Abstract
Background
Body mass index (BMI or weight in kilograms/height in meters2) is the most common metric to diagnose overweight and obesity. However, a body composition analysis more thoroughly assesses adiposity, percent body fat, lean body mass (i.e., including skeletal muscle), and sometimes bone mineral density. BMI is not an accurate assessment of body fat in individuals with increased or decreased muscle mass; the diagnostic utility of BMI in individuals is also influenced by race and sex.
Methods
Previous Obesity Pillars Roundtables addressed the diagnostic limitations of BMI, the importance of android and visceral fat (especially among those with South and East Asian ancestry), and considerations of obesity among individuals who identify as Hispanic, diverse in sexual-orientation, Black, Native American, and having ancestry from the Mediterranean and Middle East regions. This roundtable examines considerations of BMI in Black and female individuals.
Results
The panelists agreed that body composition assessment was a more accurate measure of adiposity and muscle mass than BMI. When it came to matters of race and sex, one panelist felt: “race is a social construct and not a defining biology.” Another felt that: “BMI should be a screening tool to prompt further evaluation of adiposity that utilizes better diagnostic tools for body composition.” Regarding bias and misperceptions of resistance training in female individuals, another panelist stated: “I have spent my entire medical career taking care of women and have never seen a woman unintentionally gain ‘too much’ muscle mass and bulk up from moderate strength training.”
Conclusions
Conveying the importance of race and sex regarding body composition has proven challenging, with the discussion sometimes devolving into misunderstandings or misinformation that may be perceived as racist or sexist. Body composition analysis is the ultimate diagnostic equalizer in addressing the inaccuracies and biases inherent in the exclusive use of BMI.
Keywords: Body composition, Female, Obesity, Racist, Sexist
1. Introduction
Dr. Bays
Hello. My name is Dr. Harold Bays. I am Editor-in-Chief of Obesity Pillars [official journal of the Obesity Medicine Association (OMA)], and Chief Science Officer of the OMA. I am serving as moderator for this roundtable review regarding: “Body Mass Index and Body Composition in Black and Female Individuals. Race-relevant or Racist? Sex-relevant or Sexist?”

As with other Obesity Pillars Roundtables, the opinions expressed in this roundtable reflect that of the panelists and myself, and not necessarily the opinions of the other Editors of Obesity Pillars or the OMA members or leadership. This roundtable continues the Obesity Pillars tradition of addressing clinically relevant topics in obesity medicine that are often not discussed elsewhere. Past Obesity Pillars roundtables have addressed the relevance of obesity among those who identify as Hispanic, diverse in sexual-orientation, Black, Native American [1], South Asian [2], East Asian [3], and those with ancestry from the Mediterranean and Middle East regions [4]. Prior Obesity Pillars Roundtables have discussed topics some might consider controversial, such as “Metabolic and Bariatric Surgery in Children and Adolescents” [5] and “Phentermine – Past, Present, and Future” [6]. Today, I am honored to be joined by experts, to discuss the importance of body composition, as it pertains to obesity, race, and sex. I would like to begin with introductions.
2. Introduction of expert panelists
2.1. Dr. Bays
Dr. Wharton, please provide a summary of your clinical background and your interest in body composition.

2.2. Dr. Wharton
I have a doctorate in Pharmacy and Medicine at the University of Toronto. I am medical director of the Wharton Medical Clinic, a community based internal medicine weight management and diabetes clinic. I am adjunct professor at McMaster University in Hamilton and York University in Toronto, academic staff at Women’s College Hospital, and clinical staff Hamilton Health Sciences. My research focuses are bariatric medicine and type 2 diabetes. I am co-lead authour of the 2020 Canadian Obesity Guidelines. I am involved in activism to achieve health equity in Canada. I founded the BMSA (Black Medical Students Association) at the University of Toronto in 2000. The BMSA is now a recognized mentorship organization across Canada.

2.3. Dr. Bays
Dr. Gonsahn-Bollie, please provide a summary of your clinical background your interest in body composition.
2.4. Dr. Gonsahn-Bollie
In many ways, my entire life has prepared me for working in obesity. Sadly, I witnessed how the complications of obesity debilitated my grandma, Sylvia. Although I couldn't understand it then, I now see how weight and racial bias affected her care. Eight years ago, after the birth of my son, I became a part of the "Black women have the highest rate of obesity" statistic. Sitting on the other side of obesity assessment and treatment, I painfully experienced inadequacies of obesity assessment and care. What I learned about the history of body mass index (BMI), body composition, and the diverse causes of obesity forever changed how I practice medicine. In 2015 became an active member of the Obesity Medicine Association and in 2016 took the American Board of Obesity Medicine. But my interest in the limitations of BMI dates to 2012 during my residency internship at the Office of Disease Prevention and Health Promotion (ODPHP) when I worked on the ODPHP Primary Care Obesity Tool. In 2020, while working on the COVID-19 frontlines, I launched Embrace You Weight & Wellness, a digital health company that empowers individuals with personalized metabolic tools and mindset breakthroughs for sustainable weight loss and wellness. In 2021 I released the bestseller "Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness." "Embrace You: Your Guide" was listed as Healthline.com's "Best Weight Loss Book of 2022 and included an in-depth look at individualized "healthy weight" vs. using the standard BMI. In addition to clinical care, I leverage my skills as a writer, public speaker, and trained educator to empower my peers and the public with evidence-based obesity education on social media and multiple media outlets. Lastly, I consult with organizations such as Black Health to create culturally relevant obesity tools. I am grateful to contribute to this transformational time in addressing the disparities in personalized obesity care, especially for women and minorities.

2.5. Dr. Bays
Dr. Younglove, please provide please provide a summary of your clinical background and your interest in body composition.
2.6. Dr. Younglove
I have always had a passion for women’s health - first obtaining my undergraduate degree in Women’s Studies, then pursuing a career in the field of Obstetrics & Gynecology after medical school. I discovered the field of Obesity Medicine in 2014. I initially tried to integrate both fields into a single practice, which didn’t work. I opened my own Obesity Medicine practice in 2018 and left the field of OB/GYN shortly after, although my patient population has remained primarily women. Early in my Obesity Medicine training, it was apparent that using weight or BMI as a primary metric to assess and/or measure individuals was suboptimal. Since obesity is defined as excess adiposity, to make this diagnosis and assess the impact of our treatments on patients’ adiposity, we as clinicians need to be able to measure adiposity, especially visceral adiposity. The gold standard for measuring these metrics requires very costly machines, which is not something that I could afford in a small clinic setting. However, after doing some research, I learned that we get a fairly accurate estimate of these metrics using bioelectric impedance analysis. Although we calculate BMI at every visit and note that in the chart, my team and I use total body fat, body fat percentage and waist circumference to determine whether our treatment is effective. After typing these values into spreadsheets year after year for thousands of patients, I can say with complete certainty that body composition is a far superior measure of adiposity than BMI or body weight.
3. Fat mass
3.1. Dr. Bays
According to the Obesity Medicine Association [8]:
“Obesity is defined as a chronic, progressive, relapsing, and treatable multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences”.
Obesity is a disease of increased adiposity [8,9] that increases morbidities and all-cause mortality [10]. Obesity-related heart disease, stroke, type 2 diabetes mellitus (T2DM), and cancer are among the leading causes of preventable, premature death. [https://www.cdc.gov/obesity/data/adult.html]. Beyond cardiovascular disease, obesity is secondary only to cigarette smoking as the most common preventable cause of malignant neoplasms/cancer [6]. The pathogenic potential of increased adipose tissue is due to “fat mass disease” (i.e., adverse biomechanical health consequences such as sleep apnea [11], arthritis, and congestive cardiomyopathy) and “sick fat disease” (i.e., adiposopathy leading to T2DM, hypertension, dyslipidemia, cardiovascular disease and cancer) [8,12].
Body composition can be assessed by various methods, each having advantages and disadvantages [7,13]. Compared to body mass index (BMI), body composition analysis is a better diagnostic assessment of adiposity; visceral fat is a better prognostic measure of the adverse metabolic health consequences of increased adiposity (See Table 1). Increase visceral adiposity reflects global fat dysfunction [14]. The immunopathies and endocrinopathies of adiposopathy (i.e., “sick fat” or adipose tissue dysfunction) contribute to major cardiometabolic risk factors, and increase the increased risk of cardiovascular disease [15] and cancer [6,16]. One publication suggests [17]:
“It is proposed that obesity, generally defined by an excess of body fat causing prejudice to health, can no longer be evaluated solely by the body mass index, because it represents a heterogeneous entity. Excessive amounts of visceral adipose tissue and of ectopic fat largely define the cardiovascular disease risk of overweight and moderate obesity.”
Table 1.
Obesity Medicine Association (OMA) Classifications of Percent Body Fat [7]. For the individual, a body composition analysis by an accurate method allows for a comprehensive assessment of body fat, lean body mass, and sometimes bone health as well as android/visceral fat, compared to body mass index (BMI) alone. The OMA has identified the category of “pre-obesity” based upon percent body fat criteria (i.e., not BMI criteria). Percent body fat is a diagnostic measure of adiposity. Waist circumference, android fat, and visceral fat are more validated prognostic measures of the interrelationship between adiposity and health risks (e.g., cardiovascular disease and cancer). The OMA has suggested that optimal visceral fat is less than 1 pound and optimal android fat is less than 3 pounds [7].
| Female | Male | |
|---|---|---|
| Essential fat | <15% | <10% |
| Athlete | 15–19% | 10–14% |
| Fitness | 20–24% | 15–19% |
| Acceptable | 25–29% | 20–24% |
| Pre-obesity | 30–34% | 25–29% |
| Obesity | ≥35% | ≥30% |
Thus, when determining the best measure to assess adiposity, it would seem reasonable to choose the measuring technique most accurate in providing diagnostic and prognostic information regarding health outcomes, with death being the ultimate adverse health outcome. Table 2 describes causes of death among US female and Black subgroups compared to the total US population. The most common cause of death in individuals 34–85 years of age is malignant neoplasms. The most common cause of death among those >85 years of age is cardiovascular disease. The ranking of the causes of deaths among female and Black subgroups and the US population are generally similar. However, some disparities exist. Diabetes mellitus and cerebrovascular disease rank higher as causes of death among Black individuals >65 years of age compared to White individuals. The increased ranking of death from diabetes mellitus and cerebrovascular disease may be related to the increased prevalence of obesity among Black individuals, with genetic and socioeconomic influences also contributing to the differential death rankings compared to the total US population [[18], [19], [20], [21], [22]]. Regarding females, Table 2 suggests an increased risk ranking of death due to chronic low respiratory disease in the female subgroup 34–64 years of age. These subset cause of death rankings based upon race and sex may help identify racial and sex disparities. Body composition analyses may help provide diagnostic and prognostic insights to these disparities, as well as regarding the most common causes of death (e.g., cardiovascular disease and cancer) [6,7,13].
Table 2.
US Centers for Disease Control and Prevention ranking of the most common causes of death in the US based on race and sex (year range 2010–2020). (https://wisqars.cdc.gov/data/lcd/home). Compared to the general US population, the causes of death across age ranges are generally consistent among female and Black subgroups. Exceptions include: (a) increased risk ranking of death due to chronic low respiratory disease in the female subgroup 34–64 years of age and (b) increased risk ranking of deaths due to cerebrovascular disease and diabetes among the Black subgroup 65–85 years of age.
| Total population | Females (all races) | Blacks (males and females) | |
|---|---|---|---|
| 15–34 years | (1) Unintentional injury (2) Suicide (3) Homicide |
(1) Unintentional injury (2) Suicide (3) Malignant neoplasms |
(1) Homicide (2) Unintentional injury (3) Heart disease, or suicide |
| 34–64 years | (1) Malignant neoplasms (2) Heart disease (3) Unintentional injury |
(1) Malignant neoplasms (2) Heart disease (3) Unintentional injury or chronic low respiratory disease |
(1) Malignant neoplasms (2) Heart disease (3) Unintentional injury |
| 65–85 years | (1) Malignant neoplasms (3) Heart disease (3) Chronic low respiratory disease (with cerebrovascular disease #4) |
(1) Malignant neoplasms (2) Heart disease (3) Chronic low respiratory disease (with cerebrovascular disease #4) |
(1) Malignant neoplasms (2) Heart Disease (3) Cerebrovascular disease (with diabetes mellitus #4) |
| > 85 years | (1) Heart disease (2) Malignant neoplasms (3) Alzheimer’s disease or cerebrovascular disease |
(1) Heart disease (2) Malignant neoplasms (3) Alzheimer’s disease or cerebrovascular disease |
(1) Heart disease (2) Malignant neoplasms (3) Cerebrovascular disease (with Alzheimer’s being #4) |
Dr. Wharton, before we get too granular into the intrinsic genetic differences among races and sexes regarding body composition and obesity health outcomes, what over-arching environmental, societal, or conceptual factors should we consider when discussing treatment and health outcome disparities in population subgroups [[23], [24], [25], [26]]?
3.2. Dr Wharton
Before we start, we should clarify and define a few terms. We have used the word race multiple times in this discussion and even in the title. Race is a social construct and not a defining biology. This is very important to understand. All people with more melanin are not the same. All dark people from African continent are not the same. The genetics of the biology between 2 black people from Africa are equally as dissimilar as they are similar to the genetics of a Black person from a Swedish person. Skin colour does define the social construct of race, for instance Black or White. The term “race” puts these 2 different groups in completely different interactions within society. Therefore, the discussion of body composition is not a discussion of race but more so a discussion of ethnic background or geography. Body composition in an individual from Ethiopia is completely different from that of a person from West Africa or Nigeria, which is in turn different from an African American. There is no reason to categorize the social construct of race when it comes to body composition in America. Body composition should be determined on an individual level. If we discuss body composition and geographical sense and talk about Ethiopians versus Black people from South Africa, then we would find geographic differences. When we come to America and understand that gene variation occurs all over Africa and Europe, and when we recognize there is no homogeneity when it comes to population research, I do not see any reason to connect the social construct of race with body composition. My views may differ from those on this panel and many people in America, but these are the scientific and biological facts. When it comes to gender there are differences primarily based on differing hormonal levels.
3.3. Dr. Bays
Thank you for that perspective. While recognizing their limitations, the terms “Black” and “White” are often used here, mainly because the source data reported here are derived from the US Centers for Disease Control (CDC), which utilizes the terms “Black” and “White.” https://wisqars.cdc.gov/data/lcd/home). Regarding “sex” versus “gender,” sex is intended here to reflect biologic female or male, recognizing the variation in how female or male attributes are expressed. “Gender” is intended to refer to self-identification, socially constructed attitudes, roles, feelings, behaviors, and personal experience that may not aligned with the sex recorded at birth [45]. Regarding body composition, the choice of the term “gender” versus “sex” may present challenges. In the example of transgender individuals, the variable use of hormone therapy and gender affirming surgery can vary body composition. When specifically used as cross-sex hormone therapy (as opposed to post-menopausal therapy), some reports suggest estrogens may facilitate an increase in fat mass and decrease lean body mass in transgender women, while testosterone reduces body fat and may increase lean body mass in transgender men [[45], [46], [47]].
Drs. Gonsahn-Bollie and Younglove, on the topic of females, premenopausal female individuals generally have less proportional accumulation of abdominal fat than males. Female individuals often disproportionately distribute body fat in a “pear” distribution (i.e., hip region) vs “apple” distribution (i.e., abdominal region) more often described in males. Increased abdominal fat distribution is associated with increased cardiovascular disease risk [12]. Additionally [48]:
“Despite women of African ancestry being more insulin resistant than their white counterparts, they have less visceral adipose tissue (VAT) and hepatic steatosis and more peripheral subcutaneous adipose tissue (SAT). The larger SAT adipocyte size in women of African ancestry is associated with a reduced adipogenic capacity and a higher expression of inflammatory genes compared to their White counterparts.”
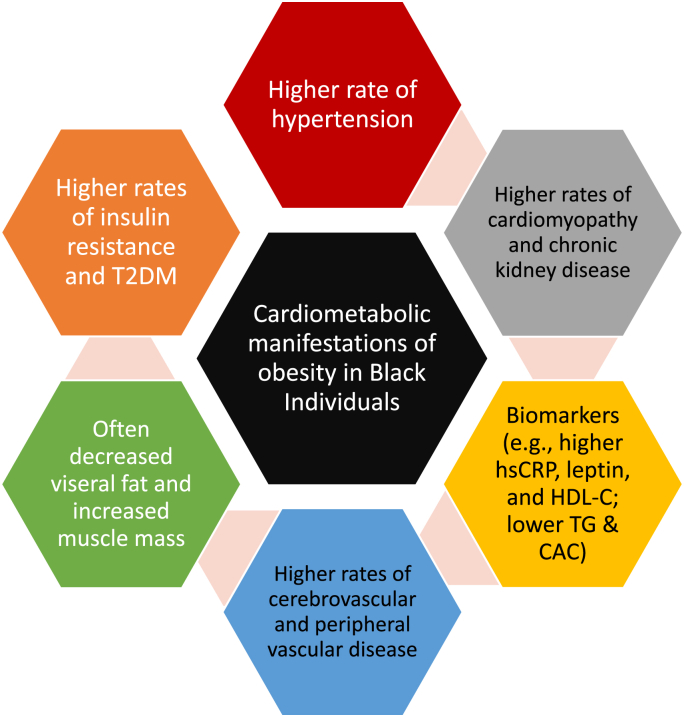
This mirrors what we find in clinical practice. When we perform body composition analyses via dual x-ray absorptiometry (DXA), it is not uncommon to find Black females with elevated percent body fat, but negligible visceral fat. Just recently, a Black female presented to us with a percent body fat of over 50%, but only moderately elevated android and visceral fat. The bottom-line is clinically meaningful racial differences exist in the body composition and pathophysiology of disease affecting Black individuals, such as cardiometabolic diseases (See Fig. 1).
Fig. 1.
Illustrative cardiometabolic manifestations of obesity in Black individuals [27]. Systemic racism may be a contributor to obesity among Black individuals as well as other People of Color [28,29]. Hypertension is highly prevalent among African Americans and contributes to disparities in stroke, heart failure, peripheral artery disease, and chronic kidney disease compared to other races [30,31]. While increased obesity prevalence is a contributor to increased hypertension among Black individuals, the increased risk for hypertension persists after adjustment for abdominal obesity [32]. The risk for type 2 diabetes mellitus (T2DM) is increased among African Americans [33], with similar risks of T2DM among Blacks occurring at relatively lower levels of visceral fat compared to Whites [34]. This supports that African Americans have potential differences from White individuals in the manner that obesity contributes to insulin resistance and T2DM [35]. In general compared to White persons, Black individuals generally have less visceral fat [36] and more skeletal muscle mass [37]. Compared to Whites, Black individuals may have increased obesity-related biomarkers of increased cardiovascular disease risk, such as highly sensitive C-reactive protein (hsCRP) [38]. Leptin levels may be higher in African American females than White females [39], and increased leptin levels in Black males and females may help identify at-risk populations regarding hypertension [40]. Compared to White persons, Black individuals may have levels of some biomarkers associated with reduced cardiovascular disease risk, such as increased high density lipoprotein cholesterol (HDL-C), reduced triglyceride (TG) levels, and reduced coronary artery calcium (CAC) scores [[41], [42], [43]]. Conversely, Black individuals may have obesity-independent increases of biomarkers of increased cardiovascular disease risk, such as increased lipoprotein (a) levels [[41], [44]].
Thus, within the context of body composition, racial and sex differences exist. Some have suggested it may be best that medical records no longer include the race of patients, because compared with White patients, an analysis of electronic health records [49]:
“Black patients had 2.54 times the odds of having at least one negative descriptor in the history and physical notes. Our findings raise concerns about stigmatizing language in the Electronic Health Records (EHR) and its potential to exacerbate racial and ethnic health care disparities”.
As a clinical trialist, I cannot imagine excluding race or sex as a baseline demographic, especially regarding obesity and cardiometabolic research. As a clinician engaged in cardiovascular disease prevention and treatment of obesity, I find integration of race and sex demographics with clinical findings and laboratory data (e.g., body composition analyses) useful towards crafting a diagnostic and treatment plan that is patient-centered and culturally sensitive. Dr. Gonsahn-Bollie, we will discuss BMI later. But specifically, regarding the recording of race in medical records, how are we best to identify racial disparities and implement race-oriented care if we no longer record the race of the patient in the medical records? My sense is that racial considerations do matter (See Fig. 1), such as how best to interpret body composition analyses among Black individuals, White individuals, and especially those of Asian descent [2,3]. How do we best balance the ability to integrate racial diagnostic data to optimize patient care, while avoiding nefarious language in the electronic health records that have the potential to exacerbate racial and ethnic health care disparities?
3.4. Dr. Gonsahn-Bollie
Racial information in health records is a complex topic requiring we synthesize multiple scientific and psychosocial perspectives to create a unified solution. I'll start by saying implicit bias is rampant and problematic in our ability to form objective assessments. Even the most well-intentioned scientist has their preferences. Unfortunately, these biases often do not work in our patients' favor. On the contrary, biases often contribute to health disparities, especially in Black patients. Therefore, removing racial labels from the forefront of patients' charts, in theory, will help clinicians form less biased "pre-assessments" of patients.
There are data in other industries and clinical studies to support this. However, from a clinical and scientific perspective, there is some utility in incorporating racial data. Ultimately, our goal is to provide the patient with the most individualized care possible. In the future of medicine, we will be able to do precise genetic and epigenetic assessments to render the most accurate care possible. However, till that day comes, we are slowly transitioning from population-based tools to precision medicine. Therefore, we are limited to using specific data points that help us better tailor care. “Race” may be an imprecise term, given, “racial,” ethnic, and geographical heterogeneity. However, given the current limitations in precision medicine, race is currently still a valid data point in specific assessments such as body composition and obesity. It is also essential for clinicians and scientists to understand that race is only one component of understanding the individual and be aware of their biases-explicit & implicit-which may impact clinical assessment. For now, racial and ethnic data is a valuable part of our clinical assessment. But it should not be at the forefront of the chart to mitigate biases influencing objective clinical assessment. Instead, medical treatment ideally should be as individualized as possible using a variety of personal attributes in addition to race/ethnicity.
3.5. Dr. Bays
Female individuals make up ∼50% of the population. It is therefore not surprising that Table 2 suggests the ranking of causes of death among females largely mirrors that of the general population (all races and all sexes). One exception might be the increased rank of chronic lung disease as a cause of death among female individuals 34–64 years of age. The rates of chronic obstructive pulmonary disease (COPD) are increasing at a rate higher in female individuals than in males, and it is said [60]:
“COPD receives scant attention as a women’s health issue, despite the fact that women are more likely to die of COPD than of breast and lung cancer combined.”
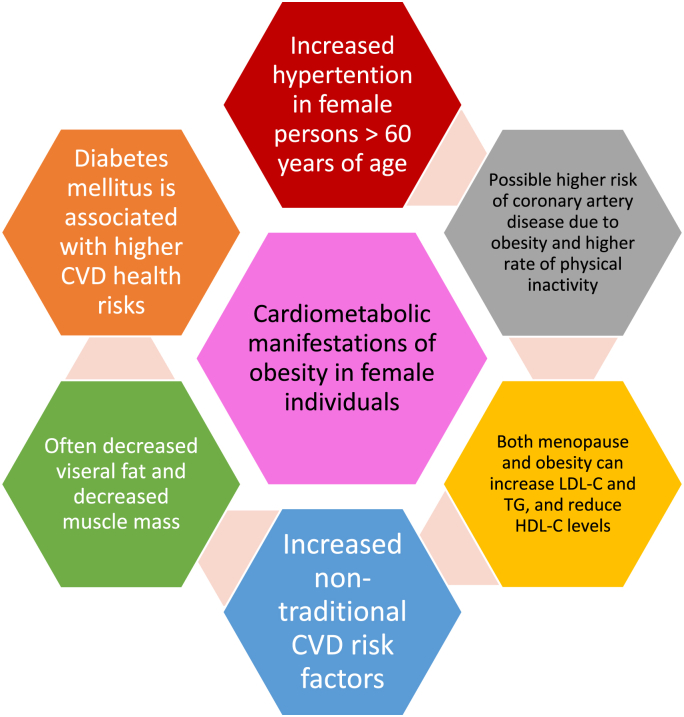
Pulmonary complications of obesity includes compromise of lung function (e.g., reduced tidal volume, reduce forced expiratory volume, daytime and night time hypoxia), disruption of innate and acquired immunity with impaired resistance to infection concurrent with a pro-inflammatory response once infection occurs, dyspnea, obstructive sleep apnea, hypoventilation/Pickwickian syndrome, asthma, and increased risk for upper respiratory tract infections (including influenza and coronavirus infections) [8]. Regarding body composition, evidence supports the association of increased abdominal visceral adipose tissue (VAT) with asthma, and higher intrathoracic VAT with impaired lung function parameters [61]. Dr. Younglove, we often hear about cancer (especially breast cancer) and heart disease risk among female individuals – which are both increased with obesity [6,8]. Fig. 2 describes unique cardiometabolic considerations in female individuals with obesity. Given its ranking as a major cause of death, why do we not hear more about the health risks of chronic obstructive lung disease among female individuals? How should obesity medicine specialists assess the importance of abdominal obesity and lung disease among female individuals with obesity?
Fig. 2.
Illustrative cardiometabolic manifestations of obesity in female compared to male individuals. As a group, female persons have a similar rate of cardiovascular disease onset 10 years of age later than males [41]. Although this is sometimes characterized as cardioprotective, cardiovascular disease (CVD) remains the second most common cause of death in younger females, the most common cause of death in older females, and the overall leading cause of death in female individuals [50] (See Table 2). Furthermore, the apparent cardioprotective effect in female individuals diminishes among those with polycystic ovary syndrome, cigarette smoking and those entering menopause [41]. Finally, any cardioprotective effect among female individuals is lost with the onset of type 2 diabetes mellitus (T2DM) [41]. Compared to males, the prevalence of hypertension is increased in female individuals over 60 years of age [50]. Compared to females without T2DM, female individuals with diabetes have 3-fold excess risk of fatal coronary artery disease [50]. Compared to males with diabetes, female individuals with diabetes have a higher risk of heart failure and higher myocardial infarction mortality rate [50]. Diabetes is a stronger risk factor for stroke and peripheral vascular disease in female than in male individuals [50]. Compared to males, obesity in female persons may have a greater association with coronary artery disease, and female individuals may be less physically active [50]. While not obesity related, older females have a higher risk for coronary artery disease due to cigarette smoking [31,50]. Female individuals often have increased subcutaneous adipose tissue, decreased visceral adipose tissue, and decreased muscle mass compared to males [51,52]. As female individuals age, abdominal/visceral fat typically increases and muscle decreases [51], increasing the risk of abdominal obesity, sarcopenia, and osteosarcopenia [9]. Compared to males, premenopausal female individuals may have lower low-density lipoprotein cholesterol (LDL-C), lower triglyceride (TG), and increased high density lipoprotein cholesterol (HDL-C) levels [53]. Both the menopause and obesity are associated with an increase in LDL-C, TG, and reduce HDL-C levels [[54], [55], [56]], all thought to increase cardiovascular disease risk. Female individuals with obesity and increased visceral fat have higher TG and lower HDL-C levels [57], and both polycystic ovary syndrome [58] and menopause [59] increase the risk of the metabolic syndrome, for which high TG and low HDL-C are 2 of the 5 components (i.e., the other components being abdominal obesity, high blood pressure, and high blood sugar) [41]. Female individuals may also have emerging non-traditional cardiovascular disease risk factors such as preterm delivery, hypertensive disorders of pregnancy, gestational diabetes, autoimmune disease, breast cancer treatment, and depression [50], many that may be obesity related.
3.6. Dr. Younglove
Chronic lung diseases are diverse in terms of etiology - some diagnoses associated with environmental triggers and others having more of a genetic predisposition. Connecting those back to diminished lung function or capacity due to obesity simply hasn’t been widely promoted - likely because the explanation isn’t simple. Until chronic pulmonary conditions get an amazing marketing campaign like the American Red Cross has done for heart disease or the Susan Komen foundation has done for breast cancer, I think it’s up to us, Obesity Medicine specialists, to keep individually educating patients and other clinicians about chronic obstructive lung disease and the association with increased abdominal adiposity.
In addition to measuring weight and BMI, I imagine most Obesity Medicine specialists obtain a waist circumference and a waist/hip ratio as part of their assessment. These values are very helpful for assessing abdominal obesity. We know that when these numbers are elevated, a person has an increased risk of heart disease. Although we don’t have the hard data to correlate these measurements with the risk of developing chronic lung disease, as clinicians, it’s not a stretch to assume that elevations in these measurements also signifies an increased risk of developing chronic obstructive lung disease. Measuring a person’s waist is often simple to do [62]. However, measuring waist circumference is best performed by adequately trained staff members utilizing the same technique at each office visit [2]. During a primary care visit, measuring waist circumference along with routine vital signs would open the door for productive conversations about excess weight and associated complications - such as the impact that excess abdominal mass has on the body’s ability to exchange air effectively.
3.7. Dr. Bays
Beyond lung disease, cardiovascular disease, and cancer, one of the major complications of obesity is obstructive sleep apnea (OSA). Those of Asian descent are at an increased risk for OSA, despite lower rates of obesity [63]. The burden of OSA symptoms is higher among African Americans, possibly related to increased rates of obesity [63]. Black individuals with OSA and metabolic syndrome are at increased risk of stroke [64]. While males are at increased risk for OSA compared to females, the consequences of OSA are at least the same, if not worse in female individuals for comparable degrees of OSA severity [65]. At least one study suggests that low-income African Americans, including females, are a high-risk group for OSA, but under-diagnosed and under-treated [66]. The Obesity Medicine Association published a Clinical Practice Statement acknowledging sleep apnea as predominantly a manifestation of “fat mass disease.” [[7], [11]]. I published a commentary of the role of adiposopathy (i.e., pathogenic inflammatory and endocrine consequences of obesity) on sleep (and vice versa) [13]. Both adiposopathy/obesity and the hypoxia of sleep apnea have inflammatory and adverse cardiovascular consequences [15,67]. Finally, increased visceral fat is associated with an increase in sleep apnea prevalence in middle-aged and older men and in postmenopausal women [68]. Dr. Younglove, regarding body composition, please provide some insight as to the importance of increased adiposity and sleep apnea in female individuals.
3.8. Dr. Younglove
As you mentioned, the data between insufficient/inadequate sleep and chronic disease is well-established [11]. There are a lot of reasons for this - some psychosocial and some medical - and they are intimately related. They also exist in a spiral - inadequate sleep often leads to weight gain, which leads to fat mass disease and adiposopathy and inflammation, which leads to OSA, which leads to inadequate sleep and so on. Although it’s obvious to us as clinicians, breaking that cycle is difficult. Most women today are overscheduled and overstressed. In addition to carrying an equal role in providing for the family (or being the sole provider, as is so often the case in low-income families), women also tend to shoulder a disproportionate amount of the daily running of the household - arranging schedules, providing meals, and managing the majority of the daily tasks required to keep everyone and everything running smoothly. Many women are also tasked with the responsibility of caring for aging relatives. Women already have so many reasons for insufficient or disrupted sleep that entertaining the idea that it could be related to a medical problem often doesn’t occur to them. There are also significant barriers to diagnosis and treatment. If a healthcare provider recommends evaluation, the inconvenience of doing a sleep study for diagnosis is an obstacle. Treatment with a cumbersome continuous positive airway pressure (CPAP) machine (if recommended) is difficult to employ if women are responsible for tending to family members’ needs during the night. Until women are better able to prioritize their needs, it’s unlikely that we are going to make huge strides in improving their sleep quality.
4. Muscle mass
4.1. Dr. Bays
In addition to limitations in accurately measuring body fat, BMI also does not account for variances in muscle mass. Unless specifically measured by body composition analyses, muscle mass is not often directly assessed in clinical practice. Creatine kinase (CK) is a muscle enzyme whose levels correlate to muscle mass [69] (Creatinine levels also correlate to muscle mass [70].). Elevated CK levels can be found in patients with increased muscle mass and increased muscle activity (e.g., especially after uncommon movement or strain of muscles, such as first-of-year gardening, moving, new exercise routine), muscle injury, drugs, ischemia, toxins, myopathies, metabolic disorders, hereditary conditions, hyper and hypothermia [71]. CK levels are often higher in men, Black individuals, muscular individuals, and athletes [69,72]. Because African American individuals generally have an increase in lean body mass (i.e., which includes skeletal muscle) compared to White individuals [73], it is often assumed the increase in CK levels in Black individuals is due to the increase in skeletal muscle. However, increased CK levels among Black persons may also be due to differential production or clearance of CK [69]. Additionally, beyond reflection of muscle mass, CK may also have other predictive value. In a study of 1405 persons of African, Asian, and European ancestry, hypertension prevalence was 39% in African vs. 29% in non-African ancestry participants vs. 41% and 27% by high and low CK tertiles; elevated CK levels better predicted a lack of blood pressure control compared to African Ancestry [74].
A common challenge facing clinicians (and clinical researchers) is the incidental finding of an elevated CK level in an asymptomatic Black individual. This is so common that I have often wondered why laboratories do not provide different normal upper range reference values of CK for Black individuals versus other races.
Adjustments for race in laboratory testing are not uncommon. In the US, estimated glomerular filtration rates (eGFR) are often adjusted for race (Table 3). That said, while it may very well be available, I cannot find a comprehensive direct comparison of body composition analyses (i.e., assessing muscle mass) of various African nations and African Americans. However, I was able to find BMI data from African nations, which reveals marked variation in the prevalence of obesity both regionally and between male and female individuals among those in four sub-Saharan African countries. Specifically, “the highest prevalence of obesity defined by BMI was observed at the three South African sites (42.3–66.6% in women and 2.81–17.5% in men) and the lowest in West Africa (1.25–4.22% in women and 1.19–2.20% in men) [75]. This suggests that regarding BMI, “Africans” are a heterogenous population.
Table 3.
Illustrative estimates of glomerular filtration rate (eGFR) in the US. Measurement of glomerular filtration rate (GFR) represents the blood filtered by the kidney, often measured by fructose-based polysaccharide (i.e., inulin) that is neither secreted nor reabsorbed across tubules. Most clinical measures use estimates of GFR via assessment of creatinine, which is a breakdown product from muscle, and thus correlates with muscle mass. Some estimates of GFR are not validated for obesity. Salazar-Corcoran includes body weight as a variable, but not commonly used in clinical practice [80]. CKD-EPI and MDRD may be indexed (adjusted) for body surface area. However, it is unclear that indexing or deindexing improves accuracy among patients with obesity [80,84,85].
| Measure of estimated glomerular filtration rate (eGFR) | Formula variables | References |
|---|---|---|
| Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is frequently used by US commercial labs. In patients with obesity, the CKD-EPI may overestimate chronic kidney disease and underestimate glomerular hyperfiltration in patients with obesity. |
Equation includes serum creatinine adjusted for age, gender, and race (Black or not Black), and not adjusted for weight. Data from non-US countries suggest the CKD-EPI equations without race-ethnicity adjustment are more appropriate. | [78,79] |
| Abbreviated Modification of Diet in Renal Disease (MDRD) equation is sometimes used in US clinical trials. CKD-EPI may be more accurate than MDRD for patients with BMI ≥30 kg/m2 and with higher eGFR (>60 ml/min/1.73 m2) |
Equation includes serum creatinine, adjusted for age, gender, and race (Black or not Black) and not adjusted for weight. It is estimated that that without the MDRD eGFR race adjustment, 3.3 million (10.4%) more Black Americans would reach a diagnostic threshold for Stage 3 chronic kidney disease, 300,000 (0.7%) more would qualify for beneficial nephrologist referral, and 31,000 (0.1%) more would become eligible for transplant evaluation and waitlist inclusion. | [[80], [81], [82]] |
| Cockcroft-Gault equation is rarely used in clinical practice, but is the historic standard used in for renally-adjusted drug dosing studies | Serum creatinine, age, and gender adjusted for body weight. Cockcroft-Gault may be inaccurate in patients with obesity and underweight. | [83] |
Similarly, Black individuals are heterogenous regarding height as well. Worldwide (Black or White), the populations with the tallest mean height in the world are from the Netherlands and the Balkan countries, while the shortest populations are central African Pygmies. Specifically regarding Black individuals, some East African populations have taller height, while Sub-Saharan African foraging and horticultural populations are shorter height (e.g., Pygmies) [76]. Total upper arm area in Baka pygmy girls and boys is less than American references [77]. This is not to suggest that a significant proportion of African Americans are from Pygmy ancestry. However, it is to suggest that that those with African ancestry may have considerable variance in body mass index, height, and muscle mass. So while I may sometimes speculate about the potential advantages of a higher reported upper limit of normal for CK levels, the heterogeneity among Black individuals makes it challenging to rationalize changing “normal” reference ranges for CK, even as CK levels are often increased among many African Americans. Dr. Wharton, as an academician and clinician, how do you recommend we sort out the elevated CK levels found so often among our asymptomatic Black patients?
4.2. Dr. Wharton
The entire population of Black people in America does not demonstrate elevated CK levels. There are likely elevated CK levels and muscle mass in certain descendants from Africa where there was higher body mass. This would be similar for most of the descendants of Arnold Schwarzenegger (a famed body builder from Austria), or any group of individuals with genetically higher amount of muscle mass.
Beyond CK levels, Black people in America are heterogenous and do not all demonstrate higher creatinine levels (i.e., a waste product of muscle that in the absence of renal insufficiency, may correlate to muscle mass). Much of the world has discarded the renal adjustment of estimated glomerular filtration Rate (eGFR) used for Black people. In Canada, we previously adjusted eGFR based on Black or not Black. This adjustment resulted in a calculation that increased the eGFR value, due to an assumption of higher muscle mass and therefore higher creatinine in Black individuals, leading to an artificially lower reading of eGFR. This has proven to be false and based on non-scientific assumptions, influenced by racist ideology. All the labs in Canada and many around the world have discarded this racist adjustment of eGFR (See Table 3).
4.3. Dr. Bays
Dr. Gonsahn-Bollie, I have read with interest your perspectives regarding the utility of BMI in the diagnosis of obesity. I have also read others who have suggested that different BMI cutoff points should be used to diagnose overweight and obesity for Black individuals. But Black persons are not a monolithic population. Per the prior discussion with Dr. Wharton above, while many Black individuals may vary from White individuals regarding body habitus (i.e., increased muscle mass and decreased visceral fat), Black individuals also differ from other Black individuals. Some may argue that a simple waist circumference measure might identify different races and different sexes at risk for cardiometabolic disease. However, while a waist circumference can reliably approximate total abdominal fat (i.e., subcutaneous abdominal fat and visceral fat) [86], the association of waist circumference with visceral fat varies based upon gender and race [87]. These are just some of the reasons why some have suggested that, with regard to the assessment of adiposity, the use of: “BMI may be considered inherently racist,” (https://www.healthline.com/nutrition/bmi-for-black-women#alternative-metrics). Given all these challenges, and given the clinical importance of accurately measuring adiposity, why should we perpetuate the notion that BMI is acceptable to assess adiposity? Why are we not preferentially using body composition analysis tools for Black and Asian individuals, as well as White, Hispanic, and other populations?
4.4. Dr. Gonsahn-Bollie
The data suggests that we should not perpetuate the notion that BMI is acceptable to assess adiposity. Instead, BMI should be a screening tool to prompt further evaluation of adiposity that utilizes better diagnostic tools for body composition. Furthermore, adiposity screening should use "specific, adjusted BMI charts" based on age, sex, race, ethnicity, obesity risk factors, and obesity-related diseases. Unfortunately, specific, adjusted BMI charts are underutilized because many clinicians-even obesity specialists-are unaware of them. Body composition analysis is standard in my practice. However, body composition analysis tools are currently not widely used because of inadequate obesity education, weight bias, and lack of access to some tools. Let's discuss each of these barriers briefly.
Inadequate obesity education: Obesity is still a "newer disease" in medicine. The American Medical Association only formally recognized obesity as a disease in 2012. Despite its inherent flaws, BMI is currently the most widely used clinical tool to diagnose obesity is BMI. Therefore, medical training must include other methods of assessing adiposity.
Weight Bias: There's lingering weight bias because of the misperception that obesity is a lifestyle choice. Weight bias impedes clinicians' ability to see beyond the BMI of the person in front of them and do a further diagnostic evaluation. Furthermore, weight bias limits much-needed funding for obesity care and treatment.
Lack of body composition analysis tools: High-end research body composition machines can be costly. Weight bias can hinder healthcare decision-makers from funding expensive body composition tools. Additionally, smaller or underfunded clinics that want them can't afford these tools.
We are living in a time of clinical transition in the diagnosis of adiposity and the disease of obesity. Unfortunately, BMI isn't the best tool. But until body composition analysis is widely available, clinicians should: utilize specific, adjusted BMI charts and waist circumference for adiposity screening and incorporate necessary laboratory testing to access the cardiometabolic effects of excess adiposity.
4 .5. Dr. Bays
Issues regarding body composition go well beyond the biological. In a prior Obesity Pillars Roundtable on Obesity and Diversity, it was stated [1]:
“Hispanics/Latinos may culturally perceive an increase in body weight as a symbol of health and wealth, with many Hispanic/Latino males reporting a preference for larger sized females as mates… education includes addressing the cultural myth in the Black Africans that obesity is a sign of wealth and healthful living, and a culture that sees abdominal/central or visceral obesity as a sign that the individual is well-fed and wealthy.
Some data suggests that Black South African women have a low perception of the threat of obesity to adverse health [88]. In a population enrolled in an intervention for overweight children, compared to White girls, Black girls perceived a larger body size as being “ideal” [89] When Black women in Maryland USA were interviewed, the participants “expressed that Black culture is more accepting of larger and curvier body types, and this reinforces a positive body image and less desire to achieve an ideal BMI” [90]. Dr. Gonsahn-Bollie, please comment on your clinical experience of the relevance of race-related body image perception, and how this applies to the management of body composition among Black individuals with overweight or obesity.
4.6. Dr. Gonsahn-Bollie
The Black diaspora celebrates curves. Losing too much weight can be viewed unfavorably. Furthermore, for many Black women being "too muscular" can be undesirable. I care for many women who view their assigned "ideal BMI" weight as "too small." Due to the biases in the standard BMI chart, perhaps our Black patients might be correct. It's essential to assess body image perception when discussing obesity & adiposity risk with all patients, especially Black women. Therefore, I approach weight/adiposity discussions from the perspective of "healthy weight" vs. "happy weight."
Healthy weight is a comprehensive, individualized adiposity assessment. As I tell patients, "Your Healthy Weight" is based on the composite of various metrics. Your Healthy Weight is a range based on the composite of specific, adjusted BMI upper weight limit, adjusted waist circumference goal; body fat percentage goal; and absence/control of the adiposity-related disease. On the other hand, happy weight is based on the person's feelings about your body. I ask, "Is your weight management important to you? Why?" "Which size do you feel most comfortable in your skin? At what size/weight are you the most comfortable." I also screen for mental health conditions impacting body image perception, such as major depression, generalized anxiety, eating disorders, disordered eating, and body dysmorphic disorder.
I also want to emphasize the goal of the discussion is not to discourage body confidence or body positivity. But to help patients see the benefits of improving their adiposity beyond cosmetics. Assessing "healthy weight" and "happy weight" enhances shared decision-making on adiposity-related goals. It can be tempting to jump into a discussion about "healthy weight." That's because we see the adverse effects of untreated adiposity and are eager to help our patients. But I can assure you from personal and professional experience we are more effective in reaching optimal health when we partner with patients to get to their "happy, healthy weight" (aka personalized body composition goals).
4.7. Dr. Bays
A decrease in muscle mass may lead to [9]:
”Sarcopenia has adverse health outcomes (e.g., reduced locomotion, frailty, reduced quality of life, osteoporosis, worsening metabolic health, and possibly an increase in obesity). Factors that contribute to sarcopenia include genetic predisposition, unhealthful nutrition or malnutrition, physical inactivity, chronic illness (especially with cachexia), neuro-degenerative diseases, hormone changes (e.g., hypercortisolism, abnormal thyroid function), medications, immobility, zero gravity, weight cycling, and age. Sarcopenia often accompanies not only an increase in percent body fat, but also a reduction in bone mineral density, sometimes termed osteosarcopenic obesity syndrome. Regarding prevalence, in an evaluation of patients evaluated for arthroplasty, 6.4% had sarcopenic obesity”.
It is not unusual to encounter (older) female individuals with “normal” BMI who have a high percent body fat but reduced muscle mass (sarcopenic obesity) [91]. Among the health risks of postmenopausal female individuals with sarcopenia are falls and vertebral fractures [92]. Conversely, BMI may be misleading in female individuals with increased muscle mass. Many of the DXA body composition analyses we perform involve female individuals engaging in body building or physique competitions. The data would suggest that body fat in female athletes extends across almost the entire range of female fatness. Some of the lowest percent body fat measurements in distance runners and body builders fall into the normal male range. Conversely, many female swimmers and strength athletes have relatively high percent body fat values, which would classify these female individuals as obese by male standards [93]. This is one of several reasons why female and male individuals have different cut-off points for assessment of adiposity (See Table 1).
These examples further illustrate how BMI as a diagnostic measure is not only often inaccurate, but potentially biased and misleading. One of the reasons female individuals who undergo resistance training seek out DXA evaluation of body composition at our research site is because they are often informed by their treating clinician that they were overweight/obese. This extraordinary and faulty conclusion is based solely upon their BMI. Of course, when measured by DXA, their percent body fat is well in the healthy range. The increase in BMI in female individuals undergoing heavy resistance training is often due to an increase in muscle mass, not an increase in body fat. We have never had a male body builder engaged in resistance training who was told he had overweight or obesity based upon BMI alone. But we have heard this from female individuals engaged in resistance training. Our clinical experience would be consistent with documented bias against muscularity in females [94]. Dr. Younglove, whether it be female persons with sarcopenia or a female individual with increased muscle mass, how do we sort out if and/or when to perform body composition analyses, especially body composition analyses that include percent body fat, android fat, visceral fat, lean body mass, and bone mineral density?
4.8. Dr. Younglove
I would love to have a research-grade body composition analysis performed on every person yearly, but that’s probably wishful thinking! Outside of a universal screening protocol, I think clinicians need to use some practical guidelines to identify people in whom body composition analysis may change treatment recommendations. Most athletes with an elevated BMI and low percent body fat are going to present as metabolically healthy (with normal vital signs and laboratory measurements) and will have a waist circumference within the normal range. People with an acceptable fitness level but with a clear gluteofemoral fat distribution (pear-shaped body type, which is more common in premenopauseal Black women than White women) who also have normal labs, vital signs, and waist circumference may also fall into this category. An astute clinician should be able to look beyond BMI. However, confirming this with a body composition analysis may help the patients mentally and emotionally, which is important. The bigger role of body composition analysis comes when assessing those patients with a normal BMI but who have metabolic dysfunction and/or an increased waist circumference. Clinicians should have a high index of suspicion for increased visceral fat and/or liver fat in these patients and confirming this via body composition analysis could be beneficial to direct the conversation toward improving health in people that don’t feel that lifestyle changes apply to them without the diagnosis of overweight or obesity.
4.9. Dr. Bays
Table 4 describes the characteristics of different muscle fiber types. This table may be relevant for athletes and their trainers, and for those taking certain types of medical board exams. Regarding muscle mass and race, infamous examples exist of claims that African Americans were successful in sports because of the genetic and generational consequences of slavery (https://en.wikipedia.org/wiki/Jimmy_Snyder_(sports_commentator). Today, folks seem less inclined to discuss muscle twitch fiber differences between Black individuals and White individuals. In one exception, an article published in 2006 by authors from the University of the West Indies in Jamaica stated [103]:
“Variations in somato-genetic patterns in muscle-fibre biology, biochemical metabolic pathways and pulmonary physiology are hypothesized to have been concentrated by natural selection over the centuries in the Afrocentric peoples displaced from West Africa to the New World. These phenotypic and genotypic characteristics are attributed to provide the athletic prowess so well documented in African-Americans. Not the least of coincidence seems to be the influence of the compensatory mechanisms on oxygen transport and its availability to the tissues, in response to the sickle cell gene. The reduced availability coupled with reduced myoglobin in the preponderant fast-twitch muscle fibres which are adapted for rapid energy (ATP) regeneration, all give a net outcome of muscle anatomical and biochemical advantages which support outstanding performances in athleticism.”
Table 4.
Fast and slow twich muscle fibers: Regarding body composition, lean body mass includes muscle mass. Muscle contraction facilitates body motion via use of the fuel of adenosine triphosphate (ATP) generated by intracellular mitochondria, and derived from body nutrients (i.e., stored carbohydrates, fats, and proteins as well as ingested food) and oxygen. The motor unit is composed of motor neuron and skeletal muscle fibers innervated by the axon, which all coordinate for muscle contraction. Muscle fibers are categorized as skeletal, cardiac, and smooth, and composed of slow and fast twitch fibers. As opposed to hemoglobin which carries oxygen in blood, myoglobin carries oxygen in muscle. While all muscle fibers can produce energy both aerobically and anaerobically, depending upon the muscle fiber, one may predominate.
| Fast twitch muscle fibers (Type 2 white muscle fibers) | Slow twitch muscle fibers (Type 1 red muscle fibers) |
|---|---|
| Faster contraction speed, more rapid fatigue, quick burst | Slower contraction speed, slower fatigue, increased endurance |
| Fast Twitch Type 2 muscle fibers are larger and used for sprinting, powerlifting, and jumping | Slow-twitch Type 1 muscle fibers are smaller and used for distance running (marathons) and endurance training |
| Muscle fibers with low myoglobin content are pale in color (e.g., "white meat" is derived from the breast muscles of ground-centered turkeys that predominantly use their breast muscles to quickly flee or fly for short distances) | Muscle fibers with high myoglobin content are red (e.g., “dark meat drumstick” from the thighs of turkeys that frequently walk) |
| Decreased capillary density | Increased capillary density |
| Decreased mitochondria number | Increased mitochondrial number |
| Decreased myoglobin with lower oxidative capacity (predominantly “anaerobic”) | Increased myoglobin with higher oxidative capacity (predominantly “aerobic”) |
Genetic analyses support that the current genetic landscape of the Americas is largely concordant with expectations derived from documentation of slave voyages [104]. One might scientifically conclude that choice selection, survival selection, and purchase selection relative to Black slavery commerce from the 1500’s to the 1900’s may conceivably have genetic and epigenetic influences body on composition relative to African Americans today – including musculoskeletal traits. To dismiss this possibility would seem to deny the atrocities and generational impact of slavery involving approximately 10 million Africans trafficked to the Americas during the slave trade [105], for which approximately 2 million did not survive the middle passage journey (https://www.statista.com/statistics/1143458/annual-share-slaves-deaths-during-middle-passage/). That said, confounders exist with genomic diversity influences of European ancestry among many African Americans. Such admixture effects may not only have genetic/epigenic implications, but also suggests the possibility that regarding African Americans in the US [106]:
“Regional differences in ancestry proportions is (because) individuals with higher European ancestry were more likely to migrate to the North and West during the Great Migration, a scenario we refer to as ancestry-biased migration.”
Given this heterogeneity, at best, genetics/epigenetics are just one of many components to athletic success (See Fig. 3). Some have suggested that attributing the success of Black athletes solely to genetic inheritance (e.g., genetic inheritance of fast muscle twitch fibers) is a “myth” [107] and potentially represents “prejudice toward and stereotyping of Blacks.” [108] Furthermore, others have suggested that from a health perspective, a disproportion of Type 2 fast twitch white muscle fibers might be potentially disadvantageous (not advantageous), in that increasing predominancy of Type 2 fast twitch muscle fibers may reduce aerobic capacity among Black individuals and women, potentially increasing the risk for obesity and related metabolic disease [109,110]. (See Table 4).
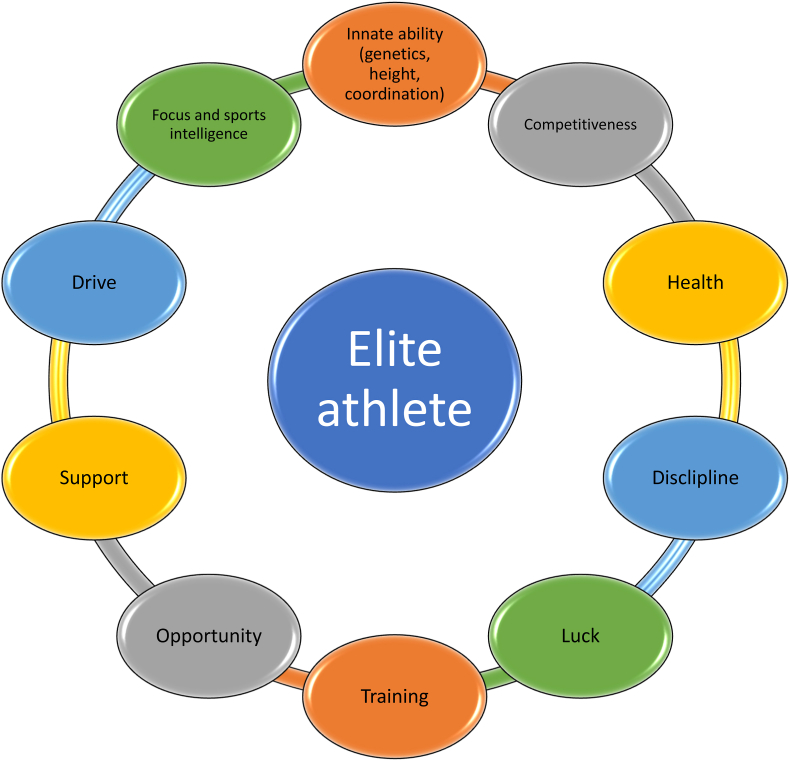
Fig. 3.
Illustrative factors that influence the success of elite athletes. Innate abilities are rarely realized unless accompanied by other factors such as training.
The bottom-line is this. The misunderstanding of muscle physiology (i.e., twitch fibers), and the misperception that increased muscle mass applies to all Black individuals, all have direct implications as to racial anticipations and interpretations of body composition. However, irrespective of potential genetic and epigenetic differences of Black individuals regarding muscle physiology, the science supports that the day-to-day clinical relevance of body composition among races and sexes is mainly centered on body fat distribution and muscle mass, all influenced by nutrition and physical activity. It is not about the speed of muscle twitch fibers. Yes, it is conceivable the proportion of fast twitch fibers and muscle-related gene variants (e.g., alpha-actinin-3 or ACTN3), may have applicability where athletic competition among elite sprint athletes is decided by seconds or milliseconds [[111], [112], [113]]. But the muscle gene variants that help explain sprint competition times among elite athletes not only occur inter-racially (i.e., between races), but intra-racially as well (i.e., within races) [114]. Moreover, genetic predisposition leading to innate ability is only one of many factors leading to athletic success. Most of us can recall intellectually or athletically gifted kids with seemingly high potential for success, who never realized their innate abilities. Other factors that would seem more important regarding the success of elite athletes include competitiveness, health, discipline, luck, opportunity, support, drive, concentration/sports intelligence, and most of all, the degree of and commitment to such things as training. (See Fig. 3). Dr. Wharton, what is your sense about the data and the science supporting the role of genetics/epigenetics in athletic achievements by Black individuals in general? When considering body composition, what is the role of genetics/epigenetic in accounting for increased muscle mass in African Americans specifically?
4.10. Dr. Wharton
All the explanations or reasons for increased athletic progress monthly plaque awards are primarily related to poverty slavery and discrimination. Just like gladiators during the Roman times, who fought their way out of slavery, minorities have had to fight their way out of poverty with athletics versus other methods. Research regarding fast twitch and slow twitch muscle in Black people versus White is flawed and they are similar genetics meaning fast twitch and slow twitch muscles amongst all populations. I believe what differs is the training and chosen profiles to elevate oneself out of poverty.
4.11. Dr. Bays
Table 5 describes some basic training guidance towards increasing muscle mass of all races and sexes. Achieving a healthier body composition often involves increasing muscle mass, or at least preserving muscle mass during weight reduction in patients with obesity. Drs. Gonsahn-Bollie, Younglove, and Wharton, when discussing body composition with patients, clinicians often encounter biased perceptions that Black individuals are inheritably athletic (see discussion above) and that female individuals have only a limited ability to gain muscle mass, or aesthetically, should not be gaining muscle mass at all. Knowing that many Black and female individuals with overweight or obesity would benefit from reduction in body fat and increase in muscle mass, what are your top 3 tips such that clinicians can best dispel these negative biases, towards the positive goal of achieving a healthier body composition?
Table 5.
Top ten training guidance towards increasing muscle mass. The most important objective in resistance training is primum non nocere (“first do not harm”). Patients who engage in resistance training should first undergo proper health evaluation, especially of their cardiovascular, pulmonary, musculoskeletal, and neurologic body systems. Afterwards, patients should learn the proper use of free weights and machines. Short-term sore muscles may be expected. Sore joints suggest poor technique. The emphasis should be to safely increase total muscle mass, which may be achieved in a more time-efficient manner by training large muscle groups (e.g., leg pressing exercises such as squats, upper-body pulling exercises such as pull-ups and upper-body pushing exercises such as bench press) [95]. Increasing total muscle mass will increase the percentage of lean body mass. Developing “core” muscles (midsection of the body, such as abdomen, back, and hips) may help healthy posture, balance stabilization, back muscle strength, and endurance [96]. For most individuals, if performed to momentary muscle failure, then low load (i.e., lower weights per set with more repetitions) and high load (i.e., heavier weights with fewer repetitions) resistance training will both help promote muscle fiber hypertrophy [97]. What matters most is the long-term adherence to a physical exercise routine, involving the strengthening of major muscle groups two or more times per week [86]. Utilizing a variety of free weights, machines, and resistance bands may reduce boredom and provide greater flexibility regarding scheduling and location. While dietary protein supplements are sometimes used by elite level athletes [98], for most individuals, adequate protein is best obtained from natural food sources [99] and overall healthful nutrition. While resistance training is important, so is dynamic physical exercise [86], which is complementary to resistance training. As with numerous other health benefits, including effects on body composition [13], healthful sleep can favorably affect the results of resistance training [100]. Conversely, in addition to improving anxiety and depression, resistance physical exercise may in turn, improve sleep quality [101]. Monitoring the progress of resistance training might best include muscle mass metrics (e.g., muscle tape measurements, body composition analyses) versus body weight or the amount of weight lifted. It is relatively common that after initiating a new resistance training program, body weight will increase due to increased muscle water retention (i.e., muscle inflammation and increased glycogen-associated water stores). The good news is that during negative caloric balance, resistance training can help mitigate muscle loss and limit the reduction in resting metabolic rate [9]. Finally, given all the available information (both accurate and inaccurate), it is best to keep in mind simple principles such as those listed above, and avoid trying to “overthink” how best to increase muscle mass, or how to employ a “quick fix” via unproven and potentially unsafe interventions such as supplements [102].
| 1. Learn proper and safe resistance training techniques |
|---|
| 2. Target large muscle groups |
| 3. Target core muscle groups |
| 4. Overload muscles |
| 5. Adhere to a routine |
| 6. Healthful nutrition (with adequate protein) and proper hydration |
| 7. Don’t forget dynamic training |
| 8. Prioritize healthful sleep |
| 9. Utilize proper metrics to assess progress |
| 10. Keep it simple and safe |
4.12. Dr. Gonsahn-Bollie
-
1.
Know your own biases: Clinicians should take the Harvard Weight Implicit Association Test to screen for weight bias. Studies show that weight bias prevents clinicians from adequately counseling patients and offering effective treatments. For Black women, these discrepancies in proper obesity counseling occur with non-Black clinicians and, to a lesser degree, Black clinicians. Awareness of your biases is a helpful first step in addressing weight bias. Furthermore, awareness also ensures you are not perpetuating the patient's biases.
-
2.
Go beyond the standard BMI: BMI is a screening tool, not a diagnostic test for adiposity. While BMI isn't the best tool, many clinicians still use it. If using BMI, utilize specific, adjusted BMI charts based on age, sex, race, ethnicity, obesity risk, and adiposity related diseases as well as waist circumference for adiposity screening. Incorporate appropriate laboratory testing to assess the cardiometabolic effects of excess adiposity and use body composition testing when available. All healthcare settings treating people with obesity or at risk for obesity should aim to have body composition testing.
-
3.
Make sure not to treat "people" but the person: Our discussion emphasizes the need to view each person we treat as an individual rather than a representation of a particular group. The racial and sex data variations emphasize that population data can inform our individualized care. However, population data is not an absolute end to individual health. As clinicians, we must utilize the appropriate tools to provide the most accurate care possible, including the essential tool of asking the patient about their perceptions and goals of care, then using shared decision-making.
4.13. Dr.Younglove
-
1.
Look beyond the data on the chart. People are so much more than the sum of their demographics and numbers. Practicing medicine is about assessing the whole person and using that information, along with the person’s goals for treatment, to create a care plan that makes sense for them as an individual. An illustrative example for this discussion, if a Black woman has more lean body mass than a White women, it doesn’t mean that they will have an easier time improving their cardiovascular fitness.
-
2.
Doing something as simple as measuring waist circumference can provide clinicians with data about disease risk. Having that information on the chart along with other vital signs may be a simple way to prompt clinicians to start conversations about visceral fat and body composition, creating an opportunity to discuss lifestyle interventions.
-
3.
Improving body composition can have benefits beyond the more common obesity-related comorbidities. Postmenopausally, women typically have a gradual loss of muscle mass and bone mass. Decreased muscle mass can result in significant issues with balance and generalized strength, which dramatically increases the risk of injury from falls. Compound this with decreased bone mass, and falls result in significant morbidity and mortality among postmenopausal women. A good strength training routine can significantly slow, if not halt, this process. It’s probably the most important thing a woman can do for overall musculoskeletal health and longevity. Regarding concerns about the potential for excessive muscle mass, I have spent my entire medical career taking care of women and have never seen a woman unintentionally gain “too much” muscle mass and bulk up from moderate strength training.
4.14. Dr. Wharton
-
1.
Race is a social construct and not biology. Ethnic variation is present but continues to diminish and has little relevance in the ethnically diverse country of America.
-
2.
There have been multiple false equations and nonscientific conclusions based on racist ideology that are being continually challenged and corrected.
-
3.
Avoid the continued attempt to identify biological difference between different races based social constructs driven by divisive ideas and structural racism. We should push back on medically assessing patients as a monolith and instead assess each patient as individuals.
5. Conclusion
Thanks to you all!
My three takeaways from this roundtable discussion are:
-
(1)
I have often heard: “We need to have a national conversation about race.” Similar sentiments are often expressed regarding sex bias. I have coauthored articles on diversity-related topics [[1], [2], [3], [4],115]. I have performed many body composition analyses in diverse patient populations. Nonetheless, after conducting research in preparation for this roundtable, and after considering the perspective of these outstanding panelists, I am humbled. I learned things I probably should have known (which seems to be true with all Obesity Pillars roundtable reviews). I am not sure that I fully agree with everything said in this roundtable. I am reasonably sure other panelists do not always agree with me. However, I feel I do have a better perspective on what I may need to reconsider. As a result, I suspect whatever perspective I have today, will likely be different tomorrow – which would seem to be a point behind these roundtable discussions.
Discussion without debate is a monologue, not a dialogue.
-
(2)
Substantially due to chromosome-mediated differences in sex organs and sex hormones, most would agree that genetic females and genetic males have biologic differences that predispose to different diseases. The respectful considerations of use of the terms “sex” (i.e., females and males) and “gender” (i.e., women and men) were previously addressed in the text above, as well as in a prior OMA Clinical Practice Statement [1]. With this caveat aside, and within the practicalities of typical patient care, individuals of different biological sexes have differences in patient presentation (i.e., biologic females are not routinely screened for prostate cancer; biologic males are not routinely screened for uterine cancer). This also includes sex differences in body composition.
Regarding race, it is often unclear the degree health disparities between Black and White individuals are attributable to genetics versus environment, socioeconomic status, and systemic racism [116]. The emergence of sickle cell genetic mutations that presumably evolved to resist against malaria in Africans [117] helps account for the substantially increased incidence of sickle cell trait and sickle cell disease among Black individuals (https://www.cdc.gov/ncbddd/sicklecell/features/keyfinding-trait.html). From a cardiometabolic standpoint, African Americans and other people of African descent have a higher incidence of hypertension and related comorbidities compared to White individuals, with several identifiable genes thought to play a role in racial differences regarding hypertension [118]. Regarding glucose metabolism compared to White Europeans, Black Africans often present with a phenotype of low insulin sensitivity and hyperinsulinaemia. With specific respect to the topic of this roundtable, Black Africans often have lower visceral adipose tissue and ectopic fat deposition and greater peripheral (gluteo-femoral) fat deposition than European counterparts [36]. Regarding lipids and compared to Whites, African Americans often have increased high density lipoprotein (HDL) cholesterol and decreased triglyceride levels potentially related to polymorphisms of identifiable regions of chromosomes 7, 8, 14, and 19 and single-nucleotide polymorphisms [42]. While these lipid profiles may seem to suggest a favorable prognosis, the clinical implications of these findings are unclear, as increased HDL cholesterol levels and lower triglycerides in Blacks may not correlate to reduced cardiovascular risk, and thus the prognostic use of these lipid parameters may underestimate cardiovascular risk in Blacks [119]. Finally, increased lipoprotein (a) is associated with increased cardiovascular disease risk, with interindividual heterogeneity being as high as over 90%. Lipoprotein (a) is genetically inherited and not substantially influenced by nutrition or physical activity] [120]. Blacks of sub-Saharan decent have higher levels of lipoprotein (a) when compared to White individuals, with people of African ancestry having the highest absolute lipoprotein (a) levels [44].
Thus, even if one concedes that health disparities between Whites and vulnerable social groups such as racial/ethnic minorities are often rooted in nonbiological factors (e.g., socioeconomic status), and even of one believes race is mainly a sociocultural construct and not a biological category, the published science supports racial genetics do play a role in human disease, with clinically applicable genetic differences that exist between Blacks and other populations. Supporting the embracement of the importance of genetics and race, some have suggested that utilization of genetic technologies may have the potential to reduce, rather than widen, health disparities [121].
-
(3)
Irrespective of biologic differences, persistent systemic racism and sexism helps account for health disparities [122,123]. That said, some misperceptions regarding sex, race, and body composition are due to the lack of sufficient factual discussions (“dialogue”) of this topic sometimes considered off limits. Regarding race, multiple published reports support increased average lean body mass and increased CK levels among Black populations. Many athletes in some of the more high-profile sports (but not all) are disproportionately Black individuals. It may therefore logically follow that when encountering a Black patient, some clinicians may assume all Black individuals are genetically predisposed to have increased muscle mass. Without a thoughtful, objective, and non-hyperbolic examination of these issues from varying points of view, such a conclusion might be less an example of “racism,” and more a lack of published literature that objectively and forthrightly discusses such matters.
We need to have that conversation.
Hopefully, the most important takeaway from this roundtable discussion is that Black individuals are not monolithic, and instead, part of a heterogenous population. Similarly, female individuals vary in their body composition, depending on genetics, race, pre or postmenopausal status, underlying illnesses, medications, and the degree and type of nutritional intake and physical activity. Given this heterogenicity between and within patient populations, this roundtable discussion further solidifies my belief of the importance of a patient-centered approach. It solidifies my belief in the importance of body composition analysis as one of the most valuable diagnostic resources available, having applicability to diverse populations such as overweight, obesity, overweight with increased muscle mass, normal weight with decreased muscle mass, all adults (and perhaps some children [124]), irrespective of age, sex, gender, race and ethnicity.
Body composition analysis is the ultimate diagnostic equalizer in addressing the inaccuracies and biases inherent in the exclusive use of BMI.
Ethical review
This Obesity Medicine Association Roundtable represents original works, with work and/or words of others appropriately cited or quoted in the submission. This submission did not involve human test subjects or volunteers. HEB was not involved in the peer review process, nor the acceptance/rejection of this submission. Responsibility for the editorial process for this article was delegated to an independent Editor and/or Associate Editor.
Disclosures
HEB’s research site performs dual X-ray absorptiometry for research protocols and management of patients.
SGB: Novo Nordisk Advisory Board, Shaklee Ambassador.
CY: No disclosures.
SW: Honoraria and Ad Boards: Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Bausch Health Canada.
Source of funding
This submission received no funding.
Acknowledgement and author contribution
HEB conceptualized the submission, wrote/sent questions to the other authors, and assisted with editing the manuscript. SW, SGB and CY responded to their assigned questions, reviewed their sections for accuracy, and gave final approval of their contribution. Special thanks to Elham Kamran (Research Coordinator, Project Manager, and Clinical Lead, Wharton Medical Clinic, Hamilton Ontario Canada) for writing assistance to Dr. Sean Wharton.
Contributor Information
Harold Edward Bays, Email: hbaysmd@outlook.com, http://www.lmarc.com.
Sylvia Gonsahn-Bollie, Email: contactus@embraceyouweightloss.com.
Courtney Younglove, Email: courtney@HeartlandWeightLoss.com.
Sean Wharton, Email: sean@whartonmedicalclinic.com.
References
- 1.Bays H.E., Muñoz-Mantilla D.X., Morgan R., Nwizu C., Garcia T.T. Obesity pillars roundtable: obesity and diversity. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bays H.E., Shrestha A., Niranjan V., Khanna M., Kambhamettu L. Obesity pillars roundtable: obesity and South Asians. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bays H.E., Ng J., Sicat J., Look M. Obesity pillars roundtable: obesity and East Asians. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bays H.E., Antoun J., Censani M., Bailony R., Alexander L. Obesity pillars roundtable: obesity and individuals from the Mediterranean region and Middle East. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuda S.E., Pratt J.S.A., Santos M., Browne A. Obesity Pillars roundtable: metabolic and bariatric surgery in children and adolescents. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus E., Bays H.E. Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays H.E., Golden A., Tondt J. Thirty obesity myths, misunderstandings, and/or oversimplifications: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global B.M.I.M.C., Di Angelantonio E., Bhupathiraju Sh N., Wormser D., Gao P., Kaptoge S., et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennings N., Golden L., Yashi N., Tondt J., Bays HE. Sleep-disordered breathing, sleep apnea, and other obesity-related sleep disorders: An Obesity Medicine Association (OMA) Clinical Practice Statement. Obesity Pillars. 2022:100043. doi: 10.1016/j.obpill.2022.100043. ISSN 2667-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bays H. Adiposopathy, "sick fat," Ockham's razor, and resolution of the obesity paradox. Curr Atherosclerosis Rep. 2014;16:409. doi: 10.1007/s11883-014-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bays H.E. Journal of the American College of Cardiology; 2022. Evaluation and practical management of increased visceral fat: should cardiologists lose sleep over it? [DOI] [PubMed] [Google Scholar]
- 14.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–351. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bays H.E. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Crudele L., Piccinin E., Moschetta A. Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients. 2021;13 doi: 10.3390/nu13062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piché M.E., Tchernof A., Després J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 18.Yako Y.Y., Echouffo-Tcheugui J.B., Balti E.V., Matsha T.E., Sobngwi E., Erasmus R.T., et al. Genetic association studies of obesity in Africa: a systematic review. Obes Rev : an official journal of the International Association for the Study of Obesity. 2015;16:259–272. doi: 10.1111/obr.12260. [DOI] [PubMed] [Google Scholar]
- 19.Gower B.A., Fowler L.A. Obesity in African-Americans: the role of physiology. J Intern Med. 2020;288:295–304. doi: 10.1111/joim.13090. [DOI] [PubMed] [Google Scholar]
- 20.Ng M.C. Genetics of type 2 diabetes in african Americans. Curr Diabetes Rep. 2015;15:74. doi: 10.1007/s11892-015-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden S.H., Yajnik C., Phatak S., Hanson R.L., Knowler W.C. Racial/ethnic differences in the burden of type 2 diabetes over the life course: a focus on the USA and India. Diabetologia. 2019;62:1751–1760. doi: 10.1007/s00125-019-4968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aambø A., Klemsdal T.O. Cardiovascular disease and diabetes in patients with African or Asian background. Tidsskr Nor Laegeforen. 2017;137 doi: 10.4045/tidsskr.16.0680. [DOI] [PubMed] [Google Scholar]
- 23.Lewis K.H., Edwards-Hampton S.A., Ard J.D. Disparities in treatment uptake and outcomes of patients with obesity in the USA. Curr Obes Rep. 2016;5:282–290. doi: 10.1007/s13679-016-0211-1. [DOI] [PubMed] [Google Scholar]
- 24.Tauqeer Z., Gomez G., Stanford F.C. Obesity in women: insights for the clinician. J Womens Health (Larchmt). 2018;27:444–457. doi: 10.1089/jwh.2016.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz A.C.E. Obesity-related cancer in women: a clinical review. Am J Nurs. 2019;119:34–40. doi: 10.1097/01.NAJ.0000577332.56265.51. [DOI] [PubMed] [Google Scholar]
- 26.Byrd A.S., Toth A.T., Stanford F.C. Racial disparities in obesity treatment. Curr Obes Rep. 2018;7:130–138. doi: 10.1007/s13679-018-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeboye B., Bermano G., Rolland C. Obesity and its health impact in Africa: a systematic review. Cardiovasc J Afr. 2012;23:512–521. doi: 10.5830/CVJA-2012-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaron D.G., Stanford F.C. Is obesity a manifestation of systemic racism? A ten-point strategy for study and intervention. J Intern Med. 2021;290:416–420. doi: 10.1111/joim.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aaron D.G., Stanford F.C. Medicine, structural racism, and systems. Soc Sci Med. 2022;298 doi: 10.1016/j.socscimed.2022.114856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnethon M.R., Pu J., Howard G., Albert M.A., Anderson C.A.M., Bertoni A.G., et al. Cardiovascular health in african Americans: a scientific statement from the American heart association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 31.Olivo R.E., Davenport C.A., Diamantidis C.J., Bhavsar N.A., Tyson C.C., Hall R., et al. Obesity and synergistic risk factors for chronic kidney disease in African American adults: the Jackson Heart Study. Nephrol Dial Transplant. 2017;33:992–1001. doi: 10.1093/ndt/gfx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs F.D. Why do black Americans have higher prevalence of hypertension? Hypertension. 2011;57:379–380. doi: 10.1161/HYPERTENSIONAHA.110.163196. [DOI] [PubMed] [Google Scholar]
- 33.Bancks M.P., Kershaw K., Carson A.P., Gordon-Larsen P., Schreiner P.J., Carnethon M.R. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA, J Am Med Assoc. 2017;318:2457–2465. doi: 10.1001/jama.2017.19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bello O., Mohandas C., Shojee-Moradie F., Jackson N., Hakim O., Alberti K., et al. Black African men with early type 2 diabetes have similar muscle, liver and adipose tissue insulin sensitivity to white European men despite lower visceral fat. Diabetologia. 2019;62:835–844. doi: 10.1007/s00125-019-4820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tay J., Goss A.M., Garvey W.T., Lockhart M.E., Bush N.C., Quon M.J., et al. Race affects the association of obesity measures with insulin sensitivity. Am J Clin Nutr. 2020;111:515–525. doi: 10.1093/ajcn/nqz309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedecke J.H., Olsson T. Pathogenesis of type 2 diabetes risk in black Africans: a South African perspective. J Intern Med. 2020;288:284–294. doi: 10.1111/joim.13083. [DOI] [PubMed] [Google Scholar]
- 37.Silva A.M., Shen W., Heo M., Gallagher D., Wang Z., Sardinha L.B., et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22:76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera A., McGuire D.K., Murphy S.A., Stanek H.G., Das S.R., Vongpatanasin W., et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 39.Azrad M., Gower B.A., Hunter G.R., Nagy T.R. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine. 2013;43:586–592. doi: 10.1007/s12020-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaze A.D., Musani S.K., Bidulescu A., Correa A., Bertoni A.G., Ahima R.S., et al. Plasma leptin and blood pressure progression in blacks. Hypertension. 2021;77:1069–1075. doi: 10.1161/HYPERTENSIONAHA.120.16174. [DOI] [PubMed] [Google Scholar]
- 41.Bays H.E., Kulkarni A., German C., Satish P., Iluyomade A., Dudum R., et al. Ten things to know about ten cardiovascular disease risk factors - 2022. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty P.B., Tang H., Feng T., Tayo B., Morrison A.C., Kardia S.L.R., et al. Variants for HDL-C, LDL-C, and triglycerides identified from admixture mapping and fine-mapping analysis in african American families. Circulation: Cardiovascul Gene. 2015;8:106–113. doi: 10.1161/CIRCGENETICS.114.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bild D.E., Detrano R., Peterson D., Guerci A., Liu K., Shahar E., et al. Ethnic differences in coronary calcification. Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 44.Reyes-Soffer G. The impact of race and ethnicity on lipoprotein(a) levels and cardiovascular risk. Curr Opin Lipidol. 2021;32:163–166. doi: 10.1097/MOL.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun Y., Kim D., Lee E.S. Effect of cross-sex hormones on body composition, bone mineral density, and muscle strength in trans women. J Bone Metab. 2021;28:59–66. doi: 10.11005/jbm.2021.28.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaver M., de Blok C.J.M., Wiepjes C.M., Nota N.M., Dekker M.J.H.J., de Mutsert R., et al. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178:163–171. doi: 10.1530/EJE-17-0496. [DOI] [PubMed] [Google Scholar]
- 47.Spanos C., Bretherton I., Zajac J.D., Cheung A.S. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: a systematic review. World J Diabetes. 2020;11:66–77. doi: 10.4239/wjd.v11.i3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goedecke J.H., Levitt N.S., Evans J., Ellman N., Hume D.J., Kotze L., et al. The role of adipose tissue in insulin resistance in women of African ancestry. J obesity. 2013;2013 doi: 10.1155/2013/952916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun M., Oliwa T., Peek M.E., Tung E.L. Negative patient descriptors: documenting racial bias in the electronic health record. Health Aff. 2022;41:203–211. doi: 10.1377/hlthaff.2021.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia M., Mulvagh S.L., Merz C.N., Buring J.E., Manson J.E. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko S.H., Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021;13 doi: 10.3390/nu13124556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nauli A.M., Matin S. Why do men accumulate abdominal visceral fat? Front Physiol. 2019;10:1486. doi: 10.3389/fphys.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Magkos F., Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torosyan N., Visrodia P., Torbati T., Minissian M.B., Shufelt C.L. Dyslipidemia in midlife women: approach and considerations during the menopausal transition. Maturitas. 2022;166:14–20. doi: 10.1016/j.maturitas.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson J.C., Crook D., Godsland I.F. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 56.Després J.P., Moorjani S., Tremblay A., Ferland M., Lupien P.J., Nadeau A., et al. Relation of high plasma triglyceride levels associated with obesity and regional adipose tissue distribution to plasma lipoprotein-lipid composition in premenopausal women. Clin Invest Med. 1989;12:374–380. [PubMed] [Google Scholar]
- 57.Leenen R., van der Kooy K., Seidell J.C., Deurenberg P. Visceral fat accumulation measured by magnetic resonance imaging in relation to serum lipids in obese men and women. Atherosclerosis. 1992;94:171–181. doi: 10.1016/0021-9150(92)90242-9. [DOI] [PubMed] [Google Scholar]
- 58.Macut D., Ognjanović S., Ašanin M., Krljanać G., Milenković T. Metabolic syndrome and myocardial infarction in women. Curr Pharmaceut Des. 2021;27:3786–3794. doi: 10.2174/1381612827666210610114029. [DOI] [PubMed] [Google Scholar]
- 59.Carr M.C. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins C.R., Chapman K.R., Donohue J.F., Roche N., Tsiligianni I., Han M.K. Improving the Management of COPD in Women. Chest. 2017;151 doi: 10.1016/j.chest.2016.10.031. 686-96. [DOI] [PubMed] [Google Scholar]
- 61.Wu T., Jahangir M.R., Mensink-Bout S.M., Klein S., Duijts L., Oei E.H.G. Visceral adiposity and respiratory outcomes in children and adults: a systematic review. Int J Obes. 2022;46:1083–1100. doi: 10.1038/s41366-022-01091-6. [DOI] [PubMed] [Google Scholar]
- 62.Fang H., Berg E., Cheng X., Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21:360–365. doi: 10.1097/MCO.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dudley K.A., Patel S.R. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96–102. doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers A.J., Kaplan I., Chung A., McFarlane S.I., Jean-Louis G. Obstructive sleep apnea risk and stroke among blacks with metabolic syndrome: results from metabolic syndrome outcome (MetSO) registry. Int J Clin Res Trials. 2020;5 doi: 10.15344/2456-8007/2020/143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wimms A., Woehrle H., Ketheeswaran S., Ramanan D., Armitstead J. Obstructive sleep apnea in women: specific issues and interventions. BioMed Res Int. 2016;2016 doi: 10.1155/2016/1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong L., Dubowitz T., Haas A., Ghosh-Dastidar M., Holliday S.B., Buysse D.J., et al. Prevalence and correlates of obstructive sleep apnea in urban-dwelling, low-income, predominantly African-American women. Sleep Med. 2020;73:187–195. doi: 10.1016/j.sleep.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maniaci A., Iannella G., Cocuzza S., Vicini C., Magliulo G., Ferlito S., et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J Clin Med. 2021:10. doi: 10.3390/jcm10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz A.R., Patil S.P., Laffan A.M., Polotsky V., Schneider H., Smith P.L. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George M.D., McGill N.-K., Baker J.F. Creatine kinase in the U.S. population: impact of demographics, comorbidities, and body composition on the normal range. Medicine. 2016;95 doi: 10.1097/MD.0000000000004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thongprayoon C., Cheungpasitporn W., Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8:E305–E311. doi: 10.21037/jtd.2016.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He B. Clinical Lipidology A Companion to Braunwald's Heart Disease Christie Ballantyne MD; 2009. Investigational agents affecting atherogenic lipoproteins; pp. 530–543. [Google Scholar]
- 72.Katiriji B., Al Jaberi M.M. Creatine kinase revisited. J Clin Neuromuscul Dis. 2001;2:158–164. doi: 10.1097/00131402-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Araujo A.B., Chiu G.R., Kupelian V., Hall S.A., Williams R.E., Clark R.V., et al. Lean mass, muscle strength, and physical function in a diverse population of men: a population-based cross-sectional study. BMC Publ Health. 2010;10:508. doi: 10.1186/1471-2458-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brewster L.M., Van Valkengoed I., van Montfrans G.A. African ancestry vs. Creatine kinase to predict hypertension control: time for a change? Am J Hypertens. 2021;34:1264–1268. doi: 10.1093/ajh/hpab114. [DOI] [PubMed] [Google Scholar]
- 75.Ramsay M., Crowther N.J., Agongo G., Ali S.A., Asiki G., Boua R.P., et al. Regional and sex-specific variation in BMI distribution in four sub-Saharan African countries: the H3Africa AWI-Gen study. Glob Health Action. 2018;11 doi: 10.1080/16549716.2018.1556561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Little M.A. Evolutionary strategies for body size. Front Endocrinol. 2020;11:107. doi: 10.3389/fendo.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rozzi F.V.R., Koudou Y., Froment A., Le Bouc Y., Botton J. Growth pattern from birth to adulthood in African pygmies of known age. Nat Commun. 2015;6:7672. doi: 10.1038/ncomms8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domislovic M., Domislovic V., Fucek M., Jelakovic A., Gellineo L., Dika Z., Jelakovic B. Should the CKD EPI Equation Be Used for Estimation of the Glomerular Filtration Rate in Obese Subjects? Kidney Blood Press Res. 2022;47(10) doi: 10.1159/000526115. Epub 2022 Sep 28. PMID: 36170804. [DOI] [PubMed] [Google Scholar]
- 79.Rocha A.D., Garcia S., Santos A.B., Eduardo J.C.C., Mesquita C.T., Lugon J.R., et al. No race-ethnicity adjustment in CKD-EPI equations is required for estimating glomerular filtration rate in the Brazilian population. Internet J Nephrol. 2020;2020 doi: 10.1155/2020/2141038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erstad B.L., Nix D.E. Assessment of kidney function in patients with extreme obesity: a narrative review. Ann Pharmacother. 2021;55:80–88. doi: 10.1177/1060028020935580. [DOI] [PubMed] [Google Scholar]
- 81.Tsai J.W., Cerdena J.P., Goedel W.C., Asch W.S., Grubbs V., Mendu M.L., et al. Evaluating the impact and rationale of race-specific estimations of kidney function: estimations from U.S. NHANES, 2015-2018. EClinicalMedicine. 2021;42 doi: 10.1016/j.eclinm.2021.101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wuerzner G., Bochud M., Giusti V., Burnier M. Measurement of glomerular filtration rate in obese patients: pitfalls and potential consequences on drug therapy. Obes Facts. 2011;4:238–243. doi: 10.1159/000329547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donker E.M., Bet P., Nurmohamed A., Serné E., Burchell George L., Friedman A.N., et al. Estimation of glomerular filtration rate for drug dosing in patients with very high or low body mass index. Clinical and Translational Science. 2022;15:2206–2217. doi: 10.1111/cts.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vlasschaert C., Thibodeau S., Parmar M.S. De-indexed estimated glomerular filtration rates: a simple step towards improving accuracy of drug dosing of renally excreted medications in moderate to severe obesity. Nephrology. 2020;25:29–31. doi: 10.1111/nep.13621. [DOI] [PubMed] [Google Scholar]
- 85.Guebre-Egziabher F., Brunelle C., Thomas J., Pelletier C.C., Normand G., Juillard L., et al. Estimated glomerular filtration rate bias in participants with severe obesity regardless of deindexation. Obesity. 2019;27:2011–2017. doi: 10.1002/oby.22574. [DOI] [PubMed] [Google Scholar]
- 86.Alexander L., Christensen S.M., Richardson L., Ingersoll A.B., Burridge K., Golden A., et al. Nutrition and physical activity: an obesity medicine association (OMA) clinical practice statement 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grundy S.M., Neeland I.J., Turer A.T., Vega G.L. Waist circumference as measure of abdominal fat compartments. J obesity. 2013;2013 doi: 10.1155/2013/454285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okop K.J., Mukumbang F.C., Mathole T., Levitt N., Puoane T. Perceptions of body size, obesity threat and the willingness to lose weight among black South African adults: a qualitative study. BMC Publ Health. 2016;16:365. doi: 10.1186/s12889-016-3028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelly N.R., Bulik C.M., Mazzeo S.E. An exploration of body dissatisfaction and perceptions of Black and White girls enrolled in an intervention for overweight children. Body Image. 2011;8:379–384. doi: 10.1016/j.bodyim.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spinner J.R. An examination of the impact of social and cultural traditions contributing to overweight and obesity among black women. J Prim Care Community Health. 2022;13 doi: 10.1177/21501319221098519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petroni M.L., Caletti M.T., Dalle Grave R., Bazzocchi A., Aparisi Gómez M.P., Marchesini G. Prevention and treatment of sarcopenic obesity in women. Nutrients. 2019;11 doi: 10.3390/nu11061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zanchetta M.B., Abdala R., Massari F., Rey P., Spivacow R., Miechi L., et al. Postmenopausal women with sarcopenia have higher prevalence of falls and vertebral fractures. Medicina (B Aires) 2021;81:47–53. [PubMed] [Google Scholar]
- 93.Vogel J.A., Friedl K.E. Body fat assessment in women. Special considerations. Sports Med. 1992;13:245–269. doi: 10.2165/00007256-199213040-00003. [DOI] [PubMed] [Google Scholar]
- 94.Musolino E.A., O'Connor B.P., Cioe J.D. Bigger isn't always better: an exploration of social perception bias against high levels of muscularity in women. J Soc Psychol. 2022;162:523–539. doi: 10.1080/00224545.2021.1927943. [DOI] [PubMed] [Google Scholar]
- 95.Iversen V.M., Norum M., Schoenfeld B.J., Fimland M.S. No time to lift? Designing time-efficient training programs for strength and hypertrophy: a narrative review. Sports Med. 2021;51:2079–2095. doi: 10.1007/s40279-021-01490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zemková E., Zapletalová L. The role of neuromuscular control of postural and core stability in functional movement and athlete performance. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.796097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grgic J. The effects of low-load vs. High-load resistance training on muscle fiber hypertrophy: a meta-analysis. J Hum Kinet. 2020;74:51–58. doi: 10.2478/hukin-2020-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stokes T., Hector A.J., Morton R.W., McGlory C., Phillips S.M. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients. 2018;10 doi: 10.3390/nu10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samal J.R.K., Samal I.R. Protein supplements: pros and cons. J Diet Suppl. 2018;15:365–371. doi: 10.1080/19390211.2017.1353567. [DOI] [PubMed] [Google Scholar]
- 100.Knowles O.E., Drinkwater E.J., Urwin C.S., Lamon S., Aisbett B. Inadequate sleep and muscle strength: implications for resistance training. J Sci Med Sport. 2018;21:959–968. doi: 10.1016/j.jsams.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 101.Kovacevic A., Mavros Y., Heisz J.J., Fiatarone Singh M.A. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Med Rev. 2018;39:52–68. doi: 10.1016/j.smrv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Tondt J., Bays H.E. Concomitant medications, functional foods, and supplements: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morrison E.Y., Cooper P.D. Some bio-medical mechanisms in athletic prowess. W Indian Med J. 2006;55:205–209. doi: 10.1590/s0043-31442006000300015. [DOI] [PubMed] [Google Scholar]
- 104.Micheletti S.J., Bryc K., Ancona Esselmann S.G., Freyman W.A., Moreno M.E., Poznik G.D., et al. Genetic consequences of the transatlantic slave trade in the Americas. Am J Hum Genet. 2020;107:265–277. doi: 10.1016/j.ajhg.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adhikari K., Chacón-Duque J.C., Mendoza-Revilla J., Fuentes-Guajardo M., Ruiz-Linares A. The genetic diversity of the Americas. Annu Rev Genom Hum Genet. 2017;18:277–296. doi: 10.1146/annurev-genom-083115-022331. [DOI] [PubMed] [Google Scholar]
- 106.Baharian S., Barakatt M., Gignoux C.R., Shringarpure S., Errington J., Blot W.J., et al. The Great migration and african-American genomic diversity. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kerr I.B. The myth of racial superiority in sports. The Hilltop Review. 2010;4 [Google Scholar]
- 108.Sheldon J.P., Jayaratne T.E., Petty E.M. White Americans' genetic explanations for a perceived race difference in athleticism: the relation to prejudice toward and stereotyping of blacks. Athletic Insight. 2007;9 [Google Scholar]
- 109.Ceaser T., Hunter G. Black and White race differences in aerobic capacity, muscle fiber type, and their influence on metabolic processes. Sports Med. 2015;45:615–623. doi: 10.1007/s40279-015-0318-7. [DOI] [PubMed] [Google Scholar]
- 110.Tanner C.J., Barakat H.A., Dohm G.L., Pories W.J., MacDonald K.G., Cunningham P.R., et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 111.Trappe S., Luden N., Minchev K., Raue U., Jemiolo B., Trappe T.A. Skeletal muscle signature of a champion sprint runner. J Appl Physiol. 2015;118:1460–1466. doi: 10.1152/japplphysiol.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roth S.M., Walsh S., Liu D., Metter E.J., Ferrucci L., Hurley B.F. The ACTN3 R577X nonsense allele is under-represented in elite-level strength athletes. Eur J Hum Genet. 2008;16:391–394. doi: 10.1038/sj.ejhg.5201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pickering C., Kiely J. ACTN3: more than just a gene for speed. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Papadimitriou I.D., Lucia A., Pitsiladis Y.P., Pushkarev V.P., Dyatlov D.A., Orekhov E.F., et al. ACTN3 R577X and ACE I/D gene variants influence performance in elite sprinters: a multi-cohort study. BMC Genom. 2016;17:285. doi: 10.1186/s12864-016-2462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Michos E.D., Reddy T.K., Gulati M., Brewer L.C., Bond R.M., Velarde G.P., et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol. 2021;8 doi: 10.1016/j.ajpc.2021.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cullen M.R., Lemeshow A.R., Russo L.J., Barnes D.M., Ababio Y., Habtezion A. Disease-specific health disparities: a targeted review focusing on race and ethnicity. Healthcare (Basel) 2022;10 doi: 10.3390/healthcare10040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Esoh K., Wonkam A. Evolutionary history of sickle-cell mutation: implications for global genetic medicine. Hum Mol Genet. 2021;30 doi: 10.1093/hmg/ddab004. R119-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zilbermint M., Hannah-Shmouni F., Stratakis C.A. Genetics of hypertension in african Americans and others of african descent. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McIntosh M.S., Kumar V., Kalynych C., Lott M., Hsi A., Chang J.L., et al. Racial differences in blood lipids lead to underestimation of cardiovascular risk in black women in a nested observational study. Glob Adv Health Med. 2013;2:76–79. doi: 10.7453/gahmj.2012.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reyes-Soffer G., Ginsberg H.N., Berglund L., Duell P.B., Heffron S.P., Kamstrup P.R., et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. 2022;42:e48–e60. doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Using genetic technologies to reduce, rather than widen, health disparities. Health Aff. 2016;35:1367–1373. doi: 10.1377/hlthaff.2015.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Javed Z., Haisum Maqsood M., Yahya T., Amin Z., Acquah I., Valero-Elizondo J., et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circul. Cardiovascul. qual outcomes. 2022;15 doi: 10.1161/CIRCOUTCOMES.121.007917. [DOI] [PubMed] [Google Scholar]
- 123.Gauci S., Cartledge S., Redfern J., Gallagher R., Huxley R., Lee C.M.Y., et al. Biology, bias, or both? The contribution of sex and gender to the disparity in cardiovascular outcomes between women and men. Curr Atherosclerosis Rep. 2022;24:701–708. doi: 10.1007/s11883-022-01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J., Zhu L., Chen Z., Liao J., Liu X. Effects and dose-response relationship of exercise training on cardiometabolic risk factors in children with obesity. J Pediatr Endocrinol Metab. 2022 Sep 27;35(10):1278–1284. doi: 10.1515/jpem-2022-0395. PMID: 36162139. [DOI] [PubMed] [Google Scholar]