Abstract
Background
Obesity is a complex disease that leads to higher morbidity and mortality and its rate in the United States is rapidly rising. Targeting obesity management is one of the cornerstones of preventive medicine. Early intervention can significantly reduce the risk of developing cardiovascular disease. While it is well known that lifestyle interventions such as healthful nutrition and routine physical activity are the first and most important step in management, some do not achieve the desired results and require further therapies.
Methods
A literature review was conducted, that included clinical documents, public scientific citations and peer review articles to evaluate anti-obesity medications, endoscopic procedures and bariatric surgeries in the management of obesity. We also included effects of these interventions on weight loss, cardiovascular disease risk reduction and side effects.
Results
This clinical review summarizes recent evidence for the different approaches in obesity management including medications, common endoscopic procedures and bariatric surgeries. For more detailed review on the different management options discussed, we recommend reviewing Obesity Medicine Association Clinical Practice Statement [1].
Conclusion
Management of obesity reduces cardiovascular risk, improves metabolic parameters and other important health outcomes. Different management approaches are available, hence, a high level of awareness of the growing epidemic of obesity is needed to ensure timely referrals to obesity medicine specialists.
1. Introduction
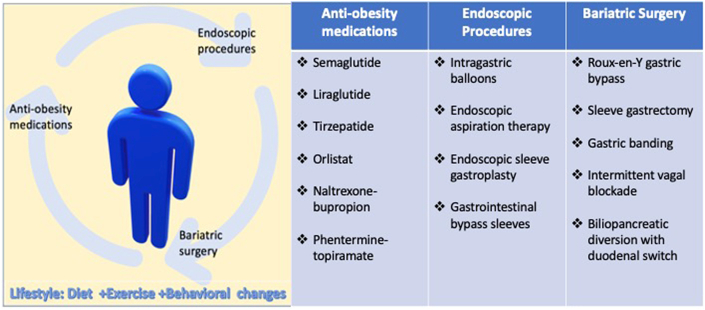
Obesity is a complex disease that is directly associated with the risk of developing dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, and sleep disorders which are well known cardiovascular disease (CVD) risk factors. Obesity also increases the risk of CVD and cardiovascular mortality independent of the other risk factors [1]. The prevalence of obesity in the United States is on the rise, reported to be as high as 42%, according to data from the Centers for Disease Control [2]. Importantly, it is one of many modifiable CVD risk factors. Lifestyle interventions, including healthful nutrition and physical activity, are first line in the management of obesity. However, other interventions including anti-obesity medications, endoscopic procedures, and bariatric surgery have been successfully utilized in patients who do not achieve desired outcomes with lifestyle modifications alone. The impact of these interventions on CVD outcomes along with cardiovascular side effects and precautions, will be reviewed in this article (see Fig. 1).
Fig. 1.
Central illustration summarizes medications, endoscopic procedures and bariatric surgery for the management of obesity discussed in the article.
2. Anti-obesity medications
2.1. Semaglutide
Glucagon-like peptide-1 (GLP-1) is an endogenous incretin produced in intestines after food intake that enhances insulin secretion and suppresses glucagon release [3]. Semaglutide is a GLP-1 receptor agonist (GLP1-RA) that has been approved for T2DM management and has been shown to reduce cardiovascular events in patients with T2DM [4]. The food and drug administration (FDA) approved semaglutide SC injection (2.4 mg once weekly) for chronic weight management in adults with obesity or overweight with at least one weight-related condition (such as high blood pressure, T2DM, or high cholesterol) on June 4, 2021 [5].
Semaglutide was evaluated in a series of clinical trials called Semaglutide Treatment Effect in People with Obesity (STEP) trials [6]. STEP 1 trial evaluated the safety and efficacy of semaglutide use for weight loss [7]. The dose of subcutaneous (SC) 2.4 mg weekly of semaglutide was studied in about 2000 patients with overweight or obesity (Body mass index (BMI) ≥27 kg/m2 in persons with ≥1 weight-related coexisting condition or ≥ BMI 30 kg/m2) without T2DM versus placebo both along with lifestyle modifications who were followed for 17 months [7,8]. Most of the participants were white (75%) and female (74%) with a mean age of 46 years. Mean change in weight was 15% vs 2.4% in semaglutide vs placebo, with an estimated treatment difference of −12.4% points (95% CI −13.4 to −11.5, P<0.001). The average reduction in body weight with semaglutide was 15.3 kg, with weight loss of ≥5% achieved by 86% in the semaglutide group versus 31% in the placebo group. Patients in the semaglutide group achieved an improvement in cardiometabolic profile compared to placebo. There was a greater reduction in waist circumference (−14 cm vs −4 cm) and BMI (−6 vs −1 kg/m2) with semaglutide vs placebo [7,8]. There was also a greater reduction in the systolic/diastolic blood pressure (−6/3 vs −1/0.4 mmHg), as well as C-reactive protein (CRP) mg/l (ratios to baseline 0.47 vs 0.85) with semaglutide vs placebo. Fasting plasma glucose (FPG) (−8 vs −0.5 mg/dl) and glycated hemoglobin (HbA1c) reductions were also greater with semaglutide compared to placebo (−0.45% vs −0.15%). Finally, semaglutide led to greater reductions in low-density lipoprotein-cholesterol (LDL-C) mg/dl (ratios to baseline 0.97 vs 1.01) and physical functioning scores [8] compared with placebo.
Transient diarrhea and nausea were the most common reported adverse events leading to discontinuation of semaglutide vs placebo (59 [4.5%] vs. 5 [0.8%], respectively) [8].
The results of the first trial to assess major adverse cardiovascular events (MACE) reduction for adults with obesity but without T2DM, the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity outcome trial (SELECT), should be released later this year [9]. This trial enrolled 17,000 adults from multiple countries with BMI ≥27 kg/m2 and established CVD, defined as one or more of the following: prior myocardial infarction (MI), prior ischemic or hemorrhagic stroke, symptomatic peripheral arterial disease with ankle-brachial index <0.85 at rest, prior peripheral arterial revascularization procedure, or amputation due to atherosclerotic disease. The following groups were excluded: patients with type 1 diabetes mellitus or T2DM, and patient with MI, stroke, hospitalization for unstable angina pectoris, or a transient ischemic within 60 days of enrollment [9,10].
2.2. Liraglutide
Liraglutide is a GLP1-RA that shares the same mechanism of action of semaglutide, which results in delayed gastric emptying [11], and appetite suppression [12,13]. The Satiety and Clinical Adiposity – Liraglutide Evidence in non-diabetic and diabetic individuals (SCALE) program was conducted to evaluate safety and efficacy of liraglutide, which included four large scale randomized multicenter phase III trials [11,[14], [15], [16], [17], [18], [19]]. The SCALE Maintenance trial enrolled 500 patients with a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with a weight-related comorbid condition who lost ≥ 5% of initial weight with a low-calorie diet were enrolled to receive liraglutide 3 mg SC daily injections vs placebo [20]. Main outcomes were percentage weight change from randomization, efficacy of liraglutide in maintaining ≥5% of initial weight loss and proportion that lost ≥ 5% of body weight after randomization [20]. At 56 weeks, the liraglutide group experienced a greater decrease in body weight compared with placebo (6% vs 0.2%, p < 0.0001) [20]. A large proportion of liraglutide-treated patients maintained the initial ≥5% weight loss (81% versus 49%), achieved ≥5% weight loss (50% vs 22%), and achieved ≥10% weight loss (26% versus 6%), compared with placebo-treated patients (p < 0.0001) [16,20].
In the SCALE-Maintenance trial, several metabolic parameters improved with Liraglutide vs placebo [20]. At 56 weeks, there was a significant difference in FPG (−9 vs −3.6 mg/dl in the liraglutide group vs placebo, respectively) with estimated treatment differences for liraglutide vs placebo −0.4 (95% CI −0.5 to −0.3, P<0.0001). There were also significant changes in HbA1c (−0.1% vs 0.1%) between liraglutide and placebo groups with treatment differences of −0.3 (95% CI −0.3 to −0.2, P<0.0001) and CRP (−20 vs −1 nmol/l) with treatment of −13.0 (95% CI −23.4 to −2.6, P=0.01). There was also a significant change in systolic/diastolic blood pressure between the two groups (+0.2/1.4 vs +2.8/1.2 mmHg) with estimated treatment differences of systolic blood pressure of −2.7 (95% CI −4.7 to −0.8, P=0.007) and diastolic blood pressure differences of −0.3 (95% CI −1.7 to 1.1, P=0.64) between liraglutide vs placebo. Net changes in lipids were of negligible magnitude [16,20].
Mild to moderate gastrointestinal side effects have been reported. Nausea was transient and mainly occurred in the first four weeks of the trial, coinciding with dose escalation [16]. Symptomatic hypoglycemia was more frequent than the placebo group (5.2% vs. 2.4%), though the difference was not statistically significant [16,19]. An increase in heart rate has also been reported with the use of liraglutide [21].
Liraglutide 3.0 mg daily SC injection has been FDA approved since December 2014 as an adjunct to a reduced calorie diet and greater physical activity for chronic weight management in adults with a BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with at least one weight-related condition [22].
GLP1-RA medications have been well studied in patients at high risk of CVD with unique benefits. One meta-analysis of 56,000 patients with T2DM on GLP1- RA showed a 12% reduction in MACE (HR 0·88; 95% CI 0·82–0·94) and a 12% reduction in all-cause mortality (HR 0.88; 95% CI 0·83–0·95). Additionally, a 9% (HR 0·91; 95% CI 0·83–0·99) reduction in admission to the hospital for heart failure was also observed [23,24]. GLP1- RA should be strongly considered in patients with existing CVD or at a high risk, irrespective of HbA1c [24]. Lower doses of GLP1-RA are recommended in older population ≥65 years to avoid hypoglycemia [24]. Liraglutide and semaglutide should be used cautiously in patients with pancreatitis, and retinopathy screening should also be undertaken. These medications are contraindicated in pregnancy or breastfeeding, or in patients with a personal or family history of multiple endocrine neoplasia type 2 or medullary thyroid cancer [24]. The SUSTAIN-6 and PIONEER-6 trials demonstrated beneficial effects of GLP1- RA on cardiovascular outcomes among those with T2DM [25,26].
2.3. Tirzepatide
Tirzepatide is a dual-glucose-dependent, insulinotropic polypeptide and GLP1- RA that is currently FDA approved for management of T2DM. The Study of Tirzepatide [LY3298176] Versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants With Type 2 Diabetes (SURPASS-2) trial demonstrated superiority to semaglutide with mean changes in HbA1c of 2.3%, and weight reductions of 11 kg with the highest dose of 15 mg at 40 weeks. There were no significant differences in LDL-C or blood pressure between the two study drugs [27]. Although tirzepatide has not been approved by the FDA for weight management in patients without T2DM, Eli Lilly has been granted fast track status by the FDA for its approval especially after the promising weight loss results [28]. The use of tirzepatide was associated with a 21% change in body weight from baseline after 72 weeks. The percentage of participants who had ≥5% weight loss was up to 91% (95% CI 88–94) with the highest dose of 15 mg weekly [29].
2.4. Orlistat
Orlistat is a gastric and pancreatic lipase inhibitor that is approved in the US and Europe for the long-term pharmacologic management of obesity [30].
Recently, Ardissino et al. conducted a large nation-wide propensity-score matched study with close to 37,000 patients with obesity who were matched on a 1:1 basis to orlistat vs placebo, followed for 6 years. MACE was lower in the orlistat group (HR 0.74; 95% CI 0.66–0.83) compared with placebo. The orlistat group had lower rates of MI with (HR 0.77; 95% CI 0.66–0.88), ischemic stroke (HR 0.68; 95% CI 0.56 to −0.84), new-onset heart failure (HR 0.79; 95% CI 0.67–0.94), as well as CKD stage III development (HR 0.78; 95% CI 0.73–0.83) and mortality (HR 0.39; 95% CI 0.36 to −0.41). There were no differences in revascularization rates (HR 1.12; 95% CI 0.91–1.38) [31].
Side effects of orlistat use are mostly gastrointestinal, including abdominal discomfort and soft oily stools. There is a possible decrease in fat-soluble vitamin absorption with long-term orlistat treatment. For example, one study noted ∼8% decrease in 25-hydroxy-D concentrations after 2 years of orlistat use [32]. Thus, vitamin level monitoring should be considered in patients taking this medication.
Orlistat 120 mg daily oral was approved by the FDA in 1999 for obesity management in conjunction with a reduced caloric diet, and to reduce the risk of regaining weight after prior weight loss and in 2007. Orlistat 60 mg was approved as a daily oral medication for over-the-counter use for weight loss in adults with overweight, 18 years and older, in conjunction with a reduced-calorie and low-fat diet [33].
In a study comparing orlistat with liraglutide, both drugs reduced weight, FPG, systolic blood pressure, LDL-C and alanine transaminase over 7 months follow up. However, weight loss was higher with liraglutide (−7.7 kg) compared with orlistat (−3.3 kg), and more individuals lost at least 5% of their baseline weight with liraglutide (64.7%) than orlistat (27.4%) [34]. Orlistat decreased FPG by 5 mg/dl, LDL-C by 9 mg/dl and systolic/diastolic blood pressure by 4/3 mmHg points [34].
2.5. Naltrexone-bupropion
Naltrexone is a water-soluble crystalline medication that is a pure opioid antagonist. Bupropion is also a water-soluble crystalline medication that is a dopamine reuptake inhibitor related to the phenylethylamines, which are known for their stimulant effects [35]. The mechanism of this combined medication on weight loss is poorly understood. Some research suggests the hypothalamic melanocortin system and the mesolimbic reward system are the potential target of this combination [36,37].
Patients from four phase III clinical trials: COR-1, COR-II, COR-BMOD and COR-DM were pooled over a 14 month follow up period. All four studies included 4536 patients and demonstrated statistically significant and clinically meaningful weight loss of approximately 5–9 kg after 52 weeks of treatment with the extended-release form of naltrexone ER/bupropion ER compared with placebo. Significant improvements in cardiometabolic markers including waist circumference, triglycerides, and high-density lipoprotein-cholesterol (HDL-C) and HbA1c [38] were also observed.
Significant reductions in HbA1c were noted in the naltrexone ER/bupropion ER group vs placebo (−0.6% vs −0.1%), with a placebo-corrected difference of 0.5% at the end of 56 weeks (p < 0.001) [38,39]. In addition, a greater percentage of patients achieved a HbA1c of <7.0% (44.1 vs 26.3%) or HbA1c of <6.5% HbA1c (20.7 vs 10.2%) in naltrexone ER/bupropion ER vs placebo groups, respectively [38,39]. Triglyceride reductions were noted in the intervention vs placebo group (−11% vs −0.8%, respectively with p=0.007). HDL-C increases were also noted in the intervention vs placebo group (+3% vs −0.3%, respectively with p<0.001) [39]. No significant change in FPG, LDL-C or CRP were observed between the two groups [39].
Most side effects are gastrointestinal, including nausea which was reported in 27%–34% of patients, and constipation (15%–24% of patients). Other side effects include, headache (14%–24%), dizziness (7%–14%), and dry mouth (8%). Some patients reported modestly higher systolic blood pressure with naltrexone ER/bupropion ER, however, there was a mean overall decrease in systolic blood pressure compared with baseline at the end of the study, with mean reductions of 3.4–11.4 mmHg [36,40].
Sposito et al. performed a systematic review and meta-analysis to examine cardiovascular outcomes in randomized controlled trials that tested naltrexone, bupropion, or the combination among patients with obesity, smoking, and other clinical conditions [41]. This analysis found that these medications, or their combination, were not associated with the incidence of MACE, and no increased risk of nonfatal MI or all-cause death was observed [41].
In 2011, the FDA declined this medication's approval because of concerns regarding long-term cardiovascular safety in adults with overweight and obesity [37]. However, the combination gained FDA approval in 2014 for patients with obesity or overweight with at least one other weight-related condition or illness, such as high blood pressure or T2DM. Clinicians should consider stopping this medication at 12 weeks if at least 5% of weight loss is not achieved [42,43].
Two cardiovascular outcome trials with naltrexone-bupropion were conducted, the LIGHT and the CONVENE trial. However, both studies were halted prematurely, thus their effects on CVD remain uncertain [44].
2.6. Phentermine-topiramate
Phentermine is an amphetamine analogue which is a sympathomimetic that increases the release of noradrenaline from presynaptic vesicles in the hypothalamus [45]. It has been approved as monotherapy for the management of adults with obesity in the US for short-term use only (up to 12 weeks) in conjunction with dietary and lifestyle modifications [46].
Topiramate isa sulfamate-substituted monosaccharide used to treat epilepsy and prevent migraines. Weight loss is thought to occur because of carbonic-anhydrase inhibition on taste but the major effect is likely γ-aminobutyric acid activity (GABA) receptor activation given the interaction between GABA and leptin pathways [47].
Phentermine and topiramate extended-release (PHEN/TPM-ER) has been approved as a once-daily combination therapy for chronic weight management in adults with obesity and overweight with at least one weight-related comorbidity as an adjunct to behavioral and lifestyle modifications [45].
Multiple trials examined the safety and efficacy of the extended-release formulation (PHEN/TPM-ER). First, the EQUIP trial (Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial) included 1267 participants with obesity (≤70 years of age and BMI ≥35 kg/m2; excluding patients with T2DM). Second, the CONQUER trial [48] (Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults) included 2487 patients with overweight and obesity (≤70 years of age; BMI ≥27 kg/m2 and ≤45 kg/m2) with at least two weight-related comorbidities, including hypertension and T2DM [48]. Third one is an extension study to CONQUER (SEQUEL study) which enrolled patients for an additional 52 weeks [49]. The fourth study included adults with T2DM who were evaluated in a 28-week extension of a 28-week double-blind, placebo-controlled phase 2 trial (56 weeks total) [50].
Taken together, these trails demonstrated that PHEN/TPM-ER was associated with significant and sustained weight loss in patients with overweight and obesity when compared with placebo. Weight loss was dose dependent and ranged from 5 to 10% weight loss from baseline at 14 months compared to 1.4% with placebo. Patients achieved at least 5% weight loss after 56 weeks of treatment and significant, sustained percentage and categorical weight loss through 108 weeks in the 2-year cohort [45].
Significant reductions in systolic and diastolic blood pressures with the higher combination dose were also noted, ranging from 7 to 8/5 mmHg mean reduction compared to baseline for PHEN/TPM-ER 7.5/46 and 15/92 doses throughout 1 and 2 years of treatment. There was a transient slight increase in the heart rate that was only significant in the highest dose of the combination drug [50,51].
PHEN/TPM-ER treatment resulted in significant reductions in serum triglycerides (reduction by 5.5–40%), HDL-C (increased by 2–20%), and LDL-C (reduction by 2–5%) vs. placebo (P < 0.05) in patients with dyslipidemia at week 56, along with a net reduction in lipid-lowering medication use. Reductions in CRP by 1–3 mg/L were also observed [52].
Theoretical increases in heart rate and blood pressure may occur with the use of phentermine, which may be due to amphetamine-like side effects based on similarities in drug pharmacology. However, short term observational studies disproved these theoretical negative cardiovascular effects, leading to its approval for short term use [53]. Phentermine is contraindicated in patients with CVD particularly in patients with high-risk conditions such asprior coronary artery bypass grafting (CABG) or stenting, history of congestive heart failure, stroke, arrhythmias, congestive heart failure [54]. Although some studies demonstrated safety of long term (>3 months) phentermine use for “low CVD risk patients”, they excluded patients with diagnoses or procedure codes for any cardiovascular outcome including MI, stroke, angina, CABG or carotid artery procedure [55]. Hence, phentermine should not be used in patients with CVD [54]. Phentermine monotherapy may be considered selectively using a patient centered approach [53,54]. Despite the precautions regarding phentermine, a retrospective cohort study suggested no increased risk of MACE in patients taking combination phentermine/topiramate [56].
Pregnancy testing is recommended prior to starting topiramate therapy for women in childbearing age due to the risk of congenital malformations [6]. PHEN/TPM-ER is currently approved at doses of 3.75 mg/23 mg, 7.5 mg/46 mg, 11.25 mg/69 mg and 15 mg/92 mg for chronic weight management in individuals age ≥12 years with obesity and overweight with at least one weight-related comorbidity [45].
2.7. Comparison between weight loss medications
The Institute for Clinical and Economic Review (ICER) finalized its report on anti-obesity medications for effectiveness and value-evidence [57]. The authors concluded that semaglutide and phentermine/topiramate had greater weight reduction than liraglutide and bupropion/naltrexone. Also, GLP1-RA (semaglutide and liraglutide) resulted in better blood sugar and blood pressure control compared to usual care but were not superior to other anti-obesity medications [57]. In addition, the studies assessing long-term outcomes data only included adults with T2DM [57], thus outcomes among individuals without T2DM remains uncertain. Furthermore, phentermine/topiramate and bupropion/naltrexone were considered the most cost effective compared to lifestyle modification alone [57].
In a network meta-analysis comparing all aforementioned classes of medications (except semaglutide and tirzepatide) head-to-head in achieving weight loss, liraglutide was associated with an OR of 5.54 (95% CrI 4.16–7.78), orlistat with an OR of 2.70 (95% CrI 2.34–3.09), naltrexone-bupropion with an OR of 3.96 (95% CrI 3.03–5.11), phentermine-topiramate an OR of 9.22 (95% CrI 6.63–12.85) for achieving at least 5% weight loss with at least 1 year of treatment. They all were associated with higher odds of achieving 10% of weight loss from baseline compared with placebo. Liraglutide was associated with achieving at least 10% weight loss in an estimated 34% of patients, orlistat with an estimated 20%, naltrexone-bupropion in an estimated 30%, and phentermine-topiramate in 54% of participants. Phentermine-topiramate was associated with the highest probability of achieving at least 5% and 10% weight loss, followed by liraglutide, naltrexone-bupropion, and lastly orlistat. Liraglutide was most likely to be discontinued due to adverse events (OR 2.95; 95% CrI 2.11–4.23; Surface Under the Cumulative RAnking curve SUCRA, 0.20) followed by naltrexone-bupropion (OR 2.64; 95% CrI 2.10–3.35; SUCRA, 0.23) [58].
Obesity pharmacotherapy should be individualized based on risk profile and amount of weight loss desired, while considering medical insurance coverage. Data from recent meta-analyses showed that the overall placebo-subtracted weight reduction with the use of anti-obesity medications for at least 12 months ranges from 2.9% to 6.8%; phentermine/topiramate (3 trials, −6.8%) liraglutide (4 trials, −5.4%), naltrexone/bupropion (5 trials, −4.0%), and orlistat (17 trials, −2.9%). However, randomized controlled trials are required to evaluate the long-term safety profile and effects on MACE [59]. Table 1A summarizes current medications for management of obesity. Table 1B summarizes the effect of anti-obesity medications on CVD risk factors.
Table 1A.
Summary of anti-obesity medications.
| Drug Name | Mechanism of action | Weight loss | Clinical trial data | Side effects | Clinical use |
|---|---|---|---|---|---|
| Semaglutide | GLP-1 receptor agonist (GLP1-RA) | Mean change in weight 15% at 17 months | STEP1 trial: 2000 patients randomized to 2.4 mg weekly Semaglutide vs placebo. At 17 months, mean weight loss 15% vs 2.4%. | Transient diarrhea and nausea | Once weekly SC injection approved by FDA 2021. |
| Liraglutide | GLP1-RA | 6.2% at 14 months | SCALE-Maintenance: 500 patients assessed. At 56 weeks Liraglutide patients achieved 6.2% weight loss vs 0.2%. | Hypoglycemia, gastrointestinal and nausea | Daily SC injections approved by FDA 2014. |
| Tirzepatide | GLP1-RA and insulinotropic polypeptide | Up to 21% at 18 months | SURPASS-2 trial showed superiority to semaglutide. Mean change in A1c 2.3%, and weight 11 kg with the highest dose of 15 mg at 40 weeks. | Gastrointestinal and nausea | SC injections once weekly approved by FDA May/2022 for T2DM. Not approved for obesity management yet but one pharmaceutical company has been granted fast track status by the FDA for its approval. |
| Orlistat | Gastric and pancreatic lipase inhibitor | Up to 5% at 3 months | Ardissino et al., 37,000 patients to Orlistat vs Placebo. At 6 years, Orlistat group had lower MACE (HR 0,74), lower MI (HR 0.77), lower stroke (HR 0.68), lower HF (HR 0.79), lower CKDIII (HR 0.78), and lower mortalitiy (HR 0.39) | Abdominal discomfort. Fat-soluble vitamin malabsorption | Daily oral medication, approved 1999, and 2002 for over the counter. |
| Naltrexone-bupropion | Centrally acting (opioid antagonist- dopamine reuptake inhibitor) | 5–9 kg at 13 months | COR-1, COR-II, COR-BMOD and COR-DM: 4536 patients to Naltrexone-bupropion vs placebo. At 52 weeks, Naltrexone-bupropion group had 5–9 kg weight loss. Sposito et al.: Naltrexone-bupropion was not associated with the incidence of MACE as compared to placebo. No statistical significance found in the incidence of nonfatal MI or all-cause death. | Nausea, constipation, headache, dizziness, dry mouth | Twice a day oral medication Approved by FDA 2014. |
| Phentermine-Topiramate ER | Amphetamine analogue- increasing GABA activity and inhibiting glutamate activity | 5–10% over 14 months | EQUIP, CONQUER, SEQUEL and 28-week extension study: analysis of the four trials showed that patients with phentermine-topiramate ER had 5–10% weight loss from baseline at of 56 weeks compared to placebo. | Possible increase in heart rate. | Daily oral medication Approved by FDA 2012. |
Table 1B.
The effect of anti-obesity medications on CVD risk factors.
| Medications | Clinical trial | LDL-C | Trial included Patients with T2DM | FPG/HbA1c (%) | Change SBP/DBP | CRP |
|---|---|---|---|---|---|---|
| Semaglutide | STEP-1 trial | Ratios to baseline 0.47 | No | −8 mg/dl/-0.45% at 68 weeks | −6/3 mmHg | ratios to baseline 0.47 |
| Liraglutide | SCALE-Maintenance trial | Small magnitude change | No | −9 mg/dl at 56 weeks | +0.2/1.4 mmHg | −20 nmol/l |
| Tirzepatide | SURPASS-2 trial | No change | Yes | −2.3% at 40 weeks | ||
| Orlistat | XENSOR study comparing orlistat to liraglutide | −9 mg/dl | Yes (20% of orlistat patients with T2DM) | −5 mg/dl | −4/3 mmHg | N/A |
| Naltrexone-bupropion | COR-1, COR-II, COR-BMOD and COR-DM trials. | No change | Yes | −0.6% at 56 weeks | Overall mean reduction of 3.4–11.4 mmHg | N/A |
| Phentermine-Topiramate | EQUIP, CONQUER, SEQUEL and 28-week extension study. | -2-5% compared to baseline | N/A | N/A | -7-8/5 mmHg at week 56. There was a transient increase in heart rate with the highest dose. | -1-3 mg/l |
3. Endoscopic procedures
Patients with BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with comorbidities should be considered for bariatric surgery. However, less than 1% of all eligible patients actually undergo any operation [60]. Bariatric endoscopy encompasses malabsorption techniques, use of space occupying devices, restrictive methods, and aspiration therapies [61]. Generally, endoscopic procedures are reserved for patients with prohibitive surgical risk (patients with CVD and other significant co-morbidities) or serve as a bridge to traditional bariatric surgery [62].
3.1. Intra-gastric balloons
This endoscopic procedure includes balloons filled with saline of different sizes and shapes, developed to aid in management of obesity for patients with a BMI 30–40 kg/m2. At 6 months, these devices can lead to reductions of 7–15% of total body weight (TBW) [62], and have been approved by the FDA [63].
The American Gastroenterological Association Practice Guidelines for obesity management cites remission of T2DM, hypertension, and dyslipidemia when using this procedure compared to a noninvasive approach. Benefits were most pronounced among patient with a FPG level >100 mg/dl, HbA1c >6.5%, and among patients with a BMI >40 kg/m2 at baseline. Results on changes in lipid profiles were mixed [64].
Advantages include reversibility, repeatability, and more significant weight loss may be achieved when used in combination with other weight reducing modalities. Higher rates of nausea and vomiting have been reported with intragastric balloons compared to a noninvasive approach [62].
3.2. Endoscopic aspiration therapy
Endoscopic aspiration therapy includes a percutaneous gastrostomy tube, known as an A-Tube, that is inserted in similar fashion to a percutaneous endoscopic gastrostomy tube (PEG) tube, then the external portion is attached to the aspiration device. This therapy is approved for patients with a BMI of 35–55 kg/m2. Long term use may result in 14–18% TBW loss after 6–24 months. Apart from reported abdominal pain and peristomal complications, this reversible therapy has minimal side effects, and can be done in the outpatient setting [61,65].
A meta-analysis including 590 patients who had aspiration therapy reported favorable effects in blood pressure: −7.8 mm Hg/−5.1 mm Hg; triglycerides: −15.8 mg/dl; HDL-C: 3.6 mg/dl; HbA1c: −1.3% at 1 year. Patients experienced 18%–19% total weight loss and 46–49% excess weight loss at 1–4 years (p<0.0001 for all) [66].
3.3. Endoscopic sleeve gastroplasty (ESG)
An endoscopic sleeve gastroplasty is created using a series of endoluminally placed sutures through the gastric wall from the pre-pyloric antrum to the gastroesophageal junction (GEJ) along the greater curvature of the stomach. ESGs are reserved for patients with a BMI of 30–40 kg/m2 [61] and may result in weight loss of 12–19% after 6–24 months [65]. These procedures usually require expertise and technical skills in specialized centers. Complications include nausea, vomiting, peri-gastric fluid collections and extra-gastric bleeding [61,65].
In a study of 91 patients undergoing ESG, 14% TBW loss was achieved at 6 months, while 18% and 21% TBW loss was achieved at 1 and 2 years, respectively. At 12 months following ESG as compared to baseline prior to ESG, there was a significant overall reduction in HbA1c (mean± SD, 6.1%±1.1% vs 5.5% ±0.48%, P=0.05). There were also significant reductions in systolic blood pressure (129 ±13.4 mm Hg vs 122.2 ±11.69 mmHg, P = 0.02) and serum triglycerides (131.84 ± 83.19 mmol/dl vs 92.36 ±39.43 mmol/dl, P =0.02). However, there was no significant change in LDL-C (P =0.79) [67].
3.4. Gastrointestinal bypass sleeves
A gastrointestinal bypass sleeve uses a liner made of Teflon that is deployed in the duodenal bulb extending into the small bowel, creating a mechanical barrier that allows food to bypass the duodenum and proximal jejunum. Duodenaljejunal and gastroduodenojejunal bypass sleeves exist as alternatives [61]. This procedure is approved for patients with BMI 35 kg/m2 and obesity related complications or BMI of ≥ 40 kg/m2. Patients achieved 49% excess weight loss at 5 years, which was sustained long term [68]. However, the overall morbidity rate is 19% at 5 years, with significant adverse events including gastroesophageal reflux disease, stricture at the gastrojejunal anastomosis, dumping, internal herniation and incisional hernia [61,68,69].
One meta-analysis showed that patients achieved 35.3% (95% CI 24.6–46.1) excess weight loss at 12 months after duodenojejunal bypass sleeve. At 12 months, HbA1c levels decreased by −0.7 (95% CI -1.76 to 0.2, P = .16) and to −1.7 (95% CI -2.5 to −0.86, P < 0.001) at 24 weeks [70].
Other small bowel endoscopic interventions include ablation technology of superficial duodenal mucosa and self-assembling magnets that can create incisionless magnetic anastomosis directing bowel contents and bypassing certain segments of the gastrointestinal system [61]. Overall, endoscopic options for management of obesity are relatively novel and may only be available in experienced centers. Long-term safety is not well established, particularly in patients with existing CVD. Thus, there is limited data or comparison studies between different endoscopic modalities in relation to CVD outcomes. Table 2A summarizes endoscopic procedures for the management of obesity. Table 2B summarizes the effect of endoscopic procedures on CVD risk factors.
Table 2A.
Summary of Endoscopic procedures in management of obesity.
| Endoscopic procedure | Mechanism | Weight loss | Side effects |
|---|---|---|---|
| Intra-gastric balloons | Space occupying balloons | 7–15% of total body weight loss at 6 months | Nausea and vomiting |
| Endoscopic aspiration therapy | Percutaneous gastrostomy tube | 14–18% total body weight loss between 6 and 24 months | Abdominal pain and peristomal complications |
| Endoscopic Sleeve Gastroplasty (ESG) | Endoluminally placed sutures through the gastric wall. | 12–19% total body weight loss at 6–24 months duration | Nausea, vomiting and peri gastric fluids collections, and extra gastric bleeding |
| Gastrointestinal Bypass Sleeves | Liner extends into small bowel to bypass the duodenum and proximal jejunum | Achieving 49% of excess weight loss at 5 years | Overall morbidity rate of 19% at 5 years due to gastroesophageal reflux disease, stricture at the gastrojejunal anastomosis, dumping, internal herniation and incisional hernia |
| Ablation technology and self-assembling magnets | Novel therapies only available in experienced centers | N/A | N/A |
Table 2B.
Endoscopic procedures effect on cardiovascular disease risk factors.
| Endoscopic Procedure | LDL-C | FPG/HbA1c | SBP/DBP |
|---|---|---|---|
| Intra-gastric balloons | Decreased | Decreased | N/A |
| Endoscopic aspiration therapy | N/A | −1.3% at 1 year | −8/5 mmHg |
| Endoscopic Sleeve Gastroplasty | No change | mean± SD, −6.1%±1.1% | Decreased |
| Gastrointestinal Bypass Sleeves | N/A | −0.7% at 1 year −1.7% at 2 years | N/A |
4. Bariatric surgery for obesity
The National Institutes of Health have set forth indications for bariatric surgery, recommended for patients with a BMI >40 kg/m2 and for patients with BMI 35–40 kg/m2 with associated comorbid conditions who failed to sustain weight loss through non-surgical means [71]. However, 30 years after these recommendations, the American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) released updates in December of 2022. These updates include consideration of metabolic and bariatric surgery (MBS) for individuals with a BMI ≥35 kg/m2, regardless of the presence, absence, or severity of co-morbidities. MBS should also be considered for individuals with a metabolic disease and BMI of 30–34.9 kg/m2 [72]. Cardiac, pulmonary and other perioperative comorbidities that affect operative risk as well as psychological risk should be assessed prior to bariatric surgery. Furthermore, bariatric surgeries can be malabsorptive, restrictive or both [73].
4.1. Roux-en-Y gastric bypass (RYGB)
A Roux-en-Y Gastric Bypass (RYGB) is a procedure in which a gastric pouch is created by dissecting the stomach into a pouch followed by A Roux-en-Y gastrojejunostomy which diverts food from the body of the stomach, duodenum, and proximal jejunum [73]. This can be performed through an open, laparoscopic, or robotic technique and is considered a malabsorptive-restrictive surgery [74]. The mortality from gastric bypass is estimated to be 0.2%, which is higher than both sleeve gastrectomy and gastric banding [75]. If done laparoscopically, there is lower morbidity and mortality [76].
Early complications include anastomosis leak which may manifest in the first 24 h, bleeding, bowel obstruction and increased thromboembolic disease, which accounts for half of the early complications [74].
Late complications include herniation, stricture of anastomosis and fistula formation. Micronutrient malabsorption has been reported with calcium, iron, vitamin B12 and thiamin [73]. Weight loss generally ranges between 12% −45% of TBW at 6 months-3 years following surgery [77], which can be maintained >10 years following surgery [73].
In an observational prospective study assessing outcomes after gastric bypass surgery, adjusted mean weight loss from baseline was −45 kg, −36 kg and −35 kg at 2, 6 and 12 years after surgery, respectively. The rate of comorbidities remission at 12 years was better with surgery as compared with the non-surgery group, with favorable effects observed in T2DM (51% vs 5%), hypertension (36% vs 14%), and LDL-C (59% vs 6%) [78]. Incidence of T2DM at 12 years was 3% in surgery group versus 26% in non-surgery group and incidence of hypertension was 16% vs 47% [78].
4.2. Sleeve gastrectomy
Sleeve gastrectomy is performed through a longitudinal resection of the stomach creating a tubular stomach based on the lesser curvature without anastomosis [73]. Sleeve gastrectomy and gastric bypass are the two most common bariatric surgeries performed in the United States [68]. Generally, patients experience slightly less weight loss with sleeve gastrectomy compared with RYGB [73].
Accelerated gastric emptying is a common risk [73]. Studies suggest that more radical antral resection leads to faster gastric emptying which may lead to reductions in the negative feedback satiety signals produced by the presence of food in the stomach, which theoretically may inhibit weight loss [79]. Vomiting, gastroesophageal reflux disease, and hernia are common complications [68].
Laparoscopic sleeve gastrectomy has shown promising long-term results. A retrospective study evaluating 578 patients with mean baseline BMI of 42.5 ± 5.5 kg/m2 who underwent this surgery experienced mean reductions in BMI and weight loss of 33 ± 6 kg/m2 and 59 ± 30%, respectively at roughly 10 years follow up [80]. There were significant remission rates reported for hypertension (52%), dyslipidemia (58%) and T2DM (72%) [80].
In a study comparing sleeve gastrectomy and gastric bypass, mean percentage excess weight loss at 5 years was 49% (95% CI 45%–52%) after sleeve gastrectomy vs 57% (95% CI 53%–61%) after gastric bypass [68]. At 5 years follow up, there was no significant difference between gastrectomy and bypass surgery for diabetes remission and discontinuing hypertension medications, but there was a trend towards higher remission rates in the bypass group [68]. LDL-C values were lower in the gastric bypass group compared with the sleeve gastrectomy group [68].
4.3. Gastric banding
Gastric banding is done by placing a band at the proximal portion of the stomach that is adjustable and connected with a subcutaneous balloon to control the rate of gastric emptying resulting in early satiety [73]. Weight loss of 16% TBW has been reported at after 3 years [81]. A review on adjustable gastric banding reported excess weight loss of 40–65% at 3 years and37-68% excess weight loss at 5 years. These results remained consistent over 15 years of follow up [82]. Complications include gastric pouch enlargement, which can be prevented by decreasing the size of the gastric pouch volume, erosion and gastroesophageal reflux disease. Techniques are rapidly evolving to mitigate these risks [83]. Notably, gastric banding is rarely performed at many institutions [84].
4.4. Intermittent vagal blockade
The vagus nerve plays an important role in satiety, metabolism, and autonomic control in upper gastrointestinal track function [85]. Intermittent vagal blockade has been developed to establish weight loss through this mechanism and is achieved through leads placed in vagal trunk. This results in vagal blockade leading to early satiety and may avoid neural adaptations compared with truncal vagatomy. This procedure has been approved by the FDA [86]. A randomized, double-blinded, placebo-controlled trial evaluated vagal blockade in the treatment of obesity, which included 8 sites across the United States. A total of 240 participants underwent vagal nerve blockade, which resulted in a 24% reduction in weight at 12 months, compared with 16% reduction in weight in the placebo group. Mild to moderate heartburn and abdominal pain were the most common side effects reported in the vagal blockade group [85].
4.5. Biliopancreatic diversion with duodenal switch (BPD-DS)
The biliopancreatic diversion with duodenal switch is done by creating a gastrectomy, followed by an anastomosis between the proximal duodenum and a portion of bypassed intestine [73]. Weight loss was slightly greater with this procedure compared to RYGB [73]. The excess weight loss at 2, 5 and 10 years was 80.6%, 69.3%, and 67.4%, respectively. This study reported 85% and 70% complete remission of T2DM rates at 2 and 5 years, respectively [87].
4.6. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S)
Biliopancreatic diversion with duodenal switch and single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) is a hypo-absorptive bariatric procedure that is generally indicated in patients with obesity and (BMI ≥ 50 kg/m2) [88]. This surgery is similar to BPD-DS, but it requires only one anastomosis [89].
There is little data on long term outcomes for patients who have undergone SADI-S. Thus, the ASMBS released a statement in 2020 cautioned against this procedure, citing a lack of evidence [90].
4.7. Cardiometabolic effects of bariatric surgery
A meta-analysis of 73 studies demonstrated consistent benefits in CVD risk factors following bariatric surgery. LDL-C was reduced by 22%, triglycerides by 32%, and HDL-C was increased by 19% at 4 years of follow up [91]. Although these studies didn't report on the use of lipid lowering therapies, the benefits of bariatric surgery on reductions in LDL-C have been shown in 62%–86% of patients [92].
Another meta-analysis demonstrated reductions in hypertension rates of 46±8% especially when BMI was reduced by 10 kg/m2 [93]. However, these impressive results may be attenuated over time, which may be due to long standing arterial wall stiffening [94].
An open label randomized controlled trial was conducted to assess diabetes control and remission rates among patients undergoing bariatric surgery with RYGB or biliopancreatic diversion vs medical management. They included patients with a BMI of ≥35 kg/m2 and concomitant T2DM. Diabetes remission was defined as an HbA1c concentration of ≤6·5% and a FPG of ≤5·6 mmol/L at 2 years without pharmacological treatment for 1 year. Remission was achieved in 50% of the surgical arm versus 0% in the medical arm (p=0·0007) [95]. One other study showed recurrence of diabetes was as high as 58% at 15 years in the gastric banding arm compared to usual care that included advanced lifestyle modifications [96].
Weight loss has been shown to decrease inflammation via reductions in inflammatory markers such as CRP, erythrocyte sedimentation rate, interleukin-6 and an increase in adiponectin, an anti-inflammatory adipokine which plays a role in insulin sensitivity [97].
Flow-mediated dilation of the vasculature, a surrogate of vascular reactivity, was studied in a systematic review that included 269 patients. There was a significant improvement in FMD (Mean difference 5.65%; 95% CI 2.87–8.03, P<0.001) at 3 months-2 years following bariatric surgery [98]. The longer the duration of reduced vascular reactivity due to obesity, the higher the chance of recurrence following bariatric surgery [73]. Hence, early referral to obesity specialists may result in better outcomes.
A systematic review and meta-analysis that included more than 29,000 patients who underwent bariatric surgery, with a follow-up 8.5 years showed that weight loss varied from 15 to 30% depending on the bariatric surgery procedure performed. It has been reported that there was a 52% reduction in mortality (OR 0.48), 54% reduction in MI (OR 0.46) and 51% reduction in stroke (OR 0.49) [99,100].
Surgical weight loss also may reduce hard cardiovascular events. In a systematic review comparing surgical to non-surgical (e.g. intensive lifestyle intervention, standard of care, or no specific therapy) weight loss, surgical weight loss led to reductions in cardiovascular mortality (HR 0.59; 95% CI 0.47–0.73, P<0.001), all-cause mortality (HR 0.55; 95% CI 0.49–0.62, P<0.001), incident heart failure (HR 0.50; 95% CI 0.38–0.66, P<0.001), MI (HR 0.58; 95% CI 0.43–0.76, P<0.001) and stroke (HR 0.64; 95% CI 0.53–0.77, P<0.001). There was no significant association with atrial fibrillation [101]. Table 3 summarizes bariatric surgery procedures in management of obesity.
Table 3.
Summary of Bariatric surgery procedures in management of obesity:
| Bariatric surgery | Mechanism | Weight loss | Side effects |
|---|---|---|---|
| Roux-en-Y Gastric Bypass (RYGB) | A gastric pouch and a bypass that diverts food from the body of the stomach, duodenum, and proximal jejunum | 12% -45% of total body weight (TBW) loss at 6 months-3 years | Malabsorption, herniation, stricture of anastomosis, fistula |
| Sleeve Gastrectomy | Stomach is resected longitudinally creating a tubular stomach. | 49% of excess body weight at 5 years | Accelerating gastric emptying |
| Gastric banding | A band placed at the proximal portion of the stomach and connected to a subcutaneous balloon | 16% TBW at 3 years | Gastric pouch enlargement, GERD and erosion |
| Intermittent vagal blockade | Early satiety stimulation by vagal blockade | 24% of excess body weight at 1 year | Heartburn and abdominal pain |
| Biliopancreatic diversion with duodenal switch | Gastrectomy and an anastomosis between proximal duodenum and a portion of bypassed intestine | 15%, 18% and 18% of excess body weight at 2, 5 and 10 years | High incidence of short- and long-term complications with high degree of malabsorption |
5. Conclusion
Obesity is a complex disease that should be evaluated by a multidisciplinary team to provide optimal care. Management decisions should be individualized based on the available data, weight loss desired, risk profile, and surgical/medical expertise. Some endoscopic and bariatric surgery options may only be performed in experienced centers, and long-term safety and efficacy data may be lacking. Outcome trials for anti-obesity medications are still ongoing in this evolving field, which will continue to expand options for patients with obesity. Although randomized controlled trial data on cardiovascular outcomes are generally poor, observational data suggests weight loss may improve cardiovascular health. While weight loss is important, sustaining weight loss is just as critical. Early referral to experienced obesity specialists is highly recommended to avoid long term morbidity and mortality related to the growing epidemic of obesity.
Authors disclosures
RH, MAR, AM, CG report no relevant disclosures.
Author contributions
RH, MAR, AM, CG reviewed, edited, and approved the document.
Ethics review
This manuscript was peer-reviewed prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author. Signed by all authors as follows.
Acknowledgements and Funding
This manuscript received no funding or editorial assistance outside of the listed authors, the journal's reviewers and editor's comments.
References
- 1.Powell-Wiley T.M., Poirier P., Burke L.E., et al. 2021. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. vol. 360. NCHS Data Brief; 2020. (Prevalence of obesity and severe obesity among adults: United States, 2017–2018). [PubMed] [Google Scholar]
- 3.Gupta V. Indian J Endocrinol Metab; 2013. Glucagon-like peptide-1 analogues: an overview. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA. Food and Drug Administration Ozempic (semaglutide) injection prescribing information, revised. 2020. https://www.accessdata.fda.gov/drugsatfda _docs/label/2020/209637s003lbl.pdf Published online 2020.
- 5.Schaffer R., Heymsfield S.B.G.T.W. Healio website; 2021. FDA approves once-weekly semaglutide for weight loss. Published online. [Google Scholar]
- 6.Bays H.E., Fitch A., Christensen S., Burridge K., Tondt J. Anti-obesity medications and investigational agents: an obesity medicine association (OMA) clinical Practice statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/J.OBPILL.2022.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Step 1: research study investigating how well semaglutide works in People suffering from overweight or obesity - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03548935 Accessed April 15, 2023.
- 8.Wilding J.P.H., Batterham R.L., Calanna S., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMOA2032183/SUPPL_FILE/NEJMOA2032183_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 9.Semaglutide effects on heart disease and stroke in patients with overweight or obesity - full text view - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03574597 Accessed April 15, 2023.
- 10.Ryan D.H., Lingvay I., Colhoun H.M., et al. Semaglutide effects on cardiovascular outcomes in People with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020 doi: 10.1016/j.ahj.2020.07.008. Published online. [DOI] [PubMed] [Google Scholar]
- 11.Blackman A., Foster G.D., Zammit G., et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the scale sleep apnea randomized clinical trial. Int J Obes. 2016 doi: 10.1038/ijo.2016.52. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Näslund E., Barkeling B., King N., et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes. 1999 doi: 10.1038/sj.ijo.0800818. Published online. [DOI] [PubMed] [Google Scholar]
- 13.Näslund E., Gutniak M., Skogar S., Rössner S., Hellström P.M. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998 doi: 10.1093/ajcn/68.3.525. Published online. [DOI] [PubMed] [Google Scholar]
- 14.Pi-Sunyer X., Astrup A., Fujioka K., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015 doi: 10.1056/nejmoa1411892. Published online. [DOI] [PubMed] [Google Scholar]
- 15.Astrup A., Carraro R., Finer N., et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012 doi: 10.1038/ijo.2011.158. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden T.A., Hollander P., Klein S., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013 doi: 10.1038/ijo.2013.120. Published online. [DOI] [PubMed] [Google Scholar]
- 17.Astrup A., Rössner S., Van Gaal L., et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009 doi: 10.1016/S0140-6736(09)61375-1. Published online. [DOI] [PubMed] [Google Scholar]
- 18.Davies M.J., Bergenstal R., Bode B., et al. JAMA - Journal of the American Medical Association; 2015. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Published online. [DOI] [PubMed] [Google Scholar]
- 19.Mehta A., Marso S.P., Neeland I.J. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. 2017 doi: 10.1002/osp4.84. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comparison of liraglutide versus placebo in weight loss maintenance in obese subjects: SCALE - maintenance - full text view - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT00781937 Accessed April 15, 2023.
- 21.Griffioen K.J., Wan R., Okun E., et al. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011 doi: 10.1093/cvr/cvq271. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FDA. FDA.
- 23.Kristensen S.L., Rørth R., Jhund P.S., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019 doi: 10.1016/S2213-8587(19)30249-9. Published online. [DOI] [PubMed] [Google Scholar]
- 24.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021 doi: 10.1016/S0140-6736(21)00536-5. Published online. [DOI] [PubMed] [Google Scholar]
- 25.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016 doi: 10.1056/nejmoa1607141. Published online. [DOI] [PubMed] [Google Scholar]
- 26.Husain M., Birkenfeld A.L., Donsmark M., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019 doi: 10.1056/nejmoa1901118. Published online. [DOI] [PubMed] [Google Scholar]
- 27.Frías J.P., Davies M.J., Rosenstock J., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. doi: 10.1056/NEJMOA2107519/SUPPL_FILE/NEJMOA2107519_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 28.Lilly receives U.S FDA Fast Track designation for tirzepatide for the treatment of adults with obesity, or overweight with weight-related comorbidities | Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-fast-track-designation-tirzepatide Accessed March 27, 2023.
- 29.Jastreboff A.M., Aronne L.J., Ahmad N.N., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022 doi: 10.1056/NEJMOA2206038/SUPPL_FILE/NEJMOA2206038_DATA-SHARING.PDF. Published online July 21. [DOI] [PubMed] [Google Scholar]
- 30.Xenical. Xenical. Package insert. Genentech; South San Francisco, CA, USA: January 2012. [Google Scholar]
- 31.Ardissino M., Vincent M., Hines O., et al. LONG-TERM cardiovascular outcomes after orlistat therapy in patients with obesity: a nationwide, propensity score matched cohort study. J Am Coll Cardiol. 2021;77(18) doi: 10.1016/s0735-1097(21)02865-5. [DOI] [PubMed] [Google Scholar]
- 32.Heck A.M., Yanovski J.A., Calis K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20(3) doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlistat (marketed as alli and xenical) information | FDA. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/orlistat-marketed-alli-and-xenical-information Accessed March 20, 2023.
- 34.Gorgojo-Martínez J.J., Basagoiti-Carreño B., Sanz-Velasco A., Serrano-Moreno C., Almodóvar-Ruiz F. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: the XENSOR Study. Int J Clin Pract. 2019;73(11) doi: 10.1111/ijcp.13399. [DOI] [PubMed] [Google Scholar]
- 35.DailyMed National Library of Medicine . Naltrexone. Bupropion SR; Jan, 2010. DailyMed national library of medicine. Published online 2010. [Google Scholar]
- 36.Greenway F.L., Fujioka K., Plodkowski R.A., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741) doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 37.Ornellas T., Chavez B. Naltrexone SR/bupropion SR (Contrave): a new approach to weight loss in obese adults. P T. 2011;36(5) [PMC free article] [PubMed] [Google Scholar]
- 38.Apovian C.M. Naltrexone/bupropion for the treatment of obesity and obesity with Type 2 diabetes. Future Cardiol. 2016;12(2) doi: 10.2217/fca.15.79. [DOI] [PubMed] [Google Scholar]
- 39.Hollander P., Gupta A.K., Plodkowski R., et al. Effects of naltrexone sustained- release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/DC13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadden T.A., Foreyt J.P., Foster G.D., et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2011;19(1) doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sposito A.C., Bonilha I., Luchiari B., et al. Cardiovascular safety of naltrexone and bupropion therapy: systematic review and meta-analyses. Obes Rev. 2021;22(6) doi: 10.1111/obr.13224. [DOI] [PubMed] [Google Scholar]
- 42.Sherman M.M., Ungureanu S., Rey J.A. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. Pharmacy and Therapeutics. 2016;41(3):164. Accessed March 22, 2023./pmc/articles/PMC4771085/ [PMC free article] [PubMed] [Google Scholar]
- 43.FDA approves weight-loss drug Contrave - harvard Health. https://www.health.harvard.edu/blog/fda-approves-weight-loss-drug-contrave-201409127431 Accessed March 20, 2023.
- 44.Another CV outcomes trial testing weight-loss drug contrave terminated early | tctmd.com. https://www.tctmd.com/news/another-cv-outcomes-trial-testing-weight-loss-drug-contrave-terminated-early Accessed April 20, 2023.
- 45.Jordan J., Astrup A., Engeli S., Narkiewicz K., Day W.W., Finer N. Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. J Hypertens. 2014;32(6) doi: 10.1097/HJH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ionamin. Package insert. Ionamin. Package insert. UCB, Inc.; Smyrna, GA, USA: December 2012. Published online 2012. [Google Scholar]
- 47.Xu Y., O'Brien W.G., Lee C.C., Myers M.G., Tong Q. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology. 2012;153(5) doi: 10.1210/en.2011-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allison D.B., Gadde K.M., Garvey W.T., et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity. 2012;20(2) doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garvey W.T., Ryan D.H., Look M., et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2) doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan J TB. Cardiovascular effects and weight loss in three 1-year, double-blind, placebo-controlled clinical trials with extended-release phentermine/topiramate in obese patients. In: 22nd annual congress of the European society of hypertension (ESH).
- 51.Jordan J, Astrup A DW. Cardiovascular safety of phentermine alone and in combination with topiramate. In: 19th annual European congress on obesity (ECO).
- 52.Davidson M.H., Tonstad S., Oparil S., Schwiers M., Day W.W., Bowden C.H. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2. Am J Cardiol. 2013;111(8) doi: 10.1016/j.amjcard.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 53.Hendricks E.J., Greenway F.L., Westman E.C., Gupta A.K. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity. 2011;19(12) doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 54.Bays H.E., Lazarus E., Primack C., Fitch A. Obesity pillars roundtable: phentermine – Past, present, and future. Obesity Pillars. 2022;3 doi: 10.1016/J.OBPILL.2022.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis K.H., Fischer H., Ard J., et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity. 2019;27(4):591–602. doi: 10.1002/OBY.22430. [DOI] [PubMed] [Google Scholar]
- 56.Ritchey M.E., Harding A., Hunter S., et al. Cardiovascular safety during and after use of phentermine and topiramate. J Clin Endocrinol Metab. 2019;104(2):513. doi: 10.1210/JC.2018-01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bays H.E. What about that 2022 ICER report on anti-obesity medications? Obesity Pillars. 2022;4 doi: 10.1016/J.OBPILL.2022.100038. [DOI] [Google Scholar]
- 58.Khera R., Murad M.H., Chandar A.K., et al. Association of pharmacological treatments for obesity withweight loss and adverse events a systematic review and meta-analysis. JAMA, J Am Med Assoc. 2016;315(22) doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tak Y.J., Lee S.Y. Anti-obesity drugs: long-term efficacy and safety: an updated review. World Journal of Men’s Health. 2020;38 doi: 10.5534/WJMH.200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imbus J.R., Voils C.I., Funk L.M. Bariatric surgery barriers: a review using andersen's model of health services use. Surg Obes Relat Dis. 2018;14(3) doi: 10.1016/j.soard.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glass J., Chaudhry A., Zeeshan M.S., Ramzan Z. New era: endoscopic treatment options in obesity-a paradigm shift. World J Gastroenterol. 2019;25(32) doi: 10.3748/wjg.v25.i32.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abu Dayyeh B.K., Edmundowicz S., Thompson C.C. Clinical Practice update: expert review on endoscopic bariatric therapies. Gastroenterology. 2017;152(4) doi: 10.1053/j.gastro.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 63.Reshape and Orbera — two gastric balloon devices for weight loss. Med Lett Drugs Ther. 2015;57(1476) [PubMed] [Google Scholar]
- 64.Muniraj T., Day L.W., Teigen L.M., et al. AGA clinical Practice Guidelines on intragastric balloons in the management of obesity. Gastroenterology. 2021;160(5):1799–1808. doi: 10.1053/J.GASTRO.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Dixon J.B., Eaton L.L., Vincent V., Michaelson R. LAP-BAND for BMI 30-40: 5-year health outcomes from the multicenter pivotal study. Int J Obes. 2016;40(2) doi: 10.1038/ijo.2015.156. [DOI] [PubMed] [Google Scholar]
- 66.Jirapinyo P., de Moura D.T.H., Horton L.C., Thompson C.C. Effect of aspiration therapy on obesity-related comorbidities: systematic review and meta-analysis. Clin Endosc. 2020;53(6):686. doi: 10.5946/CE.2019.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharaiha R.Z., Kumta N.A., Saumoy M., et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol. 2017;15(4):504–510. doi: 10.1016/J.CGH.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Salminen P., Helmio M., Ovaska J., et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss at 5 Years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi: 10.1001/JAMA.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salminen P., Helmio M., Ovaska J., et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss at 5 Years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241. doi: 10.1001/JAMA.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abu Dayyeh B.K., Kumar N., Edmundowicz S.A., et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82(3):425–438. doi: 10.1016/J.GIE.2015.03.1964. e5. [DOI] [PubMed] [Google Scholar]
- 71.Kuczmarski R.J., Flegal K.M. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5) doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 72.Eisenberg D., Shikora S.A., Aarts E., et al. American society for metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;18(12):1345–1356. doi: 10.1016/J.SOARD.2022.08.013. 2022. [DOI] [PubMed] [Google Scholar]
- 73.Wolfe B.M., Kvach E., Eckel R.H. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118(11) doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell B.G., Gupta N. Roux-en-Y gastric bypass. The SAGES Manual: A Practical Guide to Bariatric Surgery. 2022:87–100. doi: 10.1007/978-0-387-69171-8_12. Published online May 29. [DOI] [Google Scholar]
- 75.Hutter M.M., Schirmer B.D., Jones D.B., et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–422. doi: 10.1097/SLA.0B013E31822C9DAC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wehrtmann F.S., de la Garza J.R., Kowalewski K.F., et al. Learning curves of laparoscopic roux-en-Y gastric bypass and sleeve gastrectomy in bariatric surgery: a systematic review and introduction of a standardization. Obes Surg. 2020;30(2):640–656. doi: 10.1007/S11695-019-04230-7. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell J.E., Selzer F., Kalarchian M.A., et al. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis. 2012;8(5) doi: 10.1016/j.soard.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams T.D., Davidson L.E., Litwin S.E., et al. Weight and metabolic outcomes 12 Years after gastric bypass. N Engl J Med. 2017;377(12):1143–1155. doi: 10.1056/NEJMOA1700459/SUPPL_FILE/NEJMOA1700459_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sioka E., Tzovaras G., Perivoliotis K., et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract. 2018;2018 doi: 10.1155/2018/4135813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakran N., Soifer K., Hod K., et al. Long-term reported outcomes following primary laparoscopic sleeve gastrectomy. Obes Surg. 2023;33(1):117–128. doi: 10.1007/S11695-022-06365-6/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5) doi: 10.1056/nejmoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Brien P.E., Hindle A., Brennan L., et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3–14. doi: 10.1007/S11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furbetta N., Cervelli R., Furbetta F. Laparoscopic adjustable gastric banding, the past, the present and the future. Ann Transl Med. 2020;8(Suppl 1) doi: 10.21037/ATM.2019.09.17. S4-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.The lap band for weight loss is a tale of medicine gone wrong - vox. https://www.vox.com/science-and-health/2017/5/25/15659878/weight-loss-surgery-lap-band-evidence Accessed April 20, 2023.
- 85.Ikramuddin S., Blackstone R.P., Brancatisano A., et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312(9):915–922. doi: 10.1001/JAMA.2014.10540. [DOI] [PubMed] [Google Scholar]
- 86.Shikora S.A., Wolfe B.M., Apovian C.M., et al. Sustained weight loss with vagal nerve blockade but not with sham: 18-month results of the ReCharge trial. J Obes. 2015;2015 doi: 10.1155/2015/365604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorribas M., Casajoana A., Sobrino L., Admella V., Osorio J., Pujol-Gebellí J. Experience in biliopancreatic diversion with duodenal switch: results at 2, 5 and 10 years. Cir Esp. 2021;100(4):202–208. doi: 10.1016/J.CIRESP.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Pennestrì F., Sessa L., Prioli F., et al. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S): experience from a high-bariatric volume center. Langenbeck's Arch Surg. 2022;407(5):1851. doi: 10.1007/S00423-022-02501-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sánchez-Pernaute A., Rubio Herrera M.A., Pérez-Aguirre E., et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17(12):1614–1618. doi: 10.1007/S11695-007-9287-8. [DOI] [PubMed] [Google Scholar]
- 90.Kallies K., Rogers A.M. American Society for Metabolic and Bariatric Surgery updated statement on single-anastomosis duodenal switch. Surg Obes Relat Dis. 2020;16(7):825–830. doi: 10.1016/J.SOARD.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 91.Vest A.R., Heneghan H.M., Agarwal S., Schauer P.R., Young J.B. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24) doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 92.Courcoulas A.P., Christian N.J., Belle S.H., et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA, J Am Med Assoc. 2013;310(22) doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ricci C., Gaeta M., Rausa E., Asti E., Bandera F., Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25(3) doi: 10.1007/s11695-014-1442-4. [DOI] [PubMed] [Google Scholar]
- 94.Sjöström L., Lindroos A.K., Peltonen M., et al. Lifestyle, diabetes, and cardiovascular risk factors 10 Years after bariatric surgery. N Engl J Med. 2004;351(26) doi: 10.1056/nejmoa035622. [DOI] [PubMed] [Google Scholar]
- 95.Mingrone G., Panunzi S., De Gaetano A., et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 Year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997) doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 96.Carlsson L.M.S., Peltonen M., Ahlin S., et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8) doi: 10.1056/nejmoa1112082. [DOI] [PubMed] [Google Scholar]
- 97.Esposito K., Pontillo A., Di Palo C., et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. J Am Med Assoc. 2003;289(14) doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 98.Lupoli R., Di Minno M.N.D., Guidone C., et al. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: a meta-analysis of literature studies. Int J Obes. 2016;40(3) doi: 10.1038/ijo.2015.187. [DOI] [PubMed] [Google Scholar]
- 99.Sjöström L., Narbro K., Sjöström C.D., et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8) doi: 10.1056/nejmoa066254. [DOI] [PubMed] [Google Scholar]
- 100.Kwok C.S., Pradhan A., Khan M.A., et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1) doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 101.van Veldhuisen S.L., Gorter T.M., van Woerden G., et al. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehac071. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]