Abstract
Compartmental analysis of 35SO42− exchange kinetics is used to obtain SO42− fluxes and compartment contents in carrot (Daucus carota L.) storage root cells, where 2 to 5% of the SO42− taken up is reduced to organic form. The necessary curve fitting is verified by (a) consistency between `content versus time' and `rate versus time' plots of washout data; (b) agreement between loading and washout kinetics; and (c) correct identification of the fastest exchange phase as being from extracellular spaces.
Sulfate is actively transported up an electrochemical potential gradient at both plasmalemma and tonoplast. The plasmalemma influx is from 2 to 10 times higher than the tonoplast influx, is much greater than the SO42− reduction rate, and would not limit the rate of either. This is consistent with the finding that the plasmalemma influx is not regulated by internal SO42− or cysteine (Cram 1982 Plant Sci Lett, in press).
Both SO42− influxes rise with only limited saturation as the external SO42− concentration increases up to 50 millimolarity. Both effluxes appear to be passive, with extensive recycling in the plasmalemma influx pump. SO42− permeability is about 10−11 meter per second at both membranes.
The high, nonlimiting fluxes of SO42− at the plasmalemma relative to the tonoplast (found also in Lemna; Thoiron, Thoiron, Demarty, Thellier 1981 Biochim Biophys Acta 644: 24-35) contrasts with SO42− fluxes in bacteria and with Cl− fluxes in plant cells. Their implications for work on characteristics and regulation of SO42− uptake in roots and tissue cultures are discussed.
Full text
PDF
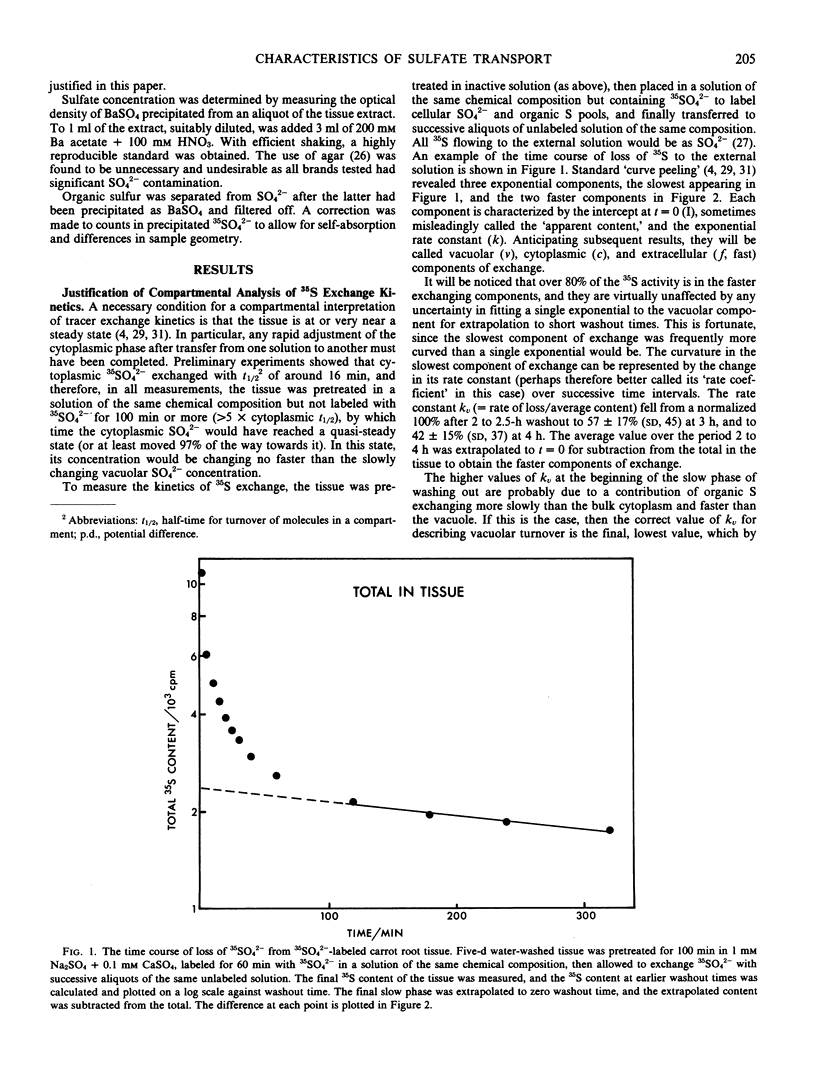
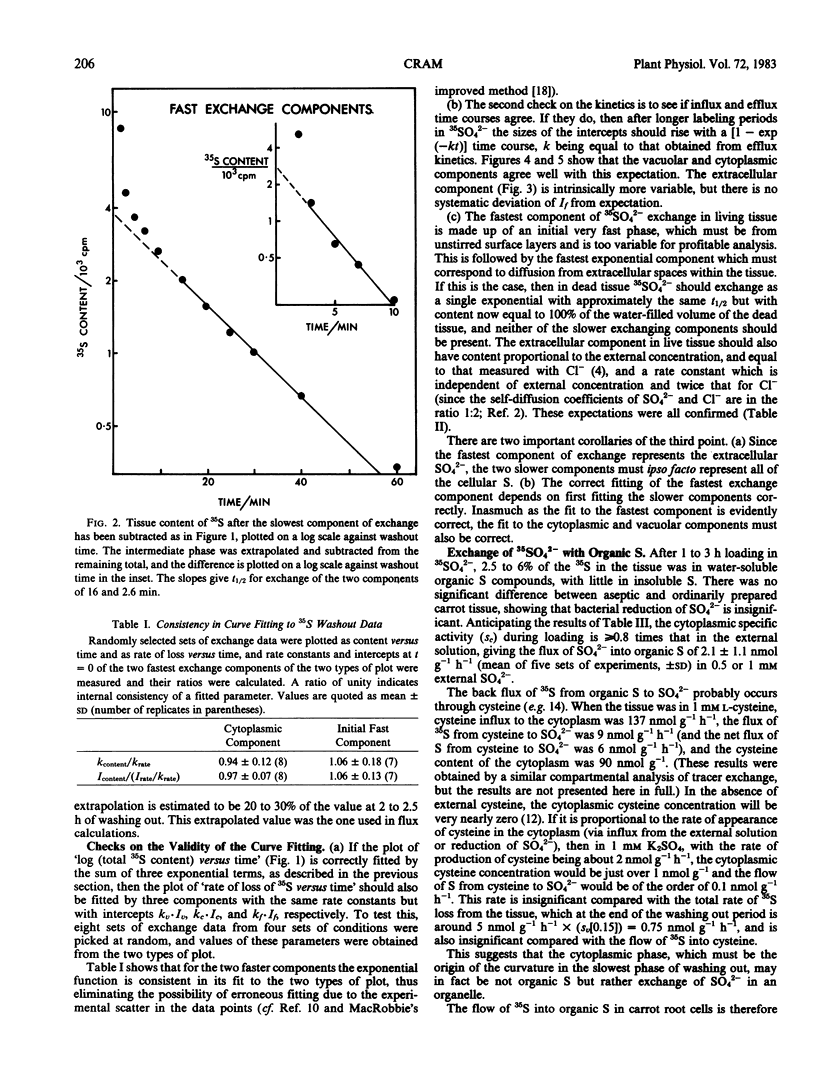
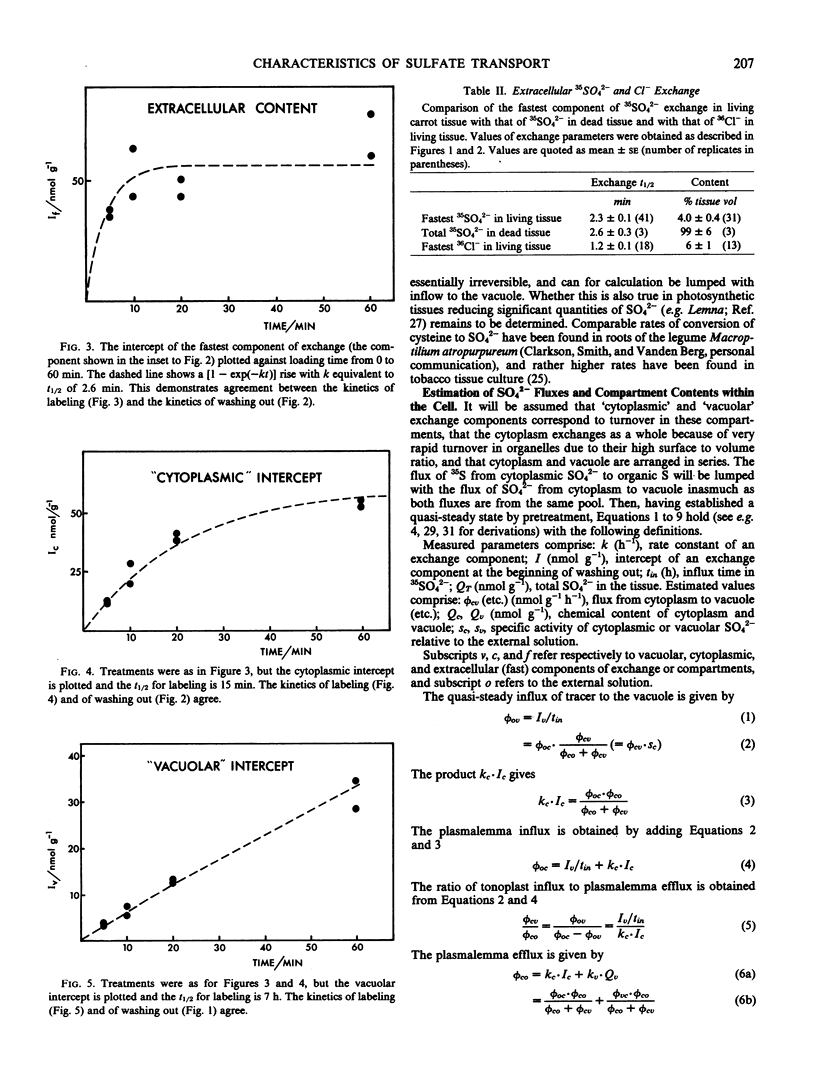




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L. Accumulation of phosphate, sulfate and sucrose by excised Phloem tissues. Plant Physiol. 1966 Mar;41(3):447–454. doi: 10.1104/pp.41.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram W. J. Compartmentation and exchange of chloride in carrot root tissue. Biochim Biophys Acta. 1968 Nov 5;163(3):339–353. doi: 10.1016/0005-2736(68)90119-3. [DOI] [PubMed] [Google Scholar]
- Cram W. J. Short term influx as a measure of influx across the plasmalemma. Plant Physiol. 1969 Jul;44(7):1013–1015. doi: 10.1104/pp.44.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Hart J. W., Filner P. Regulation of sulfate uptake by amino acids in cultured tobacco cells. Plant Physiol. 1969 Sep;44(9):1253–1259. doi: 10.1104/pp.44.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Compartmentation of Sulfur Metabolites in Tobacco Cells : USE OF EFFLUX ANALYSIS. Plant Physiol. 1981 Oct;68(4):937–940. doi: 10.1104/pp.68.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Regulation of Sulfate Assimilation in Tobacco Cells: EFFECT OF NITROGEN AND SULFUR NUTRITION ON SULFATE PERMEASE AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1980 Nov;66(5):877–883. doi: 10.1104/pp.66.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoiron A., Thoiron B., Demarty M., Thellier M. Compartmental analysis of sulphate transport in Lemna minor L., taking plant growth and sulphate metabolization into consideration. Biochim Biophys Acta. 1981 Jun 9;644(1):24–35. doi: 10.1016/0005-2736(81)90054-7. [DOI] [PubMed] [Google Scholar]


