Abstract
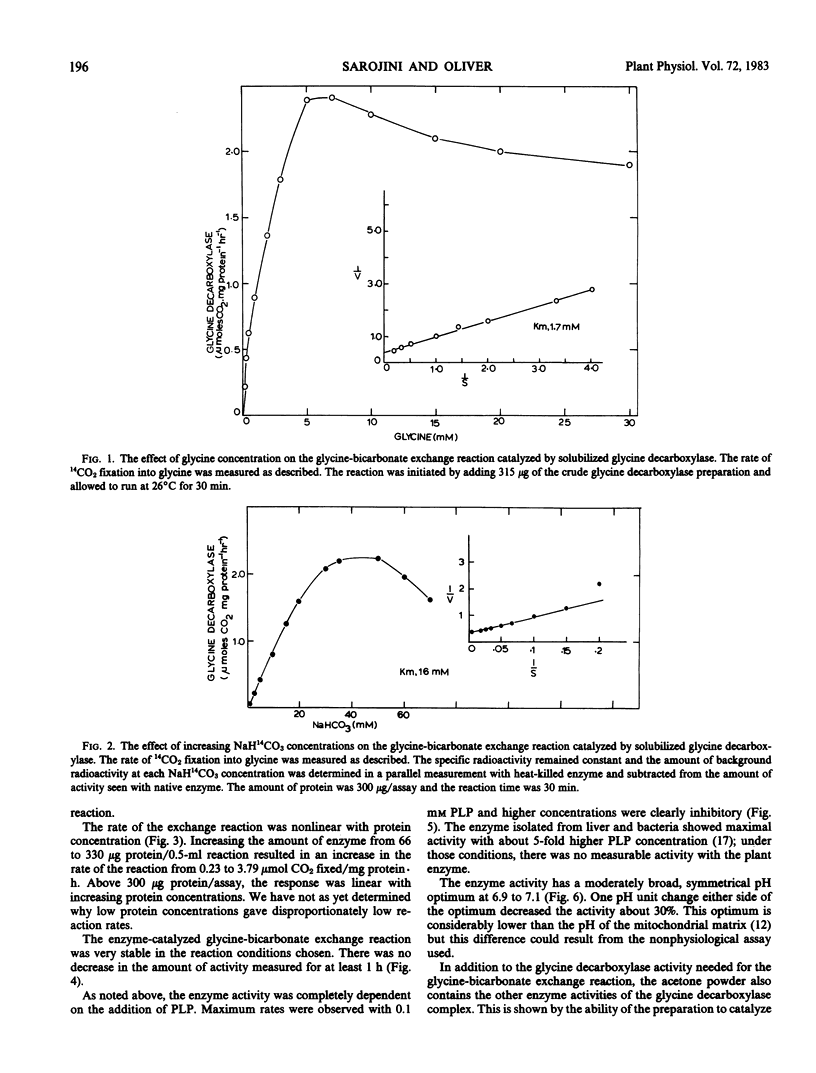
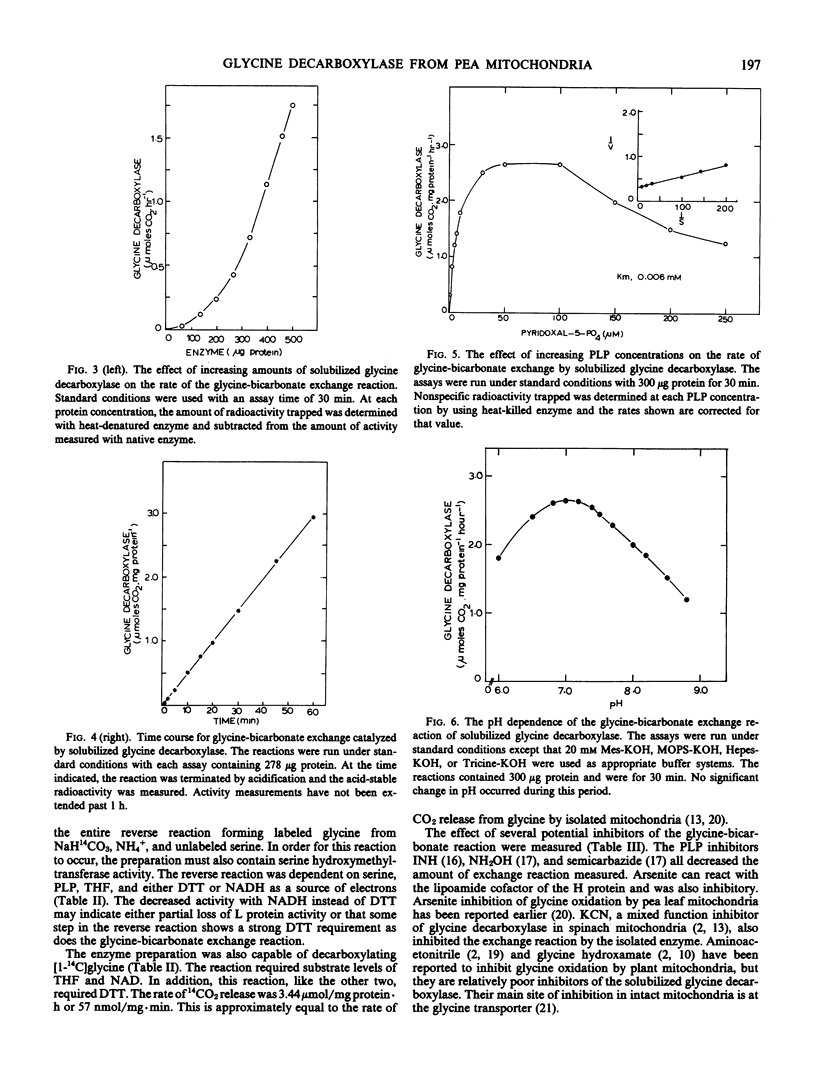
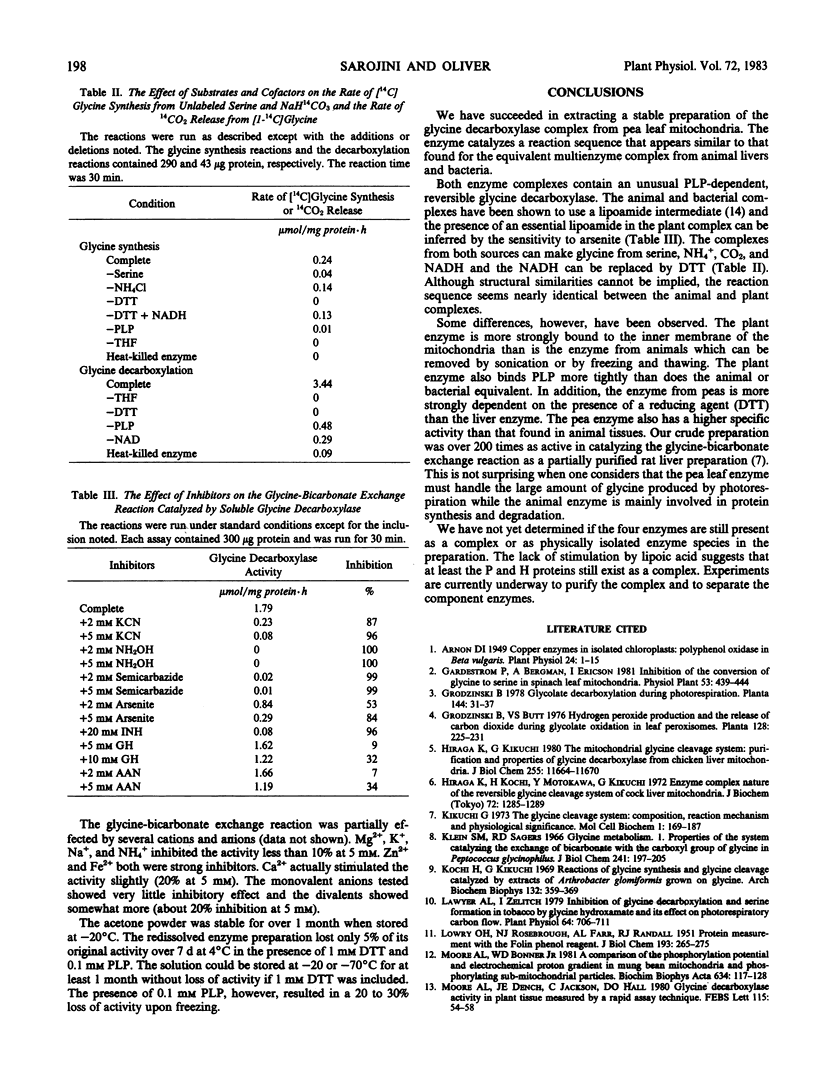
Glycine decarboxylase has been successfully solubilized from pea (Pisum sativum) leaf mitochondria as an acetone powder. The enzyme was dependent on added dithiothreitol and pyridoxal phosphate for maximal activity. The enzyme preparation could catalyze the exchange of CO2 into the carboxyl carbon of glycine, the reverse of the glycine decarboxylase reaction by converting serine, NH4+, and CO2 into glycine, and 14CO2 release from [1-14C]glycine. The half-maximal concentrations for the glycine-bicarbonate exchange reaction were 1.7 millimolar glycine, 16 millimolar NaH14CO2, and 0.006 millimolar pyridoxal phosphate. The enzyme (glycine-bicarbonate exchange reaction) was active in the assay conditions for 1 hour and could be stored for over 1 month. The enzymic mechanism appeared similar to that reported for the enzyme from animals and bacteria but some quantitative differences were noted. These included the tenacity of binding to the mitochondrial membrane, the concentration of pyridoxal phosphate needed for maximum activity, the requirement for dithiothreitol for maximum activity, and the total amount of activity present. Now that this enzyme has been solubilized, a more detailed understanding of this important step in photorespiration should be possible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D., Jr A comparison of the phosphorylation potential and electrochemical proton gradient in mung bean mitochondria and phosphorylating sub-mitochondrial particles. Biochim Biophys Acta. 1981 Jan 14;634(1):117–128. doi: 10.1016/0005-2728(81)90132-8. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Dench J. E., Jackson C., Hall D. O. Glycine decarboxylase activity in plant tissues measured by a rapid assay technique. FEBS Lett. 1980 Jun 16;115(1):54–58. doi: 10.1016/0014-5793(80)80725-3. [DOI] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism in rat liver mitochondria. V. Intramitochondrial localization of the reversible glycine cleavage system and serine hydroxymethyltransferase. Arch Biochem Biophys. 1971 Oct;146(2):461–464. doi: 10.1016/0003-9861(71)90149-4. [DOI] [PubMed] [Google Scholar]
- Sato T., Kochi H., Motokawa Y., Kawasaki H., Kikuchi G. Glycin metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J Biochem. 1969 Jan;65(1):63–70. [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Oliver D. J., Sarojini G. Simultaneous oxidation of glycine and malate by pea leaf mitochondria. Plant Physiol. 1982 Nov;70(5):1465–1469. doi: 10.1104/pp.70.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Sarojini G., Oliver D. J. Identification of a glycine transporter from pea leaf mitochondria. Biochem Biophys Res Commun. 1982 Aug;107(3):856–861. doi: 10.1016/0006-291x(82)90601-5. [DOI] [PubMed] [Google Scholar]


