Abstract
A comparative study of variable domains V4, V6, and V9 of the mitochondrial small-subunit (SSU) rRNA was carried out with the genus Agrocybe by PCR amplification of 42 wild isolates belonging to 10 species, Agrocybe aegerita, Agrocybe dura, Agrocybe chaxingu, Agrocybe erebia, Agrocybe firma, Agrocybe praecox, Agrocybe paludosa, Agrocybe pediades, Agrocybe alnetorum, and Agrocybe vervacti. Sequencing of the PCR products showed that the three domains in the isolates belonging to the same species were the same length and had the same sequence, while variations were found among the 10 species. Alignment of the sequences showed that nucleotide motifs encountered in the smallest sequence of each variable domain were also found in the largest sequence, indicating that the sequences evolved by insertion-deletion events. Determination of the secondary structure of each domain revealed that the insertion-deletion events commonly occurred in regions not directly involved in the secondary structure (i.e., the loops). Moreover, conserved sequences ranging from 4 to 25 nucleotides long were found at the beginning and end of each domain and could constitute genus-specific sequences. Comparisons of the V4, V6, and V9 secondary structures resulted in identification of the following four groups: (i) group I, which was characterized by the presence of additional P23-1 and P23-3 helices in the V4 domain and the lack of the P49-1 helix in V9 and included A. aegerita, A. chaxingu, and A. erebia; (ii) group II, which had the P23-3 helix in V4 and the P49-1 helix in V9 and included A. pediades; (iii) group III, which did not have additional helices in V4, had the P49-1 helix in V9 and included A. paludosa, A. firma, A. alnetorum, and A. praecox; and (iv) group IV, which lacked both the V4 additional helices and the P49-1 helix in V9 and included A. vervacti and A. dura. This grouping of species was supported by the structure of a consensus tree based on the variable domain sequences. The conservation of the sequences of the V4, V6, and V9 domains of the mitochondrial SSU rRNA within species and the high degree of interspecific variation found in the Agrocybe species studied open the way for these sequences to be used as specific molecular markers of the Basidiomycota.
The cultivated mushroom Agrocybe aegerita is a member of the division Basidiomycota that belongs to the order Agaricales. The mitochondrial DNA of this organism was previously cloned and mapped (13), and the complete sequence of its mitochondrial small-subunit (SSU) ribosomal DNA (rDNA) was recently obtained (4). A comparison of the SSU rRNA secondary structure with the prokaryotic model described by Neefs et al. (15) showed that three variable domains (V4, V6, and V9) have unusually long nucleotide sequences compared to the sequences of species belonging to different kingdoms (4). Alignment of the A. aegerita sequence with partial 5′ sequences from 80 basidiomycetes overlapping variable domain V4 showed that the length variations in this domain range from 22 nucleotides in Ripartitella brasiliensis to 327 nucleotides in Stropharia rugosoannulata (2, 6) and seem to be species specific. Moreover, a preliminary study of PCR products overlapping the V4 domain carried out with a few strains of A. aegerita produced similar results irrespective of the isolate.
Sequences of the V4, V6, and V9 domains are assumed to be involved in the secondary structure of the SSU rRNA and to directly interact with riboproteins to produce functional ribosomes (14, 17). As knowledge of Basidiomycota mitochondrial genes is scarce and the sequence of A. aegerita mitochondrial SSU rDNA is the only complete sequence available to date for such a gene, a study of these regions is important for understanding how mitochondrial SSU rRNA nucleotide variations occur in the Basidiomycota. This fact prompted us to investigate the lengths and sequences of the V4, V6, and V9 domains in wild isolates belonging to the genus Agrocybe.
In recent years, PCR amplification of prokaryotic 16S rRNA sequences and restriction fragment length polymorphism (RFLP) analysis of PCR products have been used to characterize bacterial species belonging to the genera Oxyphotobacteria (21), Photobacterium, and Vibrio (27); these organisms produce species-specific patterns. In contrast, in fungi most species characterizations have been based on the RFLP of the nuclear genome (for reviews, see references 3 and 8) or PCR amplification of the internal transcribed spacer located between the nuclear 18S and 28S rDNAs (11, 12, 20). Thus, given the prokaryotic origin of the mitochondrial DNA, we were particularly interested in investigating whether mitochondrial SSU rRNA sequences could also supply molecular markers for identification of fungal species.
In this study, 18-mer primers flanking variable domains V4, V6, and V9 were identified and used for PCR amplification of 42 wild isolates belonging to 10 species of the genus Agrocybe (A. aegerita, Agrocybe alnetorum, Agrocybe chaxingu, Agrocybe erebia, Agrocybe paludosa, Agrocybe dura, Agrocybe firma, Agrocybe praecox, Agrocybe pediades, and Agrocybe vervacti). Twenty-seven A. aegerita isolates were studied, and one to three isolates of the other species, obtained from different geographic regions, were used. The nucleotide sequences of all of the PCR amplification products were determined, the exact length of each domain was determined, and the nucleotide variations and alignments were compared. The secondary structure of each variable domain was established to determine the locations of sequence variations observed within the genus. A comparison of the secondary structures allowed us to determine the relationships between the species and the association of the species in different groups. A consensus tree based on the variable domain sequences was constructed by using the neighbor-joining and parsimony methods. The results of intra- and interspecies comparisons are discussed below.
MATERIALS AND METHODS
Strains and cultures.
All of the Agrocybe strains used were dikaryotic (Table 1). A. aegerita and A. chaxingu strains were grown in the dark at 26°C on petri dishes containing solid complete CYM medium (19). A. alnetorum, A. erebia, A. paludosa, A. dura, A. firma, A. praecox, A. pediades, and A. vervacti strains were grown on potato dextrose agar (39 g/liter; Sigma) in the dark at 26°C. The geographic origins of the strains used are reported in Table 1.
TABLE 1.
Lengths of variable domains V4, V6, and V9 of the mitochondrial SSU rRNA from strains belonging to 10 species of the genus Agrocybe
| Species | Straina | Geographic origin or collection no.b | Length (nucleotides) of:
|
||
|---|---|---|---|---|---|
| Domain V4 | Domain V6 | Domain V9 | |||
| A. aegerita | SM 47 | France (Agen) | 170 | 172 | 221 |
| SM 49 | Italy | 170 | 172 | 221 | |
| SM 87 10 27 | Spain (Albaladejito) | 170 | 172 | 221 | |
| SC 87 11 06 | Scotland | 170 | 172 | 221 | |
| SC 87 11 07 | Germany | 170 | 172 | 221 | |
| SC 93 02 02 | Belgium (Merelbeke) | 170 | 172 | 221 | |
| SM 51 | France (southwest) | NSc | NS | NS | |
| SM 160 | France (southwest) | NS | NS | NS | |
| SM 771 | France (southwest) | NS | NS | NS | |
| SM 75 08 01 | France (southwest) | NS | NS | NS | |
| SM 75 09 03 | France (southwest) | NS | NS | NS | |
| SM 75 10 02 | France (southwest) | NS | NS | NS | |
| SM 77 06 01 | France (southwest) | NS | NS | NS | |
| SM 84 10 05 | France (southwest) | NS | NS | NS | |
| SM 87 10 12 | France (southwest) | NS | NS | NS | |
| SM 90 06 01 | France (southwest) | NS | NS | NS | |
| SM 93 09 01 | France (southwest) | NS | NS | NS | |
| SM 93 09 02 | France (southwest) | NS | NS | NS | |
| SC 97 02 07 | France (southwest) | NS | NS | NS | |
| SM 46 | France (northwest) | NS | NS | NS | |
| SM 75 08 02 | France (northwest) | NS | NS | NS | |
| SM 75 09 02 | France (northwest) | NS | NS | NS | |
| SM 76 09 01 | France (northwest) | NS | NS | NS | |
| SM 161 | France (southeast) | NS | NS | NS | |
| SM 87 11 02 | France (southeast) | NS | NS | NS | |
| SM 50 | Czechoslovakia | NS | NS | NS | |
| SM 87 10 21 | Spain | NS | NS | NS | |
| A. alnetorum | SM 97 08 02 | CBS 440 87 | 120 | 158 | 403 |
| SM 97 08 03 | CBS 441 87 | 120 | 158 | 403 | |
| A. chaxingu | SC 96 09 03 | Thailand | 281 | 158 | 246 |
| SC 96 09 04 | Thailand | 281 | 158 | 246 | |
| A. dura | SC 93 03 01 | CBS 157 63 | 391 | 173 | 285 |
| SC 93 03 02 | ATCC 6768 | 391 | 173 | 285 | |
| A. erebia | SM 97 08 04 | CBS 206 46 | 185 | 153 | 250 |
| A. firma | SM 97 08 05 | CBS 390 79 | 122 | 244 | 453 |
| A. paludosa | SC 93 03 04 | CBS 395 79 | 119 | 175 | 415 |
| SM 97 08 06 | CBS 297 39 | 119 | 175 | 415 | |
| SM 97 08 07 | CBS 208 46 | 119 | 175 | 415 | |
| A. pediades | SM 97 08 08 | CBS 101 39 | 190 | 204 | 360 |
| A. praecox | SC 93 03 08 | CBS 396 79 | 119 | 174 | 387 |
| SM 97 08 09 | CBS 108 3959 | 119 | 174 | 387 | |
| A. vervacti | SM 97 08 10 | CBS 190 46 | 114 | 172 | 272 |
Laboratory of Molecular Genetics and Breeding of Cultivated Mushrooms designation.
CBS, Centraalbureau voor Schimmelcultures, Baarn, The Netherlands; ATCC, American Type Culture Collection, Rockville, Md.
NS, not sequenced.
Genomic DNA purification.
Total DNA was extracted from vegetative mycelia by using the N-cetyl-N,N,N-trimethylammonium bromide (CTAB) method (16) adapted to a small quantity of mycelium. Mycelium (around 0.2 g) was collected with a scalpel from an 8-day culture on solid complete CYM medium and then frozen in liquid nitrogen and crushed in a mortar. The crushed mycelium was resuspended in 0.7 ml of extraction buffer (100 mM Tris-HCl [pH 8], 2% [wt/vol] CTAB, 20 mM EDTA, 1.4 M NaCl, 2% [vol/vol] β-mercaptoethanol) and incubated for 20 min at 56°C. Then, 0.7 ml of chloroform-isoamyl alcohol (24:1, vol/vol) was added, and the two phases were mixed to obtain an emulsion. After centrifugation (9,000 × g, 15 min, 20°C), the aqueous phase was removed and then subjected to a second extraction with 0.7 ml of chloroform-isoamyl alcohol, as described above. The nucleic acids were precipitated with 0.7 ml of precipitation buffer (50 mM Tris-HCl [pH 8], 1% [wt/vol] CTAB, 10 mM EDTA, 1% [vol/vol] β-mercaptoethanol) for 30 min at room temperature. The precipitate was recovered by centrifugation (9,000 × g, 15 min, 20°C), dried, resuspended in 0.5 ml of 1 M NaCl, and incubated for 20 min at 56°C. The nucleic acids were then precipitated at room temperature by adding 2 volumes of absolute ethanol. After centrifugation (11,000 × g, 15 min, 20°C), the pellet was washed three times with 1 ml of 70% (vol/vol) ethanol to completely eliminate the excess CTAB. The pellet was dried and then resuspended in sterile distilled water. Nucleic acids were used directly for PCR amplification or stored at 4°C.
PCR amplification.
Amplification reactions were performed by using three primer pairs, V4U (CTTACTATAAGTGTTGTC) plus V4R (TATTCTACTTAGTATCTT), V6U (TTAGTCGGTCTCGGAGCA) plus V6R (TGACGACAGCCATGCAAC), and V9U (CCGTGATGAACTAACCGT) plus V9R (TTCCAGTACAAGCTACCT), to amplify the regions containing variable domains V4, V6, and V9, respectively, of the mitochondrial SSU rDNA. The PCR mixtures contained 50 mM KCl, 10 mM Tris-HCl (pH 9), 0.1% Triton X-100, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 2.5 mM MgCl2, and 3 to 5 μl of purified DNA in a final volume of 25 μl. Then 40 amplification cycles were performed with a model PTC 100 (MJ Research) thermal cycler as follows: DNA was denatured for 30 s at 95°C, annealing of primers was performed at a temperature that was 2°C less than the thermal denaturation temperature (i.e., 42, 54, and 50°C for amplification of the regions overlapping the V4, V6, and V9 domains, respectively) for 30 s, and an elongation step was performed for 30 s. The PCR products were then analyzed in a 1.5% (wt/vol) agarose gel or in a 5% polyacrylamide electrophoresis gel and were observed after ethidium bromide staining.
Purification and sequencing of PCR amplification products.
PCR amplification products were purified by using a Qiaquick PCR purification kit (Qiagen, Santa Clarita, Calif.). To 1 volume of PCR mixture 5 volumes of PB buffer was added. The solution was applied to a Qiaquick column and centrifuged (3,000 × g, 1 min, 20°C). The column was then washed with 0.75 ml of PE buffer, centrifuged as described above, and then dried by another centrifugation step (10,000 × g, 1 min, 20°C). Finally, the DNA was eluted by adding 40 μl of sterile distilled water, incubated for 1 min at room temperature, and recovered by centrifugation (10,000 × g, 1 min, 20°C). Under these conditions, all of the excess primer was removed, and the PCR amplification products could be used in sequencing reactions.
PCR products were sequenced by using a ThermoSequenase sequencing kit (United States Biochemicals, Cleveland, Ohio) as described by Sanger et al. (22) and α-33P-labeled dideoxynucleoside triphosphates. Primers V4U, V6U, and V9U were used to sequence the PCR products of variable domains V4, V6, and V9, respectively. The sequencing products were analyzed by 6% polyacrylamide gel electrophoresis and were observed after exposure to Kodak X-Omat LS film.
Phylogenetic analysis.
Sequences were aligned by using the CLUSTAL V software (7). Consensus trees were constructed by the neighbor-joining and parsimony methods from the phylogeny inference package PHYLIP (version 3.5). To infer the confidence in the branch points in the tree which was constructed, a bootstrap analysis was performed. The consensus tree obtained resulted from 100 bootstrap replicates.
RESULTS
Determination of primers for amplification of the V4, V6, and V9 domains.
To study domains V4, V6, and V9 of mitochondrial SSU rDNA in the genus Agrocybe, consensus 18-mer primers that could be used for PCR with members of this genus and/or species belonging to the division Basidiomycota were identified.
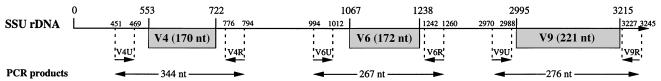
From an alignment of 80 nucleotide sequences of 5′ partial mitochondrial SSU rRNAs of members of the Basidiomycota available in databases with the corresponding nucleotide sequence of A. aegerita, two regions flanking V4 (V4U and V4R), whose nucleotide sequences appeared to be highly conserved, were identified (Fig. 1). Primer V4U was located 102 nucleotides upstream from the first base of domain V4 in a conserved region of the SSU rRNA that includes helices P19 to P21 (15). The nucleotide sequence of primer V4R, which was located 72 nucleotides downstream from V4, corresponded to the 5′ part of helix P25.
FIG. 1.
Locations of variable domains V4, V6, and V9 in the A. aegerita mitochondrial SSU rRNA gene sequence. The positions of primers are indicated by arrows. nt, nucleotides.
To identify primers that could be used to amplify variable domains V6 and V9, no other SSU rRNA sequences for Basidiomycota were available in databases. The 18-mer sequences used were sequences in flanking regions of domains V6 and V9 previously described as conserved (15). The V6U and V6R primer sequences, which were located 73 nucleotides from the first base and 22 nucleotides from the last base of the V6 domain (Fig. 1), respectively, corresponded to the 5′ parts of helices P32 and P38, respectively. The V9U and V9R primer sequences, which were located 25 nucleotides from the first base and 30 nucleotides from the last base of the V9 domain, respectively, corresponded to the 3′ parts of helix P32 and the 5′ region of helix P50, respectively.
PCR amplification and sequencing of the V4, V6, and V9 domains of 27 A. aegerita isolates.
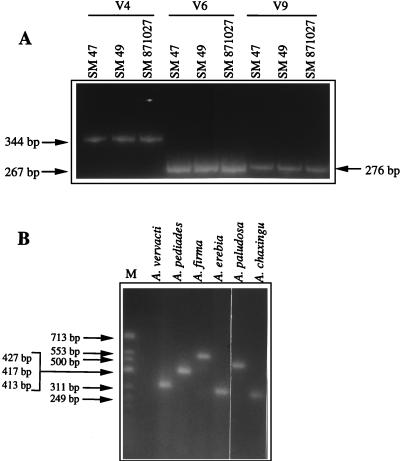
A total of 27 A. aegerita isolates from different geographic areas were used (Table 1). After ethidium bromide staining, electrophoretic analysis of PCR products that were obtained by using the three primer pairs (V4U plus V4R, V6U plus V6R, and V9U plus V9R) independently in three different reactions showed that for each variable domain, the same migration pattern was produced irrespective of the strain (Fig. 2A). However, under the electrophoresis conditions used, PCR products that differed by fewer than 20 nucleotides could not be discriminated.
FIG. 2.
(A) Polyacrylamide gel electrophoresis of the PCR products overlapping the V4, V6, and V9 domains of the mitochondrial SSU rDNA obtained from the following three A. aegerita strains from different geographic areas: SM 47 (France), SM 49 (Italy), and SM 871027 (Spain). (B) Length variations observed after agarose gel electrophoresis of the PCR product overlapping the V9 domain from the following six strains: A. vervacti SM 97 08 10, A. pediades SM 97 08 08, A. firma SM 97 08 05, A. erebia SM 97 08 04, A. paludosa SM 97 08 06, and A. chaxingu SC 96 09 03. Lane M shows the migration pattern of molecular weight marker φX174 DNA/HinfI (Promega).
The PCR products of the three variable domains were purified and sequenced for the following five strains from different geographic areas: SM 871027 from Spain, SM 871106 from Scotland, SC 871107 from Germany, SC 930202 from Belgium, and SM 49 from Italy. Alignment of the resulting nucleotide sequences showed that each of the three variable domains was the same length in all five isolates (170, 172, and 221 nucleotides for V4, V6, and V9, respectively). These lengths are identical to the lengths determined for previously sequenced strain SM 47 (Agen, France) (4). Moreover, alignment of these nucleotide sequences with the SSU rDNA sequence of SM 47 showed that the sequences were identical; i.e., no intraspecific variations were observed in any domain or in the flanking regions.
Comparisons of the sequences of the V4, V6, and V9 domains of nine species belonging to the genus Agrocybe.
Next, our study was extended to 15 wild-type strains belonging to nine other species of the genus Agrocybe (Table 1). The total DNA of each species was extracted and then subjected to PCR amplification under the same conditions as those used for the A. aegerita strains. Electrophoretic analysis of the PCR products showed that strains belonging to the same species produced identical migration patterns, while variations in size were observed among the nine species of the genus Agrocybe (Fig. 2B).
All of the PCR products were purified and sequenced in order to accurately determine the lengths of the three domains. Isolates belonging to the same species always had the same domain lengths. There was great size variation for each domain within the genus; the V9 domain lengths ranged from 246 nucleotides for A. chaxingu to 453 nucleotides for A. firma, the V6 domain lengths ranged from 153 nucleotides for A. erebia to 244 nucleotides for A. firma, and the V4 domain lengths ranged from 114 nucleotides for A. vervacti to 391 nucleotides for A. dura. Each of the nine species had a different V9 domain length (Table 1). In some cases one domain was the same length in two different species; the V4 domains of A. paludosa and A. praecox were both 119 nucleotides long, and the V6 domains of A. chaxingu and A. alnetorum were both 158 nucleotides long. In all other cases, three different lengths were observed for pairs of species.
Alignment of the nucleotide sequences showed that strains belonging to the same species had identical sequences for each domain. Moreover, a comparison of the sequences of A. paludosa and A. praecox, whose V4 domains were the same length (119 nucleotides), revealed three nucleotide differences. While the V6 domains of A. chaxingu and A. alnetorum were the same length (158 nucleotides), there were more than 70 differences in this domain in these species, so despite the identical domain lengths, the species could be discriminated on the basis of their nucleotide sequences.
The sequences located on either side of the variable domains were very similar in the 10 species. The A. aegerita sequences and the sequences of the other Agrocybe species exhibited 82 to 100% similarity in the V4, V6, and V9 flanking regions.
The sequence alignments revealed conserved sequences in each variable domain in the genus. The nucleotide motif TTGCATA, which constituted the beginning of the V6 domain, was found in all 10 Agrocybe species, as was the TTTAC motif located at the end of this domain (Fig. 3). Moreover, the first 9 nucleotides and the last 25 nucleotides of the V9 domain were conserved in the genus (Fig. 4). In the V4 domain, only the first four nucleotides and the last four nucleotides were conserved in all of the species (Fig. 5). It should be noted that all of these conserved sequences formed the base of the first helix of each domain.
FIG. 3.
Alignment of the PCR product sequences overlapping the V6 domains of 10 species of the genus Agrocybe. The beginning and end of the variable domain are indicated by arrows. Asterisks indicate nucleotides that are strictly conserved in the 10 species. The locations and putative sizes of insertion-deletion events are indicated by dashes. Nucleotides that are strictly conserved in the 10 species are enclosed in shaded boxes. The boxes labeled V6a and V6b are the locations of putative insertion-deletion events. The GenBank accession numbers for the sequences are as follows: A. aegerita, AF080410; A. alnetorum, AF080411; A. chaxingu, AF080412; A. dura, AF080413; A. erebia, AF080414; A. firma, AF080415; A. paludosa, AF080416; A. pediades, AF080417; A. praecox, AF080418; and A. vervacti, AF080419.
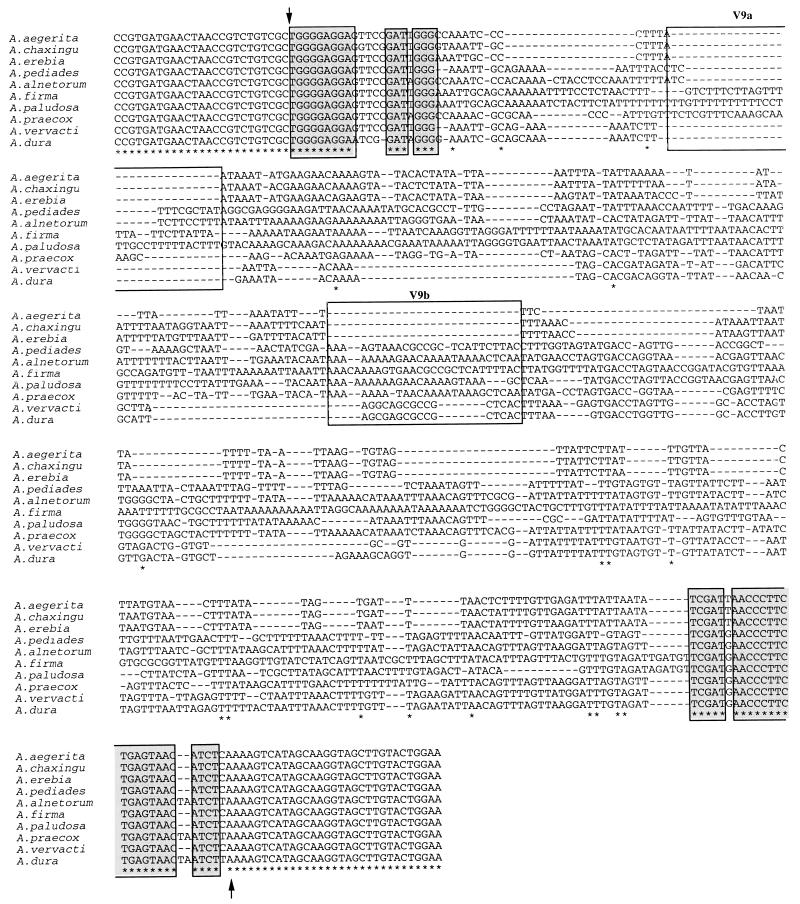
FIG. 4.
Alignment of the PCR product sequences overlapping the V9 domains of 10 species of the genus Agrocybe. The beginning and end of the variable domain of the mitochondrial SSU rDNA are indicated by arrows. Asterisks indicate nucleotides that are strictly conserved in the 10 species. The locations and putative sizes of insertion-deletion events are indicated by dashes. Nucleotides that are strictly conserved in the 10 species are enclosed in shaded boxes. The boxes labeled V9a and V9b are the locations of putative insertion-deletion events. The GenBank accession numbers for the sequences are as follows: A. aegerita, AF080420; A. alnetorum, AF080421; A. chaxingu, AF080422; A. dura, AF080423; A. erebia, AF080424; A. firma, AF080425; A. paludosa, AF080426; A. pediades, AF080427; A. praecox, AF080428; and A. vervacti, AF080429.
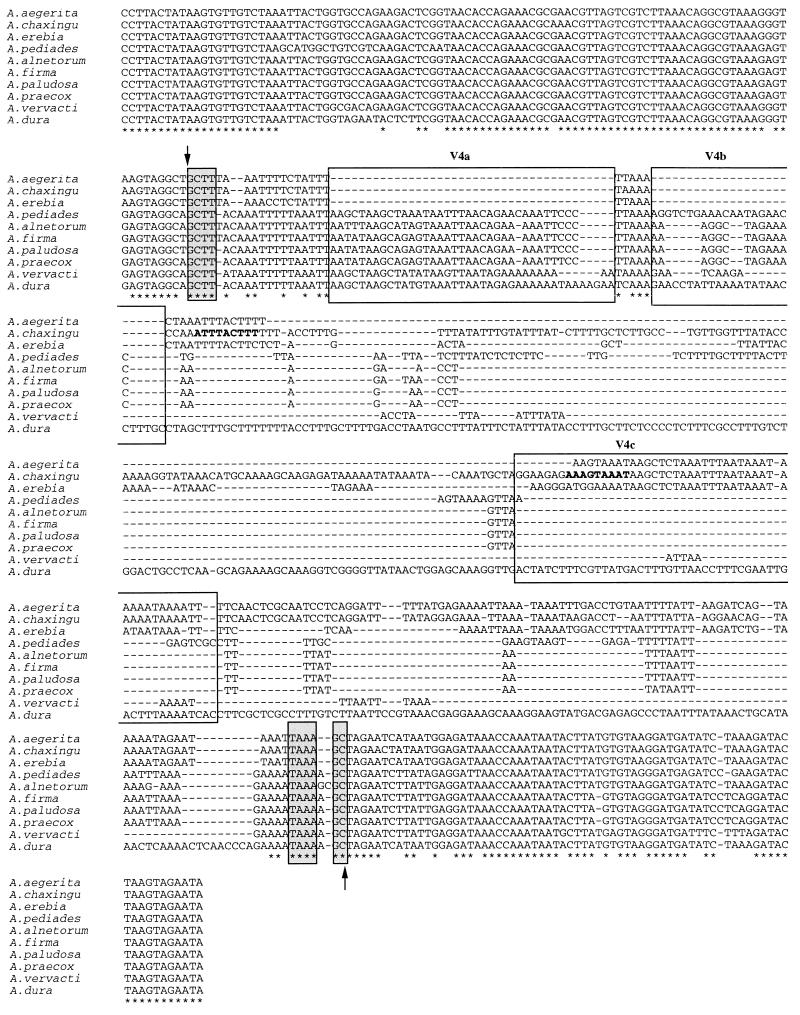
FIG. 5.
Alignment of the PCR product sequences overlapping the V4 domains of 10 species of the genus Agrocybe. The beginning and end of the variable domain are indicated by arrows. Asterisks indicate nucleotides that are strictly conserved in the 10 species. The locations and putative sizes of insertion-deletion events are indicated by dashes. Nucleotides that are strictly conserved in the 10 species are enclosed in shaded boxes. The nine nucleotides constituting an inverted repeated sequence at the boundaries of the insertion-deletion site in A. chaxingu are indicated by boldface type. The boxes labeled V4a, V4b, and V4c are the locations of putative insertion-deletion events. The GenBank accession numbers for the sequences are as follows: A. aegerita, AF080400; A. alnetorum, AF080401; A. chaxingu, AF080402; A. dura, AF080403; A. erebia, AF080404; A. firma, AF080405; A. paludosa, AF080406; A. pediades, AF080407; A. praecox, AF080408; and A. vervacti, AF080409.
Sequence variations in the V4, V6, and V9 domains in the genus Agrocybe.
In addition to the conservation of nucleotide motifs, large variations in length and sequence were observed in the domains. A comparison of the complete sequences of a variable domain (Fig. 5) showed that all of the motifs found in the shortest sequences were also present in the longest sequences, suggesting that domain variation could be due to addition or deletion of nucleotides. For example, the V4a and V4b nucleotide sequences in the V4 domain were not present in A. aegerita, A. chaxingu, and A. erebia (Fig. 5). Moreover, the V4c sequence was not present in A. alnetorum, A. firma, A. paludosa, or A. praecox, and only part of the V4c sequence was present in A. pediades. The A. chaxingu V4 domain had an additional 114-nucleotide sequence that was not present in A. aegerita; moreover, these two species had an inverted repeated 9-nucleotide sequence (ATTTACTTT) at the boundaries of the possible insertion-deletion site of these 114 nucleotides.
In the V6 domain, the V6a nucleotide sequence was not present in A. aegerita, A. chaxingu, and A. erebia, and the V6b sequence was not present in A. alnetorum, A. paludosa, and A. praecox (Fig. 3). Insertion and deletion of nucleotides in this domain appeared to be less extensive than insertion and deletion of nucleotides in V4. Indeed, the V6a sequence was only 23 nucleotides long, while, for example, the V4c sequence was 51 nucleotides long (Fig. 3 and 5). The same kinds of differences were found in the V9 sequences. The V9a sequence (31 nucleotides) was not present in A. aegerita, A. chaxingu, A. dura, A. erebia, and A. vervacti, and the V9b sequence (28 nucleotides) was not present in A. aegerita, A. chaxingu, and A. erebia (Fig. 4).
In addition to the interspecific variations due to putative insertion-deletion events, a few point mutations were observed in the remaining sequences of the 10 species. For example, when the 114 additional nucleotides located in the loop of the P23-2 helix of A. chaxingu were removed, seven point mutations differentiated the V4 domain sequences of A. aegerita and A. chaxingu; (167 nucleotides); these 7 nucleotides represented 5% of the total A. aegerita domain V4 sequence (Fig. 5). When the A. aegerita sequence was used as a basis for comparison, the numbers of point mutations ranged from 9 for A. chaxingu to 42 for A. pediades in the V6 domain and from 11 for A. chaxingu to 39 for A. pediades in the V9 domain. The point mutations were distributed throughout each whole domain sequence.
Comparison of the secondary structures of the V4, V6, and V9 domains in the genus Agrocybe.
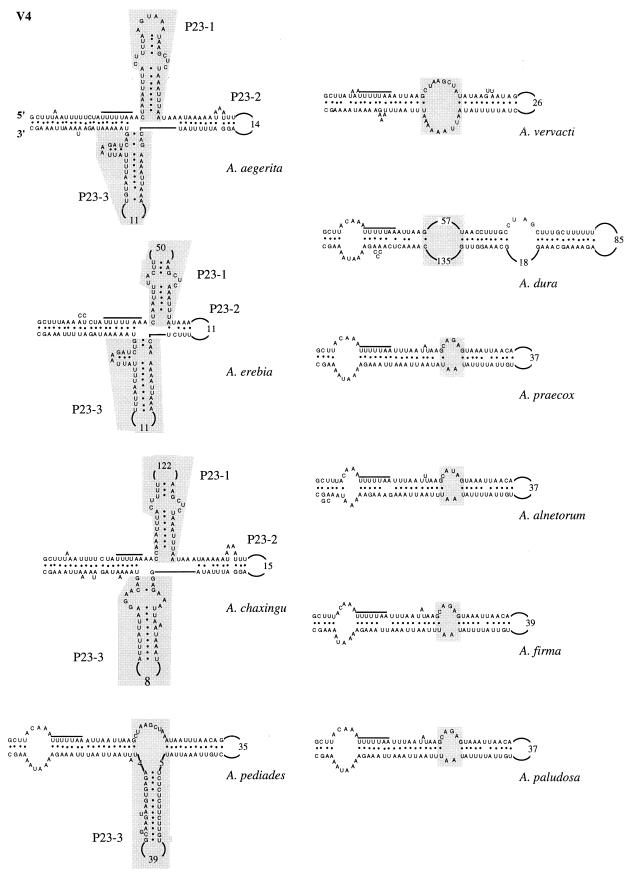
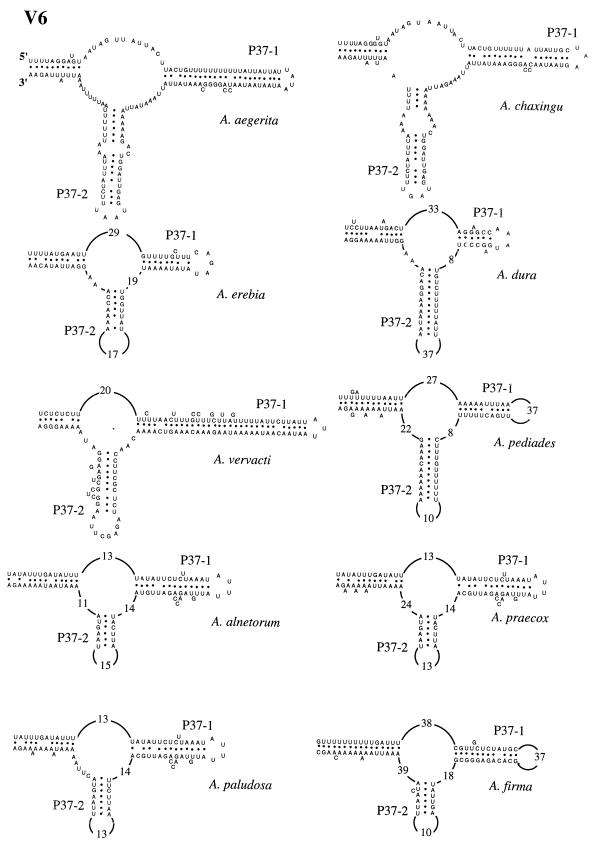
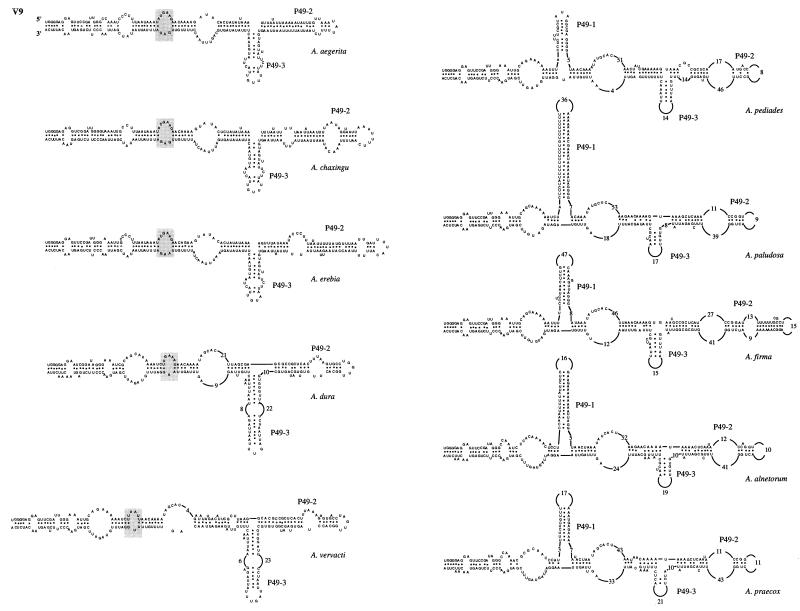
To precisely determine where the insertion-deletion events occur, the secondary structures of the V4, V6, and V9 domains of the 10 Agrocybe species were determined and then compared to the previously described secondary structure of A. aegerita mitochondrial SSU rRNA (4). In all of the species studied, the base pairings constituting the major helices of these domains were conserved. In the V4 domain, the following three types of secondary structure were distinguished based on the presence or absence of additional helices P23-1 and P23-3 (Fig. 6): (i) one type with helices P23-1 and P23-3 (A. aegerita, A. chaxingu, and A. erebia); (ii) one type having an intermediate secondary structure with only the P23-3 helix (A. pediades); (iii) and one type having a secondary domain structure comparable to that described by Neefs et al. (15), which lacked the P23-1 and P23-3 helices (A. vervacti, A. praecox, A. paludosa, A. alnetorum, A. dura, and A. firma). An internal loop was found in the last group at the putative location of the two additional helices (Fig. 6). The V6 domains of all 10 species of the genus Agrocybe had identical secondary structures (Fig. 7). The interspecific variations were due to (i) the numbers of nucleotides in the loops and (ii) the numbers of nucleotides base paired to form the two major helices, P37-1 and P37-2. Two different types of V9 secondary structure were observed (Fig. 8). One type was characterized by the presence of additional helices P49-1 and P49-3 (A. pediades, A. paludosa, A. firma, A. alnetorum, and A. praecox). In the other type the P49-1 helix was replaced by a small additional internal loop, while the P49-3 helix was present (Fig. 8). The V9 length variations observed for the species were due to the numbers of internal loops found in the P49-2 helix and to the numbers of nucleotides base paired to form helices P49-1, P49-2, and P49-3.
FIG. 6.
Secondary structure of variable domain V4 of 10 species of the genus Agrocybe. The overlined sequence is a sequence conserved in the species. Additional helices are enclosed in shaded boxes and are designated as described by Neefs et al. (15); their locations in helixless species are also indicated.
FIG. 7.
Secondary structure of variable domain V6 of 10 species of the genus Agrocybe.
FIG. 8.
Secondary structure of variable domain V9 of 10 species of the genus Agrocybe. Species that have similar secondary structures are in the same column. In the species that do not have a P49-1 helix the putative location of this helix is indicated by shaded boxes.
Relationships among the 10 species of the genus Agrocybe.
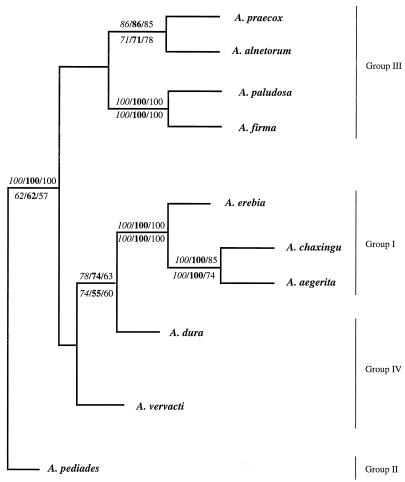
Alignments of the nucleotide sequences of the variable domains were used to construct consensus trees by the neighbor-joining and parsimony methods (PHYLIP package, version 3.5). We found that identical consensus trees were obtained when we used the nucleotide sequences of variable domains V4, V6, and V9 (Fig. 9).
FIG. 9.
Consensus tree obtained by the neighbor-joining and parsimony methods and based on the sequences of variable domains V4, V6, and V9 of 10 species of the genus Agrocybe. The bootstrap values obtained by the neighbor-joining and parsimony methods are indicated above and below the branches, respectively. The bootstrap values obtained for domains V4, V6, and V9 are indicated in italics, in boldface type, and in standard type, respectively.
Analysis of the resulting trees revealed two different ensembles related to A. pediades, which was assumed to be the most divergent species. In one ensemble, A. paludosa and A. firma were strongly associated in a subgroup (100% bootstrap support), and in the same way A. praecox was related to A. alnetorum. In the other ensemble, A. vervacti was related to A. dura, which was related to a subgroup that included A. erebia, which was associated with A. aegerita and A. chaxingu, which were strongly related to each other (100% bootstrap support). Each branch point of the consensus tree was supported by high bootstrap values obtained by either the neighbor-joining method or the parsimony method.
DISCUSSION
Conservation of the sequences of the V4, V6, and V9 domains in each species.
The nucleotide sequences of variable domains V4, V6, and V9 of the mitochondrial SSU rRNA were highly conserved in each Agrocybe species studied, irrespective of geographic origins, but the sequences were not conserved between species of the genus. The intraspecific sequence conservation observed may be linked to the fact that variable domains V4, V6, and V9 are involved in the formation of the secondary structure of the SSU rRNA and to the high degree of specificity of these sequences for correct binding of riboproteins to obtain the three-dimensional folding of the functional 30S subunit (17).
The associations between rRNA and riboproteins are well known for the 16S rRNA of Escherichia coli (1, 14, 25). In this species, it is assumed that variable domain V9 interacts with proteins S16 and S20, that variable domain V6 interacts with S19, and that variable domain V4 interacts with S16, S17, and S8. The S8 protein is important because it is thought to be the first protein to bind to the rRNA, which induces a conformational change that allows the binding of the second riboprotein (5). This suggests that nucleotide changes in the V4 domain can affect the binding of protein S8 and lead to the production of nonfunctional mitochondrial ribosomes. Moreover, the fact that the variable domain sequences are invariant within species suggests that they are under strong constraints that discourage the selection of mutations.
Interspecific variations in the domain V4, V6, and V9 sequences in the genus Agrocybe.
Comparison of domains V4, V6, and V9 in strains belonging to 10 species of the genus Agrocybe revealed interspecific variations in size and sequences due to point mutations and insertion or deletion of polynucleotides. The finding that there are repeated sequences at the insertion-deletion boundaries of the V4 domains of A. aegerita and A. chaxingu favors an interpretation based on a deletion event, but no evidence of deletion events was found in the other species studied. Sequences that were highly conserved in the genus Agrocybe were found in the V4, V6, and V9 domains. Such genus-specific motifs should be very helpful for identifying unidentified species to the genus level.
The secondary structures of the domains sequenced revealed that the insertion-deletion events preferentially occurred in the loops which were not directly involved in the secondary structures of the V4, V6, and V9 domains. This correlated with the ability of the variable domains to bind specific riboproteins involved in the three-dimensional form of the SSU of the mitochondrial ribosome, as described above.
A comparison of the secondary structures of related species showed that insertion-deletion events occurred in the same sections of the variable domains. For example, the differences in the lengths of the A. aegerita, A. chaxingu, and A. erebia V9 domains were due to insertion or deletion of less than 30 nucleotides at the end of the P49-2 helix. Moreover, the A. praecox and A. alnetorum V6 domains differed by 16 nucleotides that were located in the internal loop of this domain.
A comparison of the secondary structures allowed us to regroup the species. Using the V4 secondary structures, we distinguished three groups based on the presence or absence of additional helices P23-1 and P23-3. In addition, two distinct V9 secondary structures were identified by the presence or absence of the P49-1 helix. The V6 secondary structures were quite similar and did not allow us to group the species. Using these relationships, we identified the following four groups (Table 2): (i) group I was characterized by the presence of additional helices P23-1 and P23-3 in the V4 domain and the absence of the P49-1 helix in the V9 domain (A. aegerita, A. chaxingu, and A. erebia); (ii) group II organisms had an intermediate V4 domain secondary structure with only the P23-3 helix of domain V4 and a P49-1 helix in domain V9 (A. pediades); (iii) group III organisms did not have additional helices in domain V4 but had a P49-1 helix in domain V9 (A. paludosa, A. firma, A. alnetorum, and A. praecox); and (iv) group IV organisms lacked the P23-1, P23-3, and P49-1 helices (A. dura and A. vervacti). The three species belonging to groups II and IV (A. dura, A. vervacti, and A. pediades) had intermediate molecular organizations compared to those of the other two groups of species and could be considered links between groups I and III. This organization of the 10 species of the genus Agrocybe in four distinct groups is strengthened by the results of the phylogenetic analysis based on the sequences of variable domains V4, V6, and V9. Indeed, on the resulting consensus tree, species in the same group are shown to be closely related to each other (Fig. 9).
TABLE 2.
Grouping of 10 species of the genus Agrocybe on the basis of the secondary structures of the mitochondrial SSU rRNA V4 and V9 domains
| Group | Species | V4 domain
|
V9 domain P49-1 helix | |
|---|---|---|---|---|
| P23-1 helix | P23-3 helix | |||
| I | A. aegerita | +a | + | − |
| A. chaxingu | + | + | − | |
| A. erebia | + | + | − | |
| II | A. pediades | − | + | − |
| III | A. alnetorum | − | − | + |
| A. firma | − | − | + | |
| A. paludosa | − | − | + | |
| A. praecox | − | − | + | |
| IV | A. dura | − | − | − |
| A. vervacti | − | − | − | |
+, helix present; −, helix not present.
To date, there have been no phylogenetic studies of the genus Agrocybe, although in our study some of the groups deduced by comparing the V4, V6, and V9 secondary structures are consistent with previously reported morphological analysis data (23, 28). A. aegerita and A. erebia (group I) belong to the same subgenus, the subgenus Aporus. Moreover, A. paludosa and A. praecox (group III) are classified in the subgenus Agrocybe, section Agrocybe. A. vervacti (group IV) and A. pediades (group II) belong to the subgenus Agrocybe but to the sections Allocystide and Pedideae, respectively. The morphological groups correspond to the intermediate molecular organization of the two latter species compared to that of the A. aegerita and A. paludosa groups. However, our results emphasize some of the differences between the two morphological classifications described by Singer (23) and Watling (28). Indeed, on the basis of its SSU rRNA secondary structures A. firma is related to the A. paludosa group. Singer (23) found that A. firma belongs to the subgenus Agrocybe, like A. paludosa; in contrast, Watling (28) placed this species in the subgenus Aporus. A. dura, which is related to A. vervacti as determined in our study, is classified in the section Agrocybe by Singer and Watling.
Molecular studies of the V4, V6, and V9 domains could be a good alternative method for determining relationships between species. In recent years several mitochondrial sequences have been used in similar investigations, including investigations of the Cox I gene of Drosophila (24), Coleoptera (9), Ascomycota (18), and protista (26) and mitochondrial SSU rRNA sequences of Ascomycota (10, 18). Moreover, in view of our results obtained for the genus Agrocybe, sequences of variable domains V4, V6, and V9 of the SSU rRNA could be used as molecular markers to identify Basidiomycota species. Indeed, in contrast to RFLPs or internal transcribed spacer amplification, in which differences between isolates of the same species are observed, the lengths and sequences of the V4, V6, and V9 domains seem to be species specific. Future studies must include species belonging to other genera and families of the Basidiomycota. The results for the genus Agrocybe and preliminary assay results for other Basidiomycota species (data not shown) suggest that the three primer pairs which we used (V4U plus V4R, V6U plus V6R, and V9U plus V9R) may be ubiquitous and could be used to amplify the mitochondrial DNAs of various Basidiomycota species.
ACKNOWLEDGMENTS
This work was supported by grants from the Conseil Scientifique de l’Université Victor Segalen Bordeaux 2, the Conseil Régional d’Aquitaine, and the Institut National de la Recherche Agronomique.
We thank the Prapaisri Pitakpaivan (Department of Agriculture, Bangkok, Thailand) for providing the A. chaxingu strains and J. L. Reigne and C. Ducos for technical help.
REFERENCES
- 1.Brimacombe R, Atmajda J, Stiege W, Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16S ribosomal RNA in situ in the 30S subunit. J Mol Biol. 1988;199:115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- 2.Bruns T D, Szaro T M. Rate and mode differences between nuclear and mitochondrial small subunit rRNA genes in mushrooms. Mol Biol Evol. 1992;9:836–855. doi: 10.1093/oxfordjournals.molbev.a040760. [DOI] [PubMed] [Google Scholar]
- 3.Bruns T D, White T J, Taylor J W. Fungal molecular systematics. Annu Rev Ecol Syst. 1991;22:525–564. [Google Scholar]
- 4.Gonzalez P, Barroso G, Labarère J. DNA sequence and secondary structure of the mitochondrial small subunit ribosomal RNA coding region including a group-IC2 intron from the cultivated basidiomycete Agrocybe aegerita. Gene. 1997;184:55–63. doi: 10.1016/s0378-1119(96)00573-2. [DOI] [PubMed] [Google Scholar]
- 5.Gregory R J. Interaction of Escherichia coli ribosomal protein S8 with its binding site in ribosomal RNA and messenger RNA. J Mol Biol. 1988;204:295–307. doi: 10.1016/0022-2836(88)90577-3. [DOI] [PubMed] [Google Scholar]
- 6.Hibbet, D. S., and M. J. Donoghue. 1995. Progress toward a phylogenetic classification of the Polyporaceae through parsimony analysis of mitochondrial ribosomal DNA sequences. Can. J. Bot. 73(Suppl. 1):S853–S861.
- 7.Higgins D G, Sharp P M. Fast and sensitive multiple alignment sequence on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 8.Iraçabal B, Zervakis G, Labarère J. Molecular systematics of the genus Pleurotus: analysis of the restriction polymorphism in ribosomal DNA. Microbiology. 1995;141:1479–1490. doi: 10.1099/13500872-141-6-1479. [DOI] [PubMed] [Google Scholar]
- 9.Juan C, Oromi P, Hewitt G M. Phylogeny of the genus Hegeter (Tenebrionidae, Coleoptera) and its colonization of the Canary Islands deduced from cytochrome oxidase I mitochondrial DNA sequence. Heredity. 1996;76:392–403. doi: 10.1038/hdy.1996.57. [DOI] [PubMed] [Google Scholar]
- 10.Li K N, Rouse D I, German T L. PCR primers that allow intergeneric differentiation of ascomycetes and their applications to Verticillium spp. Appl Environ Microbiol. 1994;60:4324–4331. doi: 10.1128/aem.60.12.4324-4331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lott T J, Burns B M, Zancope-Oliveira R, Elie C M, Reiss E. Sequence analysis of the internal transcribed spacer 2 (ITS 2) from yeast species within the genus Candida. Curr Microbiol. 1998;36:63–69. doi: 10.1007/s002849900280. [DOI] [PubMed] [Google Scholar]
- 12.Molcalvo J M, Wang H H, Hseu R S. Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacer and 25S ribosomal DNA sequences. Mycologia. 1995;87:223–238. [Google Scholar]
- 13.Moulinier T, Barroso G, Labarère J. The mitochondrial genome of the basidiomycete Agrocybe aegerita: molecular cloning, physical mapping and gene location. Curr Genet. 1992;21:499–505. doi: 10.1007/BF00351660. [DOI] [PubMed] [Google Scholar]
- 14.Mueller F, Brimacombe R. A new model for the three-dimensional folding of Escherichia coli 16S ribosomal RNA. II. The RNA-protein interaction data. J Mol Biol. 1997;271:545–565. doi: 10.1006/jmbi.1997.1211. [DOI] [PubMed] [Google Scholar]
- 15.Neefs J M, Van de Peer Y, De Rijk P, Chapelle S, De Wachter R. Compilation of small ribosomal subunit RNA structures. Nucleic Acids Res. 1993;21:3025–3049. doi: 10.1093/nar/21.13.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noël T, Labarère J. Isolation of DNA from Agrocybe aegerita for construction of a genomic library in Escherichia coli. Mushroom Sci. 1987;12:187–201. [Google Scholar]
- 17.Noller H F, Lake J A. Ribosome structure and function: localization of rRNA. In: Bittar E, editor. Membrane structure and function. New York, N.Y: John Wiley & Sons, Inc.; 1984. pp. 217–297. [Google Scholar]
- 18.Paquin B, Forget L, Roewer I, Lang B F. Molecular phylogeny of Allomyces macrogynus: congruency between nuclear ribosomal RNA- and mitochondrial protein-based trees. J Mol Evol. 1995;41:657–665. doi: 10.1007/BF00175824. [DOI] [PubMed] [Google Scholar]
- 19.Raper J R, Hoffman R M. Schizophyllum commune. In: King R C, editor. Handbook of genetics. New York, N.Y: Plenum Press; 1974. pp. 597–626. [Google Scholar]
- 20.Ristaino J B, Madritch M, Trout C L, Parra G. PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora. Appl Environ Microbiol. 1998;64:948–954. doi: 10.1128/aem.64.3.948-954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudi K, Skulberg O M, Larsen F, Jakobsen K S. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol. 1997;63:2593–2599. doi: 10.1128/aem.63.7.2593-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanger F, Nicklen S, Coulson A R. Sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer R. Key world spp. Sydowia Ann Mycol. 1977;30:195–205. [Google Scholar]
- 24.Spicer G S. Phylogenetic utility of the mitochondrial cytochrome oxidase gene: molecular evolution of the Drosophila buzzatii species complex. J Mol Evol. 1995;41:749–759. doi: 10.1007/BF00173155. [DOI] [PubMed] [Google Scholar]
- 25.Stern S, Powers T, Changchien L M, Noller H F. RNA-protein interactions in 30S ribosomal subunit: folding and function of 16S rRNA. Science. 1989;244:783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- 26.Teissier L H, Van der Speck H, Gualberto J M, Grienenberger J M. The Cox I gene from Euglena gracilis: a protist mitochondrial gene without introns and genetic code modifications. Curr Genet. 1997;31:208–213. doi: 10.1007/s002940050197. [DOI] [PubMed] [Google Scholar]
- 27.Urakawa H, Kita-Tsukamoto K, Ohwada K. A new approach to separate the genus Photobacterium from Vibrio with RFLP patterns by Hha I digestion of PCR-amplified 16S rDNA. Curr Microbiol. 1998;36:171–174. doi: 10.1007/pl00006762. [DOI] [PubMed] [Google Scholar]
- 28.Watling R. Bolbitiaceae: Agrocybe, Bolbitius & Conocybe. In: Henderson D M, Orton P D, Watling R, editors. British fungus flora: agarics and boleti. Edinburgh, United Kingdom: Royal Botanic Garden; 1982. pp. 6–30. [Google Scholar]