Abstract
Background
This “Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022” is intended to provide clinicians an overview of Food and Drug Administration (FDA) approved anti-obesity medications and investigational anti-obesity agents in development.
Methods
The scientific information for this Clinical Practice Statement (CPS) is based upon published scientific citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
Results
This CPS describes pharmacokinetic principles applicable to those with obesity, and discusses the efficacy and safety of anti-obesity medications [e.g., phentermine, semaglutide, liraglutide, phentermine/topiramate, naltrexone/bupropion, and orlistat, as well as non-systemic superabsorbent oral hydrogel particles (which is technically classified as a medical device)]. Other medications discussed include setmelanotide, metreleptin, and lisdexamfetamine dimesylate. Data regarding the use of combination anti-obesity pharmacotherapy, as well as use of anti-obesity pharmacotherapy after bariatric surgery are limited; however, published data support such approaches. Finally, this CPS discusses investigational anti-obesity medications, with an emphasis on the mechanisms of action and summary of available clinical trial data regarding tirzepatide.
Conclusion
This “Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022” is one of a series of OMA CPSs designed to assist clinicians in the care of patients with pre-obesity/obesity.
Keywords: Anti-obesity medications, Investigational agents, Obesity, Semaglutide, Tirzepatide
1. Introduction
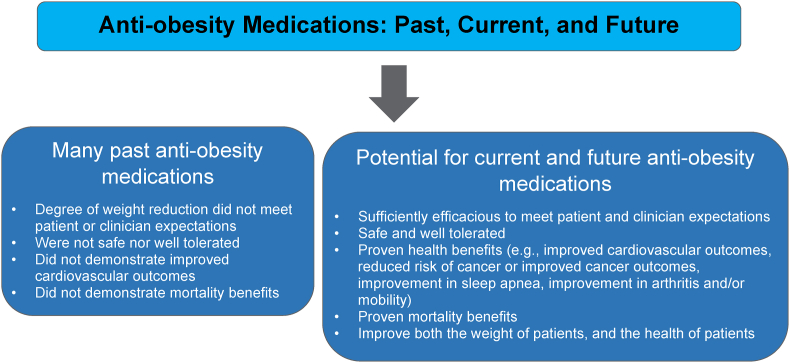
Beginning in 2013, the Obesity Medicine Association (OMA) created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees [1]. This was followed by a similar Pediatric “Obesity Algorithm” with updates approximately every two years by OMA authors. This “Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022” is one of a series of OMA Clinical Practice Statements (CPS) derived from the Obesity Algorithm designed to assist clinicians in the care of patients with the disease of obesity, with anticipation that forthcoming newer anti-obesity agents will provide additional safe and effective treatments for obesity. Finally, anti-obesity drug development is mirroring the path of past treatment of other metabolic diseases (e.g., diabetes mellitus, hypertension, and dyslipidemia), wherein proven cardiovascular disease outcome benefits will likely be the binary switch that will transform the current limited use of anti-obesity medications into standards of care for patients with obesity.
2. Overview and objectives of anti-obesity medication treatment
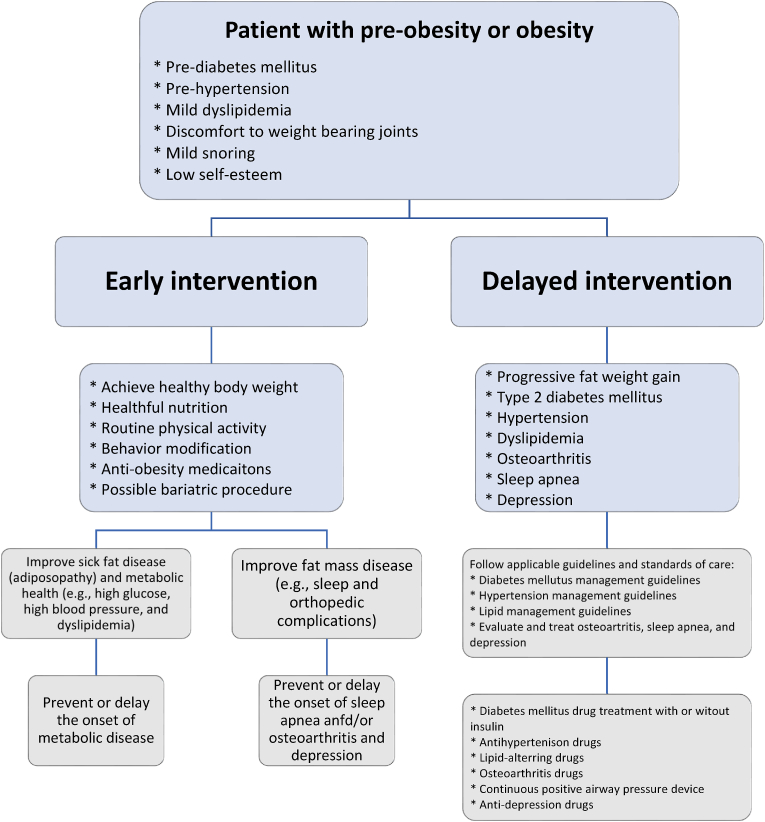
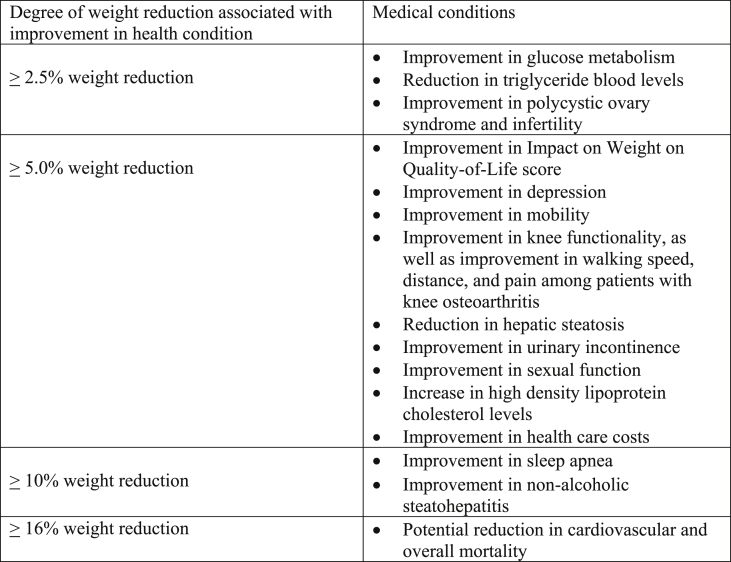
In addition to appropriate nutrition, physical activity, and healthful behavior, anti-obesity medication treatment is one of the four nonsurgical OMA pillars of obesity management. Weight reduction as little as 5–10% (or in some cases, as little as 3%) can improve both adiposopathy (“sick fat disease”) and fat mass disease [[2], [3], [4], [5]]. The purpose of anti-obesity medication treatment is to (a) serve as an adjunct to appropriate nutrition, physical activity, and healthful behavior to facilitate a more healthy body weight, (b) treat sick fat disease (adiposopathy) and its adverse cardiometabolic consequences, (c) treat fat mass diseases, (d) slow the progression of weight regain, (e) serve as an adjunct to bariatric surgery in enhancing weight reduction, and (f) generally improve the health and quality of life of patients with pre-obesity or obesity [5,6]. Table 1 describes ten takeaway messages regarding anti-obesity medications.
Table 1.
Anti-Obesity Medications. Shown are 10 takeaway messages regarding anti-obesity medications, including their mechanisms of action, approvals, illustrative side effects, and sentinel contraindications [7]. All anti-obesity medications are contraindicated in patients with hypersensitivity to the drug (e.g., anaphylaxis, angioedema), and should not be used in patients with overweight/pre-obesity or obesity who are pregnant or planning to become pregnant.
| 1. | Phentermine is an approved anti-obesity medication that is a sympathomimetic amine with possible adrenergic side effects. Phentermine is contraindicated in patients with cardiovascular disease and contraindicated in patients with uncontrolled hypertension. |
| 2. | Phentermine hydrochloride (HCl) 8–37.5 mg is marketed in the United States (US) and is generally equivalent to 6.4–30 mg of phentermine resin marketed outside the US. Phentermine HCl tablets are commonly administered at 37.5 mg once per morning, or sometimes 18.75 mg twice a day. An alternative phentermine HCl formulation is administered 8 mg (or 4 mg if using one half 8 mg tablet) three times a day before meals. Finally, phentermine HCl is also supplied as capsules or disintegrating tablets, with doses of 15 and 30 mg once per morning or 15 mg twice a day. |
| 3. | Although not consistent with the prescribing information indicated use, and while prohibited by some state laws, phentermine administration for longer than 12 weeks is supported by clinical data and opinion leaders. |
| 4. | Semaglutide is a glucagon-like peptide-1 receptor agonist. Semaglutide 2.4 mg subcutaneously per week is an approved anti-obesity medication. Semaglutide at lower subcutaneous injectable doses 0.25–2.0 mg per week and at oral doses of 7–14 mg per day, is indicated to lower blood sugar in patients with type 2 diabetes mellitus. Potential side effects include gastrointestinal adverse events (e.g., nausea). Semaglutide is contraindicated in patients with personal or family history of medullary thyroid cancer or type 2 multiple endocrine neoplasia. |
| 5. | Liraglutide is a glucagon-like peptide-1 receptor agonist approved at 3.0 mg subcutaneously per day for treatment of obesity and 1.8 mg subcutaneously per day for treatment of type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease. Side effects include possible gastrointestinal adverse events (e.g., nausea). Liraglutide is contraindicated in patients with personal or family history of medullary thyroid cancer or type 2 multiple endocrine neoplasia. |
| 6. | Phentermine/topiramate is an approved anti-obesity medication and is a combination of a sympathomimetic amine and anti-seizure/migraine medication with side effects that include paresthesias and dysgeusia; exposure to topiramate in the first trimester of pregnancy increases the risk of oral clefts (cleft lip with or without cleft palate). |
| 7. | Naltrexone/bupropion is an approved anti-obesity medication and is a combination of an opioid antagonist and an antidepressant with possible gastrointestinal side effects. It is contraindicated in patients with uncontrolled hypertension, chronic opioid use, seizure disorders, and/or abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs. |
| 8. | Orlistat is an approved anti-obesity medication and is a gastrointestinal lipase inhibitor with possible adverse experiences that include oily rectal discharge and flatus; it is contraindicated in patients with chronic malabsorption syndrome and cholestasis. |
| 9. | Semaglutide, liraglutide, and phentermine/topiramate can be taken with or without meals. Orlistat should be taken three times a day with each meal that contains fat; bupropion/naltrexone should not be taken with high-fat meals due to increased gastrointestinal absorption. |
| 10. | Biodegradable oral non-systemic superabsorbent hydrogel is an FDA-cleared weight loss aid that is made from cross-linked carboxymethylcellulose and citric acid that promotes fullness and may help to increase satiety to help with weight management. It is orally administered like a drug but regulated by the FDA as a class II medical device because it acts through mechanical modes of action. |
3. Pharmacokinetics and obesity
3.1. Drug absorption
Obesity may affect a drug's absorption, metabolism, distribution, and excretion [[8], [9], [10]]. The systemic availability of pharmacotherapeutic agents depends on how the drug is administered. Intravenous, subcutaneous, and intramuscular administration enter the systemic circulation extrahepatically, resulting in systemic bioavailability before reaching the liver for metabolism [8]. Due to increased subcutaneous fat, individuals with obesity may present challenges in locating and obtaining intravenous access and may require longer needles for intramuscular drug administration. Challenges with the route of administration are clinically relevant because intravenous and intramuscular injections generally result in faster systemic exposure compared to oral or subcutaneous administration [8].
Regarding oral absorption, tablets can be manufactured for immediate, delayed, or extended release. While capsule formulations cannot be split, they can be easier to swallow and are often used to protect drugs from degradation in the acidic environment of the stomach. While capsules can also be manufactured for immediate, delayed, or extended release, capsules also allow for fat-soluble drugs to be mixed with oil upon administration, which may enhance absorption [8].
Approximately 50% of marketed drugs are administered as ionized salts (e.g., combined with sodium, chloride, potassium, magnesium, calcium), which allow for manipulation of pharmacokinetics, improved bioavailability (e.g., enhanced disassociation in the gastrointestinal tract), greater shelf-life stability, and easier manufacturing [11]. Examples of drugs administered as salts include phentermine HCl, naltrexone HCl, and bupropion HCl [11].
An illustrative example of how drug formulation can affect absorption is phentermine hydrochloride (HCl). The portion of phentermine in a tablet or capsule is often termed “free base,” such that a tablet containing 37.5 mg of phentermine HCl is equivalent to 30 mg phentermine base [12]. While also marketed as a resin decades ago in the US, phentermine resin is now almost exclusively marketed outside the US. Complexed drugs (e.g., phentermine ion-exchange resin) often require metabolism by gastric enzymes or intestinal flora to become activated. This helps explain why phentermine resin is absorbed via the gastrointestinal tract about 3 times slower than phentermine HCl [12,13].
Other factors that may affect absorption of drugs include food and/or fat intake for orally administered drugs, and subcutaneous (SQ) blood supply for subcutaneously administered drugs. Obesity increases SQ fat and may therefore decrease SQ absorption due to reduced SQ blood flow per unit volume of SQ tissue. SQ administration of biologics is usually limited to 1–3 mL. If acceptable viscosity can be maintained, then higher doses of drugs can be achieved with higher concentration of SQ drugs. Insulin is often administered as U-100, which means it contains 100 units of insulin per milliliter. In cases where higher doses of insulin are required (>200 units), then more concentrated insulin may be beneficial for some patients with obesity, such as use of U-300 (i.e., 3 times more concentrated than U-100 insulin) or U-500 (i.e., 5 times more concentrated than U-100 insulin). Fat loss in patients with obesity and diabetes mellitus treated with insulin can sometimes require (dramatic) reductions in the insulin dose to avoid hypoglycemia, due to improved systemic insulin sensitivity coupled with potential enhancement of SQ absorption. Therefore, a proactive reduction in insulin dose is often recommended prior to aggressive weight reduction interventions (e.g., very low-calorie diets, bariatric surgery, and even before start of some anti-obesity medications). Practical applications of drug pharmacokinetics regarding anti-obesity agents include the following:
-
•
Semaglutide and liraglutide are administered SQ with or without meals. Semaglutide can also be administered as a tablet for treatment of type 2 diabetes mellitus [14,15].
-
•
Phentermine HCl is administered as a capsule or tablet with or without food, depending on the formulation [12,13].
-
•
Phentermine HCl/topiramate XR (extended release) is administered as a capsule with or without food [16].
-
•
Bupropion HCl/naltrexone HCl combination is administered as a tablet and should not be taken with high fat-meals due to enhancement of gastrointestinal absorption [17].
-
•
Orlistat is administered as a capsule and should be taken three times a day with fat-containing meals [18].
3.2. Drug metabolism (Fig. 1)
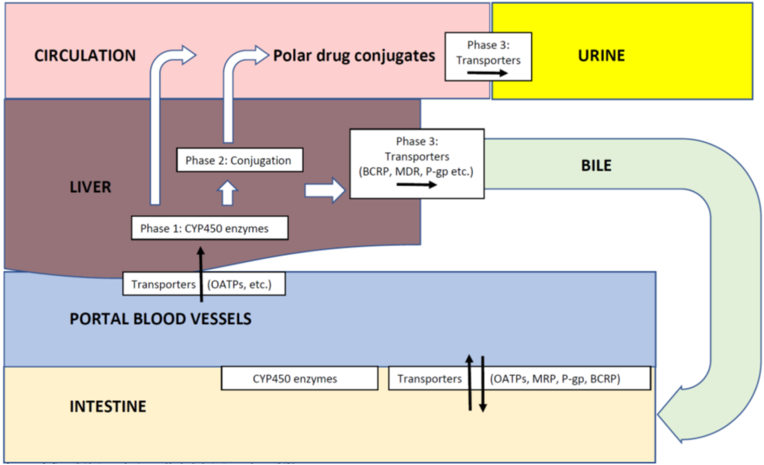
Fig. 1.
Drug Metabolism and Transport. Orally administered drugs may initially undergo metabolism in the intestine via gastrointestinal/bacterial enzymes and potentially Phase 1 metabolism in enterocytes, followed by transportation into portal vessels and liver. Afterwards, drugs may undergo Phase 1 and/or Phase 2 enzymatic alterations to form metabolites that may be excreted into the bile. If converted into polar conjugates, metabolites may be released into the circulation, and then excreted by the kidney [19].
Phase 1 drug metabolism: Oxidation, reduction, and/or hydrolysis via cytochrome P450 enzymes.
Phase 2 drug metabolism: Conjugation via glucuronidation, acetylation, glutathione conjugation, sulfate conjugation, methylation.
Phase 3 drug metabolism: Distribution and elimination of drugs mediated by transporters.
Cytochrome P450 (CYP450) proteins generally reside within cellular membranes (i.e., endoplasmic reticulum or mitochondrial membrane) and function to metabolize drugs via Phase 1 drug metabolism. The most common CYP450 isoenzyme for drug metabolism is CYP450 3A4.
Organic Anion-Transporting Polypeptides (OATP), Multidrug-Resistant-Associated Protein (MRP), P-glycoproteins (P-gp), and Breast Cancer Resistance Protein (BCRP) facilitate drug movement in and out of intestinal and hepatic cells.
Orally administered drugs undergo intestinal and hepatic pre-systemic metabolism (i.e., “first pass effect”), limiting drug systemic bioavailability. Gastrointestinal, pre-systemic metabolism involves gastrointestinal enzymes (gastric, pancreatic, and hepatic), intraluminal bacterial enzymes, Phase 1 reactions, and Phase 2 reactions [8,19].
Some orally administered drugs (especially polar drugs) are transported to the liver and excreted in the bile or urine as unchanged drug. Other drugs undergo metabolism. Phase 1 metabolism includes oxidation, reduction, and/or hydrolysis via microsomal/endoplasmic reticulum cytochrome 450 enzymes. The most common cytochrome protein (CYP) 450 isoenzyme for metabolism of drugs is CYP450 3A4 [8]. The result of Phase 1 metabolism is the alteration of the parent drug by introducing or exposing a functional group (-OH, -NH2, –SH). Usually, this converts active drugs to inactive drugs. However, some prodrugs may be converted to active metabolites via pre-systemic drug metabolism. Once a drug is affixed to a functional group, it usually becomes polar and may be excreted in the urine or bile. Alternatively, some drugs may undergo conjugation via Phase 2 metabolism (sequential to Phase 1 metabolism or via Phase 2 metabolism alone) via glucuronidation, acetylation, glutathione conjugation, sulfate conjugation, or methylation, and then released into the circulation. With some exceptions, these conjugates are highly polar (i.e., water soluble), generally inactive, and excreted in the urine [8,19].
Many clinicians are familiar with CYP450 enzymes found in tissues such as the liver and intestine. The specific CYP450 isoenzyme/s responsible for drug metabolism and drug interactions are often listed in the respective drug prescribing information. This is clinically relevant because drug metabolism can be altered by concomitant drugs that affect the same CYP450 isoenzyme, as occurs with drugs that are inducers, inhibitors, or competitive inhibitors [8]. Naltrexone HCl/Bupropion HCl is illustrative of an anti-obesity medication that undergoes metabolism via CYP isoenzymes and thus has the potential for drug interactions with other drugs metabolized by these same CYP isoenzymes [8,19].
Beyond alterations in pharmacokinetics via CYP metabolism, the circulating blood levels of drugs can also be affected via facilitation of absorption, distribution, and excretion by cellular membrane solute carrier (SLC) transporters and adenosine triphosphate (ATP)-binding cassette transporters, which include:
-
•
Organic anion transporting polypeptide-C transporter (OATP-C) is a SLC that mediates transport of mainly organic anions across cell membranes [8].
-
•
P-glycoprotein is an adenosine triphosphate (ATP)-binding cassette (ABC) transporter, also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) or cluster of differentiation 243 (CD243) [19].
-
•
Breast cancer resistance protein (BCRP/ABCG2) is an ATP-binding cassette (ABC) transporter whose name was derived because it was a protein initially cloned from a multidrug-resistant breast cancer cell line [8].
Another factor that can affect pharmacodynamics is protein binding. Once in the circulation, many drugs are bound to proteins. In general, acidic drugs bind to albumin and neutral-to-basic drugs bind to alpha1 acid glycoprotein [8]. Protein binding generally prolongs availability, prolongs duration of action, delays metabolic degradation, delays excretion (e.g., may decrease renal excretion), and may impair drug crossing of the blood brain barrier [19]. For example, semaglutide is over 99% bound to plasma albumin. Protein binding can be affected by hypoalbuminemia, severe liver disease, and nephrotic syndrome. Drugs with higher affinity to circulating protein binding will displace drugs with lower affinity. An illustrative example of the importance of protein binding in obesity medicine is that endogenous total testosterone levels are often decreased in male patients with obesity, due to reduced sex hormone binding globulin (SHBG) and/or reduction in free testosterone (free testosterone is bioactive). If total testosterone is low and free testosterone is normal, then this may be due to a reduction in SHBG due to obesity. If free testosterone is low, then this suggests obesity-mediated impairment of the hypothalamic/hypopituitary axis [20]. If not due to obesity or testicular dysfunction, then a low free testosterone could also be due to non-obesity-related hypothalamic/hypopituitary disorders that may require diagnosis and treatment, such as a hypothalamic/pituitary lesion or pituitary tumor [21,22] (See section 8.4 Hypothalamic Obesity). Polycystic ovary disease is also associated with lower levels of SHBG (possibly related to adiposopathic insulin resistance), which may contribute to higher levels of active hormones such as free androgens [8,19].
3.3. Drug distribution
Drug distribution is determined by the movement of drugs from the circulation into body tissues. Drug distribution is substantially influenced by whether the drug is water soluble (hydrophilic) or fat soluble (lipophilic).
General characteristics of hydrophilic drugs [23]:
-
•
Polar
-
•
Mainly reside in the circulation
-
•
Relatively little diffuses from the circulation across cellular membranes of body tissues
-
•
Can be moved into tissues by transporters via facilitated transport (without energy) or active transport (with energy)
-
•
Eliminated by the kidney
-
•
Dose of hydrophilic drugs might best be based on the ideal bodyweight (IBW)
General characteristics of lipophilic drugs [23]:
-
•
Non-polar
-
•
Distributes into body tissues (e.g., body fat, blood brain barrier) because in addition to proteins and carbohydrate groups, cellular membranes are substantially composed of lipids (e.g., phospholipids and cholesterol)
-
•
Because patients with obesity have more body lipids, the volume of distribution of lipophilic drugs will be increased in patients with obesity
-
•
Can also cross membranes by transporters
-
•
Dose of lipophilic drugs might best be based upon actual body weight (including body fat)
-
•
Some lipophilic drugs may have detectable blood levels years after administration [24].
It may be intuitive to think that the dosing of some drugs can be predicted based upon the drug's hydrophilicity (i.e., confined to the circulation and thus based on ideal body weight) or the drug's lipophilicity (i.e., widespread distribution in body tissues, especially body fat). However, often little systematic relationship exists between the degree of lipophilicity of markedly lipophilic drugs and their distribution in individuals with obesity. Thus, loading doses of drugs should optimally be adjusted according to data from studies carried out specifically in individuals with obesity. Similarly, adjustment of the maintenance dosage is best determined based on data-driven modifications in clearance. Drugs with narrow therapeutic and safety indexes should be used prudently, with the dose adjusted based upon drug plasma concentrations [23].
3.4. Drug excretion
Common ways systemic drugs are excreted are via the kidney, bile, lungs, and sweat glands. The term “half-life” refers to the time required to reduce the drug blood concentration by 50% after achieving steady state. Some drugs are dosed at one half-life intervals (i.e., drugs with half-lives of 8–24 hours may be dosed three, two, or one time daily). Repeated administration of a drug will achieve a steady state when the rate of drug entering the systemic circulation equals the rate of elimination, which unless initiated with a loading dose, is about 5 times the half-life of a drug. After achieving steady state, the half-life of a drug can be increased by decreasing the clearance or elimination of the drug, or by increasing the volume of distribution of the drug [23].
Regarding kidney drug excretion, measurement of glomerular filtration rate (GFR) represents the blood filtered by the kidney, often assessed by fructose-based polysaccharide (i.e., inulin) that is neither secreted nor reabsorbed across tubules. Some estimates of GFR are not validated for obesity; patients with obesity often have increased estimated GFR [25]. Complications of obesity may affect kidney excretion (e.g., renal insufficiency due to diabetes mellitus or hypertension). While the Salazar-Corcoran calculation is specific for obesity, it is not commonly used in most clinical practices. More common estimates of GFR include [25]:
-
•
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is frequently used by commercial labs, and the formula includes age, sex, race (i.e., African American), and blood creatinine (not weight)
-
•
Abbreviated Modification of Diet in Renal Disease (MDRD) equation is frequently used in clinical trials, may be less accurate at higher GFR, and the formula includes age, sex, race (i.e., Black or non-Black), and blood creatinine (not weight)
-
•
Cockcroft-Gault equation is rarely used in clinical practice, but it is a historic standard used for renally-adjusted drug dosing studies and includes parameters such as age, sex, blood creatinine, and adjustment for body mass index
Excretion has other clinical considerations when it comes to the complications of the disease of obesity. Obesity alone is a risk factor for kidney stones. Insulin resistance alters renal acid-base metabolism, lowers urine pH, and increases risk of uric acid stones [26]. Increased nutritional intake of high-oxalate foods, salt, processed foods, and animal proteins increases risk of kidney stones [26]. Orlistat increases unabsorbed intestinal fat which binds to intestinal calcium, with less calcium available to bind to intestinal oxalate. This allows for increased intestinal oxalate absorption that may ultimately cause hyperoxaluria, promoting calcium oxalate stones [27]. The topiramate component of phentermine HCl/topiramate ER decreases renal tubule reabsorption of HCO3 and decreases excretion of H, contributing to metabolic acidosis, and increased urinary pH, which may increase the risk of kidney stones [27], with the most specific reported composition being calcium phosphate stones [28]. Especially if associated with dehydration, very low-calorie diets may increase the risk of kidney stones. Gastric bypass bariatric surgery may contribute to fat malabsorption, increased gastrointestinal oxalate absorption, increased urinary oxalate excretion, decreased urinary volume, and increased risk of calcium oxalate stones [29].
Finally, urine drug testing is not uncommon for participants in anti-obesity medication clinical trials or within the workplace. Clinicians (and patients) may therefore benefit from knowing that, depending on the assay, several drugs sometimes administered to patients with obesity may test positive for amphetamine, with a partial list including amphetamine derivatives (including lisdexamfetamine), phentermine, bupropion, fluoxetine, and even metformin [30].
4. Food and Drug Administration principles
Indications for FDA-approved anti-obesity medications generally include [31]:
-
•
Patients with obesity (e.g., BMI ≥30 kg/m2)
-
•
Patients with overweight/pre-obesity (e.g., BMI ≥27 kg/m2) with presence of weight-related complications (i.e., “weight-related comorbidities”) such as type 2 diabetes mellitus, hypertension, dyslipidemia
-
•
Anti-obesity medications are contraindicated in patients with pregnancy or with hypersensitivity to the anti-obesity medications
-
•
Body mass index (BMI) is the only obesity measure listed in the prescribing information for approved uses of anti-obesity medications. However, BMI has limitations. Especially in muscular individuals or those with sarcopenia, the amount of body fat is more accurately assessed by other measures [32]. While it may be clinically appropriate to do so, the use of anti-obesity medications based upon adiposity measurements beyond BMI may be considered “off label” (i.e., not within the explicit wording of the prescribing information), potentially prompting explanation, justification, and/or appeal to health insurance payers of the anti-obesity medication.
Other general principles [31]:
-
•
Anti-obesity medications promote variable weight reduction over variable duration in individual patients with pre-obesity or obesity.
-
•
Patients have an average of approximately 5–20% weight reduction, depending on the anti-obesity medication, dose, and individual variations, with greater weight reduction in hyper-responders and less weight reduction (or even weight gain) in hypo-responders.
-
•
If an anti-obesity medication is initiated, and if the anti-obesity medication does not have a prescribing information time limitation for use, then the decision to continue or discontinue anti-obesity medication treatment is best based upon the individual patient response and clinical judgment regarding the risks of further or recurrent weight gain. Local/state laws may impose restrictions of use of anti-obesity medications, irrespective of the potential benefit to the patient and irrespective of the clinical judgment of the clinician [33].
-
•
Prescribing information guidance regarding longer-term anti-obesity medication therapy varies depending on the specific anti-obesity medication. For many anti-obesity medications, if no clinical improvement (e.g., at least 3–5% loss of baseline body weight) occurs after 12–16 weeks, then the prescribing information may recommend clinicians instruct patients to increase the dose or perhaps discontinue the anti-obesity medication. Conversely, other anti-obesity medications (e.g., semaglutide 2.4 mg weekly injection) do not have explicit stopping instructions based upon therapeutic response.
-
•
For anti-obesity medications in general, if weight regain occurs where the patient and clinician determine the medication is no longer effective, then the anti-obesity medication should be discontinued. However, if the patient achieved clinically meaningful weight reduction with the anti-obesity medication, and if the patient and clinician determine the medication is helping to avoid weight regain, then a weight reduction plateau should not necessarily be interpreted as “tolerance” to the drug. Just as with medications for other metabolic diseases (e.g., drug treatments for diabetes mellitus, hypertension, and dyslipidemia), medications for weight reduction are efficacious only if they are taken. Continued weight reduction maintenance may represent a manifestation of efficacy maintenance and should not automatically warrant the anti-obesity medication “be discontinued.”
Update to FDA Pregnancy and Lactation Labeling:
-
•
In 2014, the FDA issued its “Pregnancy and Lactation Labeling Final Rule” (PLLR), which went into effect on June 30, 2015 [34].
-
•
The PLLR removed letter pregnancy categories: A, B, C, D, and X [34].
-
•
The prescribing information materials for some anti-obesity medications continue to cite these pregnancy categories, with category X intended to convey the message that the risks of human fetal harm of a drug administered during pregnancy outweigh potential benefits
-
•
In general, anti-obesity medications are contraindicated in pregnancy and best avoided in women who are pregnant, trying to become pregnant, or breastfeeding [35,36].
5. Anti-obesity medications
5.1. Anti-obesity medication summary
See Table 2.
Table 2.
Summary of Anti-Obesity Medications. All anti-obesity medications are contraindicated in patients with hypersensitivity to the drug (e.g., anaphylaxis, angioedema), should not be used in patients planning to become pregnant or who are pregnant, and all may require downward dose adjustment of concomitant anti-diabetes medication to avoid hypoglycemia, especially in patients treated with insulin and sulfonylureas.
| Drug | Description | Main Side Effects | Illustrative Drug Interactions |
|---|---|---|---|
| Phentermine∗ [[37], [38], [39], [40], [41]] | Sympathomimetic amine approved as a weight management medication in 1959. It is a DEA Schedule IV stimulant agent approved for short-term use (12 weeks). Approved for age 17 years or older. Average weight reduction is about 3–8%.∗ | Side effects include headache, high blood pressure, rapid or irregular heart rate, overstimulation, tremor, dry mouth, and insomnia. Contraindicated in patients with cardiovascular disease, stroke, uncontrolled hypertension, within 14 days of monoamine oxidase inhibitors, hyperthyroidism, glaucoma, agitated states, or with a history of drug abuse. | May have interactions with other sympathomimetics, alcohol, adrenergic neuron blocking drugs, and possibly some anesthetic agents. Should not be taken during or within 14 days following monoamine oxidase (MAO) inhibitors. |
| Semaglutide∗∗ [42,43] | Glucagon-like peptide-1 receptor agonist that at lower injectable doses 0.25–2.0 mg per week, and at oral doses of 7–14 mg per day, is indicated to lower blood sugar in patients with type 2 diabetes mellitus. Semaglutide at 2.4 mg subcutaneously per week is approved for treatment of obesity. Average weight reduction is about 15%. |
Adverse reactions include nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distension, eructation (belching), flatulence, gastroenteritis, and gastroesophageal reflux disease. Contraindicated in patients with personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2 or known hypersensitivity to semaglutide. Warnings and precautions: acute pancreatitis, acute gallbladder disease, acute kidney injury especially in patients with severe adverse gastrointestinal reactions, diabetes retinopathy, heart rate increase, suicidal behavior and ideations. Associated with hypoglycemia in patients with type 2 diabetes treated with concomitant hypoglycemic medications such as sulfonylureas or insulin. |
May slow gastric emptying, which may impact absorption of concomitantly administered oral medication. |
| Liraglutide∗∗ [14,15,44] | Glucagon-like peptide-1 receptor agonist that is an injectable drug. At lower doses (1.8 mg per day), liraglutide is indicated to lower blood sugar among patients with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease. Liraglutide 3.0 mg per day is approved for treatment of obesity. Average weight reduction is 5–10%, especially with the liraglutide higher dose. Approved for patients 12 years or older. | Adverse reactions include nausea, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, increased lipase, and renal insufficiency. Contraindicated with personal or family history or medullary thyroid cancer or Type 2 Multiple Endocrine Neoplasia syndrome. Discontinue with suspected pancreatitis, gall bladder disease, or suicidal behavior and ideation. May promote hypoglycemia, particularly in patients with diabetes mellitus treated with insulin or sulfonylureas. | May slow gastric emptying, which may impact absorption of concomitantly administered oral medication. |
| Phentermine/topiramate [44,45] | Combination of phentermine (sympathomimetic amine, anti-obesity medication) and topiramate (used to treat seizures and migraine headaches) that is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management. DEA Schedule IV drug. Average weight reduction is 5–10%. | Can cause paresthesia (tingling or numb feelings to extremities), dizziness, dysgeusia (abnormal taste), insomnia, constipation, or dry mouth. Monitor for increased heart rate, suicidal behavior/ideation, mood and sleep disorders, cognitive impairment, metabolic acidosis, elevated creatinine, and low blood sugars in patients on anti-diabetes medications. Discontinue with acute myopia and secondary angle glaucoma. Should not be used in patients with glaucoma or hyperthyroidism. Topiramate can cause birth defects. Phentermine/topiramate should not be started until after a pregnancy test is negative. Thereafter, the FDA recommends women use effective contraception and have monthly pregnancy tests during treatment with phentermine/topiramate. (https://qsymiarems.com/) | Should not be taken during or within 14 days of monoamine oxidase inhibitors. Avoid use with alcohol, due to potentiation of depressant effects. May potentiate hypokalemia when used with non-potassium sparing diuretics. May alter hormone exposure with oral contraceptives but may not increase risk of pregnancy. |
| Naltrexone/bupropion [17,44,46,47] | Combination of naltrexone (opioid antagonist used for addictions) and bupropion (used for depression and smoking cessation) that is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management. Average weight reduction is about 5%. | Naltrexone/bupropion can cause nausea, constipation, headache, vomiting, dizziness, insomnia, dry mouth, diarrhea, and acute angle closure glaucoma. The bupropion component is marketed as an antidepressant, and antidepressants can increase the risk of suicidal thinking in children, adolescents, and young adults; monitor for suicidal thoughts and behaviors. Should not be used in patients with uncontrolled high blood pressure, seizure disorders, or drug/alcohol withdrawal. | May have drug interactions with opioid medications, anti-seizure medications, MAO inhibitors, and other drugs. Should not be taken with other bupropion or naltrexone-containing medications. Central nervous system toxicity can occur when used concomitantly with dopaminergic drugs (e.g., levodopa and amantadine). |
| Orlistat [44,48] | Gastrointestinal lipase inhibitor (i.e., impairs digestion of dietary fat) that is indicated for obesity management, including weight reduction and weight management in conjunction with a reduced-calorie diet, and for reduced risk of weight regain after weight reduction. Lower doses are approved over-the-counter. Average weight reduction is 5% of body weight. Approved for patients 12 years or older. | Side effects include oily discharge with flatus from the rectum, fecal urgency, and increased defecation and fecal incontinence, especially after fatty food intake. (May help with constipation.) May promote gallstones and kidney stones. May cause malabsorption of fat-soluble vitamins (A, D, E, K). Need to take a multivitamin daily. Contraindicated in chronic malabsorption syndrome and cholestasis. Rare cases of severe liver injury and pancreatitis. |
Cyclosporine, hormone contraceptives, seizure medications, thyroid hormones, warfarin |
∗Phentermine weight reduction efficacy reported in the medical literature depends on when and where the data were derived. The data listed for the anti-obesity agents in this table are derived from controlled trials. From the perspective of prospective controlled clinical trials, the placebo-corrected phentermine weight reduction is about 3–8% [[37], [38], [39], [40]]. In a meta-analysis of phentermine monotherapy clinical trials, the placebo-corrected reduction in body weight with phentermine was less than 4 kg [49]. Conversely, from the perspective of retrospective medical chart reviews (without placebo control), the weight reduction efficacy of phentermine is reported to range from 4% to 19% among patients commonly treated with phentermine doses of 60 mg per day (and thus doses higher than approved by the FDA) [50,51], with reports that some clinicians are known to prescribe phentermine doses as high as 112 mg per day [52].
∗∗Glucagon-like peptide-1 receptor agonists (GLP-1 RA) slow gastric emptying. Many patients with obesity have type 2 diabetes mellitus. If patients treated with GLP-1 RA develop signs, symptoms, and/or diagnostic evidence of gastroparesis, then this may prompt the clinician to discontinue the GLP-1 RA, before assuming the gastroparesis is due to diabetes mellitus neuropathy [53].
∗∗Calcitonin is a polypeptide secreted by thyroid C-cells that is involved with bone and calcium metabolism. Medullary thyroid cancer is often associated with marked elevations in calcitonin (>100 pg/mL) [54,55]. The prescribing informations for approved GLP-1 RAs do not recommend routine monitoring of calcitonin blood levels. Healthy human thyroid C cells do not express GLP 1 receptors [56]. Especially at marginal to mild elevations in calcitonin blood levels, hypercalcitoninemia is not pathognomonic of medullary thyroid cancer. Some reports suggest that mild elevation in calcitonin levels can be due to thyroid disease other than medullary thyroid cancer [54]. Especially in the absence of family history otherwise suggesting genetic predisposition to medullary thyroid cancer, and especially in the absence of abnormal thyroid gland physical findings (i.e., nodules or goiter), modestly elevated calcitonin levels are more likely due to other non-thyroid causes, such as neuroendocrine tumors [54]. Examples of common drugs that may increase calcitonin include omeprazole and other proton pump inhibitors, glucocorticoids, beta-blockers, and glucagon [54]. Calcitonin levels may also increase in patients with chronic renal disease [57].
5.2. Sympathomimetic amines
Sympathomimetic amines are stimulant compounds that reduce hunger. Examples of sympathomimetic amines include phentermine, diethylpropion, phendimetrazine, and benzphetamine. The Drug Enforcement Agency (DEA) schedules for these weight-management agents are [58]:
-
•
DEA IV for phentermine and diethylpropion
-
•
DEA III for phendimetrazine and benzphetamine
Potential adverse experiences include [[50], [59], [60], [61], [62]]:
-
•
Palpitation
-
•
Tachycardia
-
•
Increased blood pressure
-
•
Overstimulation
-
•
Tremor
-
•
Dizziness
-
•
Insomnia
-
•
Dysphoria
-
•
Headache
-
•
Dryness of mouth
-
•
Dysgeusia
-
•
Diarrhea
-
•
Constipation
-
•
Contraindicated in pregnancy
5.3. Phentermine
5.3.1. Indications and use [16]
-
•
Phentermine is a sympathomimetic amine approved by the FDA for short-term treatment of obesity, with “short-term” often interpreted as a duration of 12 weeks
-
•
It is a Drug Enforcement Agency Schedule IV drug
-
•Dose:
-
oIn the US, phentermine is almost exclusively available in the HCl formulation.
-
oPhentermine HCl tablets = 37.5 mg (or 18.75 mg for one half 37.5 mg tablet) once per morning; sometimes 18.75 mg twice a day
-
oPhentermine HCl tablet (different formulation than above) = 8 mg (or 4 mg if using one half 8 mg tablet) three times a day before meals
-
oPhentermine HCl capsules or disintegrating tablets = 15 and 30 mg once per morning or 15 mg twice a day
-
oPhentermine resin = 30 mg (or 15 mg) once per morning (not available in the US)
-
o
5.3.2. Potential Drug Interactions [16]
-
•
Monoamine Oxidase Inhibitors: Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive crisis
-
•
Alcohol: Concomitant use of alcohol with phentermine may result in an adverse cardiovascular adverse effects (e.g., increased heart rate, blood pressure changes, or chest pain)
-
•
Insulin and Oral Hypoglycemic Medications: A reduction in insulin or oral hypoglycemic medications in patients with diabetes mellitus may be required
-
•
Adrenergic Neuron Blocking Drugs: Phentermine may decrease the hypotensive effect of adrenergic neuron blocking drugs
5.3.3. Pharmacokinetics [16]
-
•
Urinary excretion may be 62–85%; use with caution when administering phentermine to patients with renal impairment
5.3.4. Most common adverse reactions [16]
The most common adverse reactions include headache, high blood pressure, rapid or irregular heart rate, overstimulation, tremor, and insomnia.
5.3.5. Contraindications [16]
-
•
Hypersensitivity
-
•
Pregnancy/nursing
-
•
History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension)
-
•
Administration during or within 14 days following the administration of monoamine oxidase inhibitors
-
•
Hyperthyroidism
-
•
Glaucoma
-
•
Agitated states
-
•
History of drug abuse
5.3.6. Warnings and precautions [16]
-
•
According to the prescribing information, the safety and efficacy of combination therapy with phentermine and any other drug products for weight reduction including prescribed drugs, over-the-counter preparations, herbal products, or serotonergic agents such as selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline, fluvoxamine, paroxetine), have not been established. Therefore, coadministration of phentermine and these drug products is not recommended.
-
•
Primary Pulmonary Hypertension (PPH) — a rare, frequently fatal disease of the lungs — has been reported to occur in patients receiving a combination of phentermine with fenfluramine or dexfenfluramine
-
•
Valvular Heart Disease: Serious regurgitant cardiac valvular disease, primarily affecting the mitral, aortic, and/or tricuspid valves, has been reported in otherwise healthy persons who had taken a combination of phentermine with fenfluramine or dexfenfluramine for weight reduction. Most evidence supports the cause of the valvulopathy was the fenfluramine or dexfenfluramine component [63].
-
•
The prescribing information states that when tolerance to the anorectant effect develops, the recommended dose should not be exceeded to increase the effect; rather, the drug should be discontinued (See Table 2)
-
•
Phentermine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle
-
•
Phentermine is related chemically and pharmacologically to amphetamine and other related stimulant drugs that have been extensively abused. The prescribing information suggests that the possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. However, in a clinical intervention trial, interruption of long-term treatment with phentermine reportedly did not induce phentermine drug craving, did not result in amphetamine-like withdrawal after abrupt treatment cessation, and did not support psychological dependence (addiction) in patients treated with phentermine for obesity [64].
-
•
Use phentermine with caution in patients with mild hypertension, with post-treatment monitoring of blood pressure recommended
-
•
Although not studied in a prospective, large, randomized, controlled, clinical outcomes trial (e.g., cardiovascular outcome trial), the use of phentermine for longer than 12 weeks is supported by other data and opinion leaders – selectively in patients with obesity at low cardiovascular disease risk [[50], [51], [52],65].
5.4. Semaglutide
5.4.1. Indications and use [42]
-
•
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist
-
•
Drug Enforcement Agency Schedule: Not a scheduled drug
-
•
Subcutaneous injection, pre-filled, 4 single-dose pen pack that delivers doses of 0.25 mg, 0.5 mg, 1.0 mg, 1.7 mg, and a pack of pens containing 2.4 mg to be administered once a week
-
•
Inject subcutaneously in the abdomen, thigh, or upper arm; the injection site and timing can be changed without dose adjustment
-
•
At injectable doses of 0.25–2.0 mg per week, and at oral doses of 7–14 mg per day, semaglutide is indicated to lower blood sugar among patients with type 2 diabetes mellitus
-
•Dosing for obesity:
-
o0.25 mg per week for 4 weeks, then;
-
o0.5 mg per week for 4 weeks, then;
-
o1.0 mg per week for 4 weeks, then;
-
o1.7 mg per week for 4 weeks, then;
-
oThen 2.4 mg per week is the maintenance dose
-
o
5.4.2. Potential Drug Interactions [42]
Semaglutide delays gastric emptying, which may impact absorption of concomitantly administered oral medications.
5.4.3. Pharmacokinetics [42]
After injection, the bioavailability of semaglutide is 89%. Maximum concentration of semaglutide is reached 1–3 days post dose. Similar exposure is achieved with subcutaneous administration of semaglutide in the abdomen, thigh, or upper arm. Semaglutide is extensively bound to plasma albumin (greater than 99%) which results in decreased renal clearance and protection from degradation. The elimination half-life is approximately 1 week; semaglutide will be present in the circulation for about 5–7 weeks after the last dose of 2.4 mg. The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain. The primary excretion routes of semaglutide-related material are via the urine and feces. Approximately 3% of the dose is excreted in the urine as intact semaglutide.
5.4.4. Most common adverse reactions [42]
The most common adverse reactions, reported in greater than or equal to 5% of patients treated with semaglutide, include nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distension, eructation, hypoglycemia in patients with type 2 diabetes (i.e., most often described in patients treated with sulfonylureas or insulin), flatulence, gastroenteritis, and gastroesophageal reflux disease.
5.4.5. Contraindications [42]
-
•
Personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia type 2
-
•
Known hypersensitivity to semaglutide or any of the excipients in semaglutide
5.4.6. Warnings and precautions [42]
-
•
Prescribing information boxed warning regarding risk of thyroid C-cell tumors: In rodents, semaglutide causes thyroid C-cell tumors at clinically relevant exposures. It is unknown whether semaglutide for obesity causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined. Semaglutide for obesity is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors.
-
•
Acute pancreatitis: Semaglutide should be discontinued if pancreatitis is suspected, and not restarted if pancreatitis is confirmed. Once pancreatitis is resolved, and if the pancreatitis was determined to be due to a cause other than semaglutide, and if that cause was successfully treated (e.g., successful surgical treatment of cholecystitis-induced pancreatitis), then restart of semaglutide may be considered, based upon clinical judgement, and followed by close monitoring for recurrent pancreatitis.
-
•
Acute gallbladder disease: If cholelithiasis is suspected, then the patient should undergo gallbladder studies, further clinical evaluation, and potential treatment (i.e., cholecystectomy)
-
•
Hypoglycemia: Concomitant use with insulin or an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing the dose of insulin secretagogue or insulin may be necessary.
-
•
Acute kidney injury, especially in patients reporting severe adverse gastrointestinal reactions or in those with renal impairment reporting severe adverse gastrointestinal reactions
-
•
Hypersensitivity (e.g., anaphylactic reactions and angioedema)
-
•
Associated with diabetes retinopathy in patients with type 2 diabetes mellitus, most described in patients with rapid and substantial lowering of high blood sugar and high baseline hemoglobin A1c [66]. Patients with type 2 diabetes mellitus administered semaglutide should have routine monitoring for potential onset or worsening of diabetes retinopathy. The greatest risk factor for early worsening of diabetes retinopathy is higher hemoglobin A1c at baseline, with other risk factors being long-term uncontrolled diabetes and previous diabetes retinopathy. Little current evidence supports that proactive gradual reduction in glycemia reduces the risk of early worsening of diabetes retinopathy [67,68]. Beyond routine eye exams, the overall clinical message is to engage in early treatment of diabetes mellitus and prevent marked elevations in blood sugar, which can often be achieved with early and aggressive management of the diabetes mellitus and obesity/adiposopathy [[69], [70], [71]].
-
•
Heart rate increase
-
•
Suicidal behavior and ideation
5.4.7. Additional information
-
•
Semaglutide SQ 0.25 mg–2 mg per week is indicated to improve glycemic control in adults with type 2 diabetes mellitus (T2DM) and to reduce the risk of major adverse cardiac events (MACE) in adults with T2DM and cardiovascular disease (SUSTAIN 6 study) [72].
-
•
Semaglutide oral administration titrated to 7–14 mg per day is indicated to treat T2DM and does not have a specific indication to reduce MACE; however, it was shown not inferior to placebo in reducing MACE in patients with T2DM (PIONEER-6) [72].
-
•
Ongoing cardiovascular outcome studies are evaluating oral semaglutide in patients with T2DM (SOUL or “Semaglutide Cardiovascular Outcomes Trial in Patients with Type 2 Diabetes”) and semaglutide 2.4 mg SQ per week in patients with obesity (SELECT or “Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity” - see Section 5.4.8. below).
5.4.8. Semaglutide STEP clinical trials (Semaglutide Treatment Effects in People with obesity)
STEP 1: Once-Weekly Semaglutide in Adults with Overweight or Obesity
[43]:
- •
68-week trial of patients with obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one weight-related complication
- •
Did not include patients with diabetes mellitus
- •
Semaglutide 2.4 mg SQ per week reduced body weight about 15%
- •
Placebo was associated with a body weight reduction of about 2.4%
STEP 2: Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial
[73]:
- •
68-week trial of patients with overweight or obesity and type 2 diabetes mellitus
- •
Semaglutide 2.4 mg SQ per week reduced body weight about 10%
- •
Placebo was associated with a body weight reduction of about 3.4%
- •
Semaglutide 2.4 mg reduced HbA1c by 1.6%; semaglutide 1.0 mg reduced HbA1c by 1.5%; placebo group had a reduction in HbA1c of 0.4%
STEP 3: Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity
[74]:
- •
68-week trial of patients with obesity or overweight with at least one weight-related complication and were administered low calorie diet and intensive behavioral therapy
- •
No diabetes mellitus
- •
First 8 weeks: Low-calorie, meal-replacement diet (1000–1200 kcal/day)
- •
Remaining 60 weeks: 1200–1800 kcal/day of conventional food
- •
Intensive behavior therapy included: (a) regular physical activity with a goal of 200 minutes of exercise per week and (b) 30 individual intensive behavioral therapy sessions with a registered dietitian
- •
Semaglutide 2.4 mg SQ per week plus intensive behavior therapy resulted in a 16% reduction in body weight
- •
Placebo plus intensive behavior therapy resulted in a 5.7% reduction in body weight
- •
The results of this STEP 3 study were compared to the prior STEP 1 study. Quote from the authors: “The STEP 1 trial examined semaglutide, 2.4 mg, combined with a less-intensive lifestyle intervention program that provided behavioral counseling visits every 4 weeks (i.e., 18 sessions in 68 weeks) and no initial low-calorie, meal-replacement diet. Participants in STEP 1 lost 14.9% of baseline weight with semaglutide at 68 weeks, compared with 2.4% for placebo plus the same lifestyle intervention. These findings suggest that the inclusion of intensive behavioral therapy plus an 8-week low-calorie diet ultimately may not contribute significant additional weight reduction beyond that achieved by semaglutide and less-intensive lifestyle intervention” [74].
STEP 4: Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity
[75]:
- •
68-week trial of patients with overweight or obesity without diabetes mellitus, focused on weight reduction maintenance
- •
For first 16 weeks, semaglutide dose was escalated to 2.4 mg SQ per week. Patients on the maintenance dose of 2.4 mg of semaglutide at week 20 were randomized to continue on semaglutide or switch to placebo for the remainder of the trial (48 weeks)
- •
From weeks 20–68, either semaglutide or placebo was administered:
STEP 5: Two-year Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity (STEP 5)
- •
Patients with obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity related complication (no diabetes mellitus)
- •
Durability of weight reduction was 2 years
- •
Semaglutide 2.4 mg SQ per week significantly reduced body weight from baseline to week 104 compared to placebo (15.2% vs. 2.6%; estimated treatment difference: 12.6%).
STEP 6: Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial
[78]:
- •
Patients in East Asia with obesity or overweight with at least one weight-related complication
- •
Compared to placebo, in adults from East Asia with obesity with or without type 2 diabetes, semaglutide 2.4 mg SQ once a week produced superior and clinically meaningful reductions in bodyweight and greater reductions in abdominal visceral fat
STEP 7: Research Study of How Well Semaglutide Works in People Living With Overweight or Obesity (STEP 7)
[79]:
- •
Patients in China, Hong Kong, Korea, and Brazil with obesity or overweight with at least one weight-related complication
- •
Ongoing
STEP 8: Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes
[80]:
- •
68-week trial of patients with obesity or overweight with at least one weight-related complication (without diabetes mellitus) comparing semaglutide 2.4 mg administered SQ once weekly versus liraglutide 3.0 mg administered SQ once daily
- •
Semaglutide: Mean weight reduction was 15.8%
- •
Liraglutide: Mean weight reduction was 6.4% (difference from semaglutide = 9.4%)
- •
Gastrointestinal adverse events were reported by 84.1% with semaglutide and 82.7% with liraglutide.
- •
Conclusion: “Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity, resulted in significantly greater weight reduction at 68 weeks.”
STEP 9: Semaglutide in knee osteoarthritis
[81]:
- •
Placebo-controlled clinical trial of semaglutide 2.4 mg SQ per week as an adjunct to a reduced-calorie diet and increased physical activity in patients with osteoarthritis of the knee
- •
Outcomes are changes in body weight and Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores at week 68
- •
Ongoing
5.4.8.1. Semaglutide Cardiovascular Outcomes Trial in Patients with type 2 diabetes (SOUL trial)
-
•
The SOUL trial is a placebo-controlled trial evaluating the effect of oral semaglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus [82].
-
•
The prior Peptide Innovation for Early Diabetes Treatment (PIONEER) 6 study in patients with type 2 diabetes demonstrated that oral semaglutide was not inferior to placebo regarding cardiovascular disease risk [82]
-
•
The prior Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) demonstrated that once-weekly subcutaneous semaglutide (0.5 or 1.0 mg) reduced cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke compared to placebo [82]
-
•
Ongoing
5.4.8.2. Semaglutide Effects on Heart Disease and Stroke in Patients with overweight or obesity (SELECT) [83]
-
•
Placebo-controlled cardiovascular outcomes trial of semaglutide 2.4 mg subcutaneously once weekly involving patients with overweight or obesity with cardiovascular disease but without diabetes mellitus
-
•
Ongoing
-
•
In addition to the SELECT trial in patients with obesity, two other ongoing trials are evaluating heart failure with preserved ejection fraction, one in patients with obesity and HFpEF (STEP-HFpEF) and the other in patients with obesity and type 2 diabetes mellitus and HFpEF (STEP-HFpEF DM)
5.5. Liraglutide
5.5.1. Indications, use, and dosing [14,15,44,[84], [85], [86]]
-
•
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist
-
•
Drug Enforcement Agency Schedule: Not a scheduled drug
-
•
Solution for subcutaneous injection, pre-filled, multi-dose pen that delivers doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg
-
•
Inject subcutaneously in the abdomen, thigh, or upper arm; the injection site and timing can be changed without dose adjustment
-
•
The lower dose of liraglutide 1.8 mg per day is approved for the treatment of type 2 diabetes mellitus (not type 1 diabetes mellitus or diabetes ketoacidosis). The recommended dose of liraglutide for treatment of obesity is 3.0 mg daily, any time of day, without regard to the timing of meals – but preferably approximately the same time each day.
-
•Dosing: slower dose titration may improve tolerability and gastrointestinal side effects:
-
oWeek 1 = 0.6 mg per day
-
oWeek 2 = 1.2 mg per day
-
oWeek 3 = 1.8 mg per day
-
oWeek 4 = 2.4 mg per day
-
oWeek 5 and onward = 3.0 mg per day
-
o
-
•
Evaluate the change in body weight after 16 weeks and discontinue liraglutide for obesity if the patient has not lost at least 4% of baseline body weight since it is unlikely that the patient will achieve and sustain clinically meaningful weight reduction with continued treatment.
-
•
The liraglutide prescribing information for treatment of type 2 diabetes mellitus (top dose 1.8 mg per day) has an indication to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease.
-
•
The liraglutide prescribing information for treatment of obesity (top dose 3.0 mg per day) has an indication as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index of 30 kg/m2 or greater, or 27 kg/m2 or greater in the presence of at least one adverse consequence of increased adiposity (e.g., type 2 diabetes mellitus, hypertension, or dyslipidemia).
-
•
Liraglutide at the 3.0 mg per day dose is not indicated to treat type 2 diabetes mellitus and should not be used with any other GLP-1 agonist. The safety of coadministration with other weight loss products has not been established.
5.5.2. Potential Drug Interactions [14,15,44,84,85]
-
•
Liraglutide delays gastric emptying. This may impact absorption of concomitantly administered oral medications.
-
•
Liraglutide has low potential for pharmacokinetic drug-to-drug interactions related to cytochrome P450 and plasma-protein binding
5.5.3. Pharmacokinetics [14,15,44,84,85]
-
•
Unlike native GLP-1, liraglutide is stable against metabolic degradation by both neutral endopeptidase and dipeptidyl peptidase IV and has a plasma half-life of 13 hours after subcutaneous administration.
-
•
Liraglutide exposures are similar among three subcutaneous injection sites (upper arm, abdomen, and thigh); absolute bioavailability of liraglutide following subcutaneous administration is approximately 55%.
-
•
Liraglutide is endogenously metabolized similar to large proteins without a specific organ as a major route of elimination.
-
•
Following a [3H]-liraglutide dose (i.e., tritium labeled), intact liraglutide is not detected in urine or feces, with only a minor part excreted as liraglutide-related metabolites in urine or feces (6% and 5%, respectively).
5.5.4. Most common adverse reactions [14,15,44,84,85]
-
•
Nausea
-
•
Hypoglycemia
-
•
Diarrhea
-
•
Constipation
-
•
Vomiting
-
•
Headache
-
•
Decreased appetite
-
•
Dyspepsia
-
•
Fatigue
-
•
Dizziness
-
•
Abdominal pain
-
•
Increased lipase
5.5.5. Contraindications [14,15,44,84,85]
-
•
Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2
-
•
Hypersensitivity to liraglutide or any product components
-
•
Pregnancy
5.5.6. Warnings [44,85]
-
•
Prescribing information boxed warning [14,15]: Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether liraglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined. Liraglutide is contraindicated in patients with a personal or family history of MTC and in patients with multiple endocrine neoplasia type 2 (MEN 2). Counsel patients regarding the potential risk of MTC with use of liraglutide and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, or persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with liraglutide (See Table 2).
-
•
Discontinue promptly if pancreatitis is suspected; do not restart if pancreatitis is confirmed [14,15]. Once pancreatitis is resolved, and if the pancreatitis was determined to be due to a cause other than liraglutide, and if that cause was successfully treated (e.g., successful surgical treatment of cholecystitis-induced pancreatitis), then restart of liraglutide may be considered, based upon clinical judgement, and followed by close monitoring for recurrent pancreatitis.
-
•
If cholelithiasis or cholecystitis are suspected, gallbladder studies and potential treatment (i.e., cholecystectomy) are indicated [14,15].
-
•
Serious hypoglycemia can occur when liraglutide is used with insulin or an insulin secretagogue (i.e., a sulfonylurea). Consider lowering the dose of anti-diabetes drugs to reduce the risk of hypoglycemia [14,15].
-
•
Monitor heart rate at regular intervals to evaluate for possible heart rate increase [44,84,85].
-
•
Renal impairment has been reported post-marketing, usually in association with nausea, vomiting, diarrhea, or dehydration, which may sometimes require hemodialysis. Use caution when initiating or escalating doses of liraglutide in patients with renal impairment [14,15].
-
•
Post-marketing reports exist regarding serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema). If these occur, then liraglutide and other suspect medications should be discontinued, and the patient should be instructed to promptly seek medical advice [14,15].
-
•
Monitor for depression or suicidal thoughts and discontinue liraglutide if symptoms develop [44,84,85].
5.5.7. Other potential benefits
-
•
Liraglutide improves many of the adiposopathic complications of obesity, such as high blood sugar, high blood pressure, and lipid parameters, via weight dependent and weight independent mechanisms [87].
-
•
One of the more common “fat mass” complications of obesity is sleep apnea. The SCALE (Satiety and Clinical Adiposity Liraglutide Evidence) Sleep Apnea clinical trial investigated the effect of liraglutide 3.0 mg on obstructive sleep apnea (OSA). The conclusion was that, as an adjunct to encouraging appropriate nutrition and physical activity, and compared to placebo, liraglutide 3.0 mg was generally well tolerated and produced significantly greater reductions in apnea-hypopnea index, body weight, systolic blood pressure, and hemoglobin A1c among patients with obesity and moderate/severe OSA [88].
-
•
Another common “fat mass” complication of obesity is osteoarthritis. In a smaller study of 168 patients with knee osteoarthritis, weight reduction with liraglutide after 52 weeks did not appear to reduce osteoarthritis knee pain [89]. However, the efficacy of weight reduction on osteoarthritis is dependent upon the severity of the disease at baseline, degree of weight reduction, outcome measured, and the length of the study (See the STEP 9 semaglutide study described above.). Other evidence supports that treating obesity in patients with co-occurring osteoarthritis improves functional status with short-term results similar to joint replacement [90,91].
5.6. Phentermine HCl/topiramate extended release
Initial approval of phentermine HCl/topiramate extended release was accompanied by an agreement with the FDA to establish a Risk Evaluation and Mitigation Strategy (REMS) program to inform prescribers and female patients about the increased risk of congenital malformations (especially orofacial clefts) in infants exposed to phentermine HCl/topiramate extended release during the first trimester of pregnancy. Completion of the FDA-mandated REMS program (https://qsymiarems.com/) may not be required by the FDA prior to prescribing phentermine HCl/topiramate extended release. However, completion of a REMS program may be required by clinician institutions.
5.6.1. Indications and use [12,13,44,45,84,85]
-
•
Drug Enforcement Agency Schedule IV drug
-
•
Phentermine is a shorter-acting sympathomimetic amine approved as monotherapy as an anti-obesity (“weight-management”) medication
-
•
Topiramate is a longer-acting neurostabilizer approved as monotherapy for seizure disorders and migraine headache prevention
-
•Doses: Once daily in the morning with or without food
-
oStarting dose = 3.75 mg/23 mg (phentermine/topiramate extended release)
-
oAfter 14-day intervals, and as clinically indicated, escalate doses to:
-
⁃Recommended dose = 7.5 mg/46 mg
-
⁃Titration dose = 11.25 mg/69 mg
-
⁃Top dose = 15 mg/92 mg
-
⁃
-
o
-
•
Patients discontinuing the top dose of phentermine HCl/topiramate extended release 15 mg/92 mg should gradually taper the dose to reduce the possibility of precipitating a seizure. The prescribing information does not specify that lower medication doses need to be tapered upon discontinuation.
5.6.2. Potential Drug Interactions [12,13,44,45,84,85]
-
•
May alter the exposure to oral contraceptives, causing irregular menstrual bleeding but not an increased risk of pregnancy. Oral contraceptives should not be discontinued if spotting occurs.
-
•
May potentiate central nervous system depressants such as alcohol. Thus, patients should avoid concomitant alcohol.
-
•
May potentiate hypokalemia of non-potassium-sparing diuretics
-
•
Phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive crisis
5.6.3. Pharmacokinetics [45,84]
-
•
Phentermine is metabolized by the liver, with most excreted by the kidney
-
•
Topiramate is excreted mainly by the kidney
5.6.4. Most common adverse reactions [12,13,44,45,84]
-
•In clinical trials, adverse reactions occurring more than or equal to 5% of the time included:
-
oParesthesia
-
oDizziness
-
oDysgeusia (taste distortion/perversion)
-
oInsomnia
-
oConstipation
-
oDry mouth
-
o
5.6.5. Laboratory abnormalities may include [12,13,45,85]
-
•
Metabolic acidosis
-
•
Elevated creatinine
-
•
Lowering of glucose levels
-
•
Hypokalemia in patients treated with non-potassium-sparing diuretics
5.6.6. Contraindications [45]
-
•
Pregnancy
-
•
Glaucoma
-
•
Hyperthyroidism
-
•
During or within 14 days of taking monoamine oxidase inhibitors
-
•
Hypersensitivity or idiosyncrasy to sympathomimetic amines
5.6.7. Warnings and precautions [12,13,44,45,84,85]
-
•
Pregnancy testing is recommended before initiating phentermine HCl/topiramate ER and monthly during therapy. Women who can become pregnant should be advised of the potential risk to a fetus and to use effective contraception during phentermine HCl/topiramate ER therapy. Guidance regarding birth defect risks and mitigation (i.e., effective contraception) can be found at: https://qsymiarems.com/(last accessed April 7, 2022).
-
•
Monitor for increased heart rate (especially patients with cardiovascular disease), depression or suicidal thoughts, acute myopia and secondary angle closure glaucoma, mood and sleep disorders, cognitive impairment (with cautioned initial use in patients operating automobiles or hazardous machinery), metabolic acidosis, elevated creatinine, and possible low blood sugar in patients treated with anti-diabetes medications.
-
•
Phentermine HCl/topiramate extended release should be discontinued or considered for discontinuation in patients with increases in adrenergic responses, such as increase in heart rate (especially in those with cardiac and/or cerebrovascular disease), suicidal behavior and ideation, acute myopia and secondary angle-closure glaucoma, unacceptable mood and sleep disorders, cognitive impairment, pregnancy, or nursing.
-
•
May increase risk of kidney stones and hypokalemia
-
•
May increase the risk of oligohidrosis and hyperthermia
5.6.8. Glaucoma
Glaucoma is damage to the optic nerve, most often with increased intraocular pressure. As opposed to the vitreous humor filling the posterior vitreous chamber, aqueous humor is the clear fluid in the anterior chamber of the eye (i.e., between the cornea and the iris). Aqueous humor is produced by the ciliary body, flows from the eye's posterior chamber, between the iris and lens, through the pupil into the anterior chamber, and then drained through trabecular meshwork between iris and cornea. Open angle glaucoma represents about 90% of glaucoma and is due to clogged drainage canals. The angle remains open between iris and cornea, and intraocular pressure increases. Angle closure glaucoma is rarer and is either primary or due to a secondary cause (e.g., drugs such as topiramate, anticholinergics, adrenergic agonists, some antidepressants, and sulfonamides) [92]. The ciliary body may become edematous or displaced, pushing the iris towards the cornea, and closing the angle between them, blocking the drainage of aqueous humor through the trabecular meshwork, increasing intraocular pressure, and causing headache and decreased vision (often emergent). In patients with onset of myopia or signs/symptoms of glaucoma (e.g., eye pain or pressure, scleral erythema, visual halos around lights, impaired vision, headache, nausea), urgent diagnosis is indicated to confirm glaucoma, type of glaucoma, and the most likely etiology. If days to weeks after start of topiramate, a patient experiences myopia and signs/symptoms of glaucoma, and if a diagnosis of drug-induced angle closure glaucoma is made, then discontinuing topiramate may result in recovery within one week if the glaucoma was due to the topiramate [93,94].
5.7. Naltrexone HCl/bupropion HCl extended release
5.7.1. Indications and use [17,44,84,85]
-
•
Naltrexone is an opioid antagonist
-
•
Bupropion is an aminoketone antidepressant with relatively weak inhibition of neuronal reuptake of norepinephrine and dopamine
-
•
Drug Enforcement Agency Schedule: Not a scheduled drug
-
•
Tablets = 8 mg/90 mg (naltrexone HCl/bupropion HCl extended release)
-
•
The tablets should not be cut, chewed, or crushed
-
•Dosing:
-
oWeek 1 = 1 tablet in AM, no tablets in PM
-
oWeek 2 = 1 tablet in AM, 1 tablet in PM
-
oWeek 3 = 2 tablets in AM, 1 tablet in PM
-
oWeek 4 and beyond = 2 tablets in AM, 2 tablets in PM (∼12 hours after AM dose)
-
o
5.7.2. Potential Drug Interactions
-
•
Should not be administered with opioids due to naltrexone component, which is an opioid receptor antagonist (all opioids should be discontinued at least 7 days prior to start of naltrexone HCl/Bupropion HCl) [84].
-
•
Monoamine oxidase inhibitors may increase the risk of hypertensive reactions when used concomitantly [17,84].
-
•Drugs metabolized by CYP2D6 may be affected [17]:
-
oBupropion inhibits CYP2D6 and can increase concentrations of antidepressants, (e.g., selective serotonin reuptake inhibitors and many tricyclics), antipsychotics (e.g., haloperidol, risperidone and thioridazine), beta-blockers (e.g., metoprolol), and type 1C antiarrhythmics (e.g., propafenone and flecainide)
-
o
-
•
Digoxin levels may be decreased [17].
-
•
Concomitant treatment with CYP2B6 inhibitors (e.g., ticlopidine or clopidogrel) can increase bupropion exposure (do not exceed one tablet twice daily when taken with CYP2B6 inhibitors) [17].
-
•
Avoid concomitant use with CYP2B6 inducers (e.g., ritonavir, lopinavir, efavirenz, carbamazepine, phenobarbital, and phenytoin), which may reduce efficacy by reducing bupropion exposure [17].
-
•
Drugs that lower seizure threshold should be used with caution [17,84].
-
•
Dopaminergic drugs (levodopa and amantadine) can cause central nervous system toxicity [17].
-
•
Naltrexone HCl/Bupropion HCl Extended Release can cause false positive urine test results for amphetamines [17].
5.7.3. Pharmacokinetics [17,44,84,85]
-
•
Both parent and the 6-beta-naltrexol metabolite are active
-
•
Naltrexone and 6-beta-naltrexol are not metabolized by cytochrome P450 enzymes
-
•
Naltrexone and its metabolites are excreted primarily by the kidney. In patients with moderate or severe renal impairment, the maximum recommended daily dose of naltrexone HCl/bupropion HCl extended release is two tablets (one tablet each morning and evening). Naltrexone HCl/bupropion HCl extended release is not recommended in patients with end-stage renal disease.
-
•
In patients with moderate hepatic impairment, the maximum recommended daily dose of naltrexone HCl/bupropion HCl extended release is two tablets (one tablet each morning and evening). Naltrexone HCl/bupropion HCl extended release is not recommended in patients with severe hepatic impairment.
-
•
CYP2B6 is the principal isozyme involved in the formation of hydroxybupropion (bupropion metabolite), whereas cytochrome P450 isozymes are not involved in the formation of the other active metabolites
-
•
Bupropion and its metabolites inhibit CYP2D6
-
•
Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively
5.7.4. Most common adverse reactions [17,44,84,85]
-
•
Nausea
-
•
Constipation
-
•
Headache
-
•
Vomiting
-
•
Dizziness
-
•
Insomnia
-
•
Dry mouth
-
•
Diarrhea
5.7.5. Contraindications [17]
-
•
Uncontrolled hypertension
-
•
Seizure disorders, anorexia nervosa or bulimia, or undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs
-
•
Use of other products containing bupropion
-
•
Chronic opioid use
-
•
During or within 14 days of taking monoamine oxidase inhibitors
-
•
Known allergy to any of its ingredients
-
•
The prescribing information recommends discontinuation of naltrexone HCl/bupropion HCl ER when pregnancy is recognized
5.7.6. Warnings [17,44,84,85]
-
•
Monitor for depression or suicidal thoughts and discontinue naltrexone HCl/bupropion HCl if these symptoms develop
-
•
Patients may experience changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, agitation, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Patients who develop neuropsychiatric adverse events should discontinue naltrexone HCl/bupropion HCl and contact a healthcare provider.
-
•
Bupropion is used for the treatment of depression. Antidepressant treatment can precipitate a manic, mixed, or hypomanic episode, with apparent increased risk in patients with bipolar disorder or who have risk factors for bipolar disorder. No activation of mania or hypomania was reported in the clinical trials of naltrexone HCl/bupropion HCl patients for treatment of obesity; however, patients receiving antidepressant medications and patients with a history of bipolar disorder or recent hospitalization because of psychiatric illness were excluded from naltrexone HCl/bupropion HCl clinical trials. Prior to initiating naltrexone HCl/bupropion Cl ER, patients should be screened for bipolar disorder and bipolar disorder risk factors. Naltrexone HCl/bupropion HCl is not approved for treating bipolar depression.
-
•Risk of seizure may be minimized by:
-
oAdhering to the recommended dosing schedule and avoiding co-administration with high-fat meals
- o
-
oBeing cautious when using in patients at risk of seizures, such as patients with cerebrovascular disorders (e.g., acute stroke, hypoxia, brain lesions), brain trauma, infections, metabolic abnormalities (e.g., hypoglycemia, electrolyte abnormalities), and excessive alcohol use [97].
-
o
-
•
Monitor blood pressure and heart rate, especially those with cardiac or cerebrovascular disease
-
•
Hepatotoxicity: Cases of hepatitis and clinically significant liver dysfunction have been observed with naltrexone exposure
-
•
Angle-closure glaucoma has occurred in patients with untreated anatomically narrow angles treated with antidepressants
-
•
Weight reduction may cause hypoglycemia in patients treated with anti-diabetes mellitus medications such as sulfonylureas or insulin. Glucose levels should be monitored
5.8. Orlistat
5.8.1. Indications and use [18,98]
-
•
Gastrointestinal lipase inhibitor
-
•
Not a Drug Enforcement Agency scheduled drug
-
•
Dose: One 120-mg capsule three times a day with each main meal containing fat (during or up to 1 hour after the meal)
-
•
An over-the-counter formulation is available at 60 mg capsule with each meal containing fat
5.8.2. Potential Drug Interactions [18,44,60,85,98]
-
•
Cyclosporine plasma levels may be reduced when orlistat is co-administered with cyclosporine; cyclosporine should be taken at least 3 hours before or after orlistat in patients taking both drugs
-
•
A 30% reduction in beta-carotene supplement absorption occurs when concomitantly administered with orlistat
-
•
Orlistat inhibits absorption of a vitamin E acetate supplements by approximately 60%
-
•
Vitamin K levels tend to decline in subjects taking orlistat; therefore, patients on chronic stable doses of warfarin who are prescribed orlistat should be monitored closely for changes in coagulation parameters
-
•
There have been post-marketing reports of hypothyroidism with orlistat and levothyroxine; levothyroxine and orlistat should be administered at least 4 hours apart
5.8.3. Pharmacokinetics [18,98]
-
•
Systemic exposure to orlistat is minimal
-
•
It is likely the metabolism of orlistat occurs mainly within the gastrointestinal wall
5.8.4. Most common adverse reactions [18,98]
Oily discharge from the rectum, flatus with discharge, increased defecation, and fecal incontinence.
5.8.5. Contraindications [18]
Orlistat is contraindicated in patients with chronic malabsorption syndrome or cholestasis, and in patients with known hypersensitivity to orlistat or to any component of this product.
5.8.6. Warnings and precautions [18,44,48,60,85]
-
•
Gastrointestinal events may increase when orlistat is taken with a diet high in fat (>30% total daily calories from fat). The daily intake of fat should be distributed over three main meals.
-
•
Patients should be strongly encouraged to take a multivitamin supplement that contains fat-soluble vitamins to ensure adequate nutrition because orlistat has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene. In addition, the levels of vitamin D and beta-carotene may be low in patients with obesity. Vitamin supplements should be taken once a day at least 2 hours before or after the administration of orlistat, such as at bedtime.
-
•
May increase risk of cholelithiasis
-
•
May increase risk of urinary oxalate and kidney stones
-
•
There have been rare post-marketing reports of severe liver injury and pancreatitis
5.9. Non-systemic superabsorbent oral hydrogel
5.9.1. Description and mechanism of action [99]
-
•
Biodegradable oral non-systemic superabsorbent hydrogel made from cross-linked carboxymethylcellulose and citric acid that promotes fullness and may help to increase satiety and help with weight management
-
•
Each capsule contains thousands of superabsorbent hydrogel particles (0.75 g per capsule), and each particle is approximately the size of a grain of salt
-
•
The capsules disintegrate in the stomach and release the enclosed hydrogel particles, which can then hydrate up to 100 times their original weight
-
•
When fully hydrated, the individual non-clustering hydrogel particles occupy about a quarter of average stomach volume
-
•
The gel particles mix with ingested foods, creating a larger volume with higher elasticity and viscosity in the stomach and small intestine, promoting satiety and fullness
-
•
The hydrogel particles are partially degraded enzymatically in the colon, releasing most of the absorbed water and subsequently being excreted in the feces
-
•
It is ingested orally similar to drugs but regulated by the FDA as a class II medical device because it acts through mechanical modes of action
5.9.2. Indications and use [99]
-
•
Indicated to aid in weight management in adults with pre-obesity (overweight) and/or obesity having a body mass index (BMI) 25–40 kg/m2 when used in conjunction with appropriate nutrition (“diet”) and physical activity (“exercise”)
-
•
Three capsules (2.25 g/dose) are administered with water 20–30 minutes before lunch and dinner
-
•
Each individual pod holds a single dose of three (3) capsules, to be administered with water before lunch and dinner/supper
-
•
Fourteen (14) pods are supplied in a weekly tube
-
•Patients should follow these steps:
-
oSwallow 3 capsules with water
-
oAfter taking the capsules, drink 2 additional glasses of water (8 fluid ounces/250 mL each), for a total of 16 ounces of water
-
oWait 20–30 minutes to begin the meal.
-
o
-
•
If a pre-meal dose is missed, the hydrogel capsules should be taken immediately after that meal
5.9.3. Potential Drug Interactions [99]
-
•
The effect of the hydrogel capsule device on all concomitant medications is not known; all medications that are taken once daily should be taken in the morning (fasting or with breakfast) or at bedtime
-
•
If a patient is taking a concomitant medication with meals or close to meals, the prescriber should consider whether the risk of incorrect dosing, especially for narrow therapeutic drugs, is outweighed by the potential benefit
-
•
For all medications that should be taken with food, the concomitant medication should be taken after the meal has started
-
•
For patients who take metformin with meals, glycemic control should be monitored after initiation of the hydrogel capsule device to determine if changes are indicated for glucose control, because the hydrogel may have an effect on metformin absorption similar to the effect of concomitant food
5.9.4. Pharmacokinetics [99]
-
•
The hydrogel capsule device passes through the digestive system, maintaining its three-dimensional structure in the stomach and small intestine before breaking down in the colon. The water is then released and reabsorbed by the body
-
•
The hydrogel capsule device particles are eliminated through normal bowel movements (not systemically absorbed)
5.9.5. Most Common Adverse Reactions [99]
-
•
Common side effects are mainly gastrointestinal, including abdominal pain, constipation, flatulence, infrequent bowel movements, abdominal distention, diarrhea, and nausea. Most of these side effects are mild or moderate in intensity, occurring within the first 3 months and resolving in 2 weeks
-
•
Clinical trials support that the adverse experiences with the hydrogel capsule device are similar to the placebo group
5.9.6. Contra-indications [99]
-
•
Pregnancy
-
•
History of allergic reaction to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium oxide
5.9.7. Warnings [99]
-
•
Keep out of reach of children
-
•
May alter the absorption of medications
-
•
Do not use after expiration date printed on the product packaging
5.9.8. Precautions [99]
-
•
Patients should contact a healthcare provider (HCP) immediately if a severe or continued adverse event occurs
-
•
If severe allergic reactions, severe abdominal pain, or severe diarrhea occur, then patients should discontinue the product and speak with an HCP
-
•
Patients with dysphagia upon swallowing other capsules are likely to have difficulty swallowing these capsules
-
•
Patients should not consume the hydrogel capsule device if the package is damaged
-
•
Capsules should be discarded if any capsules are broken, crushed, or damaged
-
•
Use with caution in patients with active gastrointestinal conditions such as gastro-esophageal reflux disease (GERD), ulcers, or heartburn
-
•Avoid using in patients with the following conditions:
-
oEsophageal anatomic anomalies, including webs, diverticula, and rings
-
oSuspected strictures (such as patients with Crohn's disease)
-
oComplications from prior gastrointestinal surgery that could affect GI transit and motility
-
o
-
•
The hydrogel capsule device is not a food substitute; it is not absorbed by the body and therefore has no nutritional or caloric value
-
•
The hydrogel capsule device should be taken under the direction of an HCP as part of a structured weight reduction program. Failure to adhere to prescribed dietary and exercise instructions may result in failure to lose weight.
5.10. Setmelanotide
5.10.1. Indications and use [100]
-
•
Setmelanotide is a melanocortin-4 receptor agonist indicated for chronic weight management in adult and pediatric patients 6 years of age and older with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency. The condition must be confirmed by genetic testing demonstrating variants in POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of variant of uncertain significance (VUS).
-
•Setmelanotide is not indicated for the treatment of patients with the following conditions, as setmelanotide would not be expected to be effective:
-
oObesity due to suspected POMC, PCSK1, or LEPR deficiency with POMC, PCSK1, or LEPR variants classified as benign or likely benign
-
oOther types of obesity not related to POMC, PCSK1 or LEPR deficiency, including obesity associated with other genetic syndromes and general (polygenic) obesity
-
o
-
•
Discontinue setmelanotide if, after 12–16 weeks of treatment, the patient has not lost at least 5% of baseline body weight or 5% of baseline BMI for patients with continued growth potential.
5.10.2. Dosing [100]
Dosage in Adult and Pediatric Patients 12 Years of Age or Older:
-
•
The starting dose is 2 mg (0.2 mL) injected subcutaneously once daily for 2 weeks. Monitor patients for gastrointestinal (GI) adverse reactions.
-
•
If the starting dose is not tolerated, reduce to 1 mg (0.1 mL) once daily. If the 1 mg once daily dose is tolerated and additional weight reduction is desired, titrate to 2 mg (0.2 mL) once daily.
-
•
If the 2 mg daily dose is tolerated, increase the dose to 3 mg (0.3 mL) once daily. If the 3 mg once daily dose is not tolerated, maintain administration of 2 mg (0.2 mL) once daily.
Dosage in Pediatric Patients 6 to Less than 12 Years of Age:
-
•
The starting dose is 1 mg (0.1 mL) injected subcutaneously once daily for 2 weeks. Monitor patients for GI adverse reactions.
-
•
If the starting dose is not tolerated, reduce to 0.5 mg (0.05 mL) once daily dose. If the 0.5 mg once daily dose is tolerated and additional weight reduction is desired, titrate to 1 mg (0.1 mL) once daily.
-
•
If the 1 mg dose is tolerated, increase the dose to 2 mg (0.2 mL) once daily
-
•
If the 2 mg once daily dose is not tolerated, reduce to 1 mg (0.1 mL) once daily. If the 2 mg once daily dose is tolerated and additional weight reduction is desired, the dose may be increased to 3 mg (0.3 mL) once daily.
5.10.3. Warnings and precautions [100]
-
•
Disturbance in sexual arousal: Spontaneous penile erections in males and sexual adverse reactions (i.e., labial hypersensitivity) in females occurred with setmelanotide. Inform patients that these events may occur and instruct patients who have an erection lasting longer than 4 hours to seek emergency medical attention.
-
•
Depression and suicidal ideation: Depression and suicidal ideation have occurred with setmelanotide. Monitor patients for new onset or worsening depression. Consider discontinuing setmelanotide if patients experience suicidal thoughts or behaviors.
-
•
Skin Pigmentation and Darkening of Pre-Existing Nevi: Setmelanotide may cause generalized increased skin pigmentation and darkening of pre-existing nevi. Perform a full body skin examination prior to initiation and periodically during treatment to monitor pre-existing and new pigmentary lesions.
-
•
Risk of Serious Adverse Reactions Due to Benzyl Alcohol Preservative in Neonates and Low Birth Weight Infants: Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions, including “gasping syndrome,” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs.
5.10.4. Adverse reactions [100]
-
•
The most common adverse reactions (incidence ≥23%) were injection site reactions, skin hyperpigmentation, nausea, headache, diarrhea, abdominal pain, back pain, fatigue, vomiting, depression, upper respiratory tract infection, and spontaneous penile erection
5.10.5. Drug interactions [100]
-
•
Setmelanotide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 (CYP), transporters, and plasma protein binding
-
•
No clinical studies evaluating the drug-drug interaction potential of setmelanotide have been conducted
5.10.6. Pharmacokinetics [100]
-
•
Elimination half-life (t½) is approximately 11 hours
-
•
Metabolized into small peptides by catabolic pathways
-
•
Approximately 39% of the administered setmelanotide dose is excreted unchanged in urine
-
•
No clinically significant differences in the pharmacokinetics of setmelanotide were observed based on sex
-
•
The effect of age 65 years or older, pregnancy, or hepatic impairment on the pharmacokinetics of setmelanotide is unknown
5.11. Metreleptin subcutaneous injection
5.11.1. Indications and use [101]
-
•
Leptin analog
-
•
Adjunct to diet as a replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy
-
•
Safety and effectiveness of metreleptin for partial lipodystrophy have not been established
-
•
Not indicated to treat patients with nonalcoholic steatohepatitis (NASH), HIV-related lipodystrophy, or metabolic disease in patients with generalized lipodystrophy
-
•
Not a DEA scheduled drug
-
•
Dose for men >40 kg: 2.5 mg daily, increase by 1.25–2.5 mg/day to a maximum 10 mg/day
-
•
Dose for women >40 kg: 5 mg daily, increase by 1.25–2.5 mg/day to a maximum 10 mg/day
5.11.2. Potential Drug Interactions [101]
-
•
May potentially alter cytochrome p450 enzymes, which should be considered regarding oral contraceptives and/or drugs with narrow therapeutic index
5.11.3. Pharmacokinetics [101]
-
•
Renal clearance may be a major route of metreleptin elimination
-
•
Anti-metreleptin antibodies with neutralizing activity may inhibit its action
5.11.4. Most common adverse reactions [101]
-
•
Headache, hypoglycemia, decreased weight, abdominal pain
5.11.5. Contra-indications [101]
-
•
Hypersensitivity
-
•
General obesity not associated with congenital leptin deficiency
5.11.6. Warnings and precautions [101]
-
•
Metreleptin is available through a REMS program (https://www.myaleptrems.com/).
-
•
T-cell lymphoma: Carefully consider the benefits and risks of treatment with metreleptin in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy.
-
•
Hypoglycemia: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary. Closely monitor blood glucose in patients on concomitant insulin or insulin secretagogue therapy.
-
•
Autoimmunity: Autoimmune disorder progression has been observed in patients treated with metreleptin. Carefully consider benefits and risks of metreleptin treatment in patients with autoimmune disease.
-
•
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with metreleptin. In patients with severe infections or loss of efficacy, clinicians should consider testing for anti-metreleptin antibodies with neutralizing activity.
-
•
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with setmelanotide
-
•
Hypersensitivity: Hypersensitivity reactions (e.g., urticaria or generalized rash) have been reported. Patient should promptly seek medical advice regarding suspected reactions
5.12. Lisdexamfetamine dimesylate
-
•Lisdexamfetamine dimesylate is a central nervous system stimulant indicated for the treatment of [102]:
-
oModerate to severe binge-eating disorder (BED)
-
oAttention deficit hyperactivity disorder (ADHD)
-
o
-
•Limitations [102]:
-
oNot indicated for weight reduction; safety and effectiveness for the treatment of obesity have not been established
-
o
-
•
Drug Enforcement Agency Schedule II drug [102].
-
•Dosing for BED: Once in the morning with or without food. Avoid afternoon doses. Capsule may be opened and mixed with yogurt, water, or orange juice (see drug interactions) [102]. Dosing for different situations includes [102]:
-
oStarting dose = 30 mg every morning for one week
-
oTitration dose = slowly titrate at 20 mg increments from 30 mg per day to 50 or 70 mg per day
-
oRecommended dose = 50–70 mg every morning
-
oSevere renal impairment: Maximum dose is 50 mg per day
-
oEnd-stage renal disease: Maximum dose is 30 mg per day
-
o
5.12.1. Potential Drug Interactions [102]
-
•Agents that alter urinary pH can alter blood levels of amphetamine
-
oAcidifying agents decrease amphetamine blood levels (e.g., ascorbic acid)
-
oAlkalinizing agents increase amphetamine blood levels (e.g., sodium bicarbonate)
-
oConcurrent administration with monoamine oxidase (MAO) inhibition may contribute to hypertensive crisis
-
o
5.12.2. Pharmacokinetics [102]
-
•
Lisdexamfetamine is rapidly absorbed from the gastrointestinal tract; it is converted to dextroamphetamine and l-lysine primarily in the blood due to the hydrolytic activity of red blood cells
-
•
Lisdexamfetamine is not metabolized by cytochrome P450 enzymes
-
•
Approximately 96% of oral dose radioactivity is recovered in the urine (42% related to amphetamine, 25% to hippuric acid, and 2% to intact lisdexamfetamine)
-
•
Plasma elimination half-life is less than one hour
5.12.3. Most common adverse reactions [102]
-
•
Anorexia
-
•
Anxiety
-
•
Decreased appetite
-
•
Decreased weight
-
•
Diarrhea
-
•
Dizziness
-
•
Dry mouth
-
•
Irritability
-
•
Insomnia
-
•
Nausea
-
•
Upper abdominal pain
-
•
Vomiting
-
•
Increased heart rate
-
•
Constipation
-
•
Feeling jittery
5.12.4. Contraindications [102]
-
•
Central nervous system stimulants (amphetamines and methylphenidate-containing products), including lisdexamfetamine dimesylate, have high potential for abuse and dependence. Risk of abuse should be assessed prior to prescribing and patients should be monitored for signs of abuse and dependence while on therapy.
-
•
Known hypersensitivity (e.g., anaphylactic reactions, Stevens-Johnson Syndrome, angioedema, and urticarial) to amphetamine products or other ingredients in lisdexamfetamine dimesylate
-
•
Use with monoamine oxidase (MAO) inhibitor or within 14 days of the last MAO inhibitor dose
5.12.5. Warnings [102]
-
•Serious cardiovascular reactions:
-
oDue to reports of sudden death in children and adolescents with serious heart problems, as well as sudden death, stroke, and myocardial infarction in adults, avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmia, or coronary artery disease.
-
o
-
•Blood pressure or heart rate increases:
-
oBlood pressure and pulse should be monitored. Benefits and risks should be considered before use in patients for whom blood pressure increases may be problematic.
-
o
-
•Psychiatric adverse reactions:
-
oMay cause psychotic or manic symptoms in patients with no prior history or exacerbation of symptoms in patients with pre-existing psychosis. Evaluate for bipolar disorder prior to stimulant use.
-
o
-
•Suppression of growth:
-
oHeight and weight should be monitored in pediatric patients during treatment.
-
o
-
•Peripheral vasculopathy, including Raynaud's phenomenon:
-
oStimulants are associated with peripheral vasculopathy, including Raynaud's phenomenon. Careful observations for digital changes are necessary during treatment with stimulants.
-
oSerotonin syndrome: Increased risk when administered with serotonergic agents (e.g., SSRI, SNRI, triptans)
-
o
5.13. Comparative efficacy of anti-obesity medications (see Charts 1 and 2)
Chart 2.
Estimated degree of mean weight reduction associated with anti-obesity medications, as well as the percent achievement of weight reduction ≥5, ≥10%, ≥15%, and ≥20%.
| Anti-obesity medication | Mean percent and categorical percent weight reductions∗ | Notes | References |
|---|---|---|---|
| Phentermine 15 mg per day (oral) | Overall mean = 7% ≥5% = 46% ≥10% = 21% ≥15% = NA |
The placebo group had a 2% mean weight reduction, with 16% and 7% achieving ≥5% and ≥10% weight reduction, respectively | [37] [38] |
| Semaglutide 2.4 mg subcutaneously once weekly | Overall mean = 15% ≥5% = 86% ≥10% = 69% ≥15% = 51% ≥20% = 32% |
The placebo group had a 2% mean weight reduction, with 32%, 12%, 5% and 2% achieving ≥5%, ≥10%, ≥15%, and ≥20% categorical weight reduction, respectively | [43] |
| Liraglutide 3.0 mg subcutaneously once daily | Overall mean = 8% ≥5% = 63% ≥10% = 33% ≥15% = 14% |
The placebo group had a 3% mean weight reduction, with 27%, 11%, and 4% achieving ≥5%, ≥10%, and ≥15% categorical weight reduction, respectively | [103] |
| Phentermine HCl/Topiramate Extended Release (oral) (top dose = phentermine 15 mg/92 mg topiramate) |
EQUATE 28-week study: [38] Overall mean = 9% ≥5% = 66% top dose ≥10% = 41% top dose ≥15% = NA SEQUEL 56-week extension study: [104] Overall mean = 10% ≥5% = 79% top dose ≥10% = 54% top dose ≥15% = 32% top dose ≥20% = 15% top dose |
EQUATE 28-week study: [38] The placebo group had a 2% mean weight reduction, with 16% and 7% achieving ≥5% and ≥10% categorical weight reduction respectively SEQUEL 56-week extension study: [104] The placebo group had a 2% mean weight reduction, with 30%, 12%, 7%, and 2% achieving ≥5%, ≥10%, ≥15% and ≥20% categorical weight reduction respectively |
[38] [104] |
| Naltrexone sustained release (SR) 32 mg/day plus bupropion SR 360 mg/day (oral) | Overall mean = 7% ≥5% = 56% ≥10% = 27% ≥15% = 10% |
The placebo group had a 2% mean weight reduction with 18%, 7%, and 2% achieving ≥5%, ≥10%, and ≥15% categorical weight reduction, respectively | [47] |
| Orlistat 120 mg three times per day (oral) | Overall mean = 9% ≥5% = 66% ≥10% = 39% ≥15% = NA |
The placebo group had a 6% weight reduction with 44% and 25% achieving >5% and >10% categorical weight reduction, respectively | [105] |
| Non-systemic Oral Hydrogel, three 2.25-g capsules before lunch and dinner (oral) | Overall mean = 6% ≥5% = 59% ≥10% = 27% ≥15% = NA |
The placebo group had a 4% mean weight reduction with 42% and 15% achieving ≥5% and ≥10% categorical weight reduction, respectively | [106] |
| Tirzepatide (subcutaneous once a week) Approved and indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus, but investigational for treatment of obesity at the time of publication ∗∗ | Overall mean = 21% (15 mg) ∗∗ ≥5% = 91% 15mg ≥20% = 57% 15mg |
The placebo group had a 3% mean weight reduction with 35% and 3% achieving ≥5% and ≥20% categorical weight reduction, respectively | ∗∗ |
NA = Not available (data was not found).
∗ The values in this chart are not intended to represent head-to-head comparisons. Data are derived from different studies. In most cases, the percent weight reductions were dose dependent. Therefore, the listed mean values may be less than the percent weight reduction with the highest doses of anti-obesity medications.
∗∗ Preliminary treatment-regimen estimand results from a News Release on April 28, 2022: https://investor.lilly.com/news-releases/news-release-details/lillys-tirzepatide-delivered-225-weight-loss-adults-obesity-or. Tirzepatide Prescribing information: https://pi.lilly.com/us/mounjaro-uspi.pdf?s=pi.
As true with pharmacotherapy for other metabolic diseases:
-
•
Anti-obesity medications vary in their weight reduction efficacy and safety
-
•
Anti-obesity medications vary in their efficacy in improving metabolic diseases
-
•
Individual variances exist in body weight responses to anti-obesity medications
-
•
Individual variances exist in metabolic disease responses to anti-obesity medications
-
•
Shared decisions regarding the most appropriate anti-obesity medications includes weight reduction efficacy, metabolic disease efficacy, safety, tolerability, indications, contraindications, limitations of use, warnings and precautions, dosing, route of administration, cost, and availability.
-
•
Anti-obesity pharmacotherapies often have therapeutic properties that improve metabolic diseases beyond weight reduction alone (i.e., weight-dependent and weight-independent effects) [87].
6. Combination anti-obesity medications
6.1. Obesity and glucose transporters
Sodium glucose transport (SGLT)-based combination therapies:
-
•
SGLT-based therapies are not approved as anti-obesity medications
-
•
Meta-analyses of the SGLT2 inhibitors dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin support modest body weight reduction (∼1–2 kg), but the weight reduction is limited among patients with type 2 diabetes [107].
Facilitative passive glucose transporters [108]:
-
•
12 isoforms (GLUT1-12)
-
•
GLUT-2: Liver, pancreatic islets, kidney, and small intestine
-
•
GLUT-4: Skeletal muscle, heart, and adipose tissue
Sodium-linked active glucose transporters [108]:
-
•
6 isoforms (SGLT1-6)
-
•
SGLT-1: Small intestine and kidney proximal tubule
-
•
SGLT-2: Proximal tubule of the kidney
-
•
Canagliflozin (SGLT2 inhibitor) can reduce body weight in patients with pre-obesity/overweight and obesity, even without diabetes mellitus [109].
-
•
SGLT-2 inhibitors contribute to lower glucose levels, promote negative caloric balance, but do not increase insulin secretion
Ketosis with SGLT2 inhibitors [108]:
-
•
Along with fasting, starvation, carbohydrate restricted diets, and poorly controlled type 1 diabetes mellitus, SGLT-2 inhibitors are associated with ketonemia
-
•
Relative or absolute deficiency of insulin and/or glucose promotes preferential metabolism of fats/triglycerides into glycerol and 3 fatty acids (via activation of adipocyte hormone sensitive lipase).
-
•
Increased free fatty acid delivery to the liver (mitochondrial oxidation) generates ketones (e.g., acetone, acetoacetone, and beta-hydroxybutyrate).
-
•
Mild ketosis due to carbohydrate restricted dietary ketosis may have favorable health effects (i.e., decreased hunger; treatment of epilepsy; fatty acids are the main fuel for the heart, which has little glycogen)
-
•
The prescribing information of SGLT-2 inhibitors describe reports of diabetes ketoacidosis in patients with type 2 diabetes mellitus
-
•
Carbohydrate restricted ketogenic diets may increase the risk of SGLT2i-induced ketoacidosis [110].
Weight loss with SGLT2 inhibitors.
-
•
Canaglifozin is illustrative of an SGLT-2 inhibitor that produces dose-dependent modest weight reduction (1–3%) in patients without diabetes due to glucosuria from impairment of proximal renal tubule glucose reabsorption. As with other SGLT-2 inhibitors, canagliflozin is associated with genital mycotic infections [109].
-
•
Lincogliflozin is an investigational combination effect, dual inhibitor of sodium/glucose cotransporter 1 (SGLT-1) and 2 (SGLT-2). Licogliflozin significantly reduces body weight. However, the magnitude of weight reduction is modest [111].
-
•
In a randomized, controlled trial in patients with pre-obesity or obesity without diabetes, canagliflozin combined with phentermine produced meaningful reductions in body weight, was generally well tolerated, and had a safety and tolerability profile consistent with that of the individual components [37].
-
•Given their respective cardiovascular outcomes benefits [112], an intriguing combination is a SGLT-2 inhibitor combined with a GLP-1 receptor agonist [113].
-
oIn a randomized, placebo-controlled trial, semaglutide once weekly was evaluated in as an add-on to SGLT-2 inhibitor therapy in patients with type 2 diabetes (SUSTAIN 9). Patients administered semaglutide had greater reductions in HbA1c (−1.42%) and bodyweight (−3.81 kg) versus placebo [114].
-
oIn a randomized, controlled trial of patients with type 2 diabetes and inadequate glycemic control while on stable metformin monotherapy, compared to dapagliflozin alone, exenatide plus dapagliflozin produced clinically relevant changes in body weight, fasting plasma glucose, 2-h post-prandial glucose, and systolic blood pressure [115].
-
o
Other reported combinations:
-
•
Authors from a single site utilizing a variety of combination anti-obesity medication therapies reported improvement in metabolic parameters and a >20% weight reduction in 74% of patients, with a caveat that further randomized controlled studies are necessary to validate their findings [116].
-
•
The combination of liraglutide and phentermine did not provide additional, clinically meaningful weight reduction in individuals who had already lost ∼13% of their initial weight with liraglutide alone [117].
-
•
A case study review of three patients with obesity and diabetes mellitus administered phentermine HCl/topiramate ER and liraglutide 3.0 mg per day suggested efficacy in weight reduction and improvement in metabolic parameters [118].
7. Anti-obesity medication treatment and bariatric surgery
-
•
Weight regain after Roux-en-Y gastric bypass surgery may be mitigated with phentermine and topiramate monotherapies or when used in combination [119].
-
•
Adjuvant weight reduction medications may halt weight regain in patients who undergo bariatric surgery. Adjuvant pharmacotherapy with anti-obesity agents such as phentermine, phentermine/topiramate extended release, or naltrexone slow-release/bupropion slow-release was more effective in patients with higher body mass index, with more than one third achieving >5% weight reduction with the addition of weight reduction medication. The conclusion was that given the low risk of medications compared with revisional surgery, adjuvant weight reduction medications may be a reasonable option in appropriate post-bariatric surgery patients [120].
-
•
Regardless of bariatric surgery type, GLP-1 receptor agonists appear to be more effective in preventing weight regain, compared to non-GLP-1 receptor agonist anti-obesity medications [121].
-
•
Aggressive clinical approaches to the patient with obesity may mitigate the need for bariatric surgery, with interventions including combination pharmacotherapy, intense surveillance with monthly body composition analysis, weekly psychotherapy, nutritional planning with a dietitian every two months, and physical exercise at least three times a week with exercises prescribed by a personal trainer at least once a month [116].
8. Investigational anti-obesity medications
Table 3 shows takeaway messages regarding anti-obesity drug development.
Table 3.
Ten Takeaway Messages Regarding Anti-Obesity Drug Development. Shown are takeaway messages regarding current and future anti-obesity drug development.
| 1. | Examples of common body tissue targets of anti-obesity drug development include the central nervous system, gastrointestinal systems, and adipose tissue, with a continuing “emerging concept that the development of anti-obesity agents must not only reduce fat mass (adiposity), but must also correct fat dysfunction (adiposopathy)” [59]. |
| 2. | Glucagon-like peptide-1 (GLP-1) is an intestinal peptide secreted by the ileum and large intestine that, after food intake, increases pancreatic insulin release (“incretin”) and decreases glucagon secretion. Therapeutic long-acting GLP-1 receptor agonists (GLP-1 RAs) reduce glucose levels, decrease hunger, increase satiety, slow gastric emptying, increase adipogenesis and lipogenesis [122] (potentially improving fat functionality and helping to correct adiposopathy), and decrease hepatic glucose production. Some GLP-1 RAs function as anti-obesity and/or anti-diabetes medications when used alone or when used as a component of dual or triple mechanistic therapeutics. |
| 3. | Glucose-dependent insulinotropic polypeptide (i.e., GIP — formerly known as gastric inhibitory peptide) is an incretin secreted by the small intestine (i.e., duodenum and jejunum) that increases pancreatic insulin release. GIP receptor agonists are a component of anti-obesity and anti-diabetes agents serving as polyagonists (i.e., promoting multiple physiologic responses). |
| 4. | Glucagon receptor (GCGR) agonists may increase glucose levels via promotion of gluconeogenesis and inhibition of insulin. While glucagon is not an incretin, GCGR agonists may reduce hunger, increase satiety, have catabolic and thermogenic effects, increase energy expenditure, increase lipolysis and fatty acid oxidation, and reduce cholesterol and triglyceride levels. Combination GCG-RA and GLP-1 receptor agonist (RA) often result in a net reduction in glucose and body weight. |
| 5. | Tirzepatide is an approved anti-diabetes agent and an investigational anti-obesity drug in development and is a unimolecular GLP-1 and GIP RA. |
| 6. | Paradoxically, dual GLP-1 RA and GIP antagonists are also in development as anti-obesity therapeutic agents. |
| 7. | Oxyntomodulin acting agents have dual GLP-1 RA and glucagon RA activity. |
| 8. | Agents that facilitate browning of adipocytes have the potential to increase energy expenditure. |
| 9. | The development of anti-obesity medications is following the path of drug development of other metabolic diseases. |
| 10. | Clinical cardiovascular outcome trial support for cardiovascular benefits is likely the binary switch that will transform the current limited use of anti-obesity medications into future standards of care for patients with obesity. |
8.1. Priorities of anti-obesity drug development: fundamentals
Table 4 describes challenges with past anti-obesity pharmacotherapies. These experiences have heightened the prioritization towards developing anti-obesity medications that are safe, generally well-tolerated, and sufficiently efficacious to meet or exceed patient and clinician expectations. Another fundamental principle cited at least since 2002 is that: “An emerging concept is that the development of anti-obesity agents must not only reduce fat mass (adiposity) but must also correct fat dysfunction (adiposopathy)” [59]. A meta-analysis of 157 microarray datasets from five independent studies that identified a meta-signature of 1511 genes that endorse the development of effective bioinformatics workflow, and further grants an indication for the acceptance of adiposopathy as the root mechanistic pathology that poses risk for development of type 2 diabetes; the concept of adiposopathy in place of metabolic syndrome will open the possibility to design drugs that will ameliorate adipose tissue dysfunction and prove to be more effective against type 2 diabetes [126].
Table 4.
Historic Adverse Consequences of Past Drug Treatments for Obesity. Since the 1800s, multiple therapies used to treat obesity have encountered unacceptable adverse side effects. Table lists historic discontinued anti-obesity therapeutics and their adverse health consequences [7,[123], [124], [125]]. None of these are currently indicated to treat obesity.
| Year | Drug | Consequence |
|---|---|---|
| 1925 - present | Thyroid | Hyperthyroidism |
| 1933 - 1938 | Dinitrophenol | Cataracts/Neuropathy/Fatal hyperthermia |
| 1947 - 1979 | Amphetamine | Addiction |
| 1965 - 1968 | Aminorex | Pulmonary Hypertension |
| 1973 - 1997 | Fenfluramine/Dexfenfluramine | Valvulopathy |
| 1976 - 2000 | Phenylpropanolamine | Strokes |
| 1920 - 2004 | Ma Huang (ephedra) | Heart attacks/stroke |
| 2006 - 2007 | Ecopipam (Dopamine) | Depression/Suicide |
| 2006 - 2009 | Rimonabant (Selective cannabinoid-1 receptor antagonist): Never approved in the US | Depression/Suicide |
| 1997 - 2009 | Sibutramine | Cardiovascular disease risk |
| 2012 - 2020 | Lorcaserin | Cancer signal (e.g., lung and pancreas) |
8.2. Priorities of anti-obesity drug development: objectives
The main objective of anti-obesity drug development is to improve the health of the patient, and includes [59]:
-
•
Improving hyperglycemia, high blood pressure, and pathogenic lipid levels
-
•
Reducing cardiovascular disease risk and improving cardiovascular disease outcomes
-
•
Reducing cancer risk and improving cancer outcomes
-
•
Improving other adverse metabolic, immunological, biomechanical (i.e., sleep apnea and osteoarthritis), and psychosocial health consequences, with improved quality of life.
-
•
Reduce mortality
-
•
Achievement of weight reduction to a clinically meaningful degree that patients and clinicians will better embrace initiation of anti-obesity medications
-
•
Maintaining weight reduction maintenance to a clinically meaningful degree that patients and clinicians will persist in adhering to long-term anti-obesity medications
8.3. Anti-obesity drug development: treatment targets
The gastrointestinal tract, central nervous system, and adipose tissue are major organ treatment targets for anti-obesity drug development. Table 5 describes illustrative gastrointestinal hormones applicable to hunger and digestion. Fig. 2 describes illustrative factors that affect the central nervous system hunger areas.
Table 5.
Illustrative Gastrointestinal Hormones. Anti-obesity drug development often involves analogue adaptations of hormones and other factors applicable to gastrointestinal responses to food intake [[128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138]].
| Where secreted | Gastrointestinal hormone | Effect of hormone on eating behavior | Effect of eating on hormone secretion | Notes: | Effect of gastric by-pass and sleeve gastrectomy |
|---|---|---|---|---|---|
| Stomach | Ghrelin (secreted by P/D1 cells) | ↑ Hunger | ↓ | ↑With fasting ↑Gastric emptying/ ↑Growth Hormone |
Ghrelin is likely to decrease with sleeve gastrectomy (not so with gastric bypass) |
| Gastrin (secreted by G cells in antrum) | ↓ Hunger | ↑ | ↑ Hydrochloric acid and pepsinogen | ↓Gastric bypass -/↑ Sleeve |
|
| Pancreasa | Insulin (secreted by pancreatic beta cells) | ↓ Hunger | ↑ | ↑Hunger with hypoglycemia ↑Glucose transporter 4 in adipose tissue/muscle, glycogenesis, lipoprotein lipase activity, lipogenesis |
↓ Insulin resistance/fasting insulin ↑ Insulin sensitivity/insulin responsiveness |
| Glucagon (secreted by pancreatic alpha cells) | ↓ Hunger | ↓ | ↑Glycogen to glucose ↑Postprandial glucagon in patients with type 2 diabetes mellitus (if glucagon not suppressed) |
Variable | |
| Pancreatic polypeptide (PP) [secreted by PP (F) cells] | ↓ Hunger | ↑ | ↓ Pancreatic exocrine secretion | Variable | |
| Amylin (secreted by pancreatic beta cells) | ↓ Hunger | ↑ | ↓ Gastric emptying, glucagon | - ↓ | |
| Somatostatin (secreted by D cells pylori antrum, duodenum, and pancreatic islets) | ↓ Hunger | ↑ | ↓ Growth hormone, gastrin, hydrochloric acid (HCl), secretin, cholecystokinin (CCK), insulin, glucagon | - ↑ | |
| Small intestine (mainly duodenum and jejunum) | Cholecystokinin (CCK) (secreted by I-cells) |
↓ Hunger | ↑ | ↑ Gall bladder contractility and bile, pancreatic enzymes; ↓ Gastric emptying |
↑ postprandial |
| Secretin (secreted by S cells) | ↓ Hunger (?) | ↑ | ↑Pancreatic bicarbonate and bile ↓ Intestinal motility & gastric acid |
Variable | |
| Glucose-dependent insulinotropic peptide (GIP; also known as Gastric Inhibitory Peptide and is secreted by K cells) | ↓ Hunger (?) | ↑ | ↑Insulin ↑Glucagon postprandial |
Variable | |
| Motilin (secreted by M or Mo cells) | ↓ Hunger | ↓ (↑ with fasting) | ↑ Gastric motility, interdigestive migratory contractions (borborygmi) | ? | |
| Ileum and/or large intestine | Fibroblast growth factor (FGF) 19 [FGF 21 is produced by the liver] (secreted by ileal cells and regulated by farnesoid X receptors - FXR) | ↓ Hunger | ↑ | ↓ Bile acids, glucose production ↑Insulin sensitivity, glycogen synthesis |
↑ |
| Glucagon like peptide-1 (secreted by ileum/colon L-cells) | ↓ Hunger | ↑ | ↑Insulin ↓Glucagon, gastric emptying |
↑ | |
| Glucagon like peptide-2 (secreted by ileum/colon L-cells) | ↓ Hunger | ↑ | ↑Glucose metabolism, intestinal mucosal growth, increases absorptive surface, epithelial brush-border nutrient transporters and digestive enzymes, intestinal blood flow, postprandial chylomicron secretion ↓ Gastrointestinal motility |
↑ | |
| Oxyntomodulin (secreted by ileum/colon L-cells) | ↓ Hunger | ↑ | ↑ GLP-1 and glucagon receptor activity ↓ Gastric acid and gastric emptying |
↑ Gastric bypass - Sleeve gastrectomy |
|
| Peptide YY (secreted by ileum/colon L-cells) | ↓ Hunger | ↑ | ↓ Gall bladder and pancreatic secretions, gastric emptying | ↑ |
Pancreatic epsilon cells produce ghrelin, with a potential increase release of pancreatic ghrelin after sleeve gastrectomy [127].
Fig. 2.
Illustrative Targets of Anti-Obesity Therapy. Factors that act on the central nervous system responsible for hunger, anabolism, and catabolism often represent targets of anti-obesity drug development [21]. Abbreviations: AgRP: Agouti-related peptide; BDNF: Brain-derived neurotrophic factor; CART: Cocaine and amphetamine regulated transcript; CB1R: Cannabinoid receptor type 1; CCK: Cholecystokinin; CNS: Central Nervous System; CRH: Corticotropin-releasing hormone; GLP-1: Glucagon like peptide – 1; MCH: Melanin concentrating hormone; MCR: Melanocortin receptor; MSH: Melanin Stimulating Hormone; NPY: Neuropeptide Y; POMC: Pro-opiomelanocortin; PYY: Peptide YY; TRH: Thyrotropin-releasing hormone.
8.4. Hypothalamic obesity
Given the central role of the hypothalamus in energy balance, anti-obesity drugs in development often target the hypothalamus. Beyond the potential for high fat food intake to promote hypothalamic inflammation [139], managing obesity due to hypothalamic trauma, surgery, or dysfunction is often challenging. The hypothalamus is in the area of the forebrain below the thalamus (i.e., “hypo”) and regulates survival functions such as feeding, fighting, fleeing, and fornication (the 4 Fs). The hypothalamus is integrated in bidirectional energy balance signaling that includes hormonal and neurologic input and output. Table 5 and Fig. 2 describe energy balance factors applicable to the hypothalamus. From a hormone input standpoint, ghrelin from the stomach increases hunger. Conversely, hormones such as insulin (i.e., from pancreatic beta cells), amylin (i.e., from pancreatic beta cells), and glucagon (i.e., from pancreatic alpha cells), CCK from the small intestine, and GLP-1, oxyntomodulin, PYY, and FGF19 from the ileum or large intestine may all reduce hunger [21]. Leptin from adipose tissue may also decrease hunger. From a hormone output standpoint, hypothalamic dysfunction may alter hormone release potentially applicable to body composition and body weight, such as corticotrophin-releasing hormone, dopamine, growth hormone-releasing hormone, somatostatin, gonadotrophin-releasing hormone, and thyrotrophin-releasing hormone [21].
In addition to hormones, afferent and efferent neurologic pathways also affect hunger. Largely through the vagus nerve, afferent stomach stretch-fibers after a meal transmit parasympathetic signals to the brain and hypothalamus to increase satiety. Hypothalamic efferent signals to other parts of the brain may affect hunger and appetite, and may affect thermogenesis, energy expenditure, increase blood pressure, and stimulate lipolysis in adipose tissue [21].
Hypothalamic dysfunction may negate post-meal negative feedback on hunger, resulting in “central nervous system starvation” and thus promote energy intake. Hypothalamic dysfunction may also reduce resting metabolic rate and reduce adipocyte lipolysis. Especially when combined with reduced mobility or a decrease in the ability to engage in recommended physical activity (e.g., those with brain dysfunction or injury), hypothalamic dysfunction can substantially contribute to positive caloric balance and increased body fat [21].
8.4.1. First order arcuate nucleus neurons
Hypothalamic satiety or anti-orexigenic hormones include proopiomelanocortin (POMC), alpha melanocyte stimulating hormone (alpha MSH), cocaine amphetamine regulated transcript (CART). Hypothalamic hunger or orexigenic hormones include neuropeptide Y (NPY) and agouti-related peptide (AgRP) [21] (See Fig. 2).
8.4.2. Second order arcuate nucleus neurons
Eating behavior is affected by secondary order messaging in the paraventricular, ventromedial, and lateral hypothalamus. Second order messengers affecting appetite/hunger include anorexigenic alpha melanocyte stimulating hormone (alpha MSH binds to the melanocortin 4 receptor/MC4R), brain-derived neurotrophic factor (BDNF), thyrotropin-releasing hormone (TRH), and corticotropin-releasing hormone (CRH), as well as orexigenic cannabinoids that affect cannabinoid brain receptors (CBR), melanin concentrating hormone (MCH), and orexin [21].
8.4.3. Causes of hypothalamic obesity
Causes of hypothalamic obesity include brain trauma, surgical excision for craniopharyngioma, pituitary adenoma with hypothalamic extension, glioma, meningioma, teratoma, germ cell tumors, and radiotherapy. Dysfunction from Prader-Willi Syndrome and mutations in leptin, leptin receptor, proopiomelanocortin (POMC), melanocortin 4 receptor, and cocaine/amphetamine regulated transcript (CART) can also contribute to hypothalamic obesity [21].
8.4.4. Hypothalamic obesity pharmacotherapy
Treatment of hypothalamic obesity includes pituitary hormone replacement when indicated. Other potential treatments include metformin, bariatric surgery, GLP-1 receptor agonists, and intra-nasal oxytocin. Setmelanotide (melanocortin-4 receptor agonist) is indicated for chronic weight management in adult and pediatric patients 6 years of age and older with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency [21].
9. Anti-obesity drugs in development
Table 6, Table 7, Table 8 show anti-obesity drugs in development, including therapies, mechanisms, and notes about each investigational agent. Fig. 3a shows the mechanistic effects of glucagon like peptide-1 (GLP-1) receptor agonism. Fig. 3b shows the mechanistic effects of glucose-dependent insulinotropic polypeptide (GIP) agonism. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8 show the paths of development of pharmacotherapy for several metabolic diseases, including diabetes mellitus, hypertension, hypercholesterolemia, and obesity. Fig. 9 shows illustrative consequences of early versus late weight management interventions (see Chart 1).
Table 6.
Anti-Obesity Drugs in Development. GLP-1 receptor agonists form the foundation of several anti-obesity drugs in development.
| Therapeutic agent | Mechanism | Notes |
|---|---|---|
| Glucagon-like peptide – 1 receptor agonist (GLP-1 RA) | Slows gastric emptying, increases satiety, decreases hunger | GLP-RA that are approved anti-obesity medications include semaglutide and liraglutide. Agents in development include efpeglenatide, danuglipron (PF-06882961), LY3502970 and oral semaglutide [140,[158], [159], [160], [161]]. |
Table 7.
Glucagon Like Peptide – 1 Receptor Agonism Combinations. Many anti-obesity drugs in development combine a GLP-1 RA with other investigational drug/s, resulting in dual or triple mechanisms of action [170].
| Glucagon Like Peptide – 1 Receptor Agonist (GLP-1 RA) PLUS | ||
|---|---|---|
| Therapeutic agent | Mechanism | Notes |
| Agonists of both GLP-1 receptor and glucose-dependent insulinotropic polypeptide (GIP) receptors, with the latter previously known as gastric inhibitory peptide | Functions as an unimolecular GLP-1 and GIP receptor agonist. While some do not recognize it as a medical term, "twincretin” is often used to describe agents that increase the activity of two incretins. | Tirzepatide is approved for treatment of type 2 diabetes mellitus, and is in development for treatment of obesity. Within its SURPASS development program (focused on type 2 diabetes), studies include the SURPASS CVOT, which is a large phase 3 clinical trial evaluating the cardiovascular outcomes of tirzepatide versus dulaglutide among patients with type 2 diabetes mellitus [[162], [163], [164]] and the SURMOUNT program for chronic weight management in patients with overweight or obesity (See Chart 3). |
| GLP-1 RA combined with GIP antagonists | GIP receptor knockout mice develop resistance toward diet-induced obesity, and those with inactivated mutations of GIP receptor have reduced body weight. Antagonizing GIP may also be advantageous in glucose control via reducing GIP-mediated glucagon secretion. | It may seem paradoxical that GLP-1 RA plus either GIP agonists (described above) or GIP antagonists may promote weight reduction and similar metabolic benefits. A possible explanation is that GIP agonists chronically activate the GIP receptor, causing desensitization and receptor internalization, thus downregulating the GIP system in a way that it mimics antagonism [157,165]. |
| GLP-1 RA combined with glucagon (GCG) receptor agonist (GCG-RA) | While glucagon may increase glucose levels via gluconeogenesis and inhibition of insulin, glucagon may also decrease hunger, increase satiety, have catabolic and thermogenic effects, increase energy expenditure, increase lipolysis and fatty acid oxidation, and reduce cholesterol and triglyceride levels. | Oxyntomodulin is an illustrative example of a unimolecular dual agonist of GLP-1 and glucagon. While glucagon alone would be expected to raise glucose levels, the net effect of GLP-1 RA and GCG-RA is weight reduction, decrease hunger, increased satiety, reduction in glucose, cholesterol, and triglyceride levels, and possible increase in energy expenditure [165,166]. Examples of GLP-1/GCG dual agonists include cotadutide, efinopegdutide, BI 456906, pemvidutide, and oxyntomodulin analogues [164]. |
| GLP-1 RA combined with agonists of GIP & GCG | Complementary actions from activation of three components (GLP-1, GIP, and GCG) | Tri-agonist [167] |
| GLP-1 RA combined with Peptide YY (PYY) | Increase satiety, decrease hunger | Some PYY agents evaluated for monotherapy of obesity resulted in unacceptable nausea and vomiting and limited weight reduction [165,168]. |
| GLP-1 RA combined with amylin | Increase satiety, decrease hunger | Amylin may promote weight reduction and increase sensitivity to leptin in patients with obesity. Cagrilintide is a long-acting amylin analogue [169,164]. |
Table 8.
Other Anti-Obesity Drug Monotherapies in Development. This table lists illustrative anti-obesity drugs in development, mechanisms of action, and applicable notes.
| Therapeutic agent | Mechanism | Notes |
|---|---|---|
| Ghrelin-O-acyltransferase (GOAT) inhibitors | GOAT activates ghrelin. Ghrelin increases hunger. GOAT inhibitors may reduce hunger and reduce body weight. | GOAT inhibitors may be the basis of future treatments for diabetes and obesity. [171] |
| Fibroblast growth factor 21 analogues and receptor/binding protein agonists | Promotes lipid catabolism, lipolysis, fatty acid oxidation, mitochondrial oxidative activity, and thermogenic energy dissipation; may promote browning of adipocytes (beige) [172] | FGF 21 agonists may reduce fat mass, reduce hyperglycaemia, reduce insulin resistance, improve dyslipidaemia, and reduce cardiovascular disorders and non-alcoholic steatohepatitis (NASH) [173,174]. |
| Macrophage inhibitory cytokine 1 (MIC-1)/Growth differentiation factor 15 (GDF15) agonists | Reduces food intake | Transforming growth factor super family cytokine that binds to the brain glial-derived neurotrophic factor (GDNF) receptor alpha-like (GFRAL) receptor [175] |
| Dopamine, serotonin, and noradrenaline reuptake inhibitor (phenyltropane family) | Increases satiety/reduces hunger | Tesofensine is a triple monoamine reuptake inhibitor [176] |
| Fatty acid desaturase 1 (FADS1) inhibitors | FADS1 helps regulate fatty acid metabolism, eicosanoid production, and inflammation | Fatty acid desaturase 1 knockout mice are lean, with improved glycemia and decreased atherogenesis |
| Therapeutics that increase energy expenditure via enhanced thermogenesis [[177], [178], [179]] | Within the mitochondria, food- derived molecules are metabolized via oxidative phosphorylation to form stored energy [adenosine triphosphate (ATP)] via the citric acid cycle or utilized to generate heat via the “futile cycle” [3]. This thermogenic process is regulated by mitochondrial uncoupling protein 1, mostly found in brown adipose tissue (BAT). Thermogenesis in BAT and other organ occurs through sympathetic, β-adrenergic receptors. | Challenges in development of agents to increase energy expenditure include how to safely limit ATP formation and activate thermogenesis, without hyperthermia and adverse cardiovascular side effects. |
| Agents that act upon factors related to brown and beige fat [(e.g., amino acid derivatives, PPAR gamma agonists, JAK inhibition, irisin, musclin, and transcription factor A mitochondrial (TFAM)] [180,181] | Increases energy expenditure. White adipocytes have a unilocular fat droplet and few mitochondria. Brown adipocytes have multilocular fat droplets, high content of mitochondria, and high levels of uncoupling protein-1. Beige adipocytes have an origin like white adipocytes but function more like brown adipocytes. | Targeting of brown adipose tissue and browning white adipose tissue (inducing white adipocytes to become beige adipocytes) may increase thermogenesis, lead to negative energy balance, and decrease glucose and triglyceride levels [181]. |
| 11beta-hydroxsteroid dehydrogenase type 1 inhibitors [182] | 11beta-HSD1 in adipose tissue converts cortisone to more active cortisol. | May reduce multiple components of the metabolic syndrome and help correct “local Cushing's syndrome” |
| Asprosin antagonists | Protein produced by adipose tissue that is orexigenic and promotes hepatic glucose production | Neonatal Progeroid Syndrome (NPS) or Marfan Lipodystrophy Syndrome have gene mutations resulting in asprosin deficiency and extreme leanness. Anti-asprosin monoclonal antibodies reduce blood glucose, appetite, and body weight [183]. |
| Phosphodiesterase-4 inhibitors | May reduce appetite, increase satiety via increased GLP-1 secretion and may also increase energy expenditure | Roflumilast is approved for treatment of chronic obstructive pulmonary disease and may reduce body weight and increase insulin sensitivity [184]. |
| Human monoclonal antibody to the myostatin/activin type II receptor (ActR II) | ActR II receptor mediates muscle loss. Loss of ActR II function mutations result in increased muscle mass of animals and humans. | Bimagrumab is a monoclonal antibody to the ActR II receptor that increases lean mass and reduces fat mass [185]. |
| G-protein coupled receptor 75 (GPR75) inhibitors | GPR75 is found in the brain and was discovered by gene sequencing [186]. Individuals with at least one inactive copy of GPR75 gene have lower body mass index and about 50% lower risk of obesity [186]. | Therapeutic inhibition of GPR75 is a potential treatment target for obesity, dyslipidemia, diabetes, cardiovascular disease, and cerebrovascular disease [187]. |
| Anti-obesity vaccines (adipose tissue antigens, somatostatin, ghrelin, glucose-dependent insulinotropic polypeptide, adenovirus36) | Utilize immunotherapy to inhibit proteins and other factors involved in energy homeostasis | Among the immunotherapy approaches towards the orexigenic ghrelin include passive and active immunization with or without virus-like particles [188,189] |
| Self-expanding polymer that when swallowed and delivered to stomach, creates a pH-sensitive super absorbent gel structure. | The encapsulated device may promote satiety via activation of the gut–brain axis thereby leading to weight loss [190]. | Study ongoing: The Effect of Epitomee Capsule on Body Weight in Patients With Overweight and Obesity With and Without Prediabetes (https://clinicaltrials.gov/ct2/show/NCT04222322) |
Fig. 3a.
Glucagon-Like Peptide-1 (GLP-1) Receptor Agonism. GLP-1 is an incretin. Analogues of glucagon-like peptide 1 serve as receptor agonists (GLP-1 RA) that are used to treat obesity, either alone or as a component of combination therapy. Some, but not all, GLP-1 RAs have clinical trial evidence supporting favorable effects on cardiovascular disease (CVD) outcomes [122,[140], [141], [142], [143], [144], [145], [146], [147]]. Abbreviations: DPP IV: Dipeptidyl Peptidase IV.
• GLP-1 receptor agnoists may improve nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH).
Fig. 3b.
Glucose-dependent insulinotropic polypeptide (GIP; previously known as gastric inhibitory peptide). GIP is an incretin. Its incretin effect is impaired in patients with type 2 diabetes mellitus. Combination glucagon-like peptide-1 receptor agonist (GLP-1 RA) and GIP RA are in development, and are illustrated by tirzepatide (Table 7 and Chart 3). [[148], [149], [150], [151], [152], [153], [154], [155]]. DPP IV: Dipeptidyl Peptidase IV. ∗ Unless combined with GLP-1, it is unclear that GIP receptor agonism alone reduces hunger and reduces body fat. However, GIP may enhance some of the effects of GLP-1 receptor agonism. The anti-nausea effects of GIP agonism may reduce the nausea adverse experiences described with GLP-1 receptor agonists [156,147]. ∗∗ In patients without diabetes, GIP increases insulin secretion and enhances deposition of fat in adipose tissues. In patients with diabetes mellitus, GIP may lose its insulinotropic effect, but retain a stimulatory effect on glucagon secretion – potentially worsening glucose levels [157]. However, as before, GIP receptor agonists may enhance some of the favorable effects of GLP-1 receptor agonists when administered concomitantly.
Fig. 4.
Development of anti-obesity medications is following the path of drug development of other metabolic diseases. Like the history of other metabolic diseases, a bias exists among many clinicians that limits pharmacotherapy to treat obesity [[201], [202], [203], [204]].
Fig. 5.
Treatment of the Disease of Diabetes Mellitus. Pharmacotherapy for diabetes mellitus began with several drugs that were poorly tolerated or that had adverse side effects. Today, more effective and better tolerated drugs are available [[205], [206], [207]].
NPH: Neutral Protamine Hagedorn.
DPP-4: Dipeptidyl-peptidase 4.
SGLT2: Sodium-glucose cotransporter-2.
GLP-1: Glucagon-like peptide-1.
Fig. 6.
Treatment of the Disease of Hypertension. Similar to the path of pharmacotherapy for diabetes mellitus, early hypertension drugs were poorly tolerated or had adverse side effects; today, more effective and well-tolerated drugs are available [208].
Fig. 7.
Treatment of the Disease of Hypercholesterolemia. In line with the development of pharmacotherapy for other metabolic diseases, the first hypercholesterolemia drugs were poorly tolerated or had adverse side effects; today, more effective and well-tolerated drugs are available [[209], [210], [211]].
Fig. 8.
Treatment of the Disease of Obesity. The development of anti-obesity medication is following the path of drug development of other metabolic diseases. Early anti-obesity drugs had limited weight reduction, were unsafe, poorly tolerated, and did not have proven health or mortality benefits. Current and future anti-obesity medications have the potential to be relatively safe, well tolerated, efficacious, and will (hopefully) prove to have health outcomes and improved mortality benefits [37,108,212].
Fig. 9.
Early versus late weight management intervention: Illustrative consequences.
Illustrative potential comparative outcomes for early versus delayed intervention in the evaluation and management of pre-obesity or obesity [84,[213], [214], [215], [216], [217], [218], [219], [220], [221]]. One of the objectives of early pharmacologic intervention for pre-obesity/obesity, is to prevent onset and complications of adiposopathic and fat mass consequences such as diabetes mellitus, hypertension, dyslipidemia, sleep apnea, arthritis, and depression (as well as other adverse health consequences of obesity such as cardiovascular disease and cancer). Lack of early intervention may lead to one or more of these complications, resulting in polypharmacy and need to follow multiple disease-oriented guidelines.
Chart 1.
Above outlines the estimated degree of mean weight reduction associated with clinically meaningful improvement in illustrative health outcomes [2].
9.1. Tirzepatide
Table 5 lists many of the more clinically relevant gastrointestinal hormones. Fig. 3a shows the mechanistic effects of glucagon like peptide-1 (GLP-1) receptor agonism. Fig. 3b shows the mechanistic effects of glucose-dependent insulinotropic polypeptide (GIP) agonism. Tirzepatide is a once weekly injectable GLP-1 and GIP receptor agonist. Both GIP and GLP-1 are incretins, which are gut peptides that enhance increased insulin secretion after oral nutrient intake [191]. Tirzepatide is approved for treatment of type 2 diabetes mellitus. It is currently undergoing development as a potential anti-obesity medication for chronic weight loss maintenance (see Chart 3).
Chart 3.
Summary of findings and weight reduction in the tirzepatide SURPASS studies for treatment of type 2 diabetes mellitus and cardiovascular disease and SURMOUNT program for obesity.
| Study and findings | Changes in body weight | Changes in hemoglobin A1c | Safety and tolerability | References |
|---|---|---|---|---|
| SURPASS 1: Compared to placebo, tirzepatide 5, 10, and 15 mg SQ per week improved hemoglobin A1c and body weight among patients with type 2 diabetes mellitus. | Reduced body weight 8 to 11% in a dose-dependent manner, compared to a 1% body weight reduction with placebo in efficacy analysis set (Baseline BMI=31.9 kg/m2) | Reduced HbA1c 1.9% to 2.1% versus placebo in efficacy analysis set (Baseline HbA1c = 7.9%) | 14% discontinued study drug and 10% discontinued study prematurely (nausea, diarrhea, vomiting; no hypoglycemia). | [192] |
| SURPASS 2: Once a week tirzepatide (5 mg, 10 mg, or 15 mg) was superior to once-a-week subcutaneous semaglutide 1.0 mg in reducing HbA1c among patients with type 2 diabetes mellitus. | Reduced body weight 7.6–11.2 kg versus 5.7 kg body weight reduction with semaglutide (Baseline BMI = 34.2 kg/m2) | Reduced HbA1c 2.01–2.30% versus a reduction of 1.86% with semaglutide (Baseline HbA1c = 8.3%) | Side effects: nausea, diarrhea, decreased appetite and vomiting – similar percentages among the four treatment groups) | [193] |
| SURPASS 3: Tirzepatide was superior to titrated insulin degludec in reducing HbA1c, reducing body weight, and had a lower risk of hypoglycemia among patients with type 2 diabetes mellitus | Reduced body weight 7.5–12.9 kg compared to a 2.3 kg increase with degludec (estimated treatment difference range of 9.8 kg–15.2 kg). (Baseline BMI = 33.6 kg/m2) |
Reduced HbA1c 1.93–2.37% versus a reduction of 1.34% with degludec (Baseline HbA1c = 8.17%) | Tirzepatide had higher rates of nausea, diarrhea, decreased appetite and vomiting, but less hypoglycemia compared to degludec | [194] |
| SURPASS 4: Tirzepatide reduced HbA1c more than titrated inulin glargine and had a lower incidence of hypoglycemia among patients with type 2 diabetes and elevated cardiovascular risk. Open label 52 to 104-week study. | Reduced body weight 6.4–10.6 kg compared to 1.7 kg increase with insulin glargine (Baseline BMI = 33 kg/m2) | Reduced HbA1c by 2.24–2.58% compared to a reduction of 1.44% with insulin glargine (Baseline HbA1c = 8.5%) |
Adjudicated major adverse cardiac events (cardiovascular death, myocardial infarction, stroke, hospitalisation for unstable angina) were not increased with tirzepatide compared to insulin glargine. | [195] |
| SURPASS 5: Tirzepatide versus placebo added to titrated insulin glargine improved glycemic control among patients with type 2 diabetes. | Reduced body weight 5.4–8.8 kg compared to increase of 1.6 kg with placebo (Baseline BMI = 33.4 kg/m2) | Reduced HbA1c by 2.1–2.4% compared to a reduction of 0.9% with placebo (Baseline HbA1c = 8.31%) | Premature discontinuations of 10%–18% in a dose- dependent manner (5, 10, 15 mg per week), vs 3% in the placebo group. Most common treatment-emergent adverse events in the tirzepatide groups vs placebo group were diarrhea (12%–21% vs 10%) and nausea (13%–18% vs 3%). | [196] |
| SURPASS 6: A Study of Tirzepatide (LY3298176) Versus Insulin Lispro (U100) in Participants With Type 2 Diabetes Inadequately Controlled on Insulin Glargine (U100) With or Without Metformin | Study ongoing | Study ongoing | Study ongoing | [197] |
| SURPASS AP COMBO: A Study of Tirzepatide (LY3298176) in Participants With Type 2 Diabetes on Metformin With or Without Sulfonylurea | Study ongoing (completed with no results yet reported) | Study ongoing (completed with no results yet reported) | Study ongoing (completed with no results yet reported) | [198] |
| SURPASS CVOT: A Study of Tirzepatide (LY3298176) Compared With Dulaglutide on Major Cardiovascular Events in Participants With Type 2 Diabetes | Study ongoing | Study ongoing | Study ongoing | [199] |
| SURMOUNT-1: A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight∗ | Study completed; publication pending∗ | Study completed; publication pending∗ | Study completed; publication pending∗ | [200] |
| SURMOUNT-2: A Study of Tirzepatide (LY3298176) in Participants With Type 2 Diabetes Who Have Obesity or Are Overweight | Study ongoing | Study ongoing | Study ongoing | https://www.clinicaltrials.gov/ct2/show/NCT04657003 |
| SURMOUNT-3: A Study of Tirzepatide (LY3298176) In Participants After A Lifestyle Weight Loss Program | Study ongoing | Study ongoing | Study ongoing | https://www.clinicaltrials.gov/ct2/show/NCT04657016 |
| SURMOUNT-4: A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight for the Maintenance of Weight Loss | Study ongoing | Study ongoing | Study ongoing | https://www.clinicaltrials.gov/ct2/show/NCT04660643 |
∗See Table 2 for preliminary efficacy findings of SURMOUNT-1.
10. Conclusions
This OMA Clinical Practice Statement on anti-obesity medications and investigational agents provides an overview of non-surgical pharmacotherapy interventions in the treatment of obesity. This “Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022” is one of a series of OMA CPSs designed to assist clinicians in the care of patients with the disease of pre-obesity/obesity.
Transparency [222]
This manuscript was largely derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. This was followed by a similar Pediatric “Obesity Algorithm,” with updates ∼ every two years by OMA authors. Authors of prior years’ version of the Obesity Algorithm are included in Supplement #1.
Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. (Supplement #1) The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
AF, SC, KB, JT, and HEB reviewed, edited, and approved the document.
Managing disclosures and dualities of interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual disclosures
HEB's research site (L-MARC Research Center) has received research grants from the following potentially applicable obesity-research related companies: Alon Medtech/Epitomee, Amgen, Boehringer Ingelheim, Eli Lilly, NovoNordisk, and Pfizer. HEB reports being a consultant for Amgen and Altimmune. AF reports being and advisor for Gelesis, NovoNordisk, Jenny Craig, Suvie, and MsMedicine. SC reports serving on the speaker's bureau for Novo Nordisk and advisor for Gelesis. KB reports serving on the speaker's bureau for Currax and Vivus, advisory board for Novo Nordisk, Currax, Gelesis, Bariatric Advantage, and is owner of Gaining Health. JT reports no relevant disclosures.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Acknowledgements and Funding
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2022.100018.
Contributor Information
Harold E. Bays, Email: hbaysmd@outlook.com.
Angela Fitch, Email: afitch@mgh.harvard.edu.
Sandra Christensen, Email: sam.chris@im-wm.com.
Karli Burridge, Email: Karli@gaininghealth.com.
Justin Tondt, Email: justintondt@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bays H.E., McCarthy W., Burridge K., Tondt J., Karjoo S., Christensen S., Ng J., Golden A., Davisson L., Richardson L. Obesity algorithm eBook. 2021. www.obesityalgorithm.orghttps://obesitymedicine.org/obesity-algorithm/ presented by the Obesity Medicine Association.
- 2.Ryan D.H., Yockey S.R. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6:187–194. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray G.A. Why do we need drugs to treat the patient with obesity? Obesity. 2013;21:893–899. doi: 10.1002/oby.20394. [DOI] [PubMed] [Google Scholar]
- 6.Stanford F.C., Alfaris N., Gomez G., Ricks E.T., Shukla A.P., Corey K.E., et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. 2017;13:491–500. doi: 10.1016/j.soard.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Drug Administration FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market?utm_campaign=FDA%20MedWatch%20-%20Belviq%2C%20Belviq%20XR%20%28lorcaserin%29%3A%20DSC%20-%20FDA%20Requests%20Withdrawal%20of%20Weight-Loss%20Drug&utm_medium=email&utm_source=Eloqua
- 8.Bays H.E., Jones P.H., Orringer C.E., Brown W.V., Jacobson T.A. National lipid association annual summary of clinical lipidology 2016. J Clin Lipidol. 2016;10:S1–S43. doi: 10.1016/j.jacl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Piscitelli S.C., Gallicano K.D. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984–996. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Lerman L.O. Obesity and renovascular disease. Am J Physiol Ren Physiol. 2015;309:F273–F279. doi: 10.1152/ajprenal.00547.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta D., Bhatia D., Dave V., Sutariya V., Varghese Gupta S. Salts of therapeutic agents: chemical, physicochemical, and biological considerations. Molecules. 2018;23 doi: 10.3390/molecules23071719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bays H.E., Gadde K.M. vol. 47. Drugs of today; Barcelona, Spain: 2011. pp. 903–914. (Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity). 1998. [DOI] [PubMed] [Google Scholar]
- 13.Bays H. Phentermine, topiramate and their combination for the treatment of adiposopathy ('sick fat') and metabolic disease. Expet Rev Cardiovasc Ther. 2010;8:1777–1801. doi: 10.1586/erc.10.125. [DOI] [PubMed] [Google Scholar]
- 14.Liraglutide prescribing information for treatment of obesity (SAXENDA) https://www.novo-pi.com/saxenda.pdf
- 15.Liraglutide prescribing information for treatment of type 2 diabetes mellitus (VICTOZA) https://www.novo-pi.com/victoza.pdf
- 16.LOMAIRA™ (phentermine hydrochloride USP) tablets, CIV) https://www.lomaira.com/Prescribing_Information.pdf
- 17.Naltrexone HCL/bupropion HCL extended release prescribing information (CONTRAVE) http://general.takedapharm.com/content/file.aspx?filetypecode=CONTRAVEPI&cacheRandomizer=c5f9d506-7c0a-4c03-b357-2a926ba14990
- 18.XENICAL® (orlistat) Capsules. https://www.xenical.com/pdf/PI_Xenical-brand_FINAL.PDF
- 19.Bays H.E., Cobble M. Individualizing treatment with statin therapy. J Fam Pract. 2018;67:S43–S48. [PubMed] [Google Scholar]
- 20.Fui M.N., Dupuis P., Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16:223–231. doi: 10.4103/1008-682X.122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abuzzahab M.J., Roth C.L., Shoemaker A.H. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;91:128–136. doi: 10.1159/000496564. [DOI] [PubMed] [Google Scholar]
- 22.Alexandraki K.I., Grossman A. Management of hypopituitarism. J Clin Med. 2019;8 doi: 10.3390/jcm8122153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 24.Gotto A.M., Jr., Cannon C.P., Li X.S., Vaidya S., Kher U., Brinton E.A., et al. Evaluation of lipids, drug concentration, and safety parameters following cessation of treatment with the cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease. Am J Cardiol. 2014;113:76–83. doi: 10.1016/j.amjcard.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Jesudason D.R., Clifton P. Interpreting different measures of glomerular filtration rate in obesity and weight loss: pitfalls for the clinician. Int J Obes. 2012;36:1421–1427. doi: 10.1038/ijo.2011.242. [DOI] [PubMed] [Google Scholar]
- 26.Carbone A., Al Salhi Y., Tasca A., Palleschi G., Fuschi A., De Nunzio C., et al. Obesity and kidney stone disease: a systematic review. Minerva Urol Nefrol. 2018;70:393–400. doi: 10.23736/S0393-2249.18.03113-2. [DOI] [PubMed] [Google Scholar]
- 27.Daudon M., Frochot V., Bazin D., Jungers P. Drug-induced kidney stones and crystalline nephropathy: pathophysiology, prevention and treatment. Drugs. 2018;78:163–201. doi: 10.1007/s40265-017-0853-7. [DOI] [PubMed] [Google Scholar]
- 28.Welch B.J., Graybeal D., Moe O.W., Maalouf N.M., Sakhaee K. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48:555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed M.H., Ahmed H.T., Khalil A.A. Renal stone disease and obesity: what is important for urologists and nephrologists? Ren Fail. 2012;34:1348–1354. doi: 10.3109/0886022X.2012.723777. [DOI] [PubMed] [Google Scholar]
- 30.Moeller K.E., Kissack J.C., Atayee R.S., Lee K.C. Mayo Clinic proceedings. Vol. 92. Mayo Clinic; 2017. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens; pp. 774–796. [DOI] [PubMed] [Google Scholar]
- 31.Bays H.E. Lorcaserin: drug profile and illustrative model of the regulatory challenges of weight-loss drug development. Expet Rev Cardiovasc Ther. 2011;9:265–277. doi: 10.1586/erc.10.22. [DOI] [PubMed] [Google Scholar]
- 32.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francavilla C., Andrick R. Obesity medicine association (OMA): advocacy update. Obes Pillars. 2022;1 [Google Scholar]
- 34.Food and Drug Administration Pregnancy and lactation labeling (drugs) final rule. December 3, 2014. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm
- 35.American College of Obstetricians and Gynecologists, Obesity and Pregnancy Frequently asked questions. https://www.acog.org/-/media/For-Patients/faq182.pdf
- 36.American College of Obstetricians Gynecologists ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121:213–217. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 37.Hollander P., Bays H.E., Rosenstock J., Frustaci M.E., Fung A., Vercruysse F., et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40:632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 38.Aronne L.J., Wadden T.A., Peterson C., Winslow D., Odeh S., Gadde K.M. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity. 2013;21:2163–2171. doi: 10.1002/oby.20584. [DOI] [PubMed] [Google Scholar]
- 39.Kim K.K., Cho H.J., Kang H.C., Youn B.B., Lee K.R. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med J. 2006;47:614–625. doi: 10.3349/ymj.2006.47.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glazer G. Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety. Arch Intern Med. 2001;161:1814–1824. doi: 10.1001/archinte.161.15.1814. [DOI] [PubMed] [Google Scholar]
- 41.Lim S., Rogers L.K., Tessler O., Mundinger G.S., Rogers C., Lau F.H. Phentermine: a systematic review for plastic and reconstructive surgeons. Ann Plast Surg. 2018;81:503–507. doi: 10.1097/SAP.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 42.Semaglutide injection 2.4 mg (WEGOVY) prescribing information. https://www.novo-pi.com/wegovy.pdf
- 43.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 44.Fujioka K. Current and emerging medications for overweight or obesity in people with comorbidities. Diabetes Obes Metabol. 2015;17:1021–1032. doi: 10.1111/dom.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phentermine HCL/topiramate extended release prescribing information (QSYMIA) http://www.vivus.com/docs/QsymiaPI.pdf
- 46.Greenway F.L., Fujioka K., Plodkowski R.A., Mudaliar S., Guttadauria M., Erickson J., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 47.Apovian C.M., Aronne L., Rubino D., Still C., Wyatt H., Burns C., et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity. 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kose M., Emet S., Akpinar T.S., Ilhan M., Gok A.F., Dadashov M., et al. An unexpected result of obesity treatment: orlistat-related acute pancreatitis. Case Rep Gastroenterol. 2015;9:152–155. doi: 10.1159/000430433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddock C.K., Poston W.S., Dill P.L., Foreyt J.P., Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metabol Disord. 2002;26:262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 50.Hendricks E.J., Greenway F.L., Westman E.C., Gupta A.K. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity. 2011;19:2351–2360. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 51.Lewis K.H., Fischer H., Ard J., Barton L., Bessesen D.H., Daley M.F., et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity. 2019;27:591–602. doi: 10.1002/oby.22430. [DOI] [PubMed] [Google Scholar]
- 52.Hendricks E.J. Off-label drugs for weight management. Diabetes Metab Syndr Obes. 2017;10:223–234. doi: 10.2147/DMSO.S95299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalas M.A., Galura G.M., McCallum R.W. Medication-induced gastroparesis: a case report. J Investig Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211051919. 23247096211051919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toledo S.P.A., Lourenço D.M., Jr., Santos M.A., Tavares M.R., Toledo R.A., Correia-Deur JEdM. Hypercalcitoninemia is not pathognomonic of medullary thyroid carcinoma. Clinics. 2009;64:699–706. doi: 10.1590/S1807-59322009000700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bethel M.A., Patel R.A., Thompson V.P., Merrill P., Reed S.D., Li Y., et al. Changes in serum calcitonin concentrations, incidence of medullary thyroid carcinoma, and impact of routine calcitonin concentration monitoring in the EXenatide study of cardiovascular event lowering (EXSCEL) Diabetes Care. 2019;42:1075–1080. doi: 10.2337/dc18-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waser B., Beetschen K., Pellegata N.S., Reubi J.C. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology. 2011;94:291–301. doi: 10.1159/000330447. [DOI] [PubMed] [Google Scholar]
- 57.Silva O.L., Becker K.L., Shalhoub R.J., Snider R.H., Bivins L.E., Moore C.F. Calcitonin levels in chronic renal disease. Nephron. 1977;19:12–18. doi: 10.1159/000180860. [DOI] [PubMed] [Google Scholar]
- 58.United States Drug Enforcement Administration Drug scheduling. https://www.dea.gov/drug-information/drug-scheduling
- 59.Bays H.E. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes Res. 2004;12:1197–1211. doi: 10.1038/oby.2004.151. [DOI] [PubMed] [Google Scholar]
- 60.Bays H., Rodbard H.W., Schorr A.B., Gonzalez-Campoy J.M. Adiposopathy: treating pathogenic adipose tissue to reduce cardiovascular disease risk. Curr Treat Options Cardiovasc Med. 2007;9:259–271. doi: 10.1007/s11936-007-0021-6. [DOI] [PubMed] [Google Scholar]
- 61.Cercato C., Roizenblatt V.A., Leanca C.C., Segal A., Lopes Filho A.P., Mancini M.C., et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes. 2009;33:857–865. doi: 10.1038/ijo.2009.124. [DOI] [PubMed] [Google Scholar]
- 62.Le Riche W.H., Van Belle G. Study of phendimetrazine bitartrate as an appetite suppressant in relation to dosage, weight loss and side effects. Can Med Assoc J. 1962;87:29–31. [PMC free article] [PubMed] [Google Scholar]
- 63.Bays H.E. Lorcaserin and adiposopathy: 5-HT2c agonism as a treatment for 'sick fat' and metabolic disease. Expet Rev Cardiovasc Ther. 2009;7:1429–1445. doi: 10.1586/erc.09.123. [DOI] [PubMed] [Google Scholar]
- 64.Hendricks E.J., Srisurapanont M., Schmidt S.L., Haggard M., Souter S., Mitchell C.L., et al. Addiction potential of phentermine prescribed during long-term treatment of obesity. Int J Obes. 2014;38:292–298. doi: 10.1038/ijo.2013.74. [DOI] [PubMed] [Google Scholar]
- 65.Apovian C.M., Aronne L.J., Bessesen D.H., McDonnell M.E., Murad M.H., Pagotto U., et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 66.Bain S.C., Klufas M.A., Ho A., Matthews D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metabol. 2019;21:454–466. doi: 10.1111/dom.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Control T.D., Group C.T.R. Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol. 1998;116:874–886. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 68.Feldman-Billard S., Larger É., Massin P. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metabol. 2018;44:4–14. doi: 10.1016/j.diabet.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 70.Bays H., Blonde L., Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expet Rev Cardiovasc Ther. 2006;4:871–895. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 71.Affinati A.H., Esfandiari N.H., Oral E.A., Kraftson A.T. Bariatric surgery in the treatment of type 2 diabetes. Curr Diabetes Rep. 2019;19:156. doi: 10.1007/s11892-019-1269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Husain M., Birkenfeld A.L., Donsmark M., Dungan K., Eliaschewitz F.G., Franco D.R., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 73.Davies M., Faerch L., Jeppesen O.K., Pakseresht A., Pedersen S.D., Perreault L., et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 74.Wadden T.A., Bailey T.S., Billings L.K., Davies M., Frias J.P., Koroleva A., et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA : J Am Med Assoc. 2021;325:1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubino D., Abrahamsson N., Davies M., Hesse D., Greenway F.L., Jensen C., et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA: J Am Med Assoc. 2021;325:1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kushner R.F., Calanna S., Davies M., Dicker D., Garvey W.T., Goldman B., et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity. 2020;28:1050–1061. doi: 10.1002/oby.22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nordisk Novo. Wegovy™ demonstrated significant and sustained weight loss in two-year study in adults with obesity. https://www.novonordisk-us.com/content/nncorp/us/en_us/media/news-archive/news-details.html?id=85378
- 78.Kadowaki T., Isendahl J., Khalid U., Lee S.Y., Nishida T., Ogawa W., Tobe K., Yamauchi T., Lim S. STEP 6 investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0. Epub 2022 Feb 4. PMID: 35131037. [DOI] [PubMed] [Google Scholar]
- 79.Novo Nordisk. Research study of how well semaglutide works in people living with overweight or obesity (STEP 7). ClinicalTrials.gov Identifier: NCT04251156.
- 80.Rubino D.M., Greenway F.L., Khalid U., O'Neil P.M., Rosenstock J., Sorrig R., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA : J Am Med Assoc. 2022;327:138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nordisk Novo. Research study looking at how well semaglutide works in people suffering from obesity and knee osteoarthritis. ClinicalTrials.gov identifier: NCT05064735. https://clinicaltrials.gov/ct2/show/NCT05064735
- 82.Nauck M.A., Quast D.R. Cardiovascular safety and benefits of semaglutide in patients with type 2 diabetes: findings from SUSTAIN 6 and PIONEER 6. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.645566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan D.H., Lingvay I., Colhoun H.M., Deanfield J., Emerson S.S., Kahn S.E., et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Garvey W.T., Mechanick J.I., Brett E.M., Garber A.J., Hurley D.L., Jastreboff A.M., et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 85.Kumar R.B., Aronne L.J. Efficacy comparison of medications approved for chronic weight management. Obesity. 2015;23(Suppl 1):S4–S7. doi: 10.1002/oby.21093. [DOI] [PubMed] [Google Scholar]
- 86.Saunders K.H., Umashanker D., Igel L.I., Kumar R.B., Aronne L.J. vol. 102. The Medical clinics of North America; 2018. pp. 135–148. (Obesity pharmacotherapy). [DOI] [PubMed] [Google Scholar]
- 87.Bays H., Pi-Sunyer X., Hemmingsson J.U., Claudius B., Jensen C.B., Van Gaal L. Liraglutide 3.0 mg for weight management: weight-loss dependent and independent effects. Curr Med Res Opin. 2017;33:225–229. doi: 10.1080/03007995.2016.1251892. [DOI] [PubMed] [Google Scholar]
- 88.Blackman A., Foster G.D., Zammit G., Rosenberg R., Aronne L., Wadden T., et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes. 2016;40:1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gudbergsen H., Overgaard A., Henriksen M., Waehrens E.E., Bliddal H., Christensen R., et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am J Clin Nutr. 2021;113:314–323. doi: 10.1093/ajcn/nqaa328. [DOI] [PubMed] [Google Scholar]
- 90.Bliddal H., Christensen R. The management of osteoarthritis in the obese patient: practical considerations and guidelines for therapy. Obes Rev. 2006;7:323–331. doi: 10.1111/j.1467-789X.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 91.Bliddal H., Leeds A.R., Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15:578–586. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu A., Khawaja A.P., Pasquale L.R., Stein J.D. A review of systemic medications that may modulate the risk of glaucoma. Eye. 2020;34:12–28. doi: 10.1038/s41433-019-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cereza G., Pedrós C., Garcia N., Laporte J.-R. Topiramate in non-approved indications and acute myopia or angle closure glaucoma. Br J Clin Pharmacol. 2005;60:578–579. doi: 10.1111/j.1365-2125.2005.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharyya K.B., Basu S. Acute myopia induced by topiramate: report of a case and review of the literature. Neurol India. 2005;53:108–109. doi: 10.4103/0028-3886.15074. [DOI] [PubMed] [Google Scholar]
- 95.Chen H.-Y., Albertson T.E., Olson K.R. Treatment of drug-induced seizures. Br J Clin Pharmacol. 2016;81:412–419. doi: 10.1111/bcp.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hedges D., Jeppson K., Whitehead P. vol. 39. Drugs of today; Barcelona, Spain: 2003. pp. 551–557. (Antipsychotic medication and seizures: a review). 1998. [DOI] [PubMed] [Google Scholar]
- 97.Trinka E., Höfler J., Zerbs A. Causes of status epilepticus. Epilepsia. 2012;53(Suppl 4):127–138. doi: 10.1111/j.1528-1167.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 98.Zenical. (orlistat) https://wwwdrugscom/pro/xenicalhtml Drugscom.
- 99.Plenity instructions for use. https://www.gelesis.com/wp-content/uploads/DEN180060_Physician_IFU_FDA_FINAL_4.9.2019Gelesis.pdf
- 100.Setmelanotide injection (IMCIVREE) prescribing information. https://www.rhythmtx.com/IMCIVREE/prescribing-information.pdf
- 101.Metreleptin (MYALEPT®) http://www.myaleptpro.com/sites/default/files/myalept_pi_sept2015_final.pdf Prescribing Information.
- 102.Lisdexamfetamine dimesylate (VYVANSE) http://pi.shirecontent.com/PI/PDFs/Vyvanse_USA_ENG.pdf Prescribing Information.
- 103.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 104.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A., et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davidson M.H., Hauptman J., DiGirolamo M., Foreyt J.P., Halsted C.H., Heber D., et al. Weight control and risk factor reduction in obese subjects treated for 2 Years with OrlistatA randomized controlled trial. JAMA : J Am Med Assoc. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 106.Greenway F.L., Aronne L.J., Raben A., Astrup A., Apovian C.M., Hill J.O., et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity. 2019;27:205–216. doi: 10.1002/oby.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai X., Yang W., Gao X., Chen Y., Zhou L., Zhang S., et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity. 2018;26:70–80. doi: 10.1002/oby.22066. [DOI] [PubMed] [Google Scholar]
- 108.Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin. 2009;25:671–681. doi: 10.1185/03007990802710422. [DOI] [PubMed] [Google Scholar]
- 109.Bays H.E., Weinstein R., Law G., Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity. 2014;22:1042–1049. doi: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamamoto M., Ide N., Kitajima S., Obayashi M., Asada K., Matsushima S., et al. [Risk of euglycemic diabetic ketoacidosis due to low-carbohydrate diet while taking empagliflozin: a case report] Yakugaku Zasshi. 2019;139:1479–1483. doi: 10.1248/yakushi.19-00120. [DOI] [PubMed] [Google Scholar]
- 111.Bays H.E., Kozlovski P., Shao Q., Proot P., Keefe D. Licogliflozin, a novel SGLT1 and 2 inhibitor: body weight effects in a randomized trial in adults with overweight or obesity. Obesity. 2020;28:870–881. doi: 10.1002/oby.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 113.Wilding J.P.H. Combination therapy for obesity. J Psychopharmacol. 2017;31:1503–1508. doi: 10.1177/0269881117737401. [DOI] [PubMed] [Google Scholar]
- 114.Zinman B., Bhosekar V., Busch R., Holst I., Ludvik B., Thielke D., et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 115.Jabbour S.A., Frias J.P., Ahmed A., Hardy E., Choi J., Sjostrom C.D., et al. Efficacy and safety over 2 Years of exenatide plus dapagliflozin in the DURATION-8 study: a multicenter, double-blind, phase 3, randomized controlled trial. Diabetes Care. 2020;43:2528–2536. doi: 10.2337/dc19-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cadegiani F.A., Diniz G.C., Alves G. Aggressive clinical approach to obesity improves metabolic and clinical outcomes and can prevent bariatric surgery: a single center experience. BMC Obes. 2017;4:9. doi: 10.1186/s40608-017-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tronieri J.S., Wadden T.A., Walsh O.A., Berkowitz R.I., Alamuddin N., Gruber K., et al. Effects of liraglutide plus phentermine in adults with obesity following 1year of treatment by liraglutide alone: a randomized placebo-controlled pilot trial. Metab, Clin Exp. 2019;96:83–91. doi: 10.1016/j.metabol.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baloch H.M., Nadolsky K. Combination of phentermine/topiramate Er and liraglutide 3 Mg for intensive therapy of severe obesity & T2Dm: a case series and brief review. AACE Clin Case Rep. 2018;4:e482–e486. [Google Scholar]
- 119.Istfan N.W., Anderson W.A., Hess D.T., Yu L., Carmine B., Apovian C.M. The mitigating effect of phentermine and topiramate on weight regain after roux-en-Y gastric bypass surgery. Obesity. 2020;28:1023–1030. doi: 10.1002/oby.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nor Hanipah Z., Nasr E.C., Bucak E., Schauer P.R., Aminian A., Brethauer S.A., et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis. 2018;14:93–98. doi: 10.1016/j.soard.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Gazda C.L., Clark J.D., Lingvay I., Almandoz J.P. Pharmacotherapies for post-bariatric weight regain: real-world comparative outcomes. Obesity. 2021;29:829–836. doi: 10.1002/oby.23146. [DOI] [PubMed] [Google Scholar]
- 122.Chen J., Zhao H., Ma X., Zhang Y., Lu S., Wang Y., et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol Biochem. 2017;42:1165–1176. doi: 10.1159/000478872. [DOI] [PubMed] [Google Scholar]
- 123.Bray G. vol. 59. Dorrance Publishing; 2007. (Battle of the bulge). [Google Scholar]
- 124.James W.P., Caterson I.D., Coutinho W., Finer N., Van Gaal L.F., Maggioni A.P., et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 125.de Andrade Mesquita L., Fagundes Piccoli G., Richter da Natividade G., Frison Spiazzi B., Colpani V., Gerchman F. Is lorcaserin really associated with increased risk of cancer? A systematic review and meta-analysis. Obes Rev. 2021;22 doi: 10.1111/obr.13170. [DOI] [PubMed] [Google Scholar]
- 126.Saxena A., Sachin K. A network biology approach for assessing the role of pathologic adipose tissues in insulin resistance using meta-analysis of microarray datasets. Curr Genom. 2018;19:630–666. doi: 10.2174/1389202919666180726125645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camacho-Ramírez A., Mayo-Ossorio M., Pacheco-García J.M., Almorza-Gomar D., Ribelles-García A., Belmonte-Núñez A., et al. Pancreas is a preeminent source of ghrelin after sleeve gastrectomy in Wistar rats. Histol Histopathol. 2020;35:801–809. doi: 10.14670/HH-18-200. [DOI] [PubMed] [Google Scholar]
- 128.Bays H.E., Jones P.H., Jacobson T.A., Cohen D.E., Orringer C.E., Kothari S., et al. Lipids and bariatric procedures part 1 of 2: scientific statement from the national Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: full report. J Clin Lipidol. 2016;10:33–57. doi: 10.1016/j.jacl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 129.Bays H., Kothari S.N., Azagury D.E., Morton J.M., Nguyen N.T., Jones P.H., et al. Lipids and bariatric procedures Part 2 of 2: scientific statement from the American Society for Metabolic and Bariatric Surgery (ASMBS), the National Lipid Association (NLA), and Obesity Medicine Association (OMA) Surg Obes Relat Dis. 2016;12:468–495. doi: 10.1016/j.soard.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 130.Dimitriadis G.K., Randeva M.S., Miras A.D. Potential hormone mechanisms of bariatric surgery. Curr Obes Rep. 2017;6:253–265. doi: 10.1007/s13679-017-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khalaf K.I., Taegtmeyer H. Clues from bariatric surgery: reversing insulin resistance to heal the heart. Curr Diabetes Rep. 2013;13:245–251. doi: 10.1007/s11892-013-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Batterham R.L., Cummings D.E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39:893–901. doi: 10.2337/dc16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meek C.L., Lewis H.B., Reimann F., Gribble F.M., Park A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 134.Nannipieri M., Baldi S., Mari A., Colligiani D., Guarino D., Camastra S., et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metabol. 2013;98:4391–4399. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 135.Sethi P., Thillai M., Nain P.S., Ahuja A., Aulakh N., Khurana P. Role of hunger hormone "Ghrelin” in long-term weight loss following laparoscopic sleeve gastrectomy. Niger J Surg. 2018;24:121–124. doi: 10.4103/njs.NJS_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma G., Nain P.S., Sethi P., Ahuja A., Sharma S. Plasma ghrelin levels after laparoscopic sleeve gastrectomy in obese individuals. Indian J Med Res. 2019;149:544–547. doi: 10.4103/ijmr.IJMR_984_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu H.C., Pang Y.C., Chen J.W., Cao J.Y., Sheng Z., Yuan J.H., et al. Systematic review and meta-analysis of the change in Ghrelin levels after roux-en-Y gastric bypass. Obes Surg. 2019;29:1343–1351. doi: 10.1007/s11695-018-03686-3. [DOI] [PubMed] [Google Scholar]
- 138.Modvig I.M., Andersen D.B., Grunddal K.V., Kuhre R.E., Martinussen C., Christiansen C.B., Ørskov C., Larraufie P., Kay R.G., Reimann F., Gribble F.M., Hartmann B., Bojsen-Møller K.N., Madsbad S., Wewer Albrechtsen N.J., Holst J.J. Secretin release after Roux-en-Y gastric bypass reveals a population of glucose-sensitive S cells in distal small intestine. Int J Obes (Lond) 2020;44(9):1859–1871. doi: 10.1038/s41366-020-0541-7. Epub 2020 Feb 3. PMID: 32015474; PMCID: PMC7445113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seong J., Kang J.Y., Sun J.S., Kim K.W. Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res (Seoul) 2019;42:383–392. doi: 10.1007/s12272-019-01138-9. [DOI] [PubMed] [Google Scholar]
- 140.Srivastava G., Apovian C. Future pharmacotherapy for obesity: new anti-obesity drugs on the horizon. Curr Obes Rep. 2018;7:147–161. doi: 10.1007/s13679-018-0300-4. [DOI] [PubMed] [Google Scholar]
- 141.Bays H.E. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating "sick fat” through improving fat function with antidiabetes therapies. Am J Cardiol. 2012;110:4B–12B. doi: 10.1016/j.amjcard.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 142.Liu R., Li N., Lin Y., Wang M., Peng Y., Lewi K., et al. Glucagon like peptide-1 promotes adipocyte differentiation via the Wnt4 mediated sequestering of beta-catenin. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo C., Huang T., Chen A., Chen X., Wang L., Shen F., et al. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz J Med Biol Res. 2016;49 doi: 10.1590/1414-431X20165826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scott R.V., Bloom S.R. Problem or solution: the strange story of glucagon. Peptides. 2018;100:36–41. doi: 10.1016/j.peptides.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanchez-Garrido M.A., Brandt S.J., Clemmensen C., Muller T.D., DiMarchi R.D., Tschop M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60:1851–1861. doi: 10.1007/s00125-017-4354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trapp S., Brierley D.I. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol. 2022;179:557–570. doi: 10.1111/bph.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Samms R.J., Coghlan M.P., Sloop K.W. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metabol: TEM (Trends Endocrinol Metab) 2020;31:410–421. doi: 10.1016/j.tem.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 148.Deacon C.F. Metabolism of GIP and the contribution of GIP to the glucose-lowering properties of DPP-4 inhibitors. Peptides. 2020;125 doi: 10.1016/j.peptides.2019.170196. [DOI] [PubMed] [Google Scholar]
- 149.Holst J.J., Rosenkilde M.M. GIP as a therapeutic target in diabetes and obesity: insight from incretin Co-agonists. J Clin Endocrinol Metabol. 2020;105:e2710–e2716. doi: 10.1210/clinem/dgaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khan R., Tomas A., Rutter G.A. Effects on pancreatic Beta and other Islet cells of the glucose-dependent insulinotropic polypeptide. Peptides. 2020;125 doi: 10.1016/j.peptides.2019.170201. [DOI] [PubMed] [Google Scholar]
- 151.Christensen M., Vedtofte L., Holst J.J., Vilsbøll T., Knop F.K. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pelle M.C., Provenzano M., Zaffina I., Pujia R., Giofre F., Luca S., et al. Role of a dual glucose-dependent insulinotropic peptide (GIP)/Glucagon-like peptide-1 receptor agonist (twincretin) in glycemic control: from pathophysiology to treatment. Life. 2021;12 doi: 10.3390/life12010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hayes M.R., Borner T., De Jonghe B.C. The role of GIP in the regulation of GLP-1 satiety and nausea. Diabetes. 2021;70:1956–1961. doi: 10.2337/dbi21-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen X., He X., Guo Y., Liu L., Li H., Tan J., et al. Glucose-dependent insulinotropic polypeptide modifies adipose plasticity and promotes beige adipogenesis of human omental adipose-derived stem cells. Faseb J. 2021;35 doi: 10.1096/fj.201903253R. [DOI] [PubMed] [Google Scholar]
- 155.Mohammad S., Patel R.T., Bruno J., Panhwar M.S., Wen J., McGraw T.E. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol Cell Biol. 2014;34:3618–3629. doi: 10.1128/MCB.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holst J.J. The incretin system in healthy humans: the role of GIP and GLP-1. Metab, Clin Exp. 2019;96:46–55. doi: 10.1016/j.metabol.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 157.Holst J.J. What combines best with GLP-1 for obesity treatment: GIP receptor agonists or antagonists? Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sharma D., Verma S., Vaidya S., Kalia K., Tiwari V. Recent updates on GLP-1 agonists: current advancements & challenges. Biomed Pharmacother. 2018;108:952–962. doi: 10.1016/j.biopha.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 159.Kalra S., Bhattacharya S., Kapoor N. Contemporary classification of glucagon-like peptide 1 receptor agonists (GLP1RAs) Diabetes Ther. 2021;12:2133–2147. doi: 10.1007/s13300-021-01113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawai T., Sun B., Yoshino H., Feng D., Suzuki Y., Fukazawa M., et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci Unit States Am. 2020;117:29959–29967. doi: 10.1073/pnas.2014879117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choe H.J., Cho Y.M. Peptidyl and non-peptidyl oral glucagon-like peptide-1 receptor agonists. Endocrinol Metab (Seoul) 2021;36:22–29. doi: 10.3803/EnM.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Min T., Bain S.C. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12:143–157. doi: 10.1007/s13300-020-00981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ryan D.H. Next generation antiobesity medications: setmelanotide, semaglutide, tirzepatide and bimagrumab: what do they mean for clinical practice? J Obes Metab Syndr. 2021;30:196–208. doi: 10.7570/jomes21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jepsen M.M., Christensen M.B. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expet Opin Emerg Drugs. 2021;26:231–243. doi: 10.1080/14728214.2021.1947240. [DOI] [PubMed] [Google Scholar]
- 165.Camilleri M., Acosta A. Combination therapies for obesity. Metab Syndr Relat Disord. 2018;16:390–394. doi: 10.1089/met.2018.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spezani R., Mandarim-de-Lacerda C.A. The current significance and prospects for the use of dual receptor agonism GLP-1/Glucagon. Life Sci. 2022;288 doi: 10.1016/j.lfs.2021.120188. [DOI] [PubMed] [Google Scholar]
- 167.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 168.Gantz I., Erondu N., Mallick M., Musser B., Krishna R., Tanaka W.K., et al. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J Clin Endocrinol Metabol. 2007;92:1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 169.Kruse T., Hansen J.L., Dahl K., Schaffer L., Sensfuss U., Poulsen C., et al. Development of cagrilintide, a long-acting amylin analogue. J Med Chem. 2021;64:11183–11194. doi: 10.1021/acs.jmedchem.1c00565. [DOI] [PubMed] [Google Scholar]
- 170.Muller T.D., Bluher M., Tschop M.H., DiMarchi R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi: 10.1038/s41573-021-00337-8. Epub 2021 Nov 23. PMID: 34815532; PMCID: PMC8609996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davis T.R., Pierce M.R., Novak S.X., Hougland J.L. Ghrelin octanoylation by ghrelin O-acyltransferase: protein acylation impacting metabolic and neuroendocrine signalling. Open Biology. 2021;11 doi: 10.1098/rsob.210080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu W., Li X., Luo Y. FGF21 in obesity and cancer: new insights. Cancer Lett. 2021;499:5–13. doi: 10.1016/j.canlet.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Geng L., Lam K.S.L., Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 174.Kim A.M., Somayaji V.R., Dong J.Q., Rolph T.P., Weng Y., Chabot J.R., et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metabol. 2017;19:1762–1772. doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- 175.Xiong Y., Walker K., Min X., Hale C., Tran T., Komorowski R., et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan8732. [DOI] [PubMed] [Google Scholar]
- 176.Li X., Bello N.T. Anorectic state of obesity medications in the United States. Are leaner times ahead? Expet Opin Pharmacother. 2020;21:167–172. doi: 10.1080/14656566.2019.1692815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen K.Y., Brychta R.J., Abdul Sater Z., Cassimatis T.M., Cero C., Fletcher L.A., et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J Biol Chem. 2020;295:1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Busiello R.A., Savarese S., Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol. 2015;6:36. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Demine S., Renard P., Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019;8 doi: 10.3390/cells8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zheng J., Xiao H., Duan Y., Song B., Zheng C., Guo Q., et al. Roles of amino acid derivatives in the regulation of obesity. Food Funct. 2021;12:6214–6225. doi: 10.1039/d1fo00780g. [DOI] [PubMed] [Google Scholar]
- 181.Jeremic N., Chaturvedi P., Tyagi S.C. Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol. 2017;232:61–68. doi: 10.1002/jcp.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gregory S., Hill D., Grey B., Ketelbey W., Miller T., Muniz-Terrera G., et al. 11β-hydroxysteroid dehydrogenase type 1 inhibitor use in human disease-a systematic review and narrative synthesis. Metab, Clin Exp. 2020;108 doi: 10.1016/j.metabol.2020.154246. [DOI] [PubMed] [Google Scholar]
- 183.Hoffmann J.G., Xie W., Chopra A.R. Energy regulation mechanism and therapeutic potential of asprosin. Diabetes. 2020;69:559–566. doi: 10.2337/dbi19-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muo I.M., MacDonald S.D., Madan R., Park S.J., Gharib A.M., Martinez P.E., et al. Early effects of roflumilast on insulin sensitivity in adults with prediabetes and overweight/obesity involve age-associated fat mass loss - results of an exploratory study. Diabetes Metab Syndr Obes. 2019;12:743–759. doi: 10.2147/DMSO.S182953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heymsfield S.B., Coleman L.A., Miller R., Rooks D.S., Laurent D., Petricoul O., et al. Effect of Bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.33457. e2033457-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Akbari P., Gilani A., Sosina O., Kosmicki J.A., Khrimian L., Fang Y.-Y., et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373 doi: 10.1126/science.abf8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murtaza B., Asghar F., Patoli D. GPR75: an exciting new target in metabolic syndrome and related disorders. Biochimie. 2022;195:19–26. doi: 10.1016/j.biochi.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 188.Na H.N., Kim H., Nam J.H. Prophylactic and therapeutic vaccines for obesity. Clin Exp Vaccine Res. 2014;3:37–41. doi: 10.7774/cevr.2014.3.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monteiro M.P. Obesity vaccines. Hum Vaccines Immunother. 2014;10:887–895. doi: 10.4161/hv.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shirin H., Richter V., Matalon S., Abramowich D., Maliar A., Shachar E., et al. Safety, tolerability and efficacy of a novel self-use biodegradable device for management of obesity. Obes Sci Pract. 2019;5:376–382. doi: 10.1002/osp4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nauck M.A., Meier J.J. Incretin hormones: their role in health and disease. Diabetes Obes Metabol. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 192.Rosenstock J., Wysham C., Frías J.P., Kaneko S., Lee C.J., Fernández Landó L., et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 193.Frías J.P., Davies M.J., Rosenstock J., Pérez Manghi F.C., Fernández Landó L., Bergman B.K., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 194.Ludvik B., Giorgino F., Jódar E., Frias J.P., Fernández Landó L., Brown K., et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 195.Del Prato S., Kahn S.E., Pavo I., Weerakkody G.J., Yang Z., Doupis J., et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 196.Dahl D., Onishi Y., Norwood P., Huh R., Bray R., Patel H., et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA : J Am Med Assoc. 2022;327:534–545. doi: 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.ClinicalTrials.gov SURPASS 6. Study of tirzepatide (LY3298176) versus insulin lispro (U100) in participants with type 2 diabetes inadequately controlled on insulin glargine (U100) with or without metformin. https://clinicaltrials.gov/ct2/show/NCT04537923
- 198.ClinicalTrials.gov SURPASS AP COMBO: a study of tirzepatide (LY3298176) in Participants with type 2 diabetes on metformin with or without sulfonylurea. https://clinicaltrials.gov/ct2/show/NCT04093752
- 199.ClinicalTrials.gov SURPASS CVOT: a study of tirzepatide (LY3298176) compared with dulaglutide on major cardiovascular events in participants with type 2 diabetes. https://www.clinicaltrials.gov/ct2/show/NCT04255433
- 200.ClinicalTrials.gov SURMOUNT-1: a study of tirzepatide (LY3298176) in participants with obesity or overweight. https://clinicaltrials.gov/ct2/show/NCT04184622
- 201.Rossini A.A. Why control blood glucose levels? Arch Surg. 1976;111:229–233. doi: 10.1001/archsurg.1976.01360210023004. [DOI] [PubMed] [Google Scholar]
- 202.Ryan C. New controversies in hypertension: questions answered, answers questioned. Compr Ther. 1992;18:20–24. [PubMed] [Google Scholar]
- 203.Thompson W.G. Cholesterol: myth or reality? South Med J. 1990;83:435–440. [PubMed] [Google Scholar]
- 204.Tobert J.A. The cholesterol controversy. BMJ. 1992;304:713. doi: 10.1136/bmj.304.6828.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bierman E.L. The oral antidiabetic agents. Am Fam Physician. 1976;13:98–104. [PubMed] [Google Scholar]
- 206.Rys P., Wojciechowski P., Rogoz-Sitek A., Niesyczynski G., Lis J., Syta A., et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649–662. doi: 10.1007/s00592-014-0698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mannucci E., Monami M., Masotti G., Marchionni N. All-cause mortality in diabetic patients treated with combinations of sulfonylureas and biguanides. Diabetes Metab Res Rev. 2004;20:44–47. doi: 10.1002/dmrr.411. [DOI] [PubMed] [Google Scholar]
- 208.Gerber J.G., Freed C.R., Nies A.S. Antihypertensive pharmacology. West J Med. 1980;132:430–439. [PMC free article] [PubMed] [Google Scholar]
- 209.Bays H.E., Ballantyne C. What's the deal with niacin development: is laropiprant add-on therapy a winning strategy to beat a straight flush? Curr Opin Lipidol. 2009;20:467–476. doi: 10.1097/MOL.0b013e3283325083. [DOI] [PubMed] [Google Scholar]
- 210.Maki K.C., Bays H.E., Dicklin M.R. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6:413–426. doi: 10.1016/j.jacl.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 211.Bays H.E., Goldberg R.B. The 'forgotten' bile acid sequestrants: is now a good time to remember? Am J Therapeut. 2007;14:567–580. doi: 10.1097/MJT.0b013e31815a69fc. [DOI] [PubMed] [Google Scholar]
- 212.Muppidi A., Zou H., Yang P.Y., Chao E., Sherwood L., Nunez V., et al. Design of potent and proteolytically stable oxyntomodulin analogs. ACS Chem Biol. 2016;11:324–328. doi: 10.1021/acschembio.5b00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bays H.E., Toth P.P., Kris-Etherton P.M., Abate N., Aronne L.J., Brown W.V., et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–383. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 214.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 215.Bays H. Adiposopathy, "sick fat,” Ockham's razor, and resolution of the obesity paradox. Curr Atherosclerosis Rep. 2014;16:409. doi: 10.1007/s11883-014-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bays H.E., Taub P.R., Epstein E., Michos E.D., Ferraro R.A., Bailey A.L., et al. Ten things to know about ten cardiovascular disease risk factors. Am J Prev Cardiol. 2021;5 doi: 10.1016/j.ajpc.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Garber A.J., Abrahamson M.J., Barzilay J.I., Blonde L., Bloomgarden Z.T., Bush M.A., et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement--executive summary. Endocr Pract. 2013;19:536–557. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA : J Am Med Assoc. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 219.American Diabetes A. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S81–S89. doi: 10.2337/dc19-S008. [DOI] [PubMed] [Google Scholar]
- 220.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., Goldberg R., Heidenreich P.A., Hlatky M.A., Jones D.W., Lloyd-Jones D., Lopez-Pajares N., Ndumele C.E., Orringer C.E., Peralta C.A., Saseen J.J., Smith S.C. Jr, Sperling L., Virani S.S., Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. Epub 2018 Nov 10. Erratum in: Circulation. 2019 Jun 18;139(25):e1182-e1186. PMID: 30586774; PMCID: PMC7403606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Landsberg L., Aronne L.J., Beilin L.J., Burke V., Igel L.I., Lloyd-Jones D., et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment--a position paper of the the Obesity Society and the American Society of Hypertension. Obesity. 2013;21:8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- 222.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.