number of steps for U.S. adults is < 5,000 steps per day. In terms of number of steps taken, < 5,000 steps per day is considered sedentary, 5,000–7,500 steps per day is low active, 7,500–10,000 steps per day is somewhat active, and ∼10,000 steps or more per day is active [121].
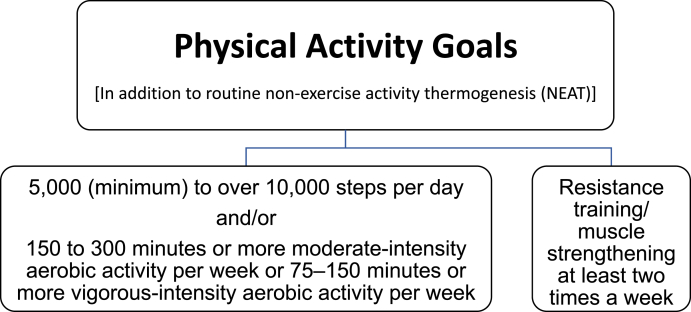
Although variable due to multiple factors, in general, one Calorie (kcal) is “burned” for every 20 steps (i.e., 4000 steps/20 = 200 Calories). 10,000 steps per day x 7 days per week x one Calorie per 20 steps = 3500 Calories burned per week. The adage of “3500 Calories per pound of fat” is a frequently referenced energy content approximation for a pound of fat. However, this calculation was developed to determine the amount of energy in one pound of fat measured via calorimetry. The amount of physical activity required to “burn 3500 Calories” depends on body weight, body efficiencies that depend on body weight, and body weight changes. During negative caloric balance and weight loss, dynamic adaptations in body energetics occur (i.e., changes in resting metabolic rate, skeletal muscle efficiencies) with greater energy expenditure and/or further reduction in energy intake required to achieve the same rate of weight reduction [122,123]. Fig. 5 describes the Obesity Medicine Physical Activity Goals, which are consistent with physical activity goals by other organizations, such as the Physical Activity Guidelines for Americans.
Fig. 5.
Obesity Medicine Association Physical Activity Goals. OMA physical activity goals include steps per day, specified exercise intensities and durations, and recommended resistance training sessions per week [118,121,124]. The OMA physical activity goals specifically include steps as a way to achieve daily, dynamic physical activity goals, with even greater aerobic activity providing additional health benefits.
8. Benefits of physical activity
8.1. Benefits of physical activity not exclusive to obesity
Physical activity has been shown to provide the following benefits not exclusive to obesity [[125], [126], [127], [128], [129], [130], [131], [132]]:
-
•
Improve metabolic health
-
•
Improve musculoskeletal health
-
•
Improve cardiovascular health
-
•
Improve pulmonary health
-
•
Improve neurological health
-
•
Improve mental health (e.g., improve mood, promote happiness & sense of well-being, reduce stress)
-
•
Improve sexual health
-
•
Improve cognitive heath
-
•
Reduce risk of cancer, and improve response to cancer treatments
8.2. Beneficial effects of physical activity to patients with obesity
Physical activity offers additional benefits to patients with obesity, including the following [126,127,133]:
-
•
Treatment of fat mass disease: Physical activity-related weight reduction, or physical activity prevention of body fat regain after weight loss, may help improve pathogenic biomechanical complications of obesity, such as sleep apnea and osteoarthritis.
-
•
Treatment of adiposopathy or sick fat disease: In addition to helping to promote weight reduction and especially chronic management of obesity, increased physical activity may potentially improve body composition, improve adiposopathic physiologic disturbances, possibly improve adipocyte function (“train” fat cells), improve insulin sensitivity, increase mitochondrial biogenesis, and increase browning (“beiging”) of fat cells.
9. Physical activity and evaluation of the patient with obesity
9.1. Essential and targeted medical evaluation to ensure safety before beginning new physical exercise program
The following evaluations are considerations before beginning a new exercise program [134]:
-
•
Assess current physical activity level
-
•
Assess readiness
-
•
Agree upon patient expectations and goals (with optional written “contract”)
-
•
Assess potential need for medical testing/evaluation (i.e., cardiac stress testing, pulmonary function tests, musculoskeletal assessment, etc.)
-
•
Assess mobility, fitness, and potential equipment needs or modifications
-
•Potential adjustment of medications
-
oBefore start of physical activity plan (e.g., diabetes and blood pressure medications)
-
oDuring implementation of physical activity plan
-
o
-
•
Optimal default backup plan
9.2. Mobility
An assessment of patient mobility will help determine the most appropriate individual exercise program. The following are recommended based on levels of mobility [135,136]:
-
•Unable to walk: Seated exercise program, arm exercises (i.e., arm cycling), swimming/aquatic exercises (e.g., shallow or deep-water exercises), resistance/gravity-mediated physical activity (e.g., seated leg raises, band exercises, dumbbells). Consider physical therapy evaluation:
-
oRehabilitation and physical therapy guided activity program
-
oSet physical activity goals.
-
o
-
•
Assess the situation for special equipment needs.
-
•
Limited mobility: Walking, swimming/aquatic exercises (e.g., shallow or deep-water exercises), resistance/gravity-mediated physical activity (e.g., seated leg raises, band exercises, dumbbells), and balance exercises (e.g., walking in a straight line, standing on one foot, standing/sitting up and down, targeting “core” muscles) are recommended. Assess the situation for special equipment needs.
-
•
No substantial limitations to mobility: The exercise/physical activity prescription plan will be driven by the patient and guided by the clinician. Assess the situation for special equipment needs.
10. Additional physical activity recommendations and tracking
10.1. Leisure time physical activity
The following activities are encouraged to promote leisure time physical activity [137,138]:
-
•
Engage in competitive sport activities involving substantial physical activity, best if on a routine basis.
-
•
Engage in non-competitive sports such as running, hiking, cycling, cross-fit training, etc.
-
•
Outdoor warm-weather physical activity in sunlight may facilitate negative caloric balance. and have other health benefits, with a caveat being the need to avoid excessive sun exposure.
-
•
Engage in physical activity sport alternatives, such as dancing.
10.2. Transportation-related and occupational Non-Exercise Activity Thermogenesis (NEAT)
Several behavior modifications related to transportation and occupation can increase NEAT, including the following recommended activities [137,138]:
-
•
Walk short distances instead of taking automated transportation.
-
•
Take stairs instead of elevators.
-
•
Carry overnight travel bags instead of using rollers (i.e., akin to a farmer's walk exercise).
-
•
Utilize an active work environment (i.e., standing desks, walking desks).
-
•
Avoid prolonged inactivity.
-
•
Take breaks from inactivity.
-
•
Walk, stand, and complete incidental movements throughout the day.
10.3. Exercise Prescription (FITTE)
The FITTE exercise prescription can help promote and measure physical activity and is defined as follows [134]:
-
•
Frequency
-
•
Intensity
-
•
Time spent
-
•
Type
-
•
Enjoyment level
10.4. Exercise Prescription (FITT-VP)
A variation on FITTE, FITT-VP, is defined as follows [139,140]:
-
•
Frequency
-
•
Intensity
-
•
Time or duration
-
•
Type or mode
-
•
Volume or total energy expenditure of the exercise
-
•
Progression of the exercise
10.5. Metabolic Equivalent Tasks (METS)
METS are used to assess the intensity of physical exercise (kcal = METS x weight x time) [141,142]:
-
•
Equal to the amount of energy expended in 1 min while lying down at rest
-
•
Equal to ∼3.5 mL of oxygen consumption per kilogram of bodyweight per minute (3.5 ml/kg/min), with oxygen consumption decreased with increased age and increased adiposity
-
•
Standing = 2 METS
-
•
Walking 4 miles per hour = 4 METS
-
•
Running 10 miles per hour = 16 METS
10.6. Tracking progress
A variety of methods and devices can be used to track physical activity-related progress, which can be helpful in motivation and goal setting [[143], [144], [145], [146], [147], [148]]:
-
•
Daily activity logs (written or electronic)
-
•
Pedometer/accelerometer logs
-
•
Dynamic training metrics (i.e., miles run, laps swam, etc.)
-
•
Resistance training metrics (i.e., muscle-circumference measurements, reps, sets, etc.)
-
•
Percent body fat measurements
11. Conclusions
This OMA Clinical Practice Statement on Nutrition and Physical Activity discusses basic principles regarding nutrition and physical activity. It is hoped that an understanding of essential nutrition and physical activity principles may help clinicians better manage patients with obesity.
Transparency [149]
This manuscript was largely derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. This was followed by a similar Pediatric “Obesity Algorithm,” with updates ∼ every two years by OMA authors. Authors of prior years’ version of the Obesity Algorithm are included in Supplement #1.
Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. (Supplement #1) The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
HEB transcribed the first draft from the 2021 OMA Adult Obesity Algorithm. LA, SMC, LR, ABI, KB, AG, SK, DC MS, and HEB then reviewed, edited, and approved the document for peer review by the OMA Board of Trustees.
Managing disclosures and dualities of interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual Disclosures
LA, SMC, LR, ABI, KB, AG, SK, DC, and MS report no disclosures. HEB reports no nutrition or physical activity disclosures.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Acknowledgements and Funding
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2021.100005.
Contributor Information
Lydia Alexander, Email: Lydia.Alexander@enarahealth.com.
Sandra M. Christensen, Email: sam.chris@im-wm.com.
Larry Richardson, Email: hawkeye@drrichardson.com.
Amy Beth Ingersoll, Email: amy.beth.ingersoll@gmail.com.
Karli Burridge, Email: Karli@gaininghealth.com.
Angela Golden, Email: npfromhome@gmail.com.
Sara Karjoo, Email: skarjoo1@jhmi.edu.
Danielle Cortez, Email: danielle@enarahealth.com.
Michael Shelver, Email: michael.shelver@enarahealth.com.
Harold Edward Bays, Email: hbaysmd@outlook.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Eslami O., Shidfar F., Dehnad A. Inverse association of long-term nut consumption with weight gain and risk of overweight/obesity: a systematic review. Nutr Res (NY) 2019;68:1–8. doi: 10.1016/j.nutres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Alonso P., Camacho-Barcia L., Bullo M., Salas-Salvado J. Nuts and dried fruits: an update of their beneficial effects on type 2 diabetes. Nutrients. 2017;9 doi: 10.3390/nu9070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins K.A., Mattes R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109:1288–1301. doi: 10.1093/ajcn/nqy381. [DOI] [PubMed] [Google Scholar]
- 4.Bays H.E. Ten things to know about ten cardiovascular disease risk factors (“ASPC Top Ten – 2020”) Am J Prev Cardiol. 2020;1:100003. doi: 10.1016/j.ajpc.2020.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flock M.R., Green M.H., Kris-Etherton P.M. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2:261–274. doi: 10.3945/an.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borradaile K.E., Halpern S.D., Wyatt H.R., Klein S., Hill J.O., Bailer B., et al. Relationship between treatment preference and weight loss in the context of a randomized controlled trial. Obesity. 2012;20:1218–1222. doi: 10.1038/oby.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy W.S., Jr., McVay M.A., Voils C.I. Effect of allowing choice of diet on weight loss--in response. Ann Intern Med. 2015;163:805–806. doi: 10.7326/L15-5159. [DOI] [PubMed] [Google Scholar]
- 8.Leavy J.M., Clifton P.M., Keogh J.B. The role of choice in weight loss strategies: a systematic review and meta-analysis. Nutrients. 2018;10 doi: 10.3390/nu10091136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of agriculture. https://fnic.nal.usda.gov/how-many-calories-are-one-gram-fat-carbohydrate-or-protein. Food and Nutrition Information Center
- 10.Douard V., Ferraris R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–E237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haber G.B., Heaton K.W., Murphy D., Burroughs L.F. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet. 1977;2:679–682. doi: 10.1016/s0140-6736(77)90494-9. [DOI] [PubMed] [Google Scholar]
- 12.Tondt J., Yancy W.S., Westman E.C. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr Res Rev. 2020:1–11. doi: 10.1017/S0954422420000050. [DOI] [PubMed] [Google Scholar]
- 13.Sacks F.M., Lichtenstein A.H., Wu J.H.Y., Appel L.J., Creager M.A., Kris-Etherton P.M., et al. Dietary fats and cardiovascular disease: a presidential advisory from the American heart association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 14.Teegala S.M., Willett W.C., Mozaffarian D. Consumption and health effects of trans fatty acids: a review. J AOAC Int. 2009;92:1250–1257. [PubMed] [Google Scholar]
- 15.Nestel P. Trans fatty acids: are its cardiovascular risks fully appreciated? Clin Therapeut. 2014;36:315–321. doi: 10.1016/j.clinthera.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Shen W., McIntosh M.K. Nutrient regulation: conjugated linoleic acid's inflammatory and browning properties in adipose tissue. Annu Rev Nutr. 2016;36:183–210. doi: 10.1146/annurev-nutr-071715-050924. [DOI] [PubMed] [Google Scholar]
- 17.Vieira S.A., McClements D.J., Decker E.A. Challenges of utilizing healthy fats in foods. Adv Nutr. 2015;6 doi: 10.3945/an.114.006965. 309S-17S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dow C.A., Stauffer B.L., Greiner J.J., DeSouza C.A. Influence of dietary saturated fat intake on endothelial fibrinolytic capacity in adults. Am J Cardiol. 2014;114:783–788. doi: 10.1016/j.amjcard.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 20.Dehghan M., Mente A., Rangarajan S., Sheridan P., Mohan V., Iqbal R., et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392:2288–2297. doi: 10.1016/S0140-6736(18)31812-9. [DOI] [PubMed] [Google Scholar]
- 21.Tapsell L.C. Fermented dairy food and CVD risk. Br J Nutr. 2015;113(Suppl 2):S131–S135. doi: 10.1017/S0007114514002359. [DOI] [PubMed] [Google Scholar]
- 22.Pollin T.I., Quartuccio M. What we know about diet, genes, and dyslipidemia: is there potential for translation? Current nutrition reports. 2013;2:236–242. doi: 10.1007/s13668-013-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 24.Jaarin K., Kamisah Y. Repeatedly heated vegetable oils and lipid peroxidation. https://www.intechopen.com/books/lipid-peroxidation/repeatedly-heated-vegetable-oils-and-lipid-peroxidation (Accessed January 6, 2019). Lipid Peroxidation Chapter 10. 2012.
- 25.Przybylski O., Aladedunye F.A. Formation of trans fats during food preparation. Can J Diet Pract Res. 2012;73:98–101. doi: 10.3148/73.2.2012.98. [DOI] [PubMed] [Google Scholar]
- 26.Wang D.D., Li Y., Chiuve S.E., Stampfer M.J., Manson J.E., Rimm E.B., et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176:1134–1145. doi: 10.1001/jamainternmed.2016.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Hruby A., Bernstein A.M., Ley S.H., Wang D.D., Chiuve S.E., et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66:1538–1548. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. [DOI] [PubMed]
- 29.Clifton P.M., Keogh J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutrition, metabolism, and cardiovascular diseases. Nutr Metabol Cardiovasc Dis. 2017;27:1060–1080. doi: 10.1016/j.numecd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 30.United States . Dietary guidelines advisory committee. Dietary guidelines for Americans, 2015-2020. eighth ed. U.S. Department of Health and Human Services and U.S. Department of Agriculture; Washington, D.C.: 2015. Department of health and human services., United States. Department of agriculture., United States. [Google Scholar]
- 31.Hargrove J.L. Does the history of food energy units suggest a solution to "Calorie confusion. Nutr J. 2007;6:44. doi: 10.1186/1475-2891-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosby A.K., Conigrave A.D., Raubenheimer D., Simpson S.J. Protein leverage and energy intake. Obes Rev : Off J Int Assoc Study Obes. 2014;15:183–191. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- 33.Gibson A.A., Seimon R.V., Lee C.M., Ayre J., Franklin J., Markovic T.P., et al. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev : Off J Int Assoc Study Obes. 2015;16:64–76. doi: 10.1111/obr.12230. [DOI] [PubMed] [Google Scholar]
- 34.Holt S.H., Miller J.C., Petocz P., Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49:675–690. [PubMed] [Google Scholar]
- 35.Arguin H., Tremblay A., Blundell J.E., Despres J.P., Richard D., Lamarche B., et al. Impact of a non-restrictive satiating diet on anthropometrics, satiety responsiveness and eating behaviour traits in obese men displaying a high or a low satiety phenotype. Br J Nutr. 2017;118:750–760. doi: 10.1017/S0007114517002549. [DOI] [PubMed] [Google Scholar]
- 36.Blundell J.E., Gibbons C., Caudwell P., Finlayson G., Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev : Off J Int Assoc Study Obes. 2015;16(Suppl 1):67–76. doi: 10.1111/obr.12257. [DOI] [PubMed] [Google Scholar]
- 37.Nedeltcheva A.V., Kilkus J.M., Imperial J., Schoeller D.A., Penev P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imaizumi H., Takahashi A., Tanji N., Abe K., Sato Y., Anzai Y., et al. The association between sleep duration and non-alcoholic fatty liver disease among Japanese men and women. Obes Facts. 2015;8:234–242. doi: 10.1159/000436997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura T., Hashimoto Y., Hamaguchi M., Obora A., Kojima T., Fukui M. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: a population-based longitudinal study. J Gastrointestin Liver Dis. 2019;28:73–81. doi: 10.15403/jgld.2014.1121.281.alc. [DOI] [PubMed] [Google Scholar]
- 40.Peng K., Lin L., Wang Z., Ding L., Huang Y., Wang P., et al. Short sleep duration and longer daytime napping are associated with non-alcoholic fatty liver disease in Chinese adults. J Diabetes. 2017;9:827–836. doi: 10.1111/1753-0407.12489. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Campoy J.M., St Jeor S.T., Castorino K., Ebrahim A., Hurley D., Jovanovic L., et al. Clinical practice guidelines for healthy eating for the prevention and treatment of metabolic and endocrine diseases in adults: cosponsored by the American Association of Clinical Endocrinologists/the American College of Endocrinology and the Obesity Society. Endocr Pract : Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists. 2013;19(Suppl 3):1–82. doi: 10.4158/EP13155.GL. [DOI] [PubMed] [Google Scholar]
- 42.Clifton P.M. Dietary treatment for obesity. Nat Clin Pract Gastroenterol Hepatol. 2008;5:672–681. doi: 10.1038/ncpgasthep1283. [DOI] [PubMed] [Google Scholar]
- 43.Brown T., Avenell A., Edmunds L.D., Moore H., Whittaker V., Avery L., et al. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev : Off J Int Assoc Study Obes. 2009;10:627–638. doi: 10.1111/j.1467-789X.2009.00641.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsai A.G., Wadden T.A. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 45.Foster G.D., Wyatt H.R., Hill J.O., Makris A.P., Rosenbaum D.L., Brill C., et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westman E.C., Feinman R.D., Mavropoulos J.C., Vernon M.C., Volek J.S., Wortman J.A., et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 47.Volek J.S., Phinney S.D., Forsythe C.E., Quann E.E., Wood R.J., Puglisi M.J., et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- 48.Tirosh A., Golan R., Harman-Boehm I., Henkin Y., Schwarzfuchs D., Rudich A., et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care. 2013;36:2225–2232. doi: 10.2337/dc12-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meckling K.A., O'Sullivan C., Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metabol. 2004;89:2717–2723. doi: 10.1210/jc.2003-031606. [DOI] [PubMed] [Google Scholar]
- 50.Schwingshackl L., Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulholland Y., Nicokavoura E., Broom J., Rolland C. Very-low-energy diets and morbidity: a systematic review of longer-term evidence. Br J Nutr. 2012;108:832–851. doi: 10.1017/S0007114512001924. [DOI] [PubMed] [Google Scholar]
- 52.Noronha J.C., Nishi S.K., Braunstein C.R., Khan T.A., Blanco Mejia S., Kendall C.W.C., et al. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2019;42:767–776. doi: 10.2337/dc18-2270. [DOI] [PubMed] [Google Scholar]
- 53.Johansson K., Sundstrom J., Marcus C., Hemmingsson E., Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes. 2014;38:279–284. doi: 10.1038/ijo.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bueno N.B., de Melo I.S., de Oliveira S.L., da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178–1187. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 55.Mansoor N., Vinknes K.J., Veierod M.B., Retterstol K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466–479. doi: 10.1017/S0007114515004699. [DOI] [PubMed] [Google Scholar]
- 56.Murphy E.A., Jenkins T.J. A ketogenic diet for reducing obesity and maintaining capacity for physical activity: hype or hope? Curr Opin Clin Nutr Metab Care. 2019;22:314–319. doi: 10.1097/MCO.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 57.Fuehrlein B.S., Rutenberg M.S., Silver J.N., Warren M.W., Theriaque D.W., Duncan G.E., et al. Differential metabolic effects of saturated versus polyunsaturated fats in ketogenic diets. J Clin Endocrinol Metabol. 2004;89:1641–1645. doi: 10.1210/jc.2003-031796. [DOI] [PubMed] [Google Scholar]
- 58.Rosenbaum M., Hall K.D., Guo J., Ravussin E., Mayer L.S., Reitman M.L., et al. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity. 2019;27:971–981. doi: 10.1002/oby.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lutas A., Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sansone M., Sansone A., Borrione P., Romanelli F., Di Luigi L., Sgro P. Effects of ketone bodies on endurance exercise. Curr Sports Med Rep. 2018;17:444–453. doi: 10.1249/JSR.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 61.Weber D.D., Aminzadeh-Gohari S., Tulipan J., Catalano L., Feichtinger R.G., Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metabol. 2019 doi: 10.1016/j.molmet.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirkpatrick C.F., Bolick J.P., Kris-Etherton P.M., Sikand G., Aspry K.E., Soffer D.E., et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin lipidology. 2019;13:689–711 e1. doi: 10.1016/j.jacl.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg I.J., Ibrahim N., Bredefeld C., Foo S., Lim V., Gutman D., et al. Ketogenic diets, not for everyone. J Clin lipidology. 2021;15:61–67. doi: 10.1016/j.jacl.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoo E.G. Sitosterolemia: a review and update of pathophysiology, clinical spectrum, diagnosis, and management. Ann Pediatr Endocrinol Metab. 2016;21:7–14. doi: 10.6065/apem.2016.21.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bays H. Ezetimibe. Expert opinion on investigational drugs. 2002;11:1587–1604. doi: 10.1517/13543784.11.11.1587. [DOI] [PubMed] [Google Scholar]
- 66.Hansel B., Carrie A., Brun-Druc N., Leclert G., Chantepie S., Coiffard A.S., et al. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis. 2014;234:162–168. doi: 10.1016/j.atherosclerosis.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 67.Bays H., Conard S., Leiter L.A., Bird S., Jensen E., Hanson M.E., et al. Are post-treatment low-density lipoprotein subclass pattern analyses potentially misleading? Lipids Health Dis. 2010;9:136. doi: 10.1186/1476-511X-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fito M., Konstantinidou V. Nutritional genomics and the Mediterranean diet's effects on human cardiovascular health. Nutrients. 2016;8:218. doi: 10.3390/nu8040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 70.Kris-Etherton P., Eckel R.H., Howard B.V., St Jeor S., Bazzarre T.L., Nutrition Committee Population Science C., et al. AHA science advisory: Lyon diet heart study. Benefits of a Mediterranean-style, national cholesterol education program/American heart association step I dietary pattern on cardiovascular disease. Circulation. 2001;103:1823–1825. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- 71.Rees K., Takeda A., Martin N., Ellis L., Wijesekara D., Vepa A., et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3:CD009825. doi: 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferro-Luzzi A., Sette S. The Mediterranean Diet: an attempt to define its present and past composition. Eur J Clin Nutr. 1989;43(Suppl 2):13–29. [PubMed] [Google Scholar]
- 73.Gorzynik-Debicka M., Przychodzen P., Cappello F., Kuban-Jankowska A., Marino Gammazza A., Knap N., et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shai I., Schwarzfuchs D., Henkin Y., Shahar D.R., Witkow S., Greenberg I., et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 75.U.S. Department of Health and Human Services . National Heart, Lung, and Blood Institute; Bethesda, MD: 2006. National institutes of health, national heart, Lung, and blood Institute. Your guide to lowering your blood pressure with DASH. NIH publication No. 06-4082. [Google Scholar]
- 76.Appel L.J., Sacks F.M., Carey V.J., Obarzanek E., Swain J.F., Miller E.R., 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA : J Am Med Assoc. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 77.Craig W.J. Health effects of vegan diets. Am J Clin Nutr. 2009;89 doi: 10.3945/ajcn.2009.26736N. 1627S-33S. [DOI] [PubMed] [Google Scholar]
- 78.Dinu M., Abbate R., Gensini G.F., Casini A., Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2016 doi: 10.1080/10408398.2016.1138447. 0. [DOI] [PubMed] [Google Scholar]
- 79.Key T.J., Fraser G.E., Thorogood M., Appleby P.N., Beral V., Reeves G., et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70 doi: 10.1093/ajcn/70.3.516s. 516s-24s. [DOI] [PubMed] [Google Scholar]
- 80.Kim H., Caulfield L.E., Rebholz C.M. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr. 2018;148:624–631. doi: 10.1093/jn/nxy019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tharrey M., Mariotti F., Mashchak A., Barbillon P., Delattre M., Fraser G.E. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47:1603–1612. doi: 10.1093/ije/dyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kahleova H., Levin S., Barnard N. Cardio-metabolic benefits of plant-based diets. Nutrients. 2017;9 doi: 10.3390/nu9080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang R.Y., Huang C.C., Hu F.B., Chavarro J.E. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J Gen Intern Med. 2016;31:109–116. doi: 10.1007/s11606-015-3390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borude S. Which is a Good diet-veg or non-veg? Faith-based vegetarianism for protection from obesity-a Myth or actuality? Obes Surg. 2019 doi: 10.1007/s11695-018-03658-7. [DOI] [PubMed] [Google Scholar]
- 85.Lara K.M., Levitan E.B., Gutierrez O.M., Shikany J.M., Safford M.M., Judd S.E., et al. Dietary patterns and incident heart failure in U.S. Adults without known coronary disease. J Am Coll Cardiol. 2019;73:2036–2045. doi: 10.1016/j.jacc.2019.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Satija A., Bhupathiraju S.N., Spiegelman D., Chiuve S.E., Manson J.E., Willett W., et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. Adults. J Am Coll Cardiol. 2017;70:411–422. doi: 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia Maldonado E., Gallego-Narbon A., Vaquero Mf. [Are vegetarian diets nutritionally adequate? A revision of the scientific evidence] Nutr Hosp. 2019;36:950–961. doi: 10.20960/nh.02550. [DOI] [PubMed] [Google Scholar]
- 88.Gupta R.K., Gangoliya S.S., Singh N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52:676–684. doi: 10.1007/s13197-013-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Expert Panel on Detection E Treatment of high blood cholesterol in A. Executive summary of the third report of the national cholesterol education program (NCEP) expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III) JAMA : J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 90.U.S. Department Of Health And Human Services . National Heart, Lung, and Blood Institute; Bethesda, MD: 2005. National institutes of health, national heart, Lung, and blood Institute. Your guide to lowering your cholesterol with TLC. NIH Publication No. 06–5235. [Google Scholar]
- 91.Scrutinio D. The potential of lifestyle changes for improving the clinical outcome of patients with coronary heart disease: mechanisms of benefit and clinical results. Rev Recent Clin Trials. 2010;5:1–13. doi: 10.2174/157488710790820508. [DOI] [PubMed] [Google Scholar]
- 92.Ornish D., Brown S.E., Scherwitz L.W., Billings J.H., Armstrong W.T., Ports T.A., et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 93.Gardner C.D., Kiazand A., Alhassan S., Kim S., Stafford R.S., Balise R.R., et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA : J Am Med Assoc. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 94.Ornish D., Scherwitz L.W., Billings J.H., Brown S.E., Gould K.L., Merritt T.A., et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA : J Am Med Assoc. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 95.Manheimer E.W., van Zuuren E.J., Fedorowicz Z., Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015;102:922–932. doi: 10.3945/ajcn.115.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jonsson T., Granfeldt Y., Ahren B., Branell U.C., Palsson G., Hansson A., et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jonsson T., Granfeldt Y., Lindeberg S., Hallberg A.C. Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes. Nutr J. 2013;12:105. doi: 10.1186/1475-2891-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabel K., Hoddy K.K., Haggerty N., Song J., Kroeger C.M., Trepanowski J.F., et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4:345–353. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris L., Hamilton S., Azevedo L.B., Olajide J., De Brun C., Waller G., et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16:507–547. doi: 10.11124/JBISRIR-2016-003248. [DOI] [PubMed] [Google Scholar]
- 100.Asif S., Morrow N.M., Mulvihill E.E., Kim K.H. Understanding dietary intervention-mediated epigenetic modifications in metabolic diseases. Front Genet. 2020;11:590369. doi: 10.3389/fgene.2020.590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Golbidi S., Daiber A., Korac B., Li H., Essop M.F., Laher I. Health benefits of fasting and caloric restriction. Curr Diabetes Rep. 2017;17:123. doi: 10.1007/s11892-017-0951-7. [DOI] [PubMed] [Google Scholar]
- 102.Kerndt P.R., Naughton J.L., Driscoll C.E., Loxterkamp D.A. Fasting: the history, pathophysiology and complications. West J Med. 1982;137:379–399. [PMC free article] [PubMed] [Google Scholar]
- 103.Lessan N., Ali T. Energy metabolism and intermittent fasting: the Ramadan perspective. Nutrients. 2019;11 doi: 10.3390/nu11051192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017;76:361–368. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- 105.Hutchison A.T., Liu B., Wood R.E., Vincent A.D., Thompson C.H., O'Callaghan N.J., et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity. 2019;27:50–58. doi: 10.1002/oby.22345. [DOI] [PubMed] [Google Scholar]
- 106.Gabel K., Kroeger C.M., Trepanowski J.F., Hoddy K.K., Cienfuegos S., Kalam F., et al. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity. 2019;27:1443–1450. doi: 10.1002/oby.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yildiran H., Mercanligil S.M. Does increasing meal frequency improve weight loss and some biochemical parameters in overweight/obese females? Nutr Hosp. 2019;36:66–72. doi: 10.20960/nh.2191. [DOI] [PubMed] [Google Scholar]
- 108.Malinowski B., Zalewska K., Wesierska A., Sokolowska M.M., Socha M., Liczner G., et al. Intermittent fasting in cardiovascular disorders-an overview. Nutrients. 2019;11 doi: 10.3390/nu11030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stice E., Davis K., Miller N.P., Marti C.N. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. 2008;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.World Health Organization Physical activity. https://www.who.int/news-room/fact-sheets/detail/physical-activity
- 111.Bays H.E., Taub P.R., Epstein E., Michos E.D., Ferraro R.A., Bailey A.L., et al. Ten things to know about ten cardiovascular disease risk factors. Am J Prev Cardiol. 2021;5:100149. doi: 10.1016/j.ajpc.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlson S.A., Fulton J.E., Schoenborn C.A., Loustalot F. Trend and prevalence estimates based on the 2008 physical activity guidelines for Americans. Am J Prev Med. 2010;39:305–313. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 114.Clark J.E. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18-65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord. 2015;14:31. doi: 10.1186/s40200-015-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Byrne N.M., Hills A.P., Hunter G.R., Weinsier R.L., Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol. 2005;99:1112–1119. doi: 10.1152/japplphysiol.00023.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 116.Barclay C.J. The basis of differences in thermodynamic efficiency among skeletal muscles. Clin Exp Pharmacol Physiol. 2017;44:1279–1286. doi: 10.1111/1440-1681.12850. [DOI] [PubMed] [Google Scholar]
- 117.Rosenbaum M., Heaner M., Goldsmith R.L., Christian Schulze P., Shukla A., Shen W., et al. Resistance training reduces skeletal muscle work efficiency in weight-reduced and non-weight-reduced subjects. Obesity. 2018;26:1576–1583. doi: 10.1002/oby.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung N., Park M.Y., Kim J., Park H.Y., Hwang H., Lee C.H., et al. Non-exercise activity thermogenesis (NEAT): a component of total daily energy expenditure. J Exerc Nutr Biochem. 2018;22:23–30. doi: 10.20463/jenb.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hamasaki H., Yanai H., Mishima S., Mineyama T., Yamamoto-Honda R., Kakei M., et al. Correlations of non-exercise activity thermogenesis to metabolic parameters in Japanese patients with type 2 diabetes. Diabetol Metab Syndrome. 2013;5:26. doi: 10.1186/1758-5996-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alessio N., Squillaro T., Monda V., Peluso G., Monda M., Melone M.A., et al. Circulating factors present in the sera of naturally skinny people may influence cell commitment and adipocyte differentiation of mesenchymal stromal cells. World J Stem Cell. 2019;11:180–195. doi: 10.4252/wjsc.v11.i3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hajna S., Ross N.A., Dasgupta K. Steps, moderate-to-vigorous physical activity, and cardiometabolic profiles. Prev Med. 2017 doi: 10.1016/j.ypmed.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Howell S., Kones R. Calories in, calories out" and macronutrient intake: the hope, hype, and science of calories. Am J Physiol Endocrinol Metab. 2017;313:E608–E612. doi: 10.1152/ajpendo.00156.2017. [DOI] [PubMed] [Google Scholar]
- 123.Hall K.D. What is the required energy deficit per unit weight loss? Int J Obes. 2008;32:573–576. doi: 10.1038/sj.ijo.0803720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., et al. The physical activity guidelines for Americans. JAMA : J Am Med Assoc. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: the evidence. CMAJ (Can Med Assoc J) : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stanford K.I., Middelbeek R.J., Goodyear L.J. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeremic N., Chaturvedi P., Tyagi S.C. Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol. 2017;232:61–68. doi: 10.1002/jcp.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomez-Pinilla F., Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fletcher G.F., Landolfo C., Niebauer J., Ozemek C., Arena R., Lavie C.J. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72:1622–1639. doi: 10.1016/j.jacc.2018.08.2141. [DOI] [PubMed] [Google Scholar]
- 130.Rezende L.F.M., Sa T.H., Markozannes G., Rey-Lopez J.P., Lee I.M., Tsilidis K.K., et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826–833. doi: 10.1136/bjsports-2017-098391. [DOI] [PubMed] [Google Scholar]
- 131.Luan X., Tian X., Zhang H., Huang R., Li N., Chen P., et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019;8:422–441. doi: 10.1016/j.jshs.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Childs E., de Wit H. Regular exercise is associated with emotional resilience to acute stress in healthy adults. Front Physiol. 2014;5:161. doi: 10.3389/fphys.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jakicic J.M., Davis K.K. vol. 34. The Psychiatric clinics of North America; 2011. pp. 829–840. (Obesity and physical activity). [DOI] [PubMed] [Google Scholar]
- 134.Meriwether R.A., Lee J.A., Lafleur A.S., Wiseman P. Physical activity counseling. Am Fam Physician. 2008;77:1129–1136. [PubMed] [Google Scholar]
- 135.Vincent H.K., Raiser S.N., Vincent K.R. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev. 2012;11:361–373. doi: 10.1016/j.arr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parr E.B., Coffey V.G., Hawley J.A. Sarcobesity': a metabolic conundrum. Maturitas. 2013;74:109–113. doi: 10.1016/j.maturitas.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 137.Garland T., Jr., Schutz H., Chappell M.A., Keeney B.K., Meek T.H., Copes L.E., et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ng S.W., Popkin B.M. Time use and physical activity: a shift away from movement across the globe. Obes Rev : Off J Int Assoc Study Obes. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bushman B.A. Determining the I (Intensity) for a FITT-VP aerobic exercise prescription. ACSM's Health & Fit J. 2014;18:4–7. [Google Scholar]
- 140.Zaleski A.L., Taylor B.A., Panza G.A., Wu Y., Pescatello L.S., Thompson P.D., et al. Coming of age: considerations in the prescription of exercise for older adults. Methodist Debakey Cardiovasc J. 2016;12:98–104. doi: 10.14797/mdcj-12-2-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lakoski S.G., Barlow C.E., Farrell S.W., Berry J.D., Morrow J.R., Jr., Haskell W.L. Impact of body mass index, physical activity, and other clinical factors on cardiorespiratory fitness (from the Cooper Center longitudinal study) Am J Cardiol. 2011;108:34–39. doi: 10.1016/j.amjcard.2011.02.338. [DOI] [PubMed] [Google Scholar]
- 142.Jette M., Sidney K., Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 143.Van Camp C.M., Hayes L.B. Assessing and increasing physical activity. J Appl Behav Anal. 2012;45:871–875. doi: 10.1901/jaba.2012.45-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Butte N.F., Ekelund U., Westerterp K.R. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc. 2012;44:S5–S12. doi: 10.1249/MSS.0b013e3182399c0e. [DOI] [PubMed] [Google Scholar]
- 145.Evenson K.R., Goto M.M., Furberg R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Activ. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mercer K., Li M., Giangregorio L., Burns C., Grindrod K. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR Mhealth Uhealth. 2016;4:e40. doi: 10.2196/mhealth.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allen L.N., Christie G.P. The emergence of personalized health technology. J Med Internet Res. 2016;18:e99. doi: 10.2196/jmir.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brickwood K.J., Watson G., O'Brien J., Williams A.D. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.