Abstract
Background
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) provides clinicians an overview of sleep-disordered breathing, (e.g., sleep-related hypopnea, apnea), and other obesity-related sleep disorders.
Methods
The scientific support for this CPS is based upon published citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
Results
Obesity contributes to sleep-disordered breathing, with the most prevalent manifestation being obstructive sleep apnea. Obesity is also associated with other sleep disorders such as insomnia, primary snoring, and restless legs syndrome. This CPS outlines the evaluation, diagnosis, and treatment of sleep apnea and other sleep disorders, as well as the clinical implications of altered circadian system.
Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on “Sleep-Disordered Breathing, Sleep Apnea, and Other Obesity-Related Sleep Disorders” is one of a series of OMA CPSs designed to assist clinicians in the care of patients with the disease of obesity.
Keywords: Clinical practice statement, Obesity, Pre-obesity, Sleep apnea, Sleep disorders
1. Introduction
Beginning in 2013, the Obesity Medicine Association (OMA) created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees [1]. This was followed by a similar Pediatric “Obesity Algorithm” with updates approximately every two years by OMA authors. This current OMA CPS regarding “Sleep-Disordered Breathing, Sleep Apnea, and Other Obesity-Related Sleep Disorders” was largely derived from the 2021 OMA Adult Obesity Algorithm and is one of a series of OMA CPSs designed to assist clinicians in the care of their patients with the disease of obesity.
In general, sleep disturbances are common among US adults, with approximately 30% experiencing 1 hour or more of sleep debt, 47% experiencing 1 hour or more of social jet lag (i.e., discrepant sleep timing between work days and free days), 30% having trouble sleeping, and 27% having daytime sleepiness [2]. Obesity can produce both “sick fat disease” (i.e., metabolic dysfunction or adiposopathy) and “fat mass disease” [3]. Among obesity-related sleep disorders (e.g., sleep-disordered breathing, primary snoring, insomnia, and restless legs syndrome) obstructive sleep apnea (OSA) is especially illustrative of sleep-disordered breathing, where the fat mass disease of obesity substantially contributes to its pathogenesis. Patients with overweight/obesity and OSA often have increased fat deposition in the tongue [4], pharyngeal airway narrowing [5], and/or increased abdominal obesity [6]. These adverse anatomical changes in sleep architecture may result in sleep deprivation, resulting in a potential increase in ghrelin levels and decrease in leptin levels, resulting in increased appetite [7,8]. This sets up a vicious cycle of impaired sleep, increased appetite, weight gain, and further sleep dysfunction. Other adverse consequences of obesity-related sleep disorders include more time to eat, desire for less healthful food choices, lower body temperature, and curbs on physical activity [9]. In summary, unhealthful dietary intake and physical inactivity may increase adiposity and promote the onset or worsening of OSA and insomnia [10]. Conversely, more healthful nutritional intake and lower body mass index (BMI) are associated with longer and more regular sleep duration [11].
2. Obesity and obstructive sleep apnea
Sleep-disordered breathing can be due to locally obstructive causes (e.g., repetitive narrowing or closure of upper airway during sleep) or due to central nervous system causes (i.e., Cheyne-Stokes breathing and centrally-mediated apneic episodes) [19,20]. Apnea, hypopnea, and respiratory effort-related sleep arousal can be diagnosed via polysomnography [19,21,22]:
Obstructive sleep apnea (OSA) is an example of sleep-disordered breathing defined as recurrent apnea or hypopnea due to complete or partial collapsed/blocked airways during sleep, resulting in intermittent hypoxemia, and autonomic fluctuation [21]. Higher BMI, greater waist circumference, and increased waist-to-height ratio are associated with an increased risk of OSA [23]. Approximately 50% of patients with OSA have obesity [19]. OSA may occur as high as 45% or more of patients with obesity [24,25]. OSA occurs in more than 40% of patients with cardiovascular disease [19,21]. Beyond obesity, other risk factors for OSA include older age, male (i.e., 2–4 times more prevalent than in females), postmenopausal females not treated with hormone replacement therapy, family history of OSA, Asian descent, narrow oropharyngeal airways, recessed jaw, brachycephalic head form, high-arched and narrow palate, large tongue, excessive throat tissue, systemic inflammation (i.e., elevated c-reactive protein, interleukin-6), and insulin and/or leptin resistance [19].
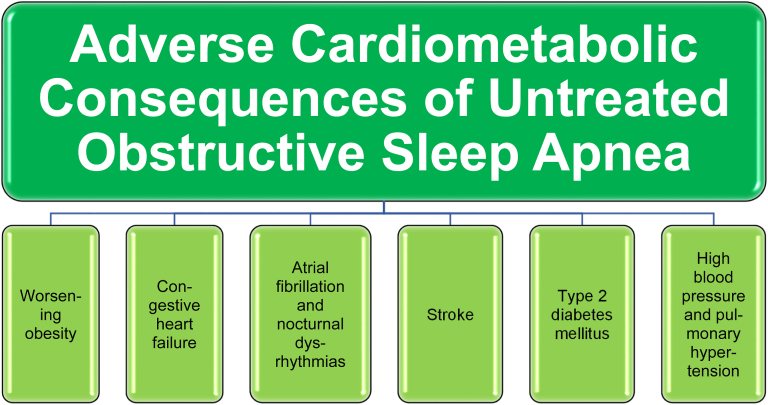
Pathophysiologic consequences of OSA include sleep disruption, swings in intrathoracic pressure, cyclical hypoxemia and reoxygenation, sympathetic system activation, endothelial dysfunction, and increased risk of thrombosis [19]. The fat mass adverse anatomic effects of obesity coupled with adiposopathic-induced arterial hypoxia may increase stress hormone release (sympathetic nervous system and cortisol), increase inflammatory cytokines, and may contribute to cardiopulmonary abnormalities and risk including peripheral edema, cardiac dysrhythmia, high blood pressure, insulin resistance, and hyperglycemia [12,13]. Poor sleep quality with OSA is linked to lower quality of life, worse mental health, and an increase in cardiovascular disease risk [20]. In short, untreated OSA may lead to worsening obesity, congestive heart failure, atrial fibrillation, nocturnal dysrhythmias, stroke, hypertension, type 2 diabetes mellitus, and pulmonary hypertension [12,13] (See Fig. 1).
Fig. 1.
Adverse Cardiometabolic Consequences of Untreated Obstructive Sleep Apnea. Shown are illustrative potential consequences of untreated obstructive sleep apnea [[12], [13], [14], [15], [16], [17]].
Another example of sleep-disordered breathing is central sleep apnea, which involves transient cessation of brainstem-mediate respiratory drive [26]. Cheyne-Stokes breathing is characterized by a gradual increase and then decrease in breathing effort and airflow with cyclical lengths of 30–60 seconds [19,27]. Central sleep apnea in can sometimes occur in premature newborns, as well as infants with brain injuries or brain masses, such as Chiari malformations (i.e., cerebellar brain tissue extension through the foramen magnum at the base of the skull into the spinal canal), or with neurologic-acting pharmacologic treatments. Compared to OSA, central sleep apnea usually occurs in older individuals, is less directly related to obesity, and more related to central nervous system disorders (e.g., central nervous system infection, brainstem/high cervical cord injury, stroke, and use of narcotics) [19]. In older individuals, central sleep apnea is associated with congestive heart failure, atrial fibrillation, stroke, high altitudes (lower oxygen), end-stage kidney disease, Parkinson's disease, multiple system dysfunction (especially if the brainstem is involved), and drug-induced apnea (e.g., opioids). Some causes of central apnea are unknown [i.e., idiopathic (primary) central sleep apnea]. Finally, some patients with OSA may develop central sleep apnea after continuous positive airway pressure (CPAP), tracheostomy, or oral appliance treatment for OSA treatment (i.e., complex sleep apnea) [27]. Thus, patients with obesity can have elements of both obstructive and central sleep apnea.
Beyond sleep-disorded breathing, obesity hypoventilation syndrome (OHS) is another obesity-related breathing disorder characterized by wakeful impairment of alveolar respiratory gas exchange, often clinically manifested by obesity, daytime sleepiness, daytime hypoventilation (decreased oxygen, increased carbon dioxide blood levels with cyanosis), and OSA [28]. Diagnosis can include Partial Pressure of Carbon Dioxide (PaCO2) >55 mmHg for >10 min or an increase in PaCO2 >10 mmHg compared to an awake supine value to a value > 50 mmHg for >10 min [28]. OHS is also commonly termed Pickwickian syndrome, named after a Charles Dickens essay (i.e., The Posthumous Papers of the Pickwick Club) where the character “Joe” had obesity and would uncontrollably fall asleep during the day [29,30]. OHS is often a diagnosis of exclusion (i.e., absence of an alternative neuromuscular, mechanical, or metabolic explanation) [28]. As opposed to OSA, OHS typically has prolonged daytime hypercapnia. That said, approximately 90% of patients with OHS have OSA [30]. OHS is often accompanied by hypertension, heart failure, coronary disease, and pulmonary hypertension [28]. Treatment for most patients with OHS and OSA usually follows treatment approaches for OSA (i.e., continuous positive airway pressure or CPAP). Treatment of the less common patient with OHS without OSA includes noninvasive ventilation (NIV) via masks with varying inspiratory and expiratory pressures that somewhat mimic mechanical ventilation [28] (i.e., sometimes termed biphasic or bilevel positive airway pressure or BPAP). NIV may also be considered in patients with OHS and milder forms of OSA, especially if initial treatment with CPAP results in an unfavorable response despite high levels of adherence [28,31].
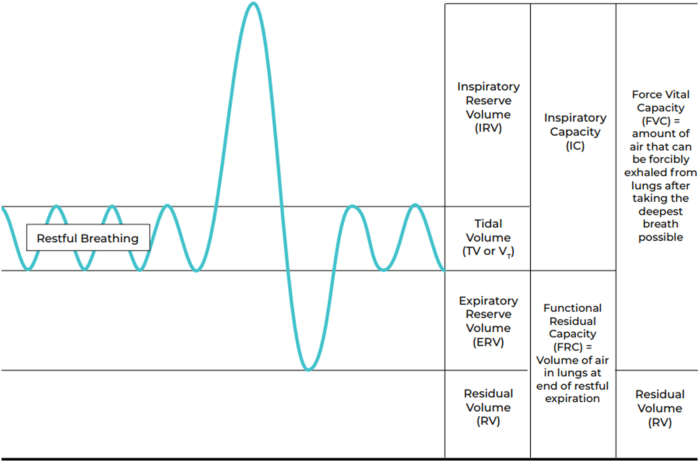
Obesity-associated anatomic and functional lung complications that contribute to obesity-related breathing disorder include compromised static lung volumes (i.e., diminished total lung capacity, functional residual capacity, residual volume), dynamic lung volumes (decreased forced vital capacity, forced expiratory volume in 0.5 second and 1.0 second), and impaired alveolar ventilation-perfusion (See Fig. 2). Weight reduction among patients with obesity often improves static lung volumes and may improve dynamic lung volumes such as improved forced vital capacity forced vital capacity [i.e., forced expiratory volume in one second (FEV1)] [32,33].
Fig. 2.
Lung volumes. Obesity can contribute to disordered breathing and hypoxia via compromised static lung volumes with obesity (i.e., diminished total lung capacity, functional residual capacity, residual volume), dynamic lung volumes (decreased forced vital capacity, forced expiratory volume in 0.5 second and 1.0 second), and impaired alveolar ventilation-perfusion. [18].
2.1. Obstructive sleep apnea: patient history
Patients with OSA may exhibit the following symptoms [12,13,29]:
-
•
Snoring (usually loudly)
-
•
Insomnia
-
•
Restless sleep
-
•
Sudden wakening with choking or gasping
-
•
Headaches
-
•
Daytime sleepiness
-
•
Fatigue
-
•
Nocturia
-
•
Motor vehicle accidents (a potential complication of sleep disorders)
-
•
Forgetfulness
-
•
Mood changes
-
•
Lack of interest in sexual behavior
-
•
Gastroesophageal reflux
2.2. Obstructive sleep apnea: physical findings
Patients with OSA may have diagnostic physical characteristics that include [12,13,[34], [35], [36]]:
-
•
Increased neck circumference (i.e., ≥17 inches/43 centimeters for men and ≥16 inches/40.5 centimeters for women)
-
•
Throat obstruction: the Mallampati Scoring System (MSS) was originally designed as an anesthesia assessment to identify patients presenting with a potentially difficult endotracheal intubation. The Modified MSS and the Friedman Tongue Position (FTP) are subjective assessments of pharyngeal opening size. The MMSS and FTP are derived by having the patient open the mouth with the tongue resting in the neutral position, and then assessing the degree of throat obstruction based upon throat anatomy (e.g., the degree the uvula and soft palate can be seen). The MMSS and FTP can be useful when assessing OSA risk; class 3 or 4 findings are most associated with OSA [37,38].
-
•
Retrognathia
-
•
Overbite
-
•
Nasal abnormalities
-
•
Macroglossia
-
•
Tonsillar hypertrophy
-
•
Lateral peritonsillar narrowing
-
•
Enlarged uvula
-
•
High and arched/narrow palate
2.3. Obstructive sleep apnea: diagnosis
2.3.1. Questionnaires that may aid in the diagnosis of OSA include: [39,40]
-
•
The Epworth Sleepiness Scale is an eight-question survey that measures daytime sleepiness (“dozing”) during 8 specified situations. The score ranges from 0 to 24 and more than 9 points suggests need for further evaluation for OSA.
-
•
STOP-BANG Questionnaire (STOP = Snoring, Tiredness, Observed apnea and high blood Pressure; BANG = BMI, Age, Neck circumference, Gender). The STOP-BANG Questionnaire may have the highest sensitivity in diagnosing mild, moderate, and severe OSA [29,40]. Each category is scored 1 point and a score of >3 points suggests a high risk for OSA [29].
-
•
The Berlin Sleep Questionnaire is a self-reported, ten question instrument that includes height, weight, age, gender, snoring behavior, observations of apnea, wake-time sleepiness or fatigue, falling asleep while driving a vehicle, obesity and hypertension. The Berlin Sleep Questionnaire has three different categories; a patient is at high risk for OSA when two of the three categories reach a high-risk score threshold [29].
2.3.2. Sleep testing
Beyond questionnaires, more formal testing for OSA includes in-lab overnight sleep studies (i.e., polysomnography), home sleep studies, and wearable technologies [12,13,21]. The apnea-hypopnea index (AHI) is the number of apnea or hypopnea events during the sleep period, divided by the number of hours of sleep. OSA definitions include:
-
•
Apnea: Cessation of airflow (reduction in peak signal excursion ≥90% of pre-event baseline) for ≥10 seconds
-
•Hypopnea:
- o One definition: ≥ 30% reduction in airflow for ≥10 seconds with (a) ≥ 4% drop in partial pressure of arterial oxygen (PaO2) or (b) cortical arousal from sleep
- o Alternative definition: ≥ 50% reduction of airflow for ≥10 seconds with (a) ≥3% oxygen desaturation or EEG microarousal or (b) if reduction in peak signal excursion ≥50% of pre-event baseline ≥4% oxygen desaturation
-
•
Mild sleep apnea: AHI of 5–15 events/hour
-
•
Moderate sleep apnea: AHI of 15–30 events/hour
-
•
Severe sleep apnea: AHI of >30 events/hour
-
•
Sleep apnea in children: AHI of 1–4.9 is mild OSA, AHI 5 to 9.9 is moderate OSA, and AHI ≥10 severe OSA
-
•Respiratory effort-related sleep arousal (RERA):
- o Increasing respiratory effort for ≥10 s leading to an arousal from sleep
- o Does not fulfill the criteria for a hypopnea or apnea [21]
Polysomnography is a multiparametric in-lab sleep test, usually performed at night at a designated sleep evaluation location, supervised by an overnight technologist. As opposed to the home testing, polysomnography measures brain activity, and thus measures sleep stages, wakefulness, sleep duration, sleep fragmentation, sleep efficiency, and sleep quality by assessing sleep and vital sign parameters during sleep. The procedure typically involves multiple wired attachments to the patient (i.e., 22 or more). Measured parameters typically include:
-
•
Respiratory effort as assessed by elastic belts to measure thoracoabdominal movements
-
•
Airflow nasal pressure and nasal temperature via pressure transducers and/or thermocouple fitted in or near the nostrils; capnography measures expired carbon dioxide
-
•
Arterial oxygen saturation via pulse oximetry
-
•
Heart rate and rhythm via electrocardiogram
-
•
Electroencephalography via surface electrodes placed on the scalp
-
•
Electrooculography via electrodes placed on the outer canthus of each eye
-
•
Electromyography via surface electrodes placed on the chin muscle (submental)
-
•
Video recording
Polysomnography scoring involves 30-second “epochs” (points of time). A split night sleep study typically includes the standard polysomnography the first half of the night (diagnostic), followed by polysomnography after implementation of continuous positive airway pressure (CPAP) for the second half of the same night (treatment titration). Optional parameters that can also be measured along with polysomnography include esophageal pH, penile tumescence, and core body temperature.
More limited evaluation of sleep apnea-relevant data can be obtained without a lab stay via wearable technologies such as a headband device and/or watch monitor that extends to a finger for peripheral arterial tonometry. Most home sleep apnea evaluations involve shipped equipment or supplied by a clinician or medical facility and are studies usually reserved for straightforward confirmation of the diagnosis of moderate to severe OSA in patients without clinically meaningful comorbidities. Measured parameters vary, but home sleep apnea testing (HSAT) may include [21]:
-
•
Airflow and breathing pattern and breathing effort via nasal breathing sensors and abdominal effort belt
-
•
Heart rate and oxygen blood level via peripheral arterial tonometry (i.e., measures arterial pulsatile volume at fingertip), pulse oximetry (i.e., measures blood oxygen), and actigraphy (i.e., measures body movement)
-
•
Snoring frequency/volume via microphone
-
•
Assessment of AHI
Home polysomnography can help confirm the diagnosis of OSA. Provided the recommendation of a more formalized and thorough lab sleep apnea test is not inevitable irrespective of the results of the home sleep apnea test, then advantages of home polysomnography included improved patient comfort, improved convenience, and reduced expense. However, patient adherence may be lower and discontinuation rates higher with home sleep apnea setups [41], limiting its potential utility [42]. If the index of suspicion for OSA is high, and if a home sleep study is negative, then this might best be confirmed by an in-lab study.
2.4. Obstructive sleep apnea treatment [21]
Treatment for OSA in patients with overweight or obesity includes reduction of fat mass via healthful nutrition, physical activity, anti-obesity medications, bariatric surgery, as well as behavior therapy to improve sleep patterns and positional therapy [21]. Other treatments include continuous positive airway pressure (CPAP), biphasic/bilevel positive airway pressure (BPAP), oral appliances such as mandibular reposition devices or tongue-retaining devices, nasal expiratory positive airway devices, adaptive servo-ventilation, and neurostimulation [19,21,43]. Interventions such as weight reduction, physical exercise and positive airway pressure therapy may improve daytime drowsiness and quality of life in patients with OSA [19,[44], [45], [46]].
Positive airway pressure (PAP) is the preferred treatment for OSA [47] Among patients with overweight/pre-obesity or obesity, weight reduction and positional therapies may be beneficial as adjunctive therapy or when PAP is not selected through shared decision-making [47,48]. Insufficient sleep contributes to weight gain through appetitive hormones resulting in increased hunger and increased energy intake, as well as changes in eating behaviors; sleep extension may reverse these unhealthful consequences, though the latter effect not always clear [49]. The American Academy of Sleep Medicine (AASM) recommends cognitive behavioral therapy for insomnia as well as other behavioral therapies to improve sleep habits [50]. The AASM found only weak evidence to support the use of prescription pharmacologic agents specifically to improve sleep as well as weak evidence against the use of over-the-counter agents such as melatonin and diphenhydramine [51]. Among patients with overweight/pre-obesity or obesity, weight loss provides symptomatic improvement in patients with OSA [52] and reduces CPAP pressure requirements in patients undergoing bariatric surgery [53]Weight loss from bariatric surgery also reduces both the prevalence and severity of OSA [54].
Non-bariatric surgery is also an option for treatment of OSA; surgical options include nasal septoplasty, adenotonsillectomy, uvulopalatoplasty, maxillomandibular advancement, laser-assisted uvulopalatoplasty, radiofrequency ablation, and palatal implants [43,55]. However, the benefit of some of these procedures may be attenuated over time, especially in patients with higher BMI [56]. Studies of implanted hypoglossal nerve stimulation devices for OSA have long-term benefit [57] for the indicated use in patients with BMI ≤32kg/m2, with evidence also supporting benefit in patients with a higher BMI [58]. In appropriately diagnosed children, OSA is often improved with tonsillectomy and adenoidectomy [59].
3. Obesity and other sleep disorders
3.1. Other obesity-related sleep disorders
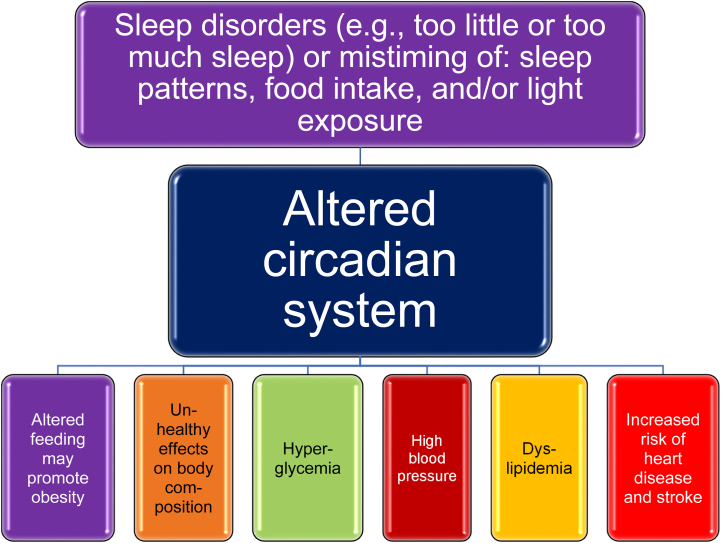
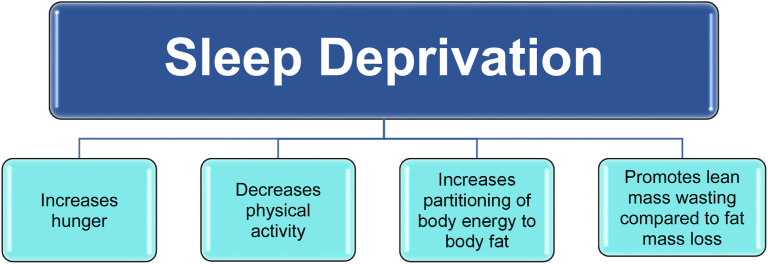
Sleep disruption, including too much sleep, too little sleep, or mistiming of sleep, may lead to an altered circadian system and the promotion of obesity via altered food intake [[60], [61], [62], [63]] (See Fig. 3). Short sleep duration (less than 6 hours per night), long sleep duration (more than 9 hours per night), and insomnia symptoms are associated with obesity and central obesity [60,64]. Sleep disruption due to obesity can promote further obesity (See Fig. 4). Sleep deprivation may increase hunger (especially for energy dense foods), decrease physical activity, increase partitioning of body energy to body fat (i.e., particularly abdominal or visceral fat), and promote lean mass wasting compared to fat mass loss (See Fig. 4) [15,49,63]. A diurnal rhythm is an endogenous or exogenous response synchronized to daytime/nighttime within a 24-hour day. A circadian rhythm (sleep/wake cycle) is an endogenously generated response synchronized to a 24-hour day [24]. Alterations in light exposure may lead to an altered circadian system [18,19]. Altered circadian rhythms may lead to a variety of negative outcomes and may promote the development of obesity, as shown in Fig. 3 [[17], [18], [19], [20]]. Delayed sleep phase syndrome is manifested by the habitual inability to fall asleep before 2 AM and may be related to circadian sleep disruption from bright light deficiency and genetic predisposition. Treatment may include bright light therapy [65].
Fig. 3.
Sleep Disruption and Obesity. Shown are the potential effects of sleep disruption on cardiometabolic risk factors and cardiovascular outcomes [[60], [61], [62], [63]].
Fig. 4.
Effects of Sleep Deprivation. Shown are the potential effects of sleep deprivation and potential contributions to obesity [63].
Obesity-related restless legs syndrome [66] can disrupt sleep or make it difficult to fall asleep. Restless legs syndrome is associated with obesity, attention deficit disorder, iron deficiency, and may be the result of autosomal dominant inheritance. Restless legs syndrome is associated with a significant decrease in quality of life [67], and the sleep disruptions caused by restless legs syndrome may lead to a worsening of obesity [61]. Prolonged dopaminergic treatment may result in a worsening of symptoms (i.e., augmentation). Other potential treatments include pregabalin, gabapentin enacarbil, oxycodone-naloxone, and iron preparations [68].
Primary snoring (i.e., simple or non-apnoeic snoring) is highly prevalent in the general population, and is an example of sleep-disordered breathing, without severe medical consequences for the snorer and co-sleeper [69]. An initial evaluation might best include: “Do you snore,” which may be the most diagnostically sensitive question when assessing the risk of snoring disorders, including OSA [34]. If severe or thought to contribute to neuropsychological impairment, then sleep studies should be considered. If OSA is diagnosed, then in addition to fat mass reduction, the OSA is best treated as noted before.
4. Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on “Sleep-Disordered Breathing, Sleep Apnea, and Other Obesity-Related Sleep Disorders” is one of a series of OMA CPSs designed to assist clinicians in better understanding the physiology and treatment of obesity. The information presented in this CPS may aid clinicians in improving the health and well-being of their patients with obesity and who may also have OSA or sleep-disordered breathing, especially those with metabolic, physiological, and psychological complications.
4.1. Transparency [70]
This manuscript was derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. This was followed by a similar Pediatric “Obesity Algorithm,” with updates approximately every two years by OMA authors. Authors of prior years’ version of the Obesity Algorithm are included in Supplement #1.
4.2. Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. (Supplement #1) The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
After receipt of a draft transcription of the applicable section of the 2021 OMA Obesity Algorithm, HEB revised and updated the manuscript. The revised manuscript was reviewed, edited, and approved by NP, JT, LG, and KY.
Managing Disclosures and Dualities of Interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Disclosures
NP reports no relevant disclosures.
LG reports no relevant disclosures.
KY reports no relevant disclosures.
JT reports no relevant disclosures.
HEB reports no relevant disclosures.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Acknowledgements and Funding
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Contributor Information
Nicholas Pennings, Email: pennings@campbell.edu.
Leslie Golden, Email: lesliemgolden0401@gmail.com.
Kanica Yashi, Email: kanica.yashi@bassett.org.
Justin Tondt, Email: justintondt@gmail.com.
Harold Edward Bays, Email: http://www.lmarc.com.
References
- 1.Bays H.E., McCarthy W., Burridge K., Tondt J., Karjoo S., Christensen S., Ng J., Golden A., Davisson L., Richardson L., Obesity Algorithm eBook https://obesitymedicine.org/obesity-algorithm/ presented by the Obesity Medicine Association. www.obesityalgorithm.org. 2021.
- 2.Di H., Guo Y., Daghlas I., Wang L., Liu G., Pan A., et al. Evaluation of sleep habits and disturbances among US adults, 2017-2020. JAMA Netw Open. 2022;5:e2240788–e. doi: 10.1001/jamanetworkopen.2022.40788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim A.M., Keenan B.T., Jackson N., Chan E.L., Staley B., Poptani H., et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelton K.E., Woodson H., Gay S., Suratt P.M. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 6.Leone N., Courbon D., Thomas F., Bean K., Jego B., Leynaert B., et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K., Tasali E., Penev P., Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bayon V., Leger D., Gomez-Merino D., Vecchierini M.F., Chennaoui M. Sleep debt and obesity. Ann Med. 2014;46:264–272. doi: 10.3109/07853890.2014.931103. [DOI] [PubMed] [Google Scholar]
- 9.Patel S.R., Hu F.B. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan X., Alen M., Cheng S.M., Mikkola T.M., Tenhunen J., Lyytikainen A., et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res. 2015;24:414–424. doi: 10.1111/jsr.12283. [DOI] [PubMed] [Google Scholar]
- 11.Dashti H.S., Follis J.L., Smith C.E., Tanaka T., Cade B.E., Gottlieb D.J., et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101:135–143. doi: 10.3945/ajcn.114.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamsuzzaman A.S., Gersh B.J., Somers V.K. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA, J Am Med Assoc. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 13.Gileles-Hillel A., Kheirandish-Gozal L., Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12:290–298. doi: 10.1038/nrendo.2016.22. [DOI] [PubMed] [Google Scholar]
- 14.Ayas N.T., Taylor C.M., Laher I. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol. 2016;31:599–605. doi: 10.1097/HCO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 15.Reutrakul S., Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabol Clin Exper. 2018;84:56–66. doi: 10.1016/j.metabol.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G., Mancusi C., Izzo R., Losi M.A., Aldo Ferrara L. Obesity and hypertensive heart disease: focus on body composition and sex differences. Diabetol Metab Syndrome. 2016;8:79. doi: 10.1186/s13098-016-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu C., Younas H., Jun J.C. Sleep apnea: an overlooked cause of lipotoxicity? Med Hypotheses. 2017;108:161–165. doi: 10.1016/j.mehy.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mafort T.T., Rufino R., Costa C.H., Lopes A.J. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscipl Respirat Med. 2016;11:28. doi: 10.1186/s40248-016-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie M.R., Linz D., Redline S., Somers V.K., Simonds A.K. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:608–624. doi: 10.1016/j.jacc.2021.05.048. [DOI] [PubMed] [Google Scholar]
- 20.Slowik J.M., Sankari A., Collen J.F. 2022. Obstructive sleep apnea. StatPearls. Treasure. Island (FL. [Google Scholar]
- 21.Yeghiazarians Y., Jneid H., Tietjens J.R., Redline S., Brown D.L., El-Sherif N., et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;144:e56–e67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 22.Shamim-Uzzaman Q.A., Singh S., Chowdhuri S. Hypopnea definitions, determinants and dilemmas: a focused review. Sleep Sci Pract. 2018;2:7. [Google Scholar]
- 23.Unal Y., Ozturk D.A., Tosun K., Kutlu G. Sleep & breathing = Schlaf & Atmung. Vol. 23. 2019. Association between obstructive sleep apnea syndrome and waist-to-height ratio; pp. 523–529. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Corral A., Caples S.M., Lopez-Jimenez F., Somers V.K. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuomilehto H., Seppa J., Uusitupa M. Obesity and obstructive sleep apnea--clinical significance of weight loss. Sleep Med Rev. 2013;17:321–329. doi: 10.1016/j.smrv.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S., Badr M.S. Central Sleep Apnea: Pathophysiologic Classification. Sleep. 2022 May 11 doi: 10.1093/sleep/zsac113. Epub ahead of print. PMID: 35551411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orr J.E., Malhotra A., Sands S.A. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22:43–52. doi: 10.1111/resp.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masa J.F., Pepin J.L., Borel J.C., Mokhlesi B., Murphy P.B., Sanchez-Quiroga M.A. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28 doi: 10.1183/16000617.0097-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbasi A., Gupta S.S., Sabharwal N., Meghrajani V., Sharma S., Kamholz S., et al. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021;14:142–154. doi: 10.5935/1984-0063.20200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah N.M., Shrimanker S., Kaltsakas G. Defining obesity hypoventilation syndrome. Breathe. 2021;17 doi: 10.1183/20734735.0089-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soghier I., Brożek J.L., Afshar M., Tamae Kakazu M., Wilson K.C., Masa J.F., et al. Noninvasive ventilation versus CPAP as initial treatment of obesity hypoventilation syndrome. Ann Am Thorac Soc. 2019;16:1295–1303. doi: 10.1513/AnnalsATS.201905-380OC. [DOI] [PubMed] [Google Scholar]
- 32.Bhammar D.M., Stickford J.L., Bernhardt V., Babb T.G. Effect of weight loss on operational lung volumes and oxygen cost of breathing in obese women. Int J Obes. 2016;40:998–1004. doi: 10.1038/ijo.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oostveen E., De Soomer K., Piedfort S., Cuypers H., Verbraecken J., Vaerenberg H. Effect of weight loss or gain on spirometry in obese adults. Eur Respir J. 2019;54:PA3916. [Google Scholar]
- 34.Gottlieb D.J., Punjabi N.M. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 35.Davies R.J., Stradling J.R. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–514. [PubMed] [Google Scholar]
- 36.Davies R.J., Ali N.J., Stradling J.R. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47:101–105. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin H.C., Lai C.C., Lin P.W., Friedman M., Salapatas A.M., Chang H.W., et al. Clinical prediction model for obstructive sleep apnea among adult patients with habitual snoring. Otolaryngol Head Neck Surg. 2019;161:178–185. doi: 10.1177/0194599819839999. [DOI] [PubMed] [Google Scholar]
- 38.Yu J.L., Rosen I. Utility of the modified Mallampati grade and Friedman tongue position in the assessment of obstructive sleep apnea. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2020;16:303–308. doi: 10.5664/jcsm.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagappa M., Liao P., Wong J., Auckley D., Ramachandran S.K., Memtsoudis S., et al. Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu H.Y., Chen P.Y., Chuang L.P., Chen N.H., Tu Y.K., Hsieh Y.J., et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Stanchina M., Lincoln J., Prenda S., Holt M., Leon I., Donat W., et al. The impact of different CPAP delivery approaches on nightly adherence and discontinuation rate in patients with obstructive sleep apnea. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2022;18:2023–2027. doi: 10.5664/jcsm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson K.G., Johnson D.C. CPAP setup by mail: we're not there yet. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2022;18:1897–1898. doi: 10.5664/jcsm.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver T.E., Calik M.W., Farabi S.S., Fink A.M., Galang-Boquiren M.T., Kapella M.C., et al. Innovative treatments for adults with obstructive sleep apnea. Nat Sci Sleep. 2014;6:137–147. doi: 10.2147/NSS.S46818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kline C.E., Reboussin D.M., Foster G.D., Rice T.B., Strotmeyer E.S., Jakicic J.M., et al. The effect of changes in cardiorespiratory fitness and weight on obstructive sleep apnea severity in overweight Adults with type 2 diabetes. Sleep. 2016;39:317–325. doi: 10.5665/sleep.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng S.S.S., Chan R.S.M., Woo J., Chan T.O., Cheung B.H.K., Sea M.M.M., et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193–1203. doi: 10.1378/chest.14-3016. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Padros C., Salord N., Alves C., Vilarrasa N., Gasa M., Planas R., et al. Effectiveness of an intensive weight-loss program for severe OSA in patients undergoing CPAP treatment: a randomized controlled trial. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2020;16:503–514. doi: 10.5664/jcsm.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil S.P., Ayappa I.A., Caples S.M., Kimoff R.J., Patel S.R., Harrod C.G. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of sleep medicine systematic review, meta-analysis, and GRADE Assessment. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2019;15:301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goyal M., Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120–124. [PMC free article] [PubMed] [Google Scholar]
- 49.Broussard J.L., Klein S. Insufficient sleep and obesity: cause or consequence. Obesity. 2022;30:1914–1916. doi: 10.1002/oby.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edinger J.D., Arnedt J.T., Bertisch S.M., Carney C.E., Harrington J.J., Lichstein K.L., et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2021;17:255–262. doi: 10.5664/jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sateia M.J., Buysse D.J., Krystal A.D., Neubauer D.N., Heald J.L. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2017;13:307–349. doi: 10.5664/jcsm.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carneiro-Barrera A., Diaz-Roman A., Guillen-Riquelme A., Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: systematic review and meta-analysis. Obes Rev : Off J Int Ass Study Obes. 2019;20:750–762. doi: 10.1111/obr.12824. [DOI] [PubMed] [Google Scholar]
- 53.Lankford D.A., Proctor C.D., Richard R. Continuous positive airway pressure (CPAP) changes in bariatric surgery patients undergoing rapid weight loss. Obes Surg. 2005;15:336–341. doi: 10.1381/0960892053576749. [DOI] [PubMed] [Google Scholar]
- 54.Peromaa-Haavisto P., Tuomilehto H., Kossi J., Virtanen J., Luostarinen M., Pihlajamaki J., et al. Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017;35:85–90. doi: 10.1016/j.sleep.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Awad M., Gouveia C., Zaghi S., Camacho M., Liu S.Y. Changing practice: trends in skeletal surgery for obstructive sleep apnea. J Cranio-Maxillo-Fac Surg. 2019;47:1185–1189. doi: 10.1016/j.jcms.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 56.He M., Yin G., Zhan S., Xu J., Cao X., Li J., et al. Long-term efficacy of uvulopalatopharyngoplasty among adult patients with obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2019;161:401–411. doi: 10.1177/0194599819840356. [DOI] [PubMed] [Google Scholar]
- 57.Woodson B.T., Strohl K.P., Soose R.J., Gillespie M.B., Maurer J.T., de Vries N., et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159:194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 58.Huntley C., Steffen A., Doghramji K., Hofauer B., Heiser C., Boon M. Upper airway stimulation in patients with obstructive sleep apnea and an elevated body mass index: a multi-institutional review. Laryngoscope. 2018;128:2425–2428. doi: 10.1002/lary.27426. [DOI] [PubMed] [Google Scholar]
- 59.Fehrm J., Nerfeldt P., Browaldh N., Friberg D. Effectiveness of adenotonsillectomy vs watchful waiting in young children with mild to moderate obstructive sleep apnea: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2020;146:647–654. doi: 10.1001/jamaoto.2020.0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan X., Chapman C.D., Cedernaes J., Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev. 2018;40:127–134. doi: 10.1016/j.smrv.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Broussard J.L., Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23:353–359. doi: 10.1097/MED.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poggiogalle E., Jamshed H., Peterson C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabol Clin Exper. 2018;84:11–27. doi: 10.1016/j.metabol.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nedeltcheva A.V., Kilkus J.M., Imperial J., Schoeller D.A., Penev P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai G.H., Theorell-Haglow J., Janson C., Svartengren M., Elmstahl S., Lind L., et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81–87. doi: 10.1016/j.sleep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Auger R.R., Burgess H.J., Emens J.S., Deriy L.V., Thomas S.M., Sharkey K.M. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med : JCSM : Off Pub Am Acad Sleep Med. 2015;11:1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin S., Zhang H., Gao T., Zhong F., Sun Y., Cai J., et al. The association between obesity and restless legs syndrome: a systemic review and meta-analysis of observational studies. J Affect Disord. 2018;235:384–391. doi: 10.1016/j.jad.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 67.Abetz L., Allen R., Follet A., Washburn T., Earley C., Kirsch J., et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Therapeut. 2004;26:925–935. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 68.Trenkwalder C., Allen R., Högl B., Clemens S., Patton S., Schormair B., et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018;17:994–1005. doi: 10.1016/S1474-4422(18)30311-9. [DOI] [PubMed] [Google Scholar]
- 69.De Meyer M.M.D., Jacquet W., Vanderveken O.M., Marks L.A.M. Systematic review of the different aspects of primary snoring. Sleep Med Rev. 2019;45:88–94. doi: 10.1016/j.smrv.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical Practice guidelines we can trust. [PubMed] [Google Scholar]