Abstract
Sexual selection has been studied as a major evolutionary driver of animal diversity for roughly 50 years. Much evidence indicates that competition for mates favors elaborate signaling traits. However, this evidence comes primarily from a few taxa, leaving sexual selection as a salient evolutionary force across Animalia largely untested. Here, we reviewed the evidence for sexual selection on communication across all animal phyla, classes, and orders with emphasis on chemoreception, the only sense shared across lifeforms. An exhaustive literature review documented evidence for sexual selection on chemosensory traits in 10 of 34 animal phyla and indications of sexual selection on chemosensory traits in an additional 13 phyla. Potential targets of sexual selection include structures and processes involved in production, delivery, and detection of chemical signals. Our review suggests sexual selection plays a widespread role in the evolution of communication and highlights the need for research that better reflects animal diversity.
Subject terms: Sexual selection, Animal behaviour
In this Perspective, the authors evaluate the evidence for sexual selection on communication across Animalia, with particular emphasis on chemosensory traits.
Introduction
Animals interact with mates and sexual rivals using diverse and often elaborate traits1. These traits are among the most striking displays of animal biodiversity (e.g., courtship dance of peacock spider2) and inspired Darwin’s theory that sexual selection arising from variation in access to mates (or gametes)3 (Box 1) acts alongside selection for survival and fecundity4. The last few decades have brought an outpouring of research on the evolution of sexual ornaments, displays, and calls5,6 and, as Darwin suggested, overwhelming evidence indicates such traits often evolve under sexual selection. Having garnered empirical support as a salient evolutionary force underlying signaling traits1, sexual selection continues to hold the keen focus of evolutionary biologists due, in part, to the hypothesis that its effects on sexual signals and preferences drive reproductive isolation and ultimately speciation7,8. As important products of sexual selection and substrates for sexual selection to drive speciation, signaling traits are often at the center of discussions on the evolution of animal biodiversity9–11.
Despite its broad scope, theory around animal signals and sexual selection has advanced largely through the intensive study of relatively few taxa. Animals such as frogs, fish, and arthropods have proven particularly useful for developing and testing models of sexual selection12,13. For example, studies on túngara frogs14, Trinidadian guppies15, and fiddler crabs16 revealed that sexual signals can evolve to exploit receivers’ sensory ecology rather than providing information about the signaler’s quality as a mate17 (e.g., male guppies have orange spots that mimic fruit18). Largely lost amongst the mechanistic details of sexual selection, however, is the fundamental question of whether sexual selection acts as an important evolutionary force across all of Animalia. Importantly, all but one (Micrognathozoa) animal phyla include species that are known to reproduce sexually and therefore could be shaped by sexual selection19. The conspicuous signaling traits that originally captured Darwin’s attention clearly illustrate a major role of sexual selection in some chordate and arthropod classes20 but whether this role is common across the animal kingdom remains largely unexplored.
In this Perspective, we review the evidence for sexual selection on traits involved in chemical communication across the animal Tree of Life. As we outline below, chemical communication is uniquely poised to be a possible target of sexual selection across all animals and therefore particularly important for evaluating the potential for sexual selection on communication at a macroevolutionary scale. Our primary objective is to review the evidence for sexual selection on signaling traits across Animalia. By focusing on chemical communication, our approach explicitly acknowledges differences in animals’ sensory biology. After establishing chemosensation as the only possible target of sexual selection on communication that is common across all of Animalia, we review (1) the evidence for sexual selection on chemosensory traits in species from all phyla, classes, and orders of animals and (2) the mechanisms (e.g., mate choice; Table 1) and targets (e.g., scent glands; Table 2) of sexual selection on chemical communication. Our goal is to encourage the field of sexual selection to have a broader scope that spans Earth’s diverse forms of animal life, and advocate that chemical communication be a key focus of the discussion.
Table 1.
| Mechanism | Example | Species |
|---|---|---|
| Scramble competition | Males with more sensilla on antennae locate females quicker86 | Mantid (Pseudomantis albofimbriata) |
| Contest competition | Eventual winning males jet urine at opponents during fights to induce defensive behaviors165 | Crayfish (Astacus leptodactylus) |
| Gamete competition | Love dart injects mucous into mates to delay remating166 | Snail (Euhadra quaesita) |
| Mate choice | Males prefer pheromones of virgin over mated females167 | Nematodes (Caenorhabditis sp.) |
| Cryptic mate choice | Females differ in the production of sperm chemoattractant and males differ in sperm chemotactic ability168,169 | Urchin (Lytechinus pictus) |
Table 2.
Example targets of potential sexual selection on chemical communication.
| Level | Trait type | Example | Species |
|---|---|---|---|
| Production | Enzyme | Female-specific expression of fatty acid elongase underlies sex pheromone production110 | Cockroach (Blattella germanica) |
| Cell | Male-specific gill cells release sex pheromone81 | Lamprey (Petromyzon marinus) | |
| Gland | Male-specific leg glands secrete sex pheromone116 | Velvet worm (Cephalofovea tomahmontis) | |
| Organ | Large kidneys and hypertrophic urinary bladders mediate pheromone signaling by dominant males120 | Tilapia (Oreochromis mossambicus) | |
| Delivery | Apparatus | Calcareous dart injects allohormone that biases paternity170 | Snail (Helix aspersa) |
| Signaling behavior | Dominant males kick feces to increase active space of odor127,128 | Rhino (Ceratotherium simum) | |
| Transmission | Accessory molecule | Proteins delay scent evaporation and extend signal duration132 | Mouse (Mus domesticus) |
| Detection | Sampling behavior | Eventual losers of fights flick sensory antennules more often to assess urine signal of competitor171 | Lobster (Nephrops norvegicus) |
| Sensory structure | Long antennae improve male mate search172 | Isopod (Asellus aquaticus) | |
| Accessory molecule | Male-specific binding proteins increases sensitivity to female pheromones173 | Moth (Chilo suppressalis) | |
| Receptor | Female-specific expression of putative pheromone receptors on sensory tentacles174 | Starfish (Acanthaster planci) | |
| Neural circuit | Sexually dimorphic neural circuit mediates sex-specific responses to a pheromone175 | Fruit fly (Drosophila melanogaster) |
Box 1 Glossary.
Sexual selection: Selection that arises from fitness differences associated with nonrandom success in the competition for access to gametes for fertilization3.
Signal: A trait of one individual (sender) that evolved to influence the physiology or behavior of another individual (receiver) after being sensed by the receiver181.
Cue: A sensory stimulus (biotic or abiotic) that triggers a response in an animal but did not evolve for communication181.
Pheromone: A molecule that evolved for signaling to conspecifics and elicit a specific reaction when detected24.
Allohormone: Molecules from one individual that are transferred directly into a conspecific and that elicit a physiological or behavioral response without being detected by external senses134,182.
Chemosensory traits as potentially universal targets of sexual selection
Despite much interest in visual and auditory signals, most animals cannot see or hear. High-resolution, image-forming eyes are present only in arthropods, chordates, and cephalopod mollusks21, and only vertebrates and some arthropods possess ears or analogs of ears22. Although some animals that lack eyes or ears can detect light and sounds21,23, the sensitivity and specificity of these channels is unlikely sufficient for communication. For example, photoreception in most animal phyla can mediate internal physiological control (e.g., circadian rhythms), directional phototaxis, and habitat orientation but not interactions with specific objects or individuals (e.g., mates)21. In contrast, all single- and multicellular organisms have chemoreceptors that allow acute sensitivity to specific chemicals24.
The capacity to sense specific molecules is a fundamental feature of life on Earth25,26. Unicellular bacteria27, archaea28, protists29, and fungi30 express membrane-bound receptors that bind specific molecules, such as those related to social conditions (e.g., quorum-sensing pheromones)31. As first suggested by JBS Haldane, the external chemoreceptors of unicellular organisms may be precursors to internal receptors that allow intercellular communication in multicellular organisms25. This basic ability to detect chemicals in the milieu surrounding cells, whether internally or externally, has given rise to specialized chemosensory cells and organs in seemingly all animals, from the nerveless poriferans and placozoans to ctenophores and cnidarians, which have nerve nets, and the bilaterians with their centralized nervous systems32,33. The mechanisms of chemoreception differ within and among taxa, and include solitary chemosensory cells, olfaction, gustation, and the vomeronasal system. However, these classifications are largely based on terrestrial vertebrates and insects and may not hold in other taxa34, especially the many groups for which our understanding of chemoreception systems is limited (e.g., ctenophores)35. Regardless, the ubiquity of chemoreception as a specific sensory channel makes it especially useful for studying the potential role of sexual selection across diverse animals.
How and where animals live further implicates chemosensory traits as common potential targets of sexual selection across higher taxonomic levels. Sexual signals evolve under selection related to animals’ ecology—specifically how individuals interact with potential mates and the environment15,36. In several phyla, individuals that already lack vision and hearing also have limited ability to interact with mates via touch as they are sessile as adults37 (with some exceptions, such as sessile barnacles with extendable penises38). Perhaps more surprising to visually oriented humans is evidence that the dominant sensory environment of animals favors communication via chemoreception39; most invertebrates40 and mammals41 are nocturnal and a large proportion of Earth’s animal diversity is found in the perennial dark of the deep sea42 and underground43. Although many animals have adaptations that allow vision in dim light44, life in the dark is often associated with a predominant role of chemoreception45–47. Numerous and interacting ecological conditions shape the evolution of signaling systems and visual, auditory, electrical, and tactile communication clearly play dominant roles in many taxa48. However, the basic sensory capabilities and ecology of many animals suggest chemoreception is the only common channel for sexual communication across higher taxonomic levels of the animal Tree of Life.
Taxonomic distribution of chemosensory traits potentially under sexual selection
Literature review
We searched for studies indicating that chemosensory traits guide sexual interactions between competitors or mates. Our primary objective was to document evidence for sexual chemosensory traits across higher taxonomic groups of Animalia (phyla n = 34, classes n = ~100, orders n = ~600). As the animal kingdom includes immensely diverse life-forms and studies on these life-forms often use different terminology, achieving our goal required a flexible and exhaustive search rather than a structured review with restricted search terms and filtering steps. We followed the taxonomy of Ruggiero et al.49 because it provided a unified classification down to the level of order and therefore allowed a taxonomically systematic search (Supplementary Data 1). To begin, we searched Google Scholar using both scientific and common names (when available), and keywords such as (but not limited to) “pheromone”, “chemical cue”, “chemosensory”, “olfactory”, and “scent”. At a minimum, we searched at the level of phyla and order. Searches for which the above keywords yielded few (or no) results were then repeated using more general keywords such as “reproductive behavior”, “mating”, and “spawning”. Often, these general searches yielded papers that were only tangentially relevant but either cited or were cited by studies that were directly relevant to our search. When available, we leveraged review papers50–54 and online resources (e.g., www.pherobase.com) that guided us to potentially relevant studies. In especially obscure taxa (e.g., Placozoans), we used Google Scholar or Google Search and no search terms other than their name to find if anything is known about how they reproduce and if chemosensory traits might be involved. Using these approaches, we searched exhaustively for the most direct evidence (see below for categories of evidence) of sexual selection on a chemosensory trait available for each order of animal. Importantly, our search was exhaustive in that we attempted to document, at a minimum, one example of a sexual chemosensory trait for each order but not exhaustive in compiling the available evidence within orders.
We included studies that fit into three categories according to the evidence they provided for sexual selection on a chemosensory trait. The first category met the criteria for sexual selection set by Andersson1: (i) evidence of a significant relationship between a trait and mating success and (ii) an identified mechanism of sexual selection, such as mate choice (Table 1). Consistent with Andersson1 and more recent literature55, this category included studies that used a proxy of mate choice (e.g., time near stimulus from a potential mate) but did not measure actual mating outcomes. To acknowledge known research biases towards certain taxa12,13,56, we also included studies in two additional categories if they indicated potential for sexual selection on a chemosensory trait but did not provide direct empirical support per the established criteria1. Specifically, studies considered to report potential for sexual selection on chemosensory traits included (i) documented behavioral or physiological responses to chemical traits of mates or competitors in a reproductive context (e.g., behavioral attraction of mature male Nautilus to female rectal extract57) or (ii) suggestions of sexual chemosensory interactions based upon indirect evidence (e.g., sexually dimorphic leg glands in centipedes58). We prioritized studies that fit in the first category, and sequentially included studies in the second and then third categories only if we were unable to find studies that met the criteria of the first category. Importantly, studies in the latter two categories do not provide direct evidence of sexual selection on chemosensory traits. Nevertheless, we included them to illustrate the state of the field across taxa and guide future work. Indeed, these relaxed criteria could produce an overestimate of the distribution of sexually selected chemosensory traits. For example, chemicals only documented to elicit responses in the opposite sex might turn out to guide sex or species recognition but not be shaped by sexual selection20 (but see ref. 59 regarding possible issues with the distinction between species recognition and mate choice).
Our review took a broad view of the chemosensory traits that guide sexual interactions. Although our primary focus was on signaling traits, we also included some chemosensory stimuli that may not fit classic definitions of signals (Box 1). In many cases, additional research is needed to determine whether a chemosensory stimulus is a signal that evolved for communication or a cue that elicits a response in receivers but did not evolve for that purpose. Indeed, traits detected by all sensory systems are often difficult to discretely categorize as cues versus signals48. For example, female goldfish (Carassius auratus) excrete hormonal metabolites via urine that attract males60; these molecules were initially considered cues that males spied on61, but subsequent research revealed females control their release of urine to facilitate communication62. In other cases, sexual chemosensory traits strain the classic definition of signals. For example, male salamanders (Plethodon shermani) release pheromone proteins that increase courtship receptivity after being smelled by females63; male frogs (Rana temporaria) produce closely related proteins suspected to also increase female receptivity but deliver them directly into females via spiny nuptial pads rather than releasing them into the environment64. Although the proteins transferred into female frogs may not fit classic definitions of a signal as they are not sensed as an external stimulus, they conceivably evolved via similar selective mechanisms and, in our opinion, are relevant to our review. We also noted studies on chemosensory traits that guide interactions between gametes (e.g., sperm chemotaxis) as they are similar targets of sexual selection in diverse animals65, though we only note these in our literature review if we were unable to find examples of chemosensory traits that guide interactions between individuals. Lastly, we did not distinguish between the various mechanisms involved in the detection of chemical traits (e.g., olfaction versus taste)24. As discussed above, a flexible and inclusive approach was necessary to support a discussion on sexual selection across the diversity of Animalia.
Potential for sexual selection on chemosensory traits across Animalia
Potential for sexual selection on chemosensory traits spans across the Tree of Life (Figs. 1 and 2 and Supplementary Data 1 and 2). Altogether, our literature review included n = 319 studies on the potential for sexual selection on chemosensory traits. Studies on animals from 10 of 34 phyla provide evidence of sexual selection on chemosensory traits that meets the criteria set by Andersson1. Among these phyla, 9 include animals with traits involved in communication (e.g., chemosensory-based mate preferences) and 1 (Echinodermata) includes animals with traits that guide gamete interactions (cryptic mate choice). An additional 6 phyla possess chemosensory traits that were found to guide interactions between mates or competitors (5 at the individual level, 1 at the gamete level), and therefore may be under sexual selection. However, a direct link between variation in these traits and mating success has yet to be established. Finally, sexual chemosensory traits have been suggested for 7 more phyla, though direct evidence for sexual communication remains lacking. Although our review focused on the animal kingdom, fungi66, bacteria67, protists68, and plants69 also have reproductive chemosensory traits that may be shaped by sexual selection. Importantly, our failure to find evidence for sexual selection on chemosensory traits in many taxa should not be interpreted as evidence against sexual selection in those taxa. Indeed, the literature shows evidence (Category 1) or potential indications (Category 2 or 3) of sexual selection on chemosensory traits across the Tree of Life and in most animal phyla (23 of 34).
Fig. 1. Examples of chemosensory traits potentially under sexual selection in animals.
a Preen gland involved in olfactory signaling in birds (Swainson’s thrush, Catharus ustulatus; photo credit: Brock and Sherri Fenton). b love dart of land snail (Bradybaena pellucida) that injects allohormone into mate to increase paternity (photo credit: Kazuki Kimura). c sensory rays of male nematode (Caenorhabditis elegans) used in chemosensation of mates (image adapted with permission from ref. 176). d inflatable scent gland of male tiger moths (Creatonotus gangis; photo credit: Darren5907/Alamy). e pulse of urine released by dominant male cichlids (Astatotilapia burtoni; image adapted with permission from ref. 177). f Male white rhino (Ceratotherium simum) kicking dung to spread chemical signal (AfriPics.com/Alamy). g Protruding teeth and swollen lips in frogs (Plectrohyla sagorum) that males use to scratch females and deliver putative pheromones (reproduced from ref. 137, BMC).
Fig. 2. Evidence and potential for sexual selection on chemosensory traits across animal phyla.
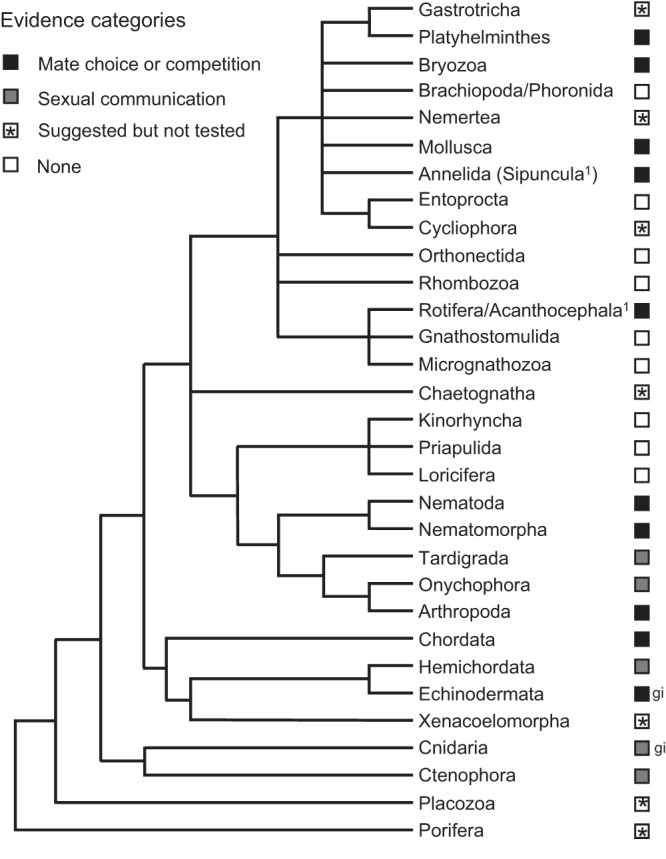
Black-filled boxes indicate phyla for which evidence suggests chemosensory traits guide mate choice or competition. Gray-filled boxes indicate phyla for which chemosensory interactions between mates or sexual rivals have been documented. Asterisks indicate phyla for which chemosensory interactions between mates or sexual rivals have been suggested but remain without direct empirical support. gi indicates the trait with the strongest support in a phylum was involved in gamete interactions. Phylogeny based on ref. 178. 1Sipuncula and Acanthocephala were considered distinct phyla in the taxonomy followed for our literature review49 but are recognized in ref. 178 as part of Annelida and Rotifera. Sexual chemical communication has been documented in Acanthocephala but remains untested in Sipuncula.
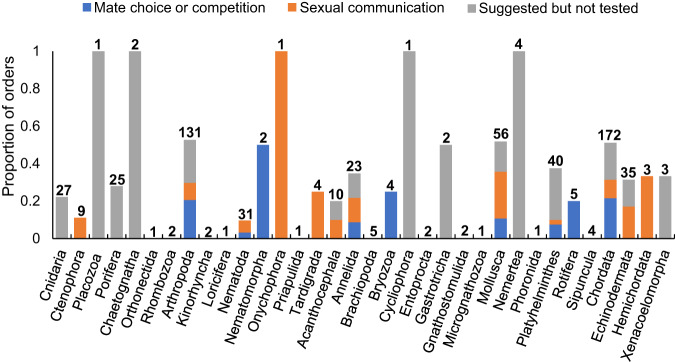
Albeit relatively broad, the taxonomic distribution of evidence for sexual selection on chemosensory traits remains shallow, with most phyla represented by relatively few classes or orders (Fig. 3 and Supplementary Data 1). In phyla with chemosensory traits that meet the criteria for sexual selection (Category 1), only Annelida, Bryozoa, Nematomorpha, and Platyhelminthes had evidence from more than half of classes, and these four phyla each have <3 recognized classes. However, most classes (≥50%) in phyla Cnidaria, Ctenophora, Porifera, Arthropoda, Nematoda, Tardigrada, Annelida, Bryozoa, Mollusca, Nemertea, Platyhelminthes, Rotifera, Chordata, Echinodermata, and Hemichordata have at least some potential indications (Category 2 or 3) of sexual selection on chemosensory traits, even if the indications are only suggestions based on indirect evidence. Phyla Placozoa, Chaetognatha, Nematomorpha, Onychophora, Cycliophora, and Gastrotricha showed potential indications of sexually selected chemosensory traits but have ≤1 recognized classes (see ref. 49; Supplementary Data 1), making the percentage of represented classes impossible or of little use to calculate. Finally, Acanthocephala and Xenacoelomorpha had indications of sexually selected chemosensory traits in <50% of classes.
Fig. 3. Proportion of orders with evidence or potential for sexual selection on chemosensory traits across phyla.
Studies are categorized according to whether they (1) report evidence that chemosensory traits guide mate choice or competition, (2) report evidence for sexual chemical communication, or (3) suggest sexual chemical communication without direct evidence. Numbers above bars indicate the number of orders within each phyla, based upon ref. 49. For clarity, only traits that mediate interactions between mates or competitors (not gametes) are included, though cryptic mate choice via gamete chemosensation has been shown or suggested in many orders of Echinodermata and Cnidaria (Supplementary Data 1).
In many taxa, the lack of evidence for sexual selection on chemosensory traits likely represents a lack of data rather than true absence. Indeed, the animal kingdom is rife with poorly understood life-forms. Phyla Loricifera, Cycliophora, and Micrognathozoa were only discovered in the last several decades70–72. Furthermore, sexual reproduction has not yet been confirmed in Micrognathozoa73 and was only recently documented in Placozoa74. The monotypic cnidarian Polypodium hydriforme, a parasite found only in eggs of a small order of fishes (Acipenseriformes), may be exclusively parthenogenetic75 and therefore not subject to sexual selection19. Coelacanths, one of only five classes of chordates for which indications of sexual selection on chemosensory traits are lacking, were thought long extinct before their rediscovery in 193876; today, basic questions about their reproductive behavior, such as how they interact with mates, remain unanswered77. Improving our basic understanding of some clades will almost certainly unveil more evidence of sexual selection on chemosensory traits.
Potential targets of sexual selection on chemosensory traits across Animalia
Sexual selection acts upon a diverse collection of chemosensory traits spanning molecules to behaviors (Table 2). Examining the specific targets of sexual selection is helpful for two primary reasons:
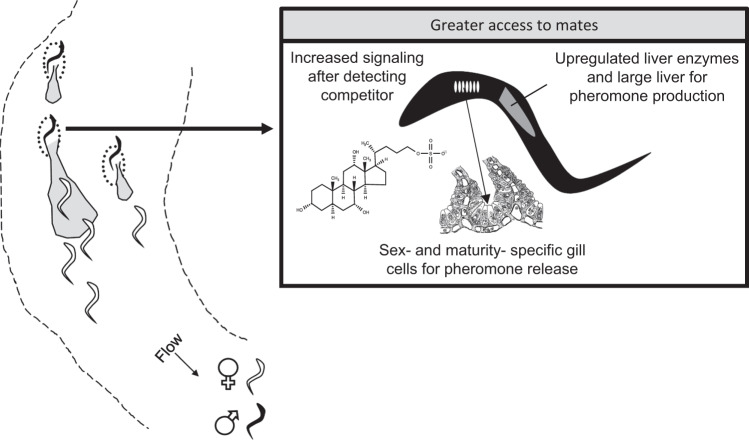
First, it shows where signatures of sexual selection might occur. By definition, sexually selected chemosensory traits influence individuals’ success at accessing mates or gametes3. This influence on mating success arises from various traits at all levels of biological organization (e.g., from cells to behaviors), not just the signal or sensory structure directly involved in sexual interactions78. For example, female preference for higher pheromone concentrations favors higher signaling rates in sea lamprey (Petromyzon marinus), but various physiological79–81 and behavioral traits82,83 that mediate pheromone production and release are likely the specific targets of sexual selection (Fig. 4). In lampreys, the traits underlying pheromone signaling show possible signatures of sexual selection, such as sexual dimorphism81 and relatively high inter-specific diversity84. Studying the various traits underlying chemical communication can be especially useful for testing potential sexual selection when the specific identity of the signal is unknown.
Fig. 4. Illustration of how sexual selection can act on various traits underlying sexual chemosensory interactions.
During spawning, female sea lamprey (Petromyzon marinus) orient towards the bile acid 3-keto petromyzonol sulfate (3kPZS). The lek-like mating system of lamprey and female preference for the more concentrated of adjacent 3kPZS plumes179 appears to generate sexual selection for upregulation of bile acid synthetic enzymes79,84, large livers (where bile acids are biosynthesized)80, and sexually dimorphic gill cells involved in 3kPZS release81. Furthermore, males increase the attractiveness of their pheromone signal by releasing more 3kPZS after exposure to a competitor83. Gill cell image adapted with permission from ref. 180.
Second, examining the targets of sexual selection can reveal the various mechanisms by which sexual selection can act upon communication across diverse taxa. Theory suggests that sexual selection acts on communication traits via several mechanisms of mate choice and competition3,6 (Table 1). Much evidence supports this theory, but the data come from relatively few animal groups1. For chemical communication, mate choice using signals that provide or indicate benefits to the choosing sex has been of particular interest85; however, other mechanisms (Table 1) of sexual selection are also important and may be more relevant for some animal groups. For example, large and elaborate sensory structures in male insects indicate a possible role of sexual selection via scramble competition (discussed below86). Indeed, most studies focus on signals that attract mates or repel competitors20 but such functions do not fully capture the diversity of sexual chemosensory traits. Below, we review the potential targets of sexual selection on chemical communication and the mechanisms by which sexual selection can act on each.
Molecular constituents
Animals use a diverse selection of molecules to interact with mates and sexual rivals24. Chemical diversity in these traits arises from differences in attributes of individual compounds, such as their class (e.g., protein vs. steroid vs. ketone)87–89, functional group90, or stereochemistry91, as well as differences in mixture constituents or proportions92. Even different concentrations of a single compound can be perceived as distinct stimuli93. High species specificity of many sexual chemical signals indicates they often diversify rapidly94. This diversity has made chemical signals an especially useful model of studying the evolution of communication94. However, the classical view on how chemical signals evolve emphasized species recognition95 and only more recently has the scope broadened to include sexual selection85,96. Nevertheless, a substantial body of evidence, largely but not exclusively from studies on insects97, shows that sexual selection can influence the molecular identity of chemical signals.
Sexual selection can act through several mechanisms to influence the identity of chemical signals. In some cases, the molecular constituents of chemical signals are closely linked to the benefits the signal provides or indicates to receivers. Insect pheromones are often molecules sequestered from needed resources85, such as the plant-derived alkaloids that deter predators and act as male sex pheromones in the moth Utetheisa ornatrix98,99. In fish, hormonal metabolites directly indicate the reproductive status of the signaler60 and peptides associated with major histocompatibility complex (MHC) molecules reflect genetic quality100. Signals can also evolve to exploit pre-existing aspects of receivers’ sensory biology without necessarily providing any benefit18, though evidence for this evolutionary mechanism of mate choice is not well-documented for chemical communication101. A few examples include prey molecules released by male beewolfs (Philanthus triangulum) and rock lizards (Iberolacerta cyreni) to attract conspecific females searching for food102,103 and a bile acid released by male sea lamprey to mimic a larval cue used to navigate to preferred habitats104. Though the above studies focus on interactions between mates, similar mechanisms act upon chemical signaling between rivals; male goldfish (Carassius auratus) mediate aggressive interactions using reproductive hormones that are likely related to their reproductive status105,106 and male Drosophila manipulate competitors using an anti-aphrodisiac pheromone that exploits a pre-existing sensory bias107. Finally, sexual selection might also drive elaboration of chemical signals if adding components increases signal information content or efficiency92,94,97, though evidence that this occurs also remains limited108. Unfortunately, a poor understanding of the specific chemical structures of chemical signals in most animals limits broad inferences about the link between their molecular composition and the mechanisms of sexual selection that act upon them94.
Traits related to the production of chemical signals
Chemical signals manifest through an assortment of molecular and physiological processes, cells, and organs that are shaped by sexual selection109. Often, sexual chemical signals are produced via sex- and stage-specific upregulation of biosynthetic enzymes or transporters79,110. These molecular processes can occur in cells with other functions or in sexually dimorphic cells that likely evolved to support chemosensory interactions between mates or sexual rivals81,111. Similarly, cells involved in chemical signaling can be dispersed throughout the body, localized to common organs, or organized into specialized glandular tissues109. Pheromone and scent glands likely shaped by sexual selection are often noted in insects and mammals24 but also occur in fish112, birds113, anurans114, reptiles115, non-insect arthropods (centipedes)58, onychophorans116, nemerteans117, gastrotrichs118, and platyhelminths119. Common organs can also have adaptations for producing or emitting sexual chemical signals (e.g., large livers80, hypertrophic urinary bladders120). Adaptations for sexual signaling in common organs, albeit more cryptic than those associated with specialized glands, may be especially widespread given many chemical signals are released via routes linked to common physiological processes (e.g., feces, urine)24.
Traits related to the delivery of chemical signals
Sexual selection can act upon physiological and behavioral traits that mediate delivery of chemical signals. Many chemical signals consist of molecules that also have non-communicative functions92 and leak out via sexual materials121, tears122, mucous123, feces124, urine125, and respiratory waste126. Release via seemingly unspecialized routes again points to selection for signals with direct links to the physiological status of signalers. As discussed above, chemicals that leak out may act as cues that receivers evolved to detect but not signals that involve any adaptations in releasers (Box 1). However, even unspecialized routes of release often involve finesse that evolved for communication; for example, dominant male white rhinos (Ceratotherium simum) defecate more often than females or nonterritorial males and kick their dung to increase the signal’s active space127,128. Controlling when and where to signal may allow signalers to deceive receivers with chemical signals that seem otherwise difficult to fake129. Alternatively, tactical signal delivery may arise via selection for signal efficiency15,130. For example, male swordtails (Xiphophorus birchmanni) urinate more often in the presence of females and orient themselves upstream of females when courting, presumably to help deliver chemical signals131. Analogous non-behavioral traits can also facilitate signal delivery; some animals produce proteins that bind chemical signals (e.g., major urinary proteins in mice) to (1) slow evaporation of the molecule, thereby extending signal duration132 or (2) release the chemical only upon arrival to the sensory organ according to its local chemical environment (e.g., pH)133.
Some sexual chemicals are delivered directly into the body without being detected by receiver’s external sensory systems (allohormones; Box 1)134. For example, males in some plethodontid salamanders open females’ skin using hypertrophic teeth and then rub their mental gland on the wound to inject directly into the blood a pheromone that increases female receptivity78. In addition to injection through skin using various methods135–137, sexual chemicals can be delivered directly through insemination138 or consumption via nuptial gifts139. Importantly, chemical traits delivered directly into receivers’ bodies are generally not considered signals, which are detected by receivers’ sensory systems134,140. However, the line between these chemicals and conventional signals can be blurry, especially in closely related species that use the same class of molecules to interact with mates but differ in whether they deliver the chemicals to sensory systems or directly into the body78. Regardless, chemicals that bypass sensory systems are important targets of sexual selection134. Across various animal phyla, including commonly studied arthropods141 but also hermaphroditic annelids, platyhelminths, and mollusks135,136,142,143, chemicals directly transferred to females prevent digestion or disposal of sperm, suppress future mating, and ultimately bias paternity. In an interesting twist, chemicals transferred to mates can subsequently be or modify chemical signals for competitors144; for example, male moths mark females with an anti-aphrodisiac that deters other males145 and inject females with substances that inhibit them from producing pheromones that attract males146. Post-copulatory gamete competition135,147 and sexual conflict136,148 are usually suggested as sources of selection on these traits, but in some cases signal efficiency137 and mate choice may also play a role134.
Traits related to the detection of chemical signals
Animals detect chemical signals using a series of molecular, physiological, and behavioral traits that are often sexually dimorphic. Darwin hypothesized that sexually dimorphic sensory capabilities arise via sexual selection when males bear the primary burden of mate search4,149. In the 150 years since, research on sexual selection has focused more on signals and attributes of receiver sensory systems that influence the evolution of signals. Nevertheless, sexual dimorphism has been reported in many taxa and for nearly all levels of chemosensory detection (Table 2), ranging from the behaviors involved in sampling chemical stimuli150 to the neural circuits involved in processing chemical stimuli151,152. Importantly, sexual dimorphism in sensory traits can arise from differences in the ecology or biology of males and females rather than competition for mates149,153,154. However, empirical evidence, especially from arthropods, supports Darwin’s hypothesis that sexual selection via scramble competition favors greater chemosensory capacity in males86,155–158. Similar selection on detection of chemicals from potential mates may also be important in broadcast spawners, some of which release gametes after exposure to chemicals in the sexual fluids of mates or competitors159 and have higher reproductive success when given the first opportunity for fertilization160. Interestingly, sexual selection may also act on chemosensory capabilities via mate choice; in moths, females may choose high-quality mates by releasing minute quantities of pheromone only detectable by males with the most sensitive olfactory systems149. Sexual selection on chemosensory detection traits is less studied than sexual selection on chemical signals but may be especially important in many animals that use chemical information during scramble competition for mates or fertilizations157.
Conclusions
Decades of empirical and theoretical research have focused on sexual selection as a major evolutionary driver of animal biodiversity. Signaling traits have been at the center of this work, as they are diverse, often appear extreme, and can lead to speciation when divergent preferences generate assortative mating. However, sexual selection as a broad and versatile evolutionary force across higher taxonomic levels of animals remains surprisingly undertested as most studies on sexual selection12,13, animal behavior56, and chemical communication94 focus on very few clades. Furthermore, most research on sexual selection has focused on communication via vision and hearing, which most animal phyla lack. In this Perspective, we reviewed the evidence for sexual selection on signaling traits across Animalia as a whole, with particular emphasis on chemosensory traits. Our review illustrates two especially important and related points, which we discuss below.
First, the broad scope of theory around sexual selection and animal signals stands in sharp contrast to the limited higher-level taxonomic distribution of supporting empirical studies. Clearly, extensive evidence supports sexual selection as a powerful evolutionary force on signaling traits in many species within a few clades1,5,6, especially some arthropods and chordates12,13,56. After searching for evidence of sexual selection on chemosensory traits across all animal phyla, classes, and orders, we found studies that meet established criteria of sexual selection1 in 10 of 34 animal phyla, and studies that report possible indications of sexual selection on chemosensory traits in an additional 13 phyla. Despite the clear potential for sexual selection on chemical signaling traits across diverse taxa, additional work is needed even in taxa for which current evidence meets established criteria of sexual selection1. Foremost is a basic need for more direct tests of sexual selection in most taxa; many studies we found only scratched the surface of how chemosensory traits could affect mate choice or competition. Even when traits clearly affect mate choice or competition, the actual strength of sexual selection on them depends on various deterministic (e.g., operational sex ratio) and random processes that underlie variation in mating success161. Our review indicates sexual selection could play a common role in the evolution of chemical communication but highlights the need for research that better reflects the diversity of animals (see Box 2).
The need for research that better represents all animals raises our second major point: chemosensory traits are arguably the primary (potential) target of sexual selection on communication when considering Animalia as a whole. A basic implication of this point is that chemosensation should be a key focus of research on sexual selection. This will ensure we do not underestimate the role of sexual selection in Animalia or predicate concepts about the prevailing mechanisms or consequences of sexual selection on sensory systems that may not be representative of many animals. Unfortunately, chemoreception is also among the least studied channels of communication12, due, in part, to the challenge of identifying the molecules that make up signals101 and the cryptic nature of most chemical signals162. Human biases further inhibit research on chemoreception162, and are exemplified by the superlatives used to characterize the traits that inspired the theory of sexual selection and continue to hold the attention of evolutionary biologists; what is an ‘extreme’ or ‘striking’ chemical signal? Emphasis on chemosensation is key to understanding sexual selection as a potentially universal and potent evolutionary force on animal communication.
Many interesting questions about sexual selection on animal communication remain unanswered20,163. We suggest one of the most fundamental of these is whether sexual selection acts as a salient evolutionary force on communication across Animalia. Admittedly, determining when this question has been answered is challenging. Nevertheless, pursuing the answer will reveal if and how the mechanisms and consequences of sexual selection differ across animals. This information is critical to develop a fuller understanding of how Earth’s animal diversity arose and to conserve this diversity in the face of rapid global change164.
Box 2 Looking forward.
Recommendations. We offer several recommendations that could help the study of sexual selection on communication to better span across Animalia.
Increase taxonomic diversity in studies of sexual selection on communication. Prioritizing taxa for future study could depend on the specific research questions being addressed, but we hope our literature review (Suppl. Data 1) will be a useful guide for identifying key knowledge gaps.
Consider the many potential targets of sexual selection. The molecular, physiological, and behavioral scaffolding underlying chemical communication can be particularly useful for studying sexual selection183 when the identity of a signal remains unknown or when the mating behavior of a species is poorly understood.
Leverage new and developing techniques. New and developing technology will likely accelerate research on chemical signals. For example, metabolomics—the global analysis of small molecules—shows promise as a powerful tool to study the molecular basis of chemosensory interactions184. Similarly, we expect other omic techniques (genomics, transcriptomics) will shed new light on the molecular and physiological processes underlying sexual selection on chemical communication.
Expand the community of researchers studying sexual selection. Achieving wider taxonomic representation is likely to rely, in large part, on researchers with specific taxonomic or technological expertise who currently focus on other research questions. We call for a broader community of scientists to study sexual selection on communication, and especially invite the attention of biologists studying animals that are not well represented in the field (see Figs. 2 and 3, Supplementary Data 1).
Future directions. Better representation across Animalia will support macroevolutionary studies that can test fundamental hypotheses about sexual selection. For example, Wiens and Tuschhoff20 provide an insightful discussion on how macroevolutionary studies could help explain the diversity of sexually selected signals and weapons. Below, we outline a few additional examples:
Sexual selection and diversification of chemosensory receptors. Chemoreceptors are encoded by some of the fastest evolving and largest gene families in the metazoan genome185 and in some species evolved under sexual selection186,187. Although chemoreceptor repertoires are well-characterized primarily in vertebrates and insects188, this list is likely to grow rapidly with the number of animal genomes sequenced189–194, putting in reach an understanding of how sexual selection shapes receiver evolution at a molecular level but macroevolutionary scale.
Sexual selection and nervous system evolution. The brain is a key player in the process of sexual selection and, for example, complicates sexual decisions by integrating inputs related to nonsexual tasks195. However, animals differ considerably in the complexity of their nervous systems, both among196 and within phyla197. How does the role of sexual selection on communication track the evolution of the nervous system in animals?
Sexual selection and speciation. The role of sexual selection in speciation has been the focus of a vibrant discussion for decades7–11. However, empirical tests of speciation by sexual selection have focused largely on arthropods and chordates9, which are among the most taxonomically rich phyla198. Greater taxonomic diversity in research on sexual selection will likely enrich discussions on the role of sexual selection in speciation.
Supplementary information
Acknowledgements
The authors were supported with funding from the Great Lakes Fishery Commission and the Great Lakes Fishery Trust. Thanks to Skye Fissette for reviewing several drafts of the paper and helping create Fig. 4, and to Leanne Grieves for providing the photo of a preen gland. Thomas Blankers and two anonymous reviewers provided insightful suggestions that greatly improved the manuscript.
Author contributions
The paper was conceived by T.J.B. and W.L., drafted by T.J.B., and revised by T.J.B. and W.L.
Peer review
Peer review information
Communications Biology thanks Ignacio Escalante, Thomas Blankers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Manuel Breuer.
Data availability
Our paper presents no new data. The results of the literature review are provided as Supplementary data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-023-05572-w.
References
- 1.Andersson, M. Sexual Selection (Princeton University Press, 1994).
- 2.Girard MB, Endler JA. Peacock spiders. Curr. Biol. 2014;24:R588–R590. doi: 10.1016/j.cub.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Shuker DM, Kvarnemo C. The definition of sexual selection. Behav. Ecol. 2021;32:781–794. doi: 10.1093/beheco/arab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwin, C. The Descent of Man, and Selection in Relation to Sex (Princeton University Press, 1871).
- 5.Andersson M, Iwasa Y. Sexual selection. Trends Ecol. Evol. 1996;11:53–58. doi: 10.1016/0169-5347(96)81042-1. [DOI] [PubMed] [Google Scholar]
- 6.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol. Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Lande R. Models of speciation by sexual selection on polygenic traits. Proc. Natl. Acad. Sci. USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West-Eberhard MJ. Sexual selection, social competition, and speciation. Q. Rev. Biol. 1983;58:155–183. doi: 10.1086/413215. [DOI] [Google Scholar]
- 9.Mendelson TC, Safran RJ. Speciation by sexual selection: 20 years of progress. Trends Ecol. Evol. 2021;36:1153–1163. doi: 10.1016/j.tree.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer HM, Ruxton GD. Signal diversity, sexual selection, and speciation. Annu. Rev. Ecol. Evol. Syst. 2015;46:573–592. doi: 10.1146/annurev-ecolsys-112414-054158. [DOI] [Google Scholar]
- 11.Servedio MR, Boughman JW. The role of sexual selection in local adaptation and speciation. Annu. Rev. Ecol. Evol. Syst. 2017;48:85–109. doi: 10.1146/annurev-ecolsys-110316-022905. [DOI] [Google Scholar]
- 12.Coleman SW. Taxonomic and sensory biases in the mate-choice literature: there are far too few studies of chemical and multimodal communication. Acta Ethol. 2009;12:45–48. doi: 10.1007/s10211-008-0050-5. [DOI] [Google Scholar]
- 13.Zuk M, Garcia-Gonzalez F, Herberstein ME, Simmons LW. Model systems, taxonomic bias, and sexual selection: beyond Drosophila. Annu. Rev. Entomol. 2014;59:321–338. doi: 10.1146/annurev-ento-011613-162014. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MJ, Fox JH, Wilczynski W, Rand AS. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature. 1990;343:66–67. doi: 10.1038/343066a0. [DOI] [PubMed] [Google Scholar]
- 15.Endler JA. Signals, signal conditions, and the direction of evolution. Am. Nat. 1992;139:S125–S153. doi: 10.1086/285308. [DOI] [Google Scholar]
- 16.Christy JH. Mimicry, mate choice, and the sensory trap hypothesis. Am. Nat. 1995;146:171–181. doi: 10.1086/285793. [DOI] [Google Scholar]
- 17.Ryan MJ, Cummings ME. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 2013;44:437–459. doi: 10.1146/annurev-ecolsys-110512-135901. [DOI] [Google Scholar]
- 18.Rodd FH, Hughes KA, Grether GF, Baril CT. A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc. R. Soc. B Biol. Sci. 2002;269:475–481. doi: 10.1098/rspb.2001.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hare RM, Simmons LW. Sexual selection and its evolutionary consequences in female animals. Biol. Rev. Camb. Philos. Soc. 2019;94:929–956. doi: 10.1111/brv.12484. [DOI] [PubMed] [Google Scholar]
- 20.Wiens JJ, Tuschhoff E. Songs versus colours versus horns: what explains the diversity of sexually selected traits? Biol. Rev. Camb. Philos. Soc. 2020;95:847–864. doi: 10.1111/brv.12593. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson D-E. The diversity of eyes and vision. Annu. Rev. Vis. Sci. 2021;7:19–41. doi: 10.1146/annurev-vision-121820-074736. [DOI] [PubMed] [Google Scholar]
- 22.Webster, D. B. Epilogue to the conference on the evolutionary biology of hearing. In The Evolutionary Biology of Hearing (eds Webster, D. B. et al.) 787–793 (Springer, 1992).
- 23.Iliff AJ, et al. The nematode C. elegans senses airborne sound. Neuron. 2021;109:3633–3646. e3637. doi: 10.1016/j.neuron.2021.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt, T. D. Pheromones and Animal Behavior: Chemical signals and Signatures (Cambridge University Press, 2014).
- 25.Haldane JBS. Animal communication and the origin of human language. Sci. Prog. 1955;43:385–401. [Google Scholar]
- 26.Hildebrand JG. Analysis of chemical signals by nervous systems. Proc. Natl. Acad. Sci. USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegde M, et al. Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J. Bacteriol. 2011;193:768–773. doi: 10.1128/JB.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlesworth, J. C., Beloe, C., Watters, C. & Burns, B. P. Quorum sensing in archaea: recent advances and emerging directions. In Biocommunication of Archaea (ed. Witzany, G.) 119–132 (Springer, Cham, 2017).
- 29.Luporini P, Vallesi A, Miceli C, Bradshaw R. Chemical signaling in ciliates. J. Eukaryot. Microbiol. 1995;42:208–212. doi: 10.1111/j.1550-7408.1995.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 30.Versele M, Lemaire K, Thevelein JM. Sex and sugar in yeast: two distinct GPCR systems. EMBO Rep. 2001;2:574–579. doi: 10.1093/embo-reports/kve132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicher D. Functional and evolutionary aspects of chemoreceptors. Front. Cell. Neurosci. 2012;6:48. doi: 10.3389/fncel.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derby, C. D. Chemoreception in aquatic invertebrates. In The senses: A Comprehensive Reference (eds Fritzsch, B. & W. Meyerhof) Vol. 3, 65–84 (Elsevier, Academic Press, 2020).
- 33.Strausfeld NJ, Hildebrand JG. Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 1999;9:634–639. doi: 10.1016/S0959-4388(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 34.Mollo E, et al. Taste and smell: a unifying chemosensory theory. Q. Rev. Biol. 2022;97:69–94. doi: 10.1086/720097. [DOI] [Google Scholar]
- 35.Sasson DA, Jacquez AA, Ryan JF. The ctenophore Mnemiopsis leidyi regulates egg production via conspecific communication. BMC Ecol. 2018;18:1–10. doi: 10.1186/s12898-018-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endler JA. Some general comments on the evolution and design of animal communication systems. Philos. Trans. R. Soc. B Biol. Sci. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- 37.Sarà M. Sessile macrofauna and marine ecosystem. Ital. J. Zool. 1986;53:329–337. [Google Scholar]
- 38.Klepal W, Barnes H, Munn E. The morphology and histology of the cirripede pemis. J. Exp. Mar. Biol. Ecol. 1972;10:243–265. doi: 10.1016/0022-0981(72)90075-5. [DOI] [Google Scholar]
- 39.Gaston KJ. Nighttime ecology: the “nocturnal problem” revisited. Am. Nat. 2019;193:481–502. doi: 10.1086/702250. [DOI] [PubMed] [Google Scholar]
- 40.Hölker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010;25:681–682. doi: 10.1016/j.tree.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Bennie JJ, Duffy JP, Inger R, Gaston KJ. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. USA. 2014;111:13727–13732. doi: 10.1073/pnas.1216063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grassle JF, Maciolek NJ. Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am. Nat. 1992;139:313–341. doi: 10.1086/285329. [DOI] [Google Scholar]
- 43.Orgiazzi, A., Bardgett, R. D. & Barrios, E. Global Soil Biodiversity Atlas (European Commission, 2016).
- 44.Warrant E. Vision in the dimmest habitats on earth. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2004;190:765–789. doi: 10.1007/s00359-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 45.Barton R, Purvis A, Harvey P. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philos. Trans. R. Soc. B Biol. Sci. 1995;348:381–392. doi: 10.1098/rstb.1995.0076. [DOI] [PubMed] [Google Scholar]
- 46.Healy S, Guilford T. Olfactory‐bulb size and nocturnality in birds. Evolution. 1990;44:339–346. doi: 10.2307/2409412. [DOI] [PubMed] [Google Scholar]
- 47.Wagner H-J. Sensory brain areas in three families of deep-sea fish (slickheads, eels and grenadiers): comparison of mesopelagic and demersal species. Mar. Biol. 2002;141:807–817. doi: 10.1007/s00227-002-0892-8. [DOI] [Google Scholar]
- 48.Bradbury, J. W. & Vehrencamp, S. L. Principles of Animal Communication (Sinauer Associates, 1998).
- 49.Ruggiero MA, et al. A higher level classification of all living organisms. PLoS ONE. 2015;10:e0119248. doi: 10.1371/journal.pone.0119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bone LW. Reproductive chemical communication of helminths. I. Platyhelminthes. Int. J. Invert. Reprod. 1982;5:261–268. doi: 10.1080/01651269.1982.10553477. [DOI] [Google Scholar]
- 51.Bone LW. Reproductive chemical communication of helminths. II. Aschelminthes. Int. J. Invert. Reprod. 1982;5:311–321. doi: 10.1080/01651269.1982.10553484. [DOI] [Google Scholar]
- 52.Grieves LA, et al. Olfactory camouflage and communication in birds. Biol. Rev. Camb. Philos. Soc. 2022;97:1193–1209. doi: 10.1111/brv.12837. [DOI] [PubMed] [Google Scholar]
- 53.Brown, R. E. & Macdonald, D. W. Social Odours in Mammals (Oxford University Press, 1985).
- 54.Mayer, M. S. & McLaughlin, J. R. Handbook of Insect Pheromones and Sex Attractions (CRC Press, 1991).
- 55.Dougherty LR. Designing mate choice experiments. Biol. Rev. Camb. Philos. Soc. 2020;95:759–781. doi: 10.1111/brv.12586. [DOI] [PubMed] [Google Scholar]
- 56.Rosenthal MF, Gertler M, Hamilton AD, Prasad S, Andrade MC. Taxonomic bias in animal behaviour publications. Anim. Behav. 2017;127:83–89. doi: 10.1016/j.anbehav.2017.02.017. [DOI] [Google Scholar]
- 57.Westermann B, Beuerlein K. Y-maze experiments on the chemotactic behaviour of the tetrabranchiate cephalopod Nautilus pompilius (Mollusca) Mar. Biol. 2005;147:145–151. doi: 10.1007/s00227-005-1555-3. [DOI] [Google Scholar]
- 58.Sombke A, Müller CH. When SEM becomes a deceptive tool of analysis: the unexpected discovery of epidermal glands with stalked ducts on the ultimate legs of geophilomorph centipedes. Front. Zool. 2021;18:1–19. doi: 10.1186/s12983-021-00402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendelson TC, Shaw KL. The (mis) concept of species recognition. Trends Ecol. Evol. 2012;27:421–427. doi: 10.1016/j.tree.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Stacey, N. Hormonally derived pheromones in teleost fishes. In Fish Pheromones and Related Cues (eds Sorensen, P. W. & B. D. Wisenden) 33–88 (John Wiley & Sons, 2015).
- 61.Sørensen P, Scott A. The evolution of hormonal sex pheromones in teleost fish: poor correlation between the pattern of steroid release by goldfish and olfactory sensitivity suggests that these cues evolved as a result of chemical spying rather than signal specialization. Acta Physiol. Scand. 1994;152:191–205. doi: 10.1111/j.1748-1716.1994.tb09799.x. [DOI] [PubMed] [Google Scholar]
- 62.Appelt CW, Sorensen PW. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim. Behav. 2007;74:1329–1338. doi: 10.1016/j.anbehav.2007.02.032. [DOI] [Google Scholar]
- 63.Houck L, et al. A new vertebrate courtship pheromone, PMF, affects female receptivity in a terrestrial salamander. Anim. Behav. 2007;73:315–320. doi: 10.1016/j.anbehav.2006.07.008. [DOI] [Google Scholar]
- 64.Willaert B, et al. Frog nuptial pads secrete mating season-specific proteins related to salamander pheromones. J. Exp. Biol. 2013;216:4139–4143. doi: 10.1242/jeb.086363. [DOI] [PubMed] [Google Scholar]
- 65.Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150541. doi: 10.1098/rstb.2015.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson CL, Hartwell LH. Courtship in S. cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell. 1990;63:1039–1051. doi: 10.1016/0092-8674(90)90507-B. [DOI] [PubMed] [Google Scholar]
- 67.Dunny GM, Brown BL, Clewell DB. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gillard J, et al. Metabolomics enables the structure elucidation of a diatom sex pheromone. Angew. Chem. Int. Ed. 2013;52:854–857. doi: 10.1002/anie.201208175. [DOI] [PubMed] [Google Scholar]
- 69.Frenkel J, Vyverman W, Pohnert G. Pheromone signaling during sexual reproduction in algae. Plant J. 2014;79:632–644. doi: 10.1111/tpj.12496. [DOI] [PubMed] [Google Scholar]
- 70.Funch P, Kristensen RM. Cycliophora is a new phylum with affinities to Entoprocta and Ectoprocta. Nature. 1995;378:711–714. doi: 10.1038/378711a0. [DOI] [Google Scholar]
- 71.Kristensen RM. Loricifera, a new phylum with Aschelminthes characters from the meiobenthos. Z. f.ür. zoologische Systematik und Evolutionsforschung. 1983;21:163–180. doi: 10.1111/j.1439-0469.1983.tb00285.x. [DOI] [Google Scholar]
- 72.Kristensen R, Funch P. Micrognathozoa: a new class with complicated jaws like those of Rotifera and Gnathostomulida. J. Morphol. 2000;246:1–49. doi: 10.1002/1097-4687(200010)246:1<1::AID-JMOR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 73.Sørensen, M.V. & Kristensen, R. M. Micrognathozoa. In Gastrotricha and Gnathifera (ed. Schmidt-Rhaesa, A.) Vol. 3, 197–216 (De Gruyter, 2014).
- 74.Schierwater B, DeSalle R. Placozoa. Curr. Biol. 2018;28:R97–R98. doi: 10.1016/j.cub.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 75.Raikova, E. V. Life cycle, cytology, and morphology of Polypodium hydriforme, a coelenterate parasite of the eggs of acipenseriform fishes. J. Parasitol. 80, 1–22 (1994). [PubMed]
- 76.Smith JLB. A living coelacanthid fish from South Africa. Nature. 1939;143:748–750. doi: 10.1038/143748a0. [DOI] [Google Scholar]
- 77.Lampert KP, et al. Single-male paternity in coelacanths. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms3488. [DOI] [PubMed] [Google Scholar]
- 78.Watts RA, et al. Stabilizing selection on behavior and morphology masks positive selection on the signal in a salamander pheromone signaling complex. Mol. Biol. Evol. 2004;21:1032–1041. doi: 10.1093/molbev/msh093. [DOI] [PubMed] [Google Scholar]
- 79.Brant CO, Chung-Davidson Y-W, Li K, Scott AM, Li W. Biosynthesis and release of pheromonal bile salts in mature male sea lamprey. BMC Biochem. 2013;14:1–11. doi: 10.1186/1471-2091-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buchinger TJ, et al. Increased pheromone signaling by small male sea lamprey has distinct effects on female mate search and courtship. Behav. Ecol. Sociobiol. 2017;71:1–8. doi: 10.1007/s00265-017-2384-3. [DOI] [Google Scholar]
- 81.Siefkes MJ, Scott AP, Zielinski B, Yun S-S, Li W. Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biol. Reprod. 2003;69:125–132. doi: 10.1095/biolreprod.102.014472. [DOI] [PubMed] [Google Scholar]
- 82.Fissette SD, et al. Diel patterns of pheromone release by male sea lamprey. Integr. Comp. Biol. 2021;61:1795–1810. doi: 10.1093/icb/icab190. [DOI] [PubMed] [Google Scholar]
- 83.Fissette SD, Bussy U, Huerta B, Buchinger TJ, Li W. Evidence that male sea lamprey increase pheromone release after perceiving a competitor. J. Exp. Biol. 2020;223:jeb226647. doi: 10.1242/jeb.226647. [DOI] [PubMed] [Google Scholar]
- 84.Buchinger TJ, et al. Intra-and interspecific variation in production of bile acids that act as sex pheromones in lampreys. Physiol. Biochem. Zool. 2019;92:463–472. doi: 10.1086/705278. [DOI] [PubMed] [Google Scholar]
- 85.Johansson BG, Jones TM. The role of chemical communication in mate choice. Biol. Rev. Camb. Philos. Soc. 2007;82:265–289. doi: 10.1111/j.1469-185X.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 86.Jayaweera A, Barry KL. Male antenna morphology and its effect on scramble competition in false garden mantids. Sci. Nat. 2017;104:1–9. doi: 10.1007/s00114-017-1494-0. [DOI] [PubMed] [Google Scholar]
- 87.Rollmann SM, Houck LD, Feldhoff RC. Proteinaceous pheromone affecting female receptivity in a terrestrial salamander. Science. 1999;285:1907–1909. doi: 10.1126/science.285.5435.1907. [DOI] [PubMed] [Google Scholar]
- 88.Yoshida M, Murata M, Inaba K, Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA. 2002;99:14831–14836. doi: 10.1073/pnas.242470599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeeck E, Hardege J, Bartels‐Hardege H, Wesselmann G. Sex pheromone in a marine polychaete: determination of the chemical structure. J. Exp. Zool. 1988;246:285–292. doi: 10.1002/jez.1402460308. [DOI] [Google Scholar]
- 90.Li K, Buchinger TJ, Li W. Discovery and characterization of natural products that act as pheromones in fish. Nat. Prod. Rep. 2018;35:501–513. doi: 10.1039/C8NP00003D. [DOI] [PubMed] [Google Scholar]
- 91.Mori K. Significance of chirality in pheromone science. Bioorg. Med. Chem. 2007;15:7505–7523. doi: 10.1016/j.bmc.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 92.Steiger S, Schmitt T, Schaefer HM. The origin and dynamic evolution of chemical information transfer. Proc. R. Soc. B Biol. Sci. 2011;278:970–979. doi: 10.1098/rspb.2010.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaae R, Shorey H, Gaston LK. Pheromone concentration as a mechanism for reproductive isolation between two lepidopterous species. Science. 1973;179:487–488. doi: 10.1126/science.179.4072.487. [DOI] [PubMed] [Google Scholar]
- 94.Symonds MR, Elgar MA. The evolution of pheromone diversity. Trends Ecol. Evol. 2008;23:220–228. doi: 10.1016/j.tree.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Löfstedt C. Moth pheromone genetics and evolution. Philos. Trans. R. Soc. B Biol. Sci. 1993;340:167–177. doi: 10.1098/rstb.1993.0055. [DOI] [Google Scholar]
- 96.De Pasqual C, Groot AT, Mappes J, Burdfield-Steel E. Evolutionary importance of intraspecific variation in sex pheromones. Trends Ecol. Evol. 2021;36:848–859. doi: 10.1016/j.tree.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Steiger S, Stökl J. The role of sexual selection in the evolution of chemical signals in insects. Insects. 2014;5:423–438. doi: 10.3390/insects5020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eisner T, Meinwald J. The chemistry of sexual selection. Proc. Natl. Acad. Sci. USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- 100.Milinski M, et al. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl. Acad. Sci. USA. 2005;102:4414–4418. doi: 10.1073/pnas.0408264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yohe LR, Brand P. Evolutionary ecology of chemosensation and its role in sensory drive. Curr. Zool. 2018;64:525–533. doi: 10.1093/cz/zoy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herzner G, Schmitt T, Linsenmair KE, Strohm E. Prey recognition by females of the European beewolf and its potential for a sensory trap. Anim. Behav. 2005;70:1411–1418. doi: 10.1016/j.anbehav.2005.03.032. [DOI] [Google Scholar]
- 103.Rodríguez-Ruiz G, López P, Martín J. Possible reproductive benefits to female Carpetan rock lizards of pre-sensory bias towards chemical signals. Biol. J. Linn. Soc. 2019;127:787–799. doi: 10.1093/biolinnean/blz056. [DOI] [Google Scholar]
- 104.Buchinger TJ, Wang H, Li W, Johnson NS. Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc. R. Soc. B Biol. Sci. 2013;280:20131966. doi: 10.1098/rspb.2013.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poling KR, Fraser EJ, Sorensen PW. The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comp. Biochem. Physiol. B Biochem. 2001;129:645–651. doi: 10.1016/S1096-4959(01)00361-X. [DOI] [PubMed] [Google Scholar]
- 106.Sorensen P, Pinillos M, Scott A. Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. Gen. Comp. Endocrinol. 2005;140:164–175. doi: 10.1016/j.ygcen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Ng SH, et al. Pheromone evolution and sexual behavior in Drosophila are shaped by male sensory exploitation of other males. Proc. Natl. Acad. Sci. USA. 2014;111:3056–3061. doi: 10.1073/pnas.1313615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baeckens S, Martín J, García-Roa R, Van Damme R. Sexual selection and the chemical signal design of lacertid lizards. Zool. J. Linn. Soc. 2018;183:445–457. doi: 10.1093/zoolinnean/zlx075. [DOI] [Google Scholar]
- 109.Brückner A, Parker J. Molecular evolution of gland cell types and chemical interactions in animals. J. Exp. Biol. 2020;223:jeb211938. doi: 10.1242/jeb.211938. [DOI] [PubMed] [Google Scholar]
- 110.Pei X-J, et al. Modulation of fatty acid elongation in cockroaches sustains sexually dimorphic hydrocarbons and female attractiveness. PLoS Biol. 2021;19:e3001330. doi: 10.1371/journal.pbio.3001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma, P. W. & Ramaswamy, S. B. Biology and ultrastructure of sex pheromone-producing tissue. In Insect Biochemistry and Molecular Biology (eds Blomquist, G. & R. Vogt) 19–51 (Elsevier, 2003).
- 112.Barata EN, et al. Putative pheromones from the anal glands of male blennies attract females and enhance male reproductive success. Anim. Behav. 2008;75:379–389. doi: 10.1016/j.anbehav.2007.05.018. [DOI] [Google Scholar]
- 113.Whittaker DJ, Hagelin JC. Female-based patterns and social function in avian chemical communication. J. Chem. Ecol. 2021;47:43–62. doi: 10.1007/s10886-020-01230-1. [DOI] [PubMed] [Google Scholar]
- 114.Bossuyt F, et al. Multiple independent recruitment of sodefrin precursor-like factors in anuran sexually dimorphic glands. Mol. Biol. Evol. 2019;36:1921–1930. doi: 10.1093/molbev/msz115. [DOI] [PubMed] [Google Scholar]
- 115.Mason RT, Parker MR. Social behavior and pheromonal communication in reptiles. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2010;196:729–749. doi: 10.1007/s00359-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 116.Eliott S, Tait N, Briscof D. A pheromonal function for the crural glands of the onychophoran Cephalofovea tomahmontis (Onychophora: Peripatopsidae) J. Zool. 1993;231:1–9. doi: 10.1111/j.1469-7998.1993.tb05348.x. [DOI] [Google Scholar]
- 117.Roe P, Norenburg JL. Morphology and taxonomic distribution of a newly discovered feature, postero-lateral glands, in pelagic nemerteans. Hydrobiologia. 2001;456:133–144. doi: 10.1023/A:1013008723656. [DOI] [Google Scholar]
- 118.Schnier J, et al. Ultrastructure of the epidermal gland system of Tetranchyroderma suecicum Boaden, 1960 (Gastrotricha: Macrodasyida) indicates a defensive function of its exudate. Zoomorphology. 2019;138:443–462. doi: 10.1007/s00435-019-00462-4. [DOI] [Google Scholar]
- 119.Kearn G, Whittington I. Sperm transfer in monogenean (platyhelminth) parasites. Acta Parasitol. 2015;60:567–600. doi: 10.1515/ap-2015-0082. [DOI] [PubMed] [Google Scholar]
- 120.Keller-Costa T, et al. Muscular hypertrophy of urinary bladders in dominant tilapia facilitates the control of aggression through urinary signals. Behaviour. 2012;149:953–975. doi: 10.1163/1568539X-00003023. [DOI] [Google Scholar]
- 121.Zizzari ZV, et al. Love at first sniff: a spermatophore-associated pheromone mediates partner attraction in a collembolan species. Anim. Behav. 2017;124:221–227. doi: 10.1016/j.anbehav.2016.12.015. [DOI] [Google Scholar]
- 122.Haga S, et al. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 123.Johannesson K, et al. Male discrimination of female mucous trails permits assortative mating in a marine snail species. Evolution. 2008;62:3178–3184. doi: 10.1111/j.1558-5646.2008.00510.x. [DOI] [PubMed] [Google Scholar]
- 124.Nisa Ramiro C, et al. Chemosensory discrimination of male age by female Psammodromus algirus lizards based on femoral secretions and feces. Ethology. 2019;125:802–809. doi: 10.1111/eth.12934. [DOI] [Google Scholar]
- 125.Rajagopal T, Archunan G, Geraldine P, Balasundaram C. Assessment of dominance hierarchy through urine scent marking and its chemical constituents in male blackbuck Antelope cervicapra, a critically endangered species. Behav. Process. 2010;85:58–67. doi: 10.1016/j.beproc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 126.Cartolano MC, Babcock EA, McDonald MD. Evidence that Gulf toadfish use pulsatile urea excretion to communicate social status. Physiol. Behav. 2020;227:113182. doi: 10.1016/j.physbeh.2020.113182. [DOI] [PubMed] [Google Scholar]
- 127.Marneweck C, Jürgens A, Shrader A. Ritualised dung kicking by white rhino males amplifies olfactory signals but reduces odour duration. J. Chem. Ecol. 2018;44:875–885. doi: 10.1007/s10886-018-0988-3. [DOI] [PubMed] [Google Scholar]
- 128.Marneweck C, Jürgens A, Shrader AM. The role of middens in white rhino olfactory communication. Anim. Behav. 2018;140:7–18. doi: 10.1016/j.anbehav.2018.04.001. [DOI] [Google Scholar]
- 129.Christy, J. H. & Rittschof, D. Deception in visual and chemical communication in crustaceans. In Chemical Communication in Crustaceans (eds Breithaupt, T. & M. Thiel) 313–333 (Springer, 2010).
- 130.MacGillavry T, Spezie G, Fusani L. When less is more: coy display behaviours and the temporal dynamics of animal courtship. Proc. R. Soc. B Biol. Sci. 2023;290:20231684. doi: 10.1098/rspb.2023.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rosenthal GG, Fitzsimmons JN, Woods KU, Gerlach G, Fisher HS. Tactical release of a sexually-selected pheromone in a swordtail fish. PLoS ONE. 2011;6:e16994. doi: 10.1371/journal.pone.0016994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hurst J, Robertson D, Tolladay U, Beynon R. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim. Behav. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- 133.Lazar J, Rasmussen L, Greenwood DR, Bang I-S, Prestwich GD. Elephant albumin: a multipurpose pheromone shuttle. Chem. Biol. 2004;11:1093–1100. doi: 10.1016/j.chembiol.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 134.Koene JM, Ter Maat A. Allohormones: a class of bioactive substances favoured by sexual selection. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2001;187:323–326. doi: 10.1007/s003590100214. [DOI] [PubMed] [Google Scholar]
- 135.Chase R, Blanchard KC. The snail’s love-dart delivers mucus to increase paternity. Proc. R. Soc. B Biol. Sci. 2006;273:1471–1475. doi: 10.1098/rspb.2006.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koene JM, Pförtner T, Michiels NK. Piercing the partner’s skin influences sperm uptake in the earthworm Lumbricus terrestris. Behav. Ecol. Sociobiol. 2005;59:243–249. doi: 10.1007/s00265-005-0030-y. [DOI] [Google Scholar]
- 137.Schulte LM, Martel A, Cruz-Elizalde R, Ramírez-Bautista A, Bossuyt F. Love bites: male frogs (Plectrohyla, Hylidae) use teeth scratching to deliver sodefrin precursor-like factors to females during amplexus. Front. Zool. 2021;18:1–14. doi: 10.1186/s12983-021-00445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 139.Gwynne DT. Sexual conflict over nuptial gifts in insects. Annu. Rev. Entomol. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- 140.Ruther J, Steidle JL. “Allohormones”: a new class of bioactive substances or old wine in new skins? J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002;188:161–162. doi: 10.1007/s00359-002-0288-8. [DOI] [PubMed] [Google Scholar]
- 141.Wigby S, et al. The Drosophila seminal proteome and its role in postcopulatory sexual selection. Philos. Trans. R. Soc. B Biol. Sci. 2020;375:20200072. doi: 10.1098/rstb.2020.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kimura K, Shibuya K, Chiba S. The mucus of a land snail love-dart suppresses subsequent matings in darted individuals. Anim. Behav. 2013;85:631–635. doi: 10.1016/j.anbehav.2012.12.026. [DOI] [Google Scholar]
- 143.Patlar B, Weber M, Temizyürek T, Ramm SA. Seminal fluid-mediated manipulation of post-mating behavior in a simultaneous hermaphrodite. Curr. Biol. 2020;30:143–149. e144. doi: 10.1016/j.cub.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Thomas ML. Detection of female mating status using chemical signals and cues. Biol. Rev. Camb. Philos. Soc. 2011;86:1–13. doi: 10.1111/j.1469-185X.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- 145.Hosseini SA, et al. Experimental evidence for chemical mate guarding in a moth. Sci. Rep. 2016;6:1–6. doi: 10.1038/srep38567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kingan TG, Bodnar WM, Raina AK, Shabanowitz J, Hunt DF. The loss of female sex pheromone after mating in the corn earworm moth Helicoverpa zea: identification of a male pheromonostatic peptide. Proc. Natl. Acad. Sci. USA. 1995;92:5082–5086. doi: 10.1073/pnas.92.11.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wigby S, et al. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 2009;19:751–757. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 149.Elgar MA, Johnson TL, Symonds MR. Sexual selection and organs of sense: Darwin’s neglected insight. Anim. Biol. 2019;69:63–82. doi: 10.1163/15707563-00001046. [DOI] [Google Scholar]
- 150.Loudon C, Koehl M. Sniffing by a silkworm moth: wing fanning enhances air penetration through and pheromone interception by antennae. J. Exp. Biol. 2000;203:2977–2990. doi: 10.1242/jeb.203.19.2977. [DOI] [PubMed] [Google Scholar]
- 151.Stowers L, Logan DW. Sexual dimorphism in olfactory signaling. Curr. Opin. Neurobiol. 2010;20:770–775. doi: 10.1016/j.conb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Williams AT, Verhulst EC, Haverkamp A. A unique sense of smell: development and evolution of a sexually dimorphic antennal lobe–a review. Entomol. Exp. Appl. 2022;170:303–318. doi: 10.1111/eea.13145. [DOI] [Google Scholar]
- 153.Allen CE, Zwaan BJ, Brakefield PM. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 2011;56:445–464. doi: 10.1146/annurev-ento-120709-144828. [DOI] [PubMed] [Google Scholar]
- 154.Shine R. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 1989;64:419–461. doi: 10.1086/416458. [DOI] [PubMed] [Google Scholar]
- 155.Hanks LM, Millar JG, Paine TD. Body size influences mating success of the eucalyptus longhorned borer (Coleoptera: Cerambycidae) J. Insect Behav. 1996;9:369–382. doi: 10.1007/BF02214016. [DOI] [Google Scholar]
- 156.Holwell G, Barry K, Herberstein M. Mate location, antennal morphology, and ecology in two praying mantids (Insecta: Mantodea) Biol. J. Linn. Soc. 2007;91:307–313. doi: 10.1111/j.1095-8312.2007.00788.x. [DOI] [Google Scholar]
- 157.Lefebvre F, Limousin M, Caubet Y. Sexual dimorphism in the antennae of terrestrial isopods: a result of male contests or scramble competition? Can. J. Zool. 2000;78:1987–1993. doi: 10.1139/z00-128. [DOI] [Google Scholar]
- 158.Johnson TL, Symonds MR, Elgar MA. Sexual selection on receptor organ traits: younger females attract males with longer antennae. Sci. Nat. 2017;104:44. doi: 10.1007/s00114-017-1466-4. [DOI] [PubMed] [Google Scholar]
- 159.Lin CY, Tung CH, Yu JK, Su YH. Reproductive periodicity, spawning induction, and larval metamorphosis of the hemichordate acorn worm Ptychodera flava. J. Exp. Zool. B Mol. Dev. Evol. 2016;326:47–60. doi: 10.1002/jez.b.22665. [DOI] [PubMed] [Google Scholar]
- 160.Marshall D, Evans J. Does egg competition occur in marine broadcast‐spawners? J. Evol. Biol. 2005;18:1244–1252. doi: 10.1111/j.1420-9101.2005.00947.x. [DOI] [PubMed] [Google Scholar]
- 161.Jennions M, Kokko H, Klug H. The opportunity to be misled in studies of sexual selection. J. Evol. Biol. 2012;25:591–598. doi: 10.1111/j.1420-9101.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- 162.Caves EM, Nowicki S, Johnsen S. Von Uexküll revisited: addressing human biases in the study of animal perception. Integr. Comp. Biol. 2019;59:1451–1462. doi: 10.1093/icb/icz073. [DOI] [PubMed] [Google Scholar]
- 163.Jones AG, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl. Acad. Sci. USA. 2009;106:10001–10008. doi: 10.1073/pnas.0901129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Candolin U. Mate choice in a changing world. Biol. Rev. Camb. Philos. Soc. 2019;94:1246–1260. doi: 10.1111/brv.12501. [DOI] [PubMed] [Google Scholar]
- 165.Breithaupt T, Eger P. Urine makes the difference: chemical communication in fighting crayfish made visible. J. Exp. Biol. 2002;205:1221–1231. doi: 10.1242/jeb.205.9.1221. [DOI] [PubMed] [Google Scholar]
- 166.Shibuya K, Chiba S, Kimura K. Sexual inactivation induced by the mucus that covers land snail love darts: sexual selection and evolution of allohormones in hermaphrodites. J. Exp. Biol. 2022;225:jeb238782. doi: 10.1242/jeb.238782. [DOI] [PubMed] [Google Scholar]
- 167.Borne F, Kasimatis KR, Phillips PC. Quantifying male and female pheromone-based mate choice in Caenorhabditis nematodes using a novel microfluidic technique. PLoS ONE. 2017;12:e0189679. doi: 10.1371/journal.pone.0189679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hussain YH, Guasto JS, Zimmer RK, Stocker R, Riffell JA. Sperm chemotaxis promotes individual fertilization success in sea urchins. J. Exp. Biol. 2016;219:1458–1466. doi: 10.1242/jeb.134924. [DOI] [PubMed] [Google Scholar]
- 169.Hussain YH, Sadilek M, Salad S, Zimmer RK, Riffell JA. Individual female differences in chemoattractant production change the scale of sea urchin gamete interactions. Dev. Biol. 2017;422:186–197. doi: 10.1016/j.ydbio.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 170.Koene JM. Tales of two snails: sexual selection and sexual conflict in Lymnaea stagnalis and Helix aspersa. Integr. Comp. Biol. 2006;46:419–429. doi: 10.1093/icb/icj040. [DOI] [PubMed] [Google Scholar]
- 171.Katoh, E., Johnson, M. & Breithaupt, T. Fighting behaviour and the role of urinary signals in dominance assessment of Norway lobsters, Nephrops norvegicus. Behaviour145, 1447–1464 (2008).
- 172.Bertin A, Cezilly F. Sexual selection, antennae length and the mating advantage of large males in Asellus aquaticus. J. Evol. Biol. 2003;16:491–500. doi: 10.1046/j.1420-9101.2003.00536.x. [DOI] [PubMed] [Google Scholar]
- 173.Chang H, et al. Pheromone binding proteins enhance the sensitivity of olfactory receptors to sex pheromones in Chilo suppressalis. Sci. Rep. 2015;5:13093. doi: 10.1038/srep13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jönsson M, et al. Sex-specific expression of pheromones and other signals in gravid starfish. BMC Biol. 2022;20:1–18. doi: 10.1186/s12915-022-01491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Datta SR, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 176.Hajduskova M, Jindra M, Herman MA, Asahina M. The nuclear receptor NHR-25 cooperates with the Wnt/β-catenin asymmetry pathway to control differentiation of the T seam cell in C elegans. J. Cell Sci. 2009;122:3051–3060. doi: 10.1242/jcs.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maruska KP, Fernald RD. Contextual chemosensory urine signaling in an African cichlid fish. J. Exp. Biol. 2012;215:68–74. doi: 10.1242/jeb.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Telford MJ, Budd GE, Philippe H. Phylogenomic insights into animal evolution. Curr. Biol. 2015;25:R876–R887. doi: 10.1016/j.cub.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 179.Johnson NS, Yun S-S, Thompson HT, Brant CO, Li W. A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc. Natl. Acad. Sci. USA. 2009;106:1021–1026. doi: 10.1073/pnas.0808530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morris R. Some aspects of the structure and cytology of the gills of Lampetra fluviatilis. J. Cell Sci. 1957;3:473–485. doi: 10.1242/jcs.s3-98.44.473. [DOI] [Google Scholar]
- 181.Ruxton G, Schaefer H. Resolving current disagreements and ambiguities in the terminology of animal communication. J. Evol. Biol. 2011;24:2574–2585. doi: 10.1111/j.1420-9101.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 182.Koene JM, Ter Maat A. The distinction between pheromones and allohormones. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002;188:163–164. doi: 10.1007/s00359-002-0289-7. [DOI] [Google Scholar]
- 183.Weiss K, Herzner G, Strohm E. Sexual selection and the evolution of male pheromone glands in philanthine wasps (Hymenoptera, Crabronidae) BMC Evol. Biol. 2017;17:1–20. doi: 10.1186/s12862-017-0963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuhlisch C, Pohnert G. Metabolomics in chemical ecology. Nat. Prod. Rep. 2015;32:937–955. doi: 10.1039/C5NP00003C. [DOI] [PubMed] [Google Scholar]
- 185.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat. Rev. Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 186.Hickner PV, et al. Molecular signatures of sexual communication in the phlebotomine sand flies. PLoS Negl. Trop. Dis. 2020;14:e0008967. doi: 10.1371/journal.pntd.0008967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wyer CA, Cator LJ, Hollis B. Release from sexual selection leads to rapid genome-wide evolution in Aedes aegypti. Curr. Biol. 2023;33:1351–1357. e1355. doi: 10.1016/j.cub.2023.02.031. [DOI] [PubMed] [Google Scholar]
- 188.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Ann. Rev. Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 189.Churcher AM, Taylor JS. The antiquity of chordate odorant receptors is revealed by the discovery of orthologs in the cnidarian Nematostella vectensis. Genome Biol. Evol. 2011;3:36–43. doi: 10.1093/gbe/evq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cummins SF, et al. Candidate chemoreceptor subfamilies differentially expressed in the chemosensory organs of the mollusc Aplysia. BMC Biol. 2009;7:1–20. doi: 10.1186/1741-7007-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Marquet N, et al. Holothurians have a reduced GPCR and odorant receptor-like repertoire compared to other echinoderms. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-60167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roberts RE, et al. Identification of putative olfactory G-protein coupled receptors in Crown-of-Thorns starfish, Acanthaster planci. BMC Genom. 2017;18:1–15. doi: 10.1186/s12864-017-3793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sania RE, et al. A new subfamily of ionotropic glutamate receptors unique to the echinoderms with putative sensory role. Mol. Ecol. 2021;30:6642–6658. doi: 10.1111/mec.16206. [DOI] [PubMed] [Google Scholar]
- 194.Thomas JH, Robertson HM. The Caenorhabditis chemoreceptor gene families. BMC Biol. 2008;6:1–17. doi: 10.1186/1741-7007-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ryan, M. J. Darwin, sexual selection, and the brain. Proc. Natl. Acad. Sci. USA. 118, e2008194118 (2021). [DOI] [PMC free article] [PubMed]
- 196.Arendt D, Tosches MA, Marlow H. From nerve net to nerve ring, nerve cord and brain—evolution of the nervous system. Nat. Rev. Neurosci. 2016;17:61–72. doi: 10.1038/nrn.2015.15. [DOI] [PubMed] [Google Scholar]
- 197.Holland LZ. The origin and evolution of chordate nervous systems. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20150048. doi: 10.1098/rstb.2015.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mora C, Tittensor DP, Adl S, Simpson AG, Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;9:e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our paper presents no new data. The results of the literature review are provided as Supplementary data.