Abstract
Objective
Binge eating disorder (BED) is the most common eating disorder, and yet only one pharmacotherapy (lisdexamfetamine), which has known abuse-potential, is FDA-approved. Topiramate is also commonly prescribed off-label for binge eating but has many contraindications. In contrast, the glucagon-like peptide-1 (GLP1) analog semaglutide has profound effects on central satiety signaling leading to reduced food intake, and has been approved for the treatment of obesity based on its efficacy and safety profile. Semaglutide would thus seem to be a potential candidate for the treatment of BED.
Methods
This open-label study examined the effects of semaglutide on Binge Eating Scale (BES) scores in individuals with BED. Patients were divided into three groups: those prescribed semaglutide, those prescribed either lisdexamphetamine or topiramate, and those prescribed a combination of semaglutide with lisdexamphetamine or topiramate.
Results
Patients receiving semaglutide only exhibited greater reductions in BES scores compared to the other groups. Combined pharmacotherapy with both semaglutide and the other anti-obesity medications did not result in greater reductions in BES scores compared to the semaglutide-only group. Findings were similar in patients with moderate/severe BED, as well as the full sample.
Conclusion
The therapeutic effects of semaglutide in binge eating disorder warrant further investigation.
Keywords: Binge eating disorder, GLP-1 agonist, Semaglutide
1. Introduction
Binge Eating Disorder (BED) is the most common of eating disorders, with a lifetime prevalence of approximately 2.8% [1], and approximately 36% comorbidity with obesity [2]. Clinically, BED presents as a lack of control during binge episodes characterized by time-limited hyperphagia during which thousands of calories can be consumed [3]. These binge episodes are often psychologically disturbing, with patients experiencing significant embarrassment, guilt, or disgust associated with the episode. [4].
Lisdexamfetamine (Vyvanse®, Takeda), is the only FDA-approved pharmacotherapy for BED. It is a prodrug that is converted in the body to dextroamphetamine, causing the reverse transport of dopamine and noradrenaline into the synaptic cleft, thereby potentiating postsynaptic firing of catecholaminergic neurons [5,6]. Through mechanisms that are not fully understood, this results in a reduction in appetite. In addition to lisdexamfetamine, other medications are often prescribed for BED. Most notably, the antiepileptic topiramate (Topamax®, Janssen), has also been shown to be effective in randomized double-blind placebo controlled clinical trials, and is commonly prescribed off-label for binge eating [7], although it has many known contraindications including fatigue, cognitive impairment, metabolic acidosis, and interactions with other medications or alcohol. Because of their effects on appetite, both lisdexamfetamine and topiramate are commonly prescribed as anti-obesity medications [8].
In the past decade, glucagon-like peptide-1 (GLP-1) modulators have garnered attention for their efficacy in ameliorating type 2 diabetes and effectiveness as weight loss medications. In particular, semaglutide is an effective and FDA-approved treatment for obesity (Ozempic®, Novo Nordisk) and weight reduction (Wegovy®, Novo Nordisk). Delivered once weekly via subcutaneous injection, semaglutide alters gastric and hepatic function [9], but its most profound effects are on central satiety signaling. GLP-1 receptors are found in key appetite-regulating regions of the central nervous system, and pharmacological treatment with GLP-1 analogues modulates the downstream release of hunger and reward-related neurotransmitters in the hypothalamus and striatum in rodents [10]. Intraperitoneal administration of GLP-1 reduces hedonic feeding in a mouse-model of BED [10,11]. Likewise, in humans, treatment with semaglutide is associated with a significant reduction in emotional eating, which is a known contributor to BED [12].
Based on the extant pre-clinical and human data, Semaglutide would thus seem to be an excellent candidate for treating BED. As a first step in assessing this hypothesis, the present open-label study examined the effects of semaglutide on Binge Eating Scale (BES) scores in patients with moderate to severe BED. To contextualize the effects observed with this new pharmacological treatment, we compared changes in BES scores in the semaglutide treated group to a group of matched BED patients prescribed either lisdexamphetamine or topiramate, as well as a group that received both semaglutide and other anti-obesity medications.
2. Methods
A retrospective chart review identified 98 patients attending an obesity medicine and bariatric surgery clinic from June 2021 to December 2022. The study was inclusive of women and minorities. Forty-eight patients were identified as likely having moderate to severe BED as defined by intake visit with the Binge Eating Scale (BES), a validated psychiatric instrument [13]. The BES is a validated 16-item questionnaire that assesses binge eating severity, with scores > 16 indicating at least “moderate” binge eating symptomatology, and scores >26 indicating “severe” binge eating.
There were three groups of participants: patients receiving semaglutide only (n = 19); patients receiving semaglutide plus another anti-obesity medication (either topiramate or lisdexamphetamine) (n=13); or patients receiving the alternative anti-obesity medications (AOM) but not receiving semaglutide (n=16). Patients on other types of GLP-1 agonist medications were excluded from the analysis. The following data were collected via chart review: patient age, gender, medical history, medications, doses of medications, dates of initiation of medications, the amount of weight loss at 30, 90, and 180 days after treatment, and the starting and ending weight, BMI, and BES score with date and individual answers. The above information was also collected for patients with probable mild/minimal BED (BES scores <17), with their results included in supplemental figures. The retrospective study design was approved by the institutional review board of the University of Oklahoma Center for Health Sciences.
Based on our interest in identifying the impact of semaglutide on moderate to severe BED, a one-way analysis of covariance (ANCOVA) was initially conducted in the subsample of patients with a moderate or severe initial BES score (n=48). The independent variable had three levels: patients treated with semaglutide (SEMA_ONLY), a combination of semaglutide and other anti-obesity medications (SEMA + OAOM), and other anti-obesity medications only (OAOM). The dependent variable was mean change in BES score between baseline and follow-up. Covariates included the patient's self-reported gender, initial BES score, and time in days between baseline and follow-up. The ANCOVA was repeated for the full sample of patients that included those with a low initial BES score (N=98). Tukey's HSD post hoc analyses were used to determine which groups differed significantly. All analyses were conducted in R [14] using the car [15] and multcomp [16] packages.
3. Results
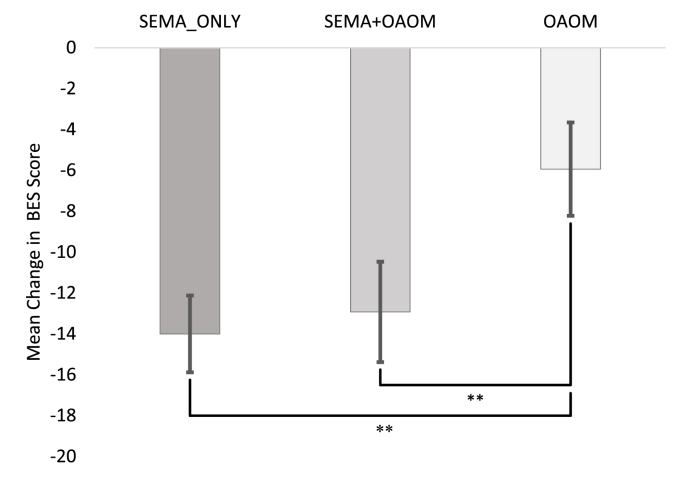
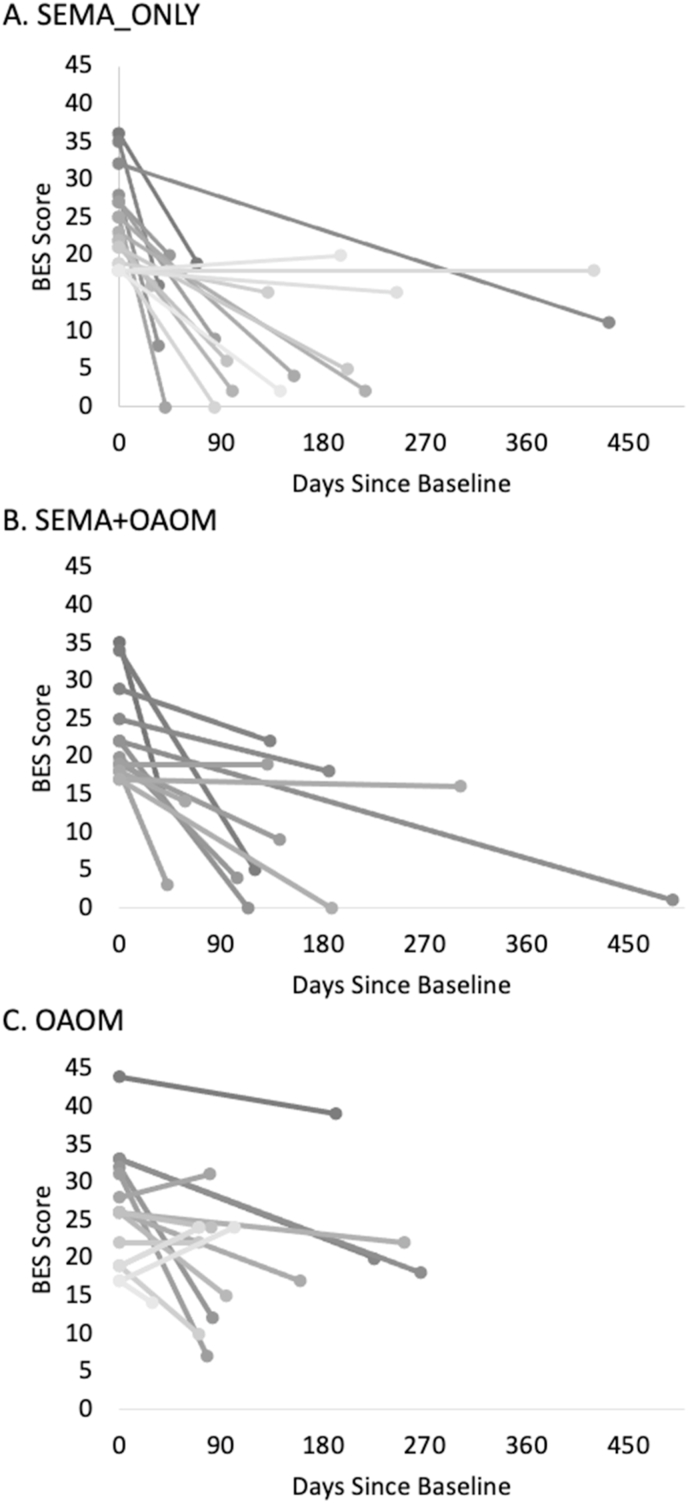
Descriptive statistics are presented in Table 1 for the subsample of participants with moderate/severe BES score subsample (see Supplemental Table S1 for the full sample). In the moderate/severe BES score subsample, the ANCOVA revealed a significant effect of treatment type on BES: F(2,42)=8.02, p<.01. Tukey's HSD post hoc testing revealed a significant difference in the mean change in BES score between the SEMA_ONLY group and the OAOM group (p<.01) as well as between the SEMA + OAOM group and the OAOM group (p<.01) (see Fig. 1). The mean change in BES score between the SEMA_ONLY group and SEMA_OAOM group was not significantly different. On average, BES scores decreased by 14 points in the SEMA_ONLY group, 12.9 points in the SEMA + OAOM group, and 5.9 points in the OAOM group. Fig. 2A–C shows each patient's change in BES score from baseline to follow-up for each treatment group.
Table 1.
Moderate/severe initial BES score patient characteristics.
| SEMA Only | SEMA + OAOM | OAOM | |
|---|---|---|---|
| n | 19 | 13 | 16 |
| Female | 17 | 10 | 14 |
| Race/Ethnicity | White 15 Black 3 American Indian 1 | White 5 American Indian 3 | White 9 Black 1 American Indian 1 Multiracial 1 |
| Age (yrs) M [SD, range] | 43.5 [13.6, 22-74] | 43.1 [9.8, 32-64] | 39.6 [12.8, 21-67] |
| Initial weight (lbs) M [SD, range] | 257.6 [58.1, 162.4-360.6] | 327.1 [127.6, 209.9-606.4] | 268.4 [57.7, 179.5-358.2] |
| Total body weight loss (lbs) M [SD, range] | 22.5 [14.3, 6.4-52.6] | 53 [64.2, 1.3-224.9] | 13.2 [20.1, -23.54-67.1] |
| Baseline BES Score M [SD, range] | 23.89 [5.7, 18-36] | 22.7 [6.3, 17-35] | 26.1 [7.4, 17-44] |
| Average Change in BES Score M [SD, range] | 14 [8.2, -2-25] | 12.9 [8.9, 0-29] | 5.9 [9.1, -7-24] |
| Prescribed Vyvanse | 0 | 2 | 1 |
| Prescribed Topiramate | 0 | 13 | 12 |
| Prescribed both Vyvanse and Topiramate | 0 | 2 | 1 |
Abbreviations: M=mean; SD=standard deviation.
Fig. 1.
Mean change in BES score for patients with moderate or severe initial BES scores treated with semaglutide only (SEMA_ONLY, n = 19), a combination of semaglutide and other anti-obesity medications (SEMA + OAOM, n = 13), and other anti-obesity medications only (OAOM, n = 16). n = 48. ∗∗p <.01. Error bars = standard error of the mean.
Fig. 2.
Change in BES score from baseline to follow-up for patients with moderate or severe initial BES scores. (A) Patients treated with semaglutide only (SEMA_ONLY), (B) patients treated with a combination of semaglutide and other anti-obesity medications (SEMA + OAOM), (C) and patients treated with other anti-obesity medications (OAOM).
The second ANCOVA conducted using the full sample of patients showed findings that mirrored those observed in the subsample of moderate/severe BED patients, with a significant effect of treatment type on BES score: F(2,92)=10.1, p<.001. Similarly, Tukey's HSD post hoc analysis showed a significant difference in mean change in BES score between the SEMA_ONLY group and the OAOM group (p<.001) as well as a significant difference in mean change in BES score between the SEMA + OAOM group and the OAOM group (p<.001, see Supplemental Fig. S1). The mean change in BES score between the SEMA_ONLY group and SEMA + OAOM was not significantly different. On average, BES scores decreased by 7.9 points in the full sample of SEMA_ONLY patients, 8.8 points in the full sample of SEMA + OAOM patients, and 4.8 points in the full sample of OAOM patients. Individual changes in BES scores from baseline to follow-up are shown in Supplemental Figs. S2A–S2C.
4. Discussion
The present open-label retrospective cohort study evaluated the effects of semaglutide on binge eating symptoms in patients with moderate to severe levels of binge eating. Treatment with semaglutide resulted in a significantly greater reduction in Binge Eating Scale (BES) scores compared to compared to those receiving lisdexamfetamine and topiramate, two common anti-obesity medications used to treat BED. Combined pharmacotherapy with both semaglutide and other anti-obesity medications did not result in greater reductions in BES scores compared to semaglutide-only group.
These findings are consistent with previous studies showing that GLP-1 agonists, including semaglutide, have an effect on central satiety signaling, which may play a role in reducing binge eating symptoms. [17] A study by Da Porto and colleagues [18] found that treatment with the GLP-1 analogue dulaglutide was associated with a greater reduction in BES scores relative to gliclazide, a sulfonylurea compound that increases insulin but is not known to affect brain reward circuitry or appetite. Additionally a short term 3 month pilot study of liraglutide by Robert et al. has shown promise in reducing BED symptoms [19]. The present findings thus make the important contribution of providing the first evidence in humans that GLP-1 analogue Semaglutide may be effective in reducing binge eating symptomatology, and possibly more effective than other commonly prescribed medications known to alter reward neurocircuitry and appetitive behaviors.
The results of this study have important implications for future neuroscientific studies on the mechanism of action of GLP-1 agonists in the treatment of BED. Neurons with GLP-1 receptors are found throughout the mesolimbic dopamine pathway and hypothalamic sub-nuclei [20], and extensive preclinical research demonstrates that GLP-1 analogues such as semaglutide have their effects on eating behavior primarily through modulation of these central nervous system targets [21]. We expect that the reductions in binge eating symptomatology observed here likely arise from semaglutide's effects on these CNS circuits, but further research is needed to determine the precise mechanism by which GLP-1 agonists reduce binge eating symptoms. Future studies should investigate the optimal dosing and duration of treatment with semaglutide, as well as potential interactions with other medications commonly prescribed for BED and/or obesity.
5. Conclusion
BED is a common psychiatric condition that can affect cardiovascular mortality of patients with obesity, with limited current treatment options. The findings from this study provide a rationale for future randomized clinical trials to assess the efficacy and safety of semaglutide in the treatment of BED. Overall, the present study adds to the growing body of evidence supporting the use of GLP-1 agonists, including semaglutide, in the treatment of BED. Semaglutide may be a promising pharmacological treatment option for patients with moderate to severe BED, and further research is needed to fully elucidate the mechanisms underlying its therapeutic effects.
Funding
There were no sources of funding for this manuscript.
Declaration of competing interest
Dr Richards is on Speaker Bureau for Rhythm Pharmaceuticals and Novo Nordisk and is on Advisory Board for Rhythm Pharmaceuticals. The other authors report no disclosures to declare.
Acknowledgements
The authors would like to thank the participants for contributing to this study. Individual deidentified participant data is available upon request and implementation of the appropriate institutional agreements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2023.100080.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hudson J.I., Hiripi E., Pope H.G., Jr., Kessler R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatr. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R.C., Berglund P.A., Chiu W.T., Deitz A.C., Hudson J.I.…Xavier M. The prevalence and correlates of binge eating disorder in the world Health organization world mental Health surveys. Biol Psychiatr. 2013;73(9):904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giel K.E., Bulik C.M., Fernandez-Aranda F., Hay P., Keski-Rahkonen A., Schag K.…Zipfel S. Binge eating disorder. Nat Rev Dis Prim. 2022;8(1):16. doi: 10.1038/s41572-022-00344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . fifth ed. 2013. Diagnostic and statistical manual of mental disorders. [DOI] [Google Scholar]
- 5.Bello N.T., Yeomans B.L. Safety of pharmacotherapy options for bulimia nervosa and binge eating disorder. Expet Opin Drug Saf. 2018;17(1):17–23. doi: 10.1080/14740338.2018.1395854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths K.R., Yang J., Touyz S.W., Hay P.J., Clarke S.D., Korgaonkar M.S.…Kohn M.R. Understanding the neural mechanisms of lisdexamfetamine dimesylate (LDX) pharmacotherapy in Binge Eating Disorder (BED): a study protocol. Journal of Eating Disorders. 2019;7(1):1–10. doi: 10.1186/s40337-019-0253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElroy, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatr. 2003;160(2):255–261. doi: 10.1176/appi.ajp.160.2.255. [DOI] [PubMed] [Google Scholar]
- 8.Brownley K.A., Berkman N.D., Peat C.M., Lohr K.N., Cullen K.E., Bann C.M., Bulik C.M. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 2016;165(6):409–420. doi: 10.7326/M15-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailey M.J., Moran T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metabol. 2013;24(2):85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward O.R., Gribble F.M., Reimann F., Lewis J.E. Gut peptide regulation of food intake–evidence for the modulation of hedonic feeding. J Physiol. 2022;600(5):1053–1078. doi: 10.1113/JP280581. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi E., Yasoshima Y., Shimura T. Systemic administration of anorexic gut peptide hormones impairs hedonic-driven sucrose consumption in mice. Physiol Behav. 2017;171:158–164. doi: 10.1016/j.physbeh.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Nicolau J., Pujol A., Tofé S., Bonet A., Gil A. Short term effects of semaglutide on emotional eating and other abnormal eating patterns among subjects living with obesity. Physiol Behav. 2022;257 doi: 10.1016/j.physbeh.2022.113967. [DOI] [PubMed] [Google Scholar]
- 13.Gormally J.I.M., Black S., Daston S., Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: a language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 15.Fox J., Weisberg S. third ed. Sage; Thousand Oaks CA: 2019. An R companion to applied regression.https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- 16.Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 17.Holst J.J. Incretin hormones and the satiation signal. Int J Obes. 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Porto A., Casarsa V., Colussi G., Catena C., Cavarape A., Sechi L. Dulaglutide reduces binge episodes in type 2 diabetic patients with binge eating disorder: a pilot study. Diabetes Metabol Syndr: Clin Res Rev. 2020;14(4):289–292. doi: 10.1016/j.dsx.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Robert S.A., Rohana A.G., Shah S.A., Chinna K., Wan Mohamud W.N., Kamaruddin N.A. Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide - a pilot study. Obes Res Clin Pract. 2015 May-Jun;9(3):301–304. doi: 10.1016/j.orcp.2015.03.005. Epub 2015 Apr 11. PMID: 25870084. [DOI] [PubMed] [Google Scholar]
- 20.Van Bloemendaal L., Ten Kulve J.S., La Fleur S.E., Ijzerman R.G., Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1–T16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 21.Drucker D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metabol. 2022;57 doi: 10.1016/j.molmet.2021.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.