Abstract
Background
Previous Obesity Medicine Association (OMA) Clinical Practice Statements (CPS) included topics such as behavior modification, motivational interviewing, and eating disorders, as well as the effect of concomitant medications on weight gain/reduction (i.e., including psychiatric medications). This OMA CPS provides clinicians a more focused overview of stress and psychiatric disease as they relate to obesity.
Methods
The scientific support for this CPS is based upon published citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
Results
Topics in this CPS include the relationship between psychological stress and obesity, including both acute and chronic stress. Additionally, this CPS describes the neurobiological pathways regarding stress and addiction-like eating behavior and explores the relationship between psychiatric disease and obesity, with an overview of psychiatric medications and their potential effects on weight gain and weight reduction.
Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on stress and psychiatric disease is one of a series of OMA CPSs designed to assist clinicians in the care of patients with the disease of obesity. Knowledge of stress, addiction-like eating behavior, psychiatric disease, and effects of psychiatric medications on body weight may improve the care obesity medicine clinicians provide to their patients with obesity.
Keywords: Clinical practice statement, Depression, Obesity, Pre-obesity, Psychiatric disease, Stress
1. Introduction
Beginning in 2013, the Obesity Medicine Association (OMA) created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees [1]. This was followed by a similar Pediatric “Obesity Algorithm” with updates approximately every two years by OMA authors. This current “Stress, Psychiatric Disease, and Obesity: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022” was derived from the 2021 OMA Adult Obesity Algorithm and is one of a series of OMA CPSs designed to assist clinicians in the care of their patients with the disease of obesity. Illustrative examples of prior published OMA CPS potentially applicable and complementary to this topic include:
-
•
Behavior, motivational interviewing, eating disorders, and obesity management technologies: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022 [2].
-
•
Concomitant medications, functional foods, and supplements: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022 [3].
2. Mental stress and obesity
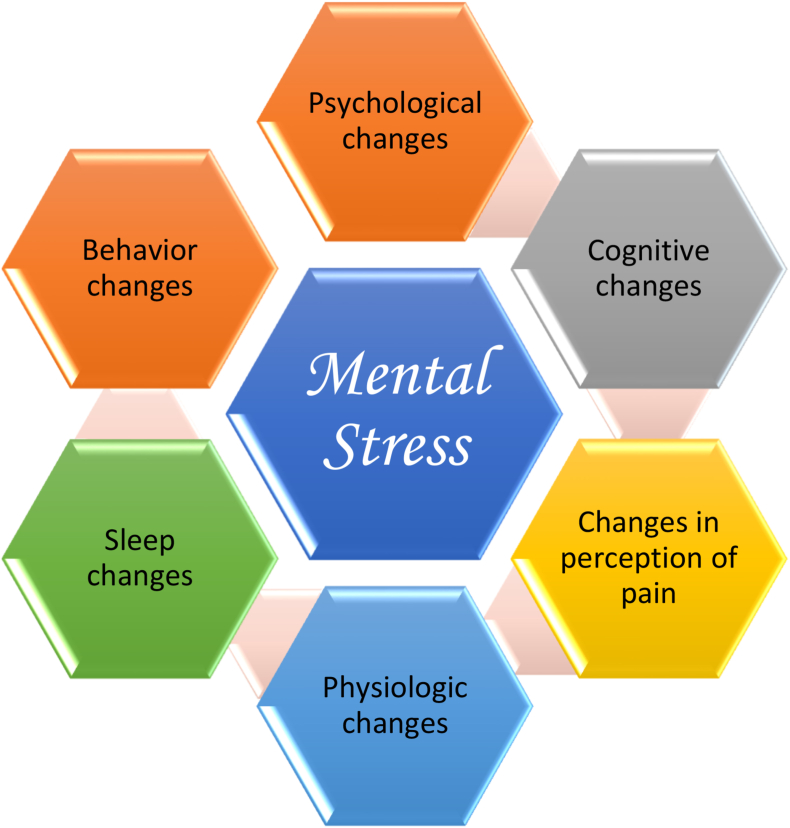
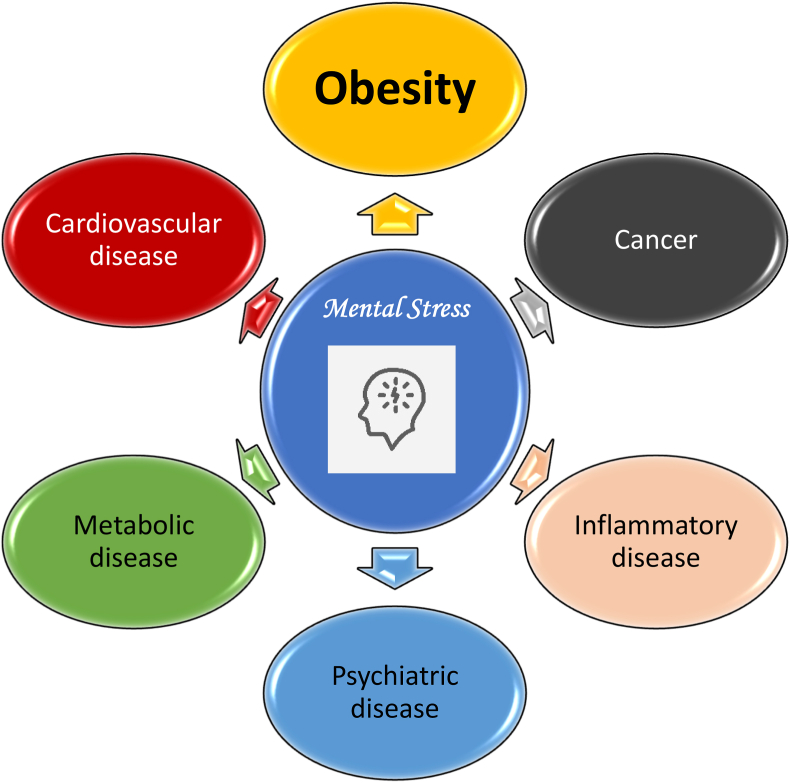
Stress involves intrinsic or extrinsic stimuli that evoke biological responses [14]. Biologic stress can be mental and/or physical (See Fig. 1). Mental stress contributes to the most common medical conditions encountered by clinicians and their patients (e.g., obesity, cardiovascular disease, metabolic diseases, cancer, inflammatory diseases, and psychiatric diseases (See Fig. 2). Similar to obesity and other pathogenic processes, mental stress can promote adverse health effects (See Fig. 3).
Fig. 1.
Illustrative Clinical Manifestations of Mental Stress. Biologic stress can be physical and/or mental. Mental stress can influence behavior and affect psychological well-being. Many adaptive responses to acute stress are favorable towards enhancing survival. (i.e., ”fight or flight”). Conversely, chronic mental stress (i.e., “submit and stay”) is generally unfavorable and may increase allostatic load involving biologic alterations that weaken stress-related adaptive processes and increase susceptibility to disease [4]. Mental stress can promote changes in sleep, cognition, and perception of pain. Finally, beyond the contribution to obesity, mental stress can contribute to adverse physiologic changes in body systems, via generation of reactive oxygen species, as well as acute and chronic effects upon the endocrine and immune systems, potentially resulting in alterations in cardiopulmonary function, metabolic processes, increased risk of inflammatory diseases [5], cardiovascular disease [6,7], and cancer [7,8] (See Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Fig. 2.
Effects of mental stress on human disease. The endocrinopathies and immunopathies of mental stress can worsen or increase the risk of chronic diseases such as obesity, cardiovascular disease, cancer, metabolic diseases (e.g., hypertension, diabetes mellitus), inflammatory and autoimmune diseases (e.g., lupus, rheumatoid arthritis), and psychiatric diseases (e.g., depression, anxiety) [7,9,10].
Fig. 3.
Reactive oxygen species (ROS) creation and adverse clinical consequences (Adapted from Ref. [11]). Obesity contributes to mental stress [12]; mental stress contributes to obesity [13]. Both obesity [14] and mental stress can help generate reactive oxygen species that adversely affects endothelial function [15], promotes DNA damage [16], promotes cancer [17], increases the degree of perivascular fibrosis [18], and influences multiple biological aging pathways [19].
DNA: Deoxynucleic acid.
Endogenous promoters: includes NADPH oxidase in the cellular plasma membranes, myeloperoxidases in the lysosome, and electronic transport in the mitochondria.
NADPH: nicotinamide adenine dinucleotide phosphate.
2.1. Psychological or mental stress
Chronic mental stress can change behavior and adversely affect body system functions [4] (See Fig. 1, Fig. 2, Fig. 3). “Stress” can be experimentally defined as a state of threatened homeostasis during which adaptive processes are activated to produce physiologic and behavior change [56]. “Stress” can be clinically defined as a physical, emotional, or psychological strain reaction to an experience. “Stress” can be objectively measured (e.g., thermal changes, noise, carbon dioxide challenge, emotional challenges) [56]. The best practice measures and validated instruments regarding psychologic and physiologic stress depend on the type of stress, timing of stress, and individual patient presentation [57]. For example, an “acute stress” experience may differ from an “acute stress disorder.” An “acute stress” experience can reflect an isolated momentary experience (e.g., sudden injury, illness, disaster, argument, criticism, or unexpected news or events) [58]. Regarding stress as a diagnosable disorder, “acute stress disorder” is defined by the Diagnostic and Statistical Manual of Mental Disorders as a reaction no less than 3 days and no more than 4 weeks [59].
Chronic stress may cause sustained strain on biologic systems, with the cumulative burden of adverse life events contributing to an “allostatic load,” with allostasis (i.e., allo = different and stasis = standing still) representing body responses to stressors with the intent to achieve an equilibrium. Chronic stress is intended to reflect physiologic and/or emotional responses to a more prolonged event, or persistently recurring events (e.g., prolonged illness or pain, long-term and sustained adverse emotional response to an adverse personal, family, and/or workplace environment) [60] (See Fig. 1).
Cognitive abilities may improve or worsen during acute stress but tend to worsen during cases of prolonged mental stress [4]. Mental stress can adversely affect sleep, and sleep disruption can contribute to body fat and visceral fat accumulation [61]. While the effect of acute stress is affected by individual stress responsiveness (with high stress responders potentially having increased perceived pain), increase in pain tolerance may occur with acute stress, especially among low stress responders [62]. Diminished pain tolerance may also occur with chronic mental stress [63]. From a psychologic and behavior standpoint, chronic mental stress may lead emotional eaters to consume less healthful, ultra-processed, hyperpalatable foods. These “comfort” foods may provide short-term relief from anxiety and depression through the release of neurochemicals associated with pleasure (i.e., dopamine) [2,4], with hedonic eating often surpassing homeostatic needs, and thus contributing to obesity [64,65]. Individuals experiencing chronic mental stress may find the sources of their stress (e.g., work, family, or other issues) overtake their nutritional and physical activity-related goals and lead to impairment of self-regulation regarding food consumption [2,54]. Among the physiologic changes are the linkage of stress-induced neuroendocrine and immune disruption [53].
2.2. Mental stress: Acute and chronic endocrine responses
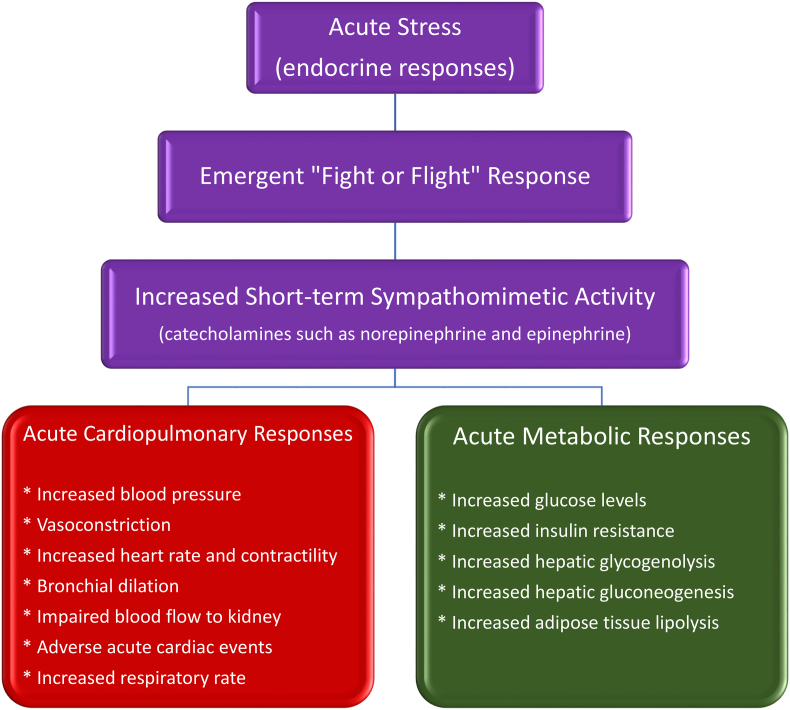
Acute mental stress can elicit shorter-term “fight or flight” endocrine/hormone responses (See Fig. 4). Increased sympathomimetic activity from acute stress can provide favorable, shorter-term adrenergic stress responses such as improved cognition, increased tolerance to pain, and improved physiologic functions [4]. Short-term metabolic responses to increased norepinephrine (i.e., noradrenaline, mainly from nerve endings) and epinephrine (i.e., adrenaline, mainly from the adrenal medulla) [66] include a potential increase in glucose levels (increased insulin resistance, increased hepatic glycogenolysis, and increased hepatic gluconeogenesis) and increased adipose tissue lipolysis [4,51,52,55], increasing readily available nutrient fuels. Among potentially beneficial effects of the “fight or flight” reaction to acute stress are cardiopulmonary responses that include increased blood pressure, increased heart rate, increased heart contractility, and bronchial dilation [4,51]. While these responses may be advantageous regarding “fight or flight,” acute psychosocial stress (e.g., cancer diagnosis, sudden financial loss) can also have unfavorable effects, such as an increased risk of acute cardiovascular events [4] and increased respiratory rate leading to dyspnea [5]. While acute stress is usually associated with decreased hunger, acute stress may contribute to emotional eating, especially for those with underlying chronic mental stress [4].
Fig. 4.
Acute stress and potential endocrine responses. Among the potential effects of acute mental stress include alterations in glandular (e.g., adrenal) and nervous system catecholamine hormone release, that can affect cardiopulmonary responses, metabolic processes, and other body systems. While “fight or flight” responses are often characterized as favorable (e.g., improved cognition, improved cardiopulmonary and muscular function, and increased tolerance to pain), acute psychosocial stress (e.g., cancer diagnosis, sudden financial loss) can increase the risk of acute cardiovascular complications (e.g., myocardial infarction, left ventricular dysfunction, and cardiac dysrhythmias) [20]. Similarly, acute stress can also contribute to increased blood sugar [21] and increased respiratory rate resulting in dyspnea [22]. Finally, while acute stress typically decreases hunger, those under chronic mental stress may engage in emotional eating when encountering acute mental stress [4].
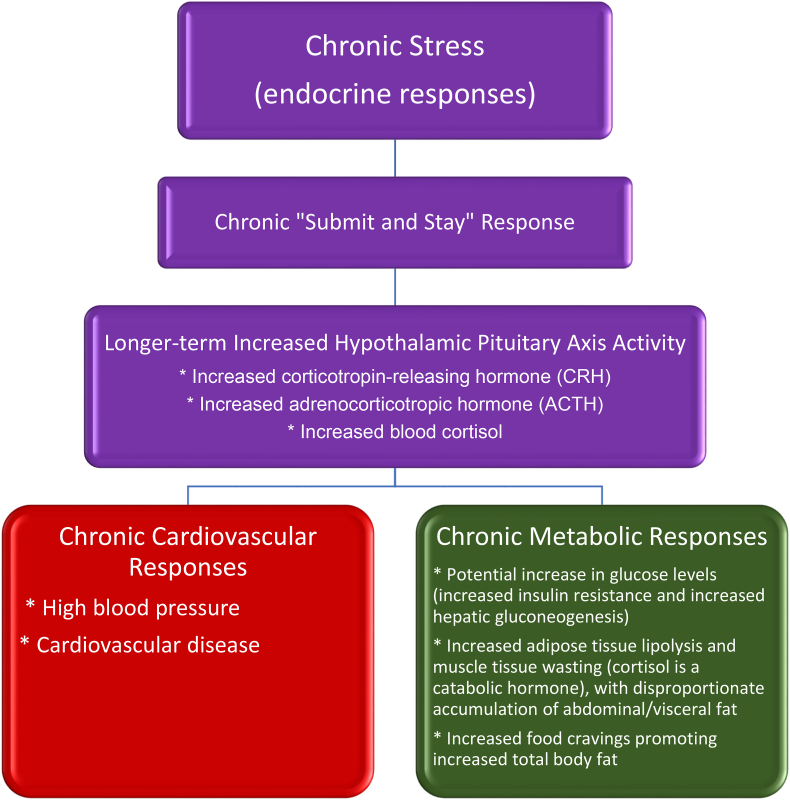
Chronic mental stress can elicit longer-term “submit and stay” hormonal responses (See Fig. 5). Chronic mental stress can lead to unfavorable hormonal and adverse health consequences, such as increased anxiety and depression, behavior change, diminished cognition, poor sleep patterns, reduced tolerance to pain, and adverse physiologic/metabolic processes [4,[51], [52], [53]]. This chronic response corresponds to increased hypothalamic pituitary axis activity [4,51,52]. The endocrine response to prolonged/chronic psychological or medical stress involves increased release of stress hormones such as corticotropin-releasing hormone, adrenocorticotropic hormone, and cortisol [4,51]. In fact, experimental evaluation of “stress” often involves measurement of cortisol in saliva, blood, urine, or hair [67]. The metabolic response to increased cortisol may include increased insulin resistance and increased hepatic gluconeogenesis leading to hyperglycemia (i.e., pre-diabetes or diabetes mellitus) [4,51]. Additionally, a metabolic response to chronic mental stress may include increased blood pressure and cardiovascular disease. Chronic mental stress can facilitate increased caloric intake due to increased food cravings, and increase adipose tissue lipolysis and muscle tissue wasting (because cortisol is a catabolic hormone) with disproportionate accumulation of abdominal fat [4,51,[68], [69], [70], [71]]. Finally, weight bias can contribute to chronic psychological stress [72], with weight discrimination commonly encountered in institutions such as policy construct, healthcare, media, workplaces, education [73], and society in general. Clinically, weight bias may manifest by further weight gain, contributing to a “cyclic obesity/weight-biased stigma” model [74].
Fig. 5.
Chronic stress and potential endocrine responses. Among the potential physiologic effects of chronic mental stress include alterations in hormone release that affect cardiac function (e.g., high blood pressure [23], cardiovascular events, and heart failure [20]). Mental stress can also adversely affect processes involving glucose and fat metabolism [15], as well as increase food cravings [4]. Potential contributors to mental stress include major life changes, adverse life encounters, adverse life situations, adverse socioeconomic factors [20], as well as encounters of adverse body weight stigma [24].
2.3. Medical stress: Immune responses
Medical or psychological/mental stress can affect both the innate and adaptive immune responses (See Fig. 6). Innate immunity involves nonspecific body defense mechanisms that are activated short-term (i.e., within hours) and include physical barriers such as skin, chemicals in blood (i.e., complement proteins and cytokines), and immune cells that attack foreign cells in the body. Among the immune cells involved in innate immunity are dendritic leukocytes, neutrophils, monocytes/macrophages, eosinophils, basophils, and mast cells. Immunity can also be derived from the complement system and killer cells, which have both innate and adaptive traits [75,76]. Adaptive immunity is mainly lymphocyte driven, antigen specific, and acquired during exposure during a lifetime. The immune response with adaptive immunity is longer-term than innate immunity (i.e., onset days to weeks). Adaptive immunity includes cytotoxin-producing T-cells and antibody-producing B cells.
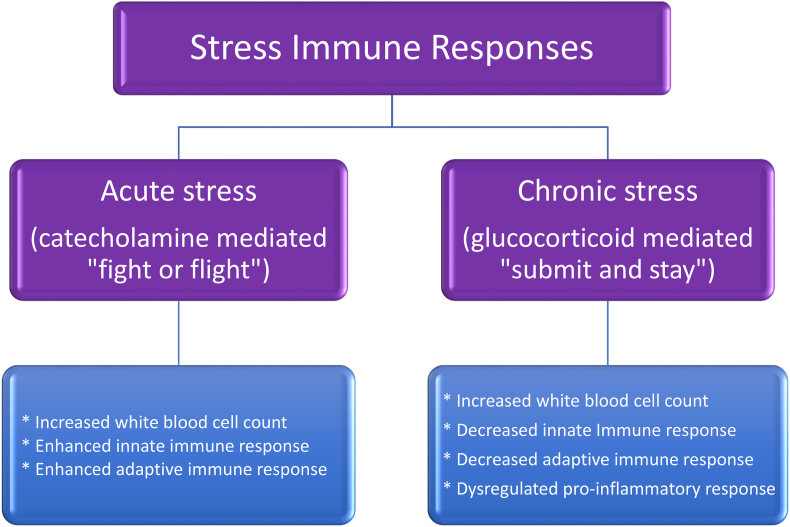
Fig. 6.
Acute and chronic stress immune responses. The immunologic reactions to stress stimuli depend on neuroendocrine mediation. Acute mental stress can elicit a catecholamine-mediated increase in white blood cell count (i.e., possibly due to vascular demargination or mobilization of immune cells from the bone marrow) [[25], [26], [27]], reflecting a potential increase in nonspecific innate immune response (i.e., nonspecific rapid activation and trafficking of dendritic leukocytes, neutrophils, monocytes/macrophages, eosinophils, basophils, and mast cells). Immunity also involves the complement system and cytokine release from natural killer cells, both which may function as innate and adaptive immune response. Acute mental stress may also enhance adaptive immune response (i.e., T-cell cytokines and B-cell antibodies) [28]. Chronic mental stress is often associated with increased hypothalamic pituitary axis activity resulting in increased corticotropin-releasing hormone, increased adrenocorticotropin, and increased glucocorticoids such as cortisol. In contrast to acute mental stress that may enhance immune function [29], chronic mental stress can impair adaptive immune responses. While chronic stress can increase proliferation of monocytes and neutrophils, chronic mental stress may decrease lymphocytes (such as T cells and B cells) [28,30,31] and thus decrease adaptive immune responses. Paradoxically, chronic stress can also facilitate dysregulated lymphocyte responses (i.e., deranged release of cytokines), resulting in unfavorable proinflammatory reactions helping to account for the association of chronic mental stress with inflammatory and autoimmune diseases [7,29].
2.3.1. Mental stress: Acute and chronic immune response
Immune effects due to stress can be mixed, but, in general, an acute stress response may enhance both innate and adaptive immune responses [4,51,77] (See Fig. 6). In contrast to acute mental stress, prolonged mental stress response may dysregulate the immune response (See Fig. 6). Those under prolonged stress show an increased total white blood cell count, suggesting increased systemic inflammation [78]. Prolonged stress may also impair immunity by promoting a decrease in cytokine-producing T lymphocytes and decrease in antibody-producing B lymphocytes [30,79], all resulting in impaired adaptive immunity (See Fig. 6). The concurrent impairment of immune response to pathogens and dysregulated pro-inflammatory response helps explain the poor outcomes of certain diseases among patients with obesity, such as lung infections (See Fig. 7). Patients with obesity often struggle with chronic stress and often have underlying lung dysfunction (i.e., due to fat mass disease), predisposing them to additional or more severe pulmonary disease. Upon exposure to a pathogen, severe and potentially life-threatening lung disease [24] can occur when immunity to the infection is impaired (i.e., both by obesity and chronic mental stress), and especially when compounded by dysregulated pro-inflammatory immune response (i.e., “cytokine storm”) [25].
Fig. 7.
Physical stress and lung disease in patients with obesity. In addition to mental stress, common physical stressors can also adversely affect the health of patients with obesity (e.g., acute cardiovascular events, cancer, sleep apnea, osteoarthritis, and lung infection). The adverse health effects of mental stress are often the result of background predisposition to disease, followed by an introduction of an unhealthful stressor, eliciting a pathogenic body response (sometimes exaggerated) leading to an adverse clinical outcome. Physical stress can follow a similar pattern. The figure illustrates a common clinical scenario of an obesity-related predisposed disease (e.g., lung dysfunction), worsened by the added stressor of upper respiratory tract infection in a patient with obesity-mediated impaired immunity, resulting in severe and potentially life-threatening lung disease [32], compounded by dysregulated immune response (i.e., “cytokine storm”) [33].
2.4. Chronic mental stress and eating behavior
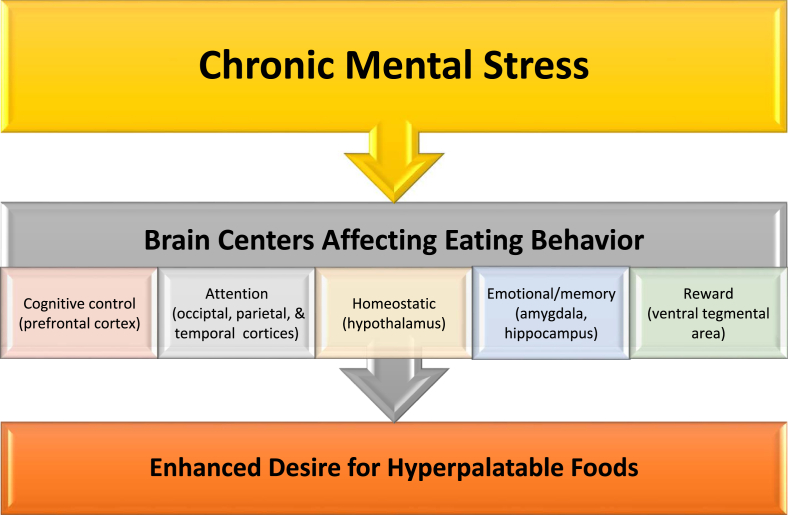
Physiologically, eating food serves to provide calories and nutrients for essential body functions. Eating is necessary for survival (i.e., caloric and nutrient homeostasis). Presumably to facilitate the essential behavior of eating, food intake is facilitated by stimulation of the brain's pleasure and reward centers. An obesogenic environment emerges with the manufacturing and advertising of foods that are low in micronutrients, low in fiber, high in calories, high in fat, high in sugar (i.e., added sweeteners), and high in salt. Consumption of nutrient poor, hyperpalatable foods often target various areas of the brain in a way that overwhelms cognitive control (See Fig. 8). Chronic mental stress can compound this obesogenic environment via increased attention to food eating cues, integration with emotions, and prioritizing the stimulation of the reward center [80] (See Fig. 9). Obesity, adiposopathy, and metabolic disease can contribute to worsening chronic physical and mental stress, further worsening endocrinopathies, immunopathies, and adverse behavior, leading to further increases in body fat, worsening of adiposopathy, all potentially leading to an adiposopathic stress cycle [4,52,55] (See Fig. 10).
Fig. 8.
Differential effects of healthful whole foods versus unhealthful ultra-processed / hyperpalatable foods. Consumption of a variety of natural whole foods provides healthful macro and micronutrients, fiber, and are often modest in calories. Eating healthful whole foods may result in a balanced stimulation of multiple areas of the brain related to alleviating hunger, and promoting satiety, pleasure, and reward, in a way that is homeostatic, not excessive, and in a way that does not overwhelm cognitive restraints. Other foods undergo ultra-processing [34]. Ultra-processed foods often undergo manufacturing research development and advertisement investment prioritized towards the creation of hyperpalatable foods. Consumption of ultra-processed hyperpalatable foods may result in the unbalanced stimulation of pleasure and reward brain centers (i.e., independent of hunger, irrespective of satiation, and beyond the control of cognitive restraint), often resulting in body systemic exposure to unhealthful hedonistic foods that are low in micronutrients, low in fiber, high in calories, high in fat, high in sugar (i.e., added sweeteners), high in salt, and lower in cost compared to more healthful foods, all contributing to addiction-like eating behavior [[35], [36], [37], [38]].
Fig. 9.
Chronic psychological/mental stress and eating behavior. Eating behavior is mediated by multiple brain areas and processes. The research, manufacturing, advertisement, availability, lower cost, and consumption of hyperpalatable ultra-processed foods may contribute to increased adiposity by facilitating central nervous system-mediated food seeking behavior, that in some cases, may lead to addiction-like eating behavior. Chronic mental stress can accentuate obesogenic potential by effects upon the cerebrum (i.e., frontal, parietal, occipital, and temporal lobes), limbic system (i.e., hypothalamus, thalamus, amygdala, hippocampus), and the ventral tegmental area (midbrain which sends dopaminergic neural projections to both the cortical and limbic areas) leading to altered eating behaviors and an enhanced desire for hyperpalatable foods (i.e., containing high fat and high sugar) [4,39,40].
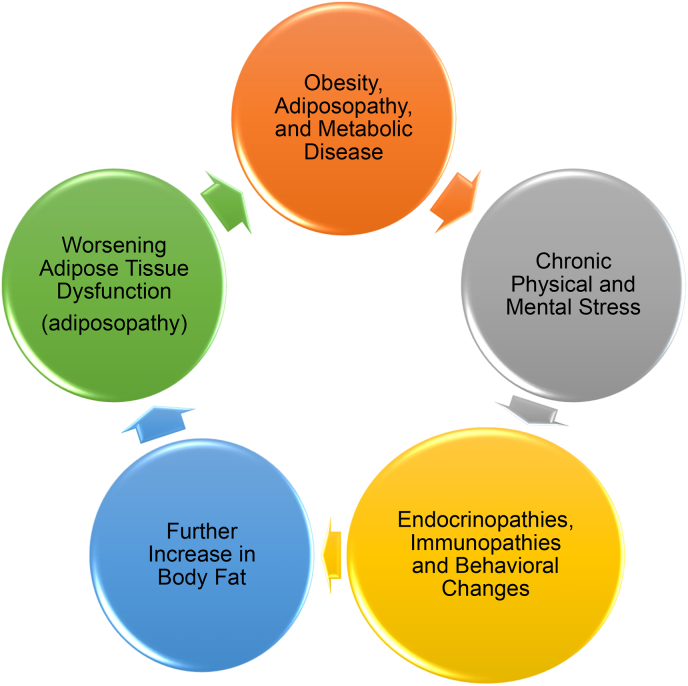
Fig. 10.
The adiposopathic stress cycle [1]. Obesity and its adverse health complications may increase physical and mental stress, which may contribute to unhealthful behavior, endocrinopathies, and immunopathies, which, in turn, may further worsen obesity and its complications, resulting in an adiposopathic stress cycle.
Specifically, behaviors such as eating can be influenced by the effects of mental stress on various parts of the brain (See Fig. 9). The cerebral cortex (i.e., gray matter of the cerebrum), is a portion of the outer layer of the brain that can be divided into frontal, parietal, occipital, and temporal lobes. The frontal lobe includes the prefrontal cortex, which is responsible for higher-level function, and which can influence cognitive control over behaviors such as eating. Touch of food is mainly processed by the parietal lobe, which also coordinates somatosensory information input from the occipital lobe (vision), temporal lobe (hearing), as well as the olfactory bulb (smell) and insular cortex (taste). The increased activation of these sensory processes lead to higher level attention to food and eating environment surroundings, thus potentially influencing appetite and eating behavior. The limbic system (i.e., hypothalamus, amygdala, hippocampus, and thalamus) controls automatic primitive reactions, such as food, fight, flight, and fornication (4F's). Hunger (as potentially opposed to appetite) is driven mainly by the hypothalamus. Eating that is prompted by emotion and meal-related pleasure memories are driven by the amygdala and hippocampus regions, respectively. The thalamus relays sensory information to the cerebral cortex for processing.
Chronic mental stress can impact the cerebrum through priority replacement: personal, work, or emotional priorities may overtake priorities relative to nutrition, physical activity, and/or health [40]. Self-regulation can be undermined by failures to transcend overwhelming temptations, negative moods, resource depletion (“decision fatigue” wherein daily choices or self-regulating emotions over an extended period of time may impair resistance regarding the temptation to eat appetizing foods), and when minor lapses in self-control snowball into self-regulatory collapse [40,81]. While not a stress disorder, Gourmand Syndrome is illustrative of how cerebral disorders may affect eating behaviors. Gourmand Syndrome occurs with damage to the right frontal lobe, which often takes place either due to trauma or stroke. After injury to the right frontal lobe, patients may experience compulsions related to eating and often exhibit a passion for gourmet foods [82].
Just as chronic stress-induced endocrinopathies and immunopathies may adversely affect the higher level cerebrum [4], the same adverse effects can occur with the lower level, more primitive limbic system – all leading to altered eating behaviors and an enhanced desire for hyperpalatable foods [4,4,39,40,51,52] (See Fig. 9). Areas of the limbic system especially described as potentially contributory to obesity include inflammation of the hypothalamus (and possibly thalamus) [83,84].
The mesolimbic dopamine pathway (i.e., reward pathway) includes the ventral tegmental area (VTA) that connects to other parts of the brain via (a) mesocortical pathways to the cerebral cortex (e.g., to the prefrontal lobe and other areas involved with motivation, emotion, and executive function) and (b) limbic pathways (e.g., to the nucleus accumbens and other areas involved with the reward system). Dopamine is an example of neurotransmitter often associated with facilitation of happiness (with others being serotonin, norepinephrine, and endorphins) [85]. Stimuli such as hyperpalatable food intake (as well as music, sexual activity, opiates, and alcohol) may increase VTA dopamine secretion, facilitate pleasure, and improve mood [86]. Excessive brain exposure to glucocorticoids due to chronic mental stress may affect the brain reward center in a manner that may promote binge eating and intake of hyperpalatable foods even when individuals are satiated [87,88]. Hypersecretion of glucocorticoids (i.e., as may occur with chronic stress) are also linked to symptoms of anxiety and depression [89], with anxiety and depression contributing to adverse eating behaviors potentially leading to preobesity/overweight or obesity [90].
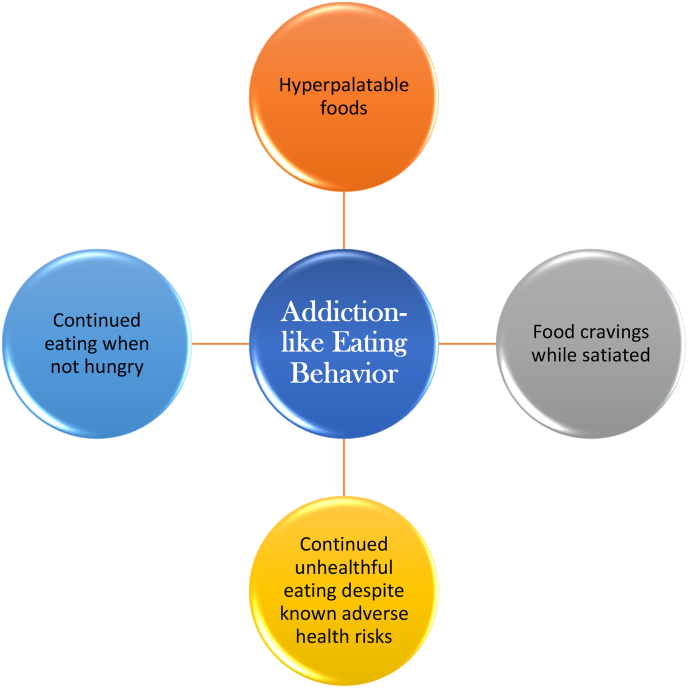
A reason why ultra-processed, hyperpalatable foods are especially problematic in the patient with obesity and mental stress, is because acute consumption may induce pleasure and reward [91] (See Fig. 11). However, chronic consumption of hyperpalatable food may disrupt and possibly desensitize the brain's reward system (i.e., reward deficiency syndrome) [49]. Chronic hyperpalatable food intake may disrupt appetite regulation and lead to reward hyposensitivity, in a way that prompts recurrent consumption of “junk food” to “feed” the impaired reward system, clinically manifested by compulsive eating [91]. When patients continue to eat hyperpalatable, ultra-processed, calorie dense, nutrient poor, high fat, high sugar, and high salt foods, despite lack of hunger, despite being satiated, and despite the cognitive recognition that such consumption is unhealthful and potentially life-threatening, then this may reasonably be characterized as addiction-like eating behavior [[35], [36], [37], [38]]. The pathways and clinical challenges of addiction-like eating behavior are not dissimilar to other addictions such as opioid and alcohol addictions [80]. Specifically, an addiction can be defined as a neuropsychologic disorder resulting in persistent and intense urges to engage in adverse conduct or unhealthful behavior. Some individuals find illicit drug use, alcohol consumption, or cigarette smoking pleasurable. However, they employ cognitive control to override and stop such activities, when these activities are harmful to themselves, family, or friends. Individuals who persist with harmful behaviors, despite clear harm to themselves, family, and friends, are often characterized as being “addicted.” Among patients with obesity, continued unhealthful eating despite known adverse health risks might also be reasonably characterized as displaying an addiction-like eating behavior [[35], [36], [37], [38]] (See Fig. 12).
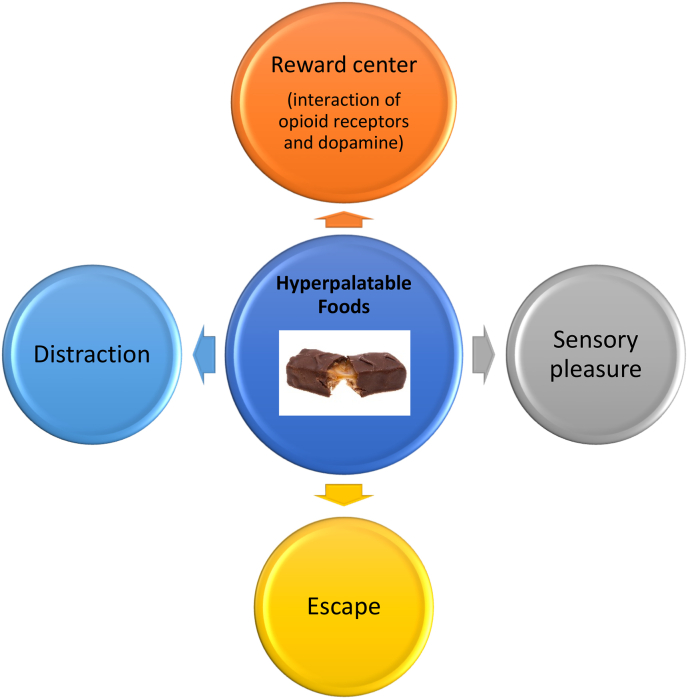
Fig. 11.
Effects of hyperpalatable foods on mental processes. Hyperpalatable “junk foods” are typically ultra-processed and unhealthful (i.e., calorie dense, nutrient poor, and contain high fat, high sugar, and high salt) [41]. Consumption of hyperpalatable, unhealthful junk food may help sooth mental stress and depression on a short-term basis by (a) stimulating the reward center (i.e., ventral tegmental area) [42] via release of hypothalamic endorphins and activating mu-opioid receptors (MOR) that enhance dopamine release (i.e. “feel good” hormone). Over time, MOR dopamine interactions may become dysfunctional with diminished opioid-dependent rewarding effects of eating, which may prompt even more consumption of hyperpalatable foods to achieve the same pleasurable effects (i.e., addition-like eating behavior) [[43], [44], [45]]; (b) fulfilling food pleasure cues (e.g., sensory pleasures such as food appearance, odor, taste, and texture [46]; and (c) submitting to the distraction of visualized hyperpalatable foods (e.g., advertisement or strategic placement in grocery stores) towards a desire for personal escape (e.g., combat boredom and temporarily improve depressed mood) [47,48].
Fig. 12.
Addiction-like Eating Behavior [[35], [36], [37], [38]]. The neurobiologic pathways and clinical manifestations of patients with addiction-like eating behavior are analogous to other addictions (e.g., addiction to opioids, alcohol, heavy cigarette smoking, and gambling) [[35], [36], [37], [38],49]. While hunger and eating are largely mediated by more primitive parts of the brain such as the hypothalamus, the ability to resist overeating requires prioritization by higher cognitive functions (See Fig. 8). Patients with obesity have impaired dopaminergic pathways that otherwise regulate reward sensitivity and cognitive control [50]. Failure of higher brain functions to override other brain-mediated consumption (eating) of hyperpalatable, unhealthful foods, despite lack of hunger, despite being satiated, and despite known adverse health risks, might reasonably be characterized as an addiction-like behavior [[35], [36], [37], [38]] (See Fig. 8).
In short, just as mental stress may predispose individuals to adverse health-risk behaviors such as illicit drug use, alcohol consumption, and cigarette smoking, so might chronic mental stress promote unhealthful intake of highly palatable, nutrient-poor food that may mitigate stress symptoms via activation of the endogenous opioid (reward) system, reduction of the hypothalamic-pituitary-adrenal (HPA) axis stress response, as well as via stimulation of sensory pleasure, distraction, and escape effects [41]. Chronic mental stress leading to the chronic consumption of unhealthful hyperpalatable foods may contribute to addiction-like eating behavior and contribute to obesity (See Fig. 11, Fig. 12).
3. Obesity and psychiatric disease
Among the most common psychiatric disorders encountered in clinical practice are anxiety disorders (general anxiety, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder, and social phobia), mood disorders (e.g., depression, bipolar disorder), and psychosis (e.g., schizophrenia), as well as the consequences of dementia and eating disorders.
Causes of mood or affective disorders include genetic predisposition, alcohol and drug abuse, and post-traumatic events such as adverse childhood experiences [107]. The pathogenesis of psychiatric disease and basis of pharmacologic treatment of psychiatric disease mostly involve neurotransmitters [108]. Given that many antiobesity medications also act upon neurotransmitters and given that some antiobesity medications can alter the metabolism of psychiatric drugs, then before prescribing, clinicians might best be aware of potential interactions (including drug interactions) with psychiatric medications [109]. For example, phentermine and the phentermine/topiramate combination agent should not be taken with 14 days of monoamine oxidase inhibitors due to the risk of hypertensive crisis. The naltrexone/bupropion combination agent has several potential drug interactions, including potential drug interactions with psychiatric medications such as monoamine oxidase inhibitors, antidepressants (e.g., selective serotonin reuptake inhibitors and many tricyclics), and antipsychotics (e.g., haloperidol, risperidone and thioridazine) [109]. While glucagon-like peptide-1 receptor agonists may slow gastric emptying, existing data suggests that such agents can be safely used in patients with psychiatric disease and may help treat the weight gain often found with antipsychotic medications [110]. Conversely, the concomitant use of some antidepressants may diminish the weight reduction effects of glucagon-like peptide-1 receptor agonists [111].
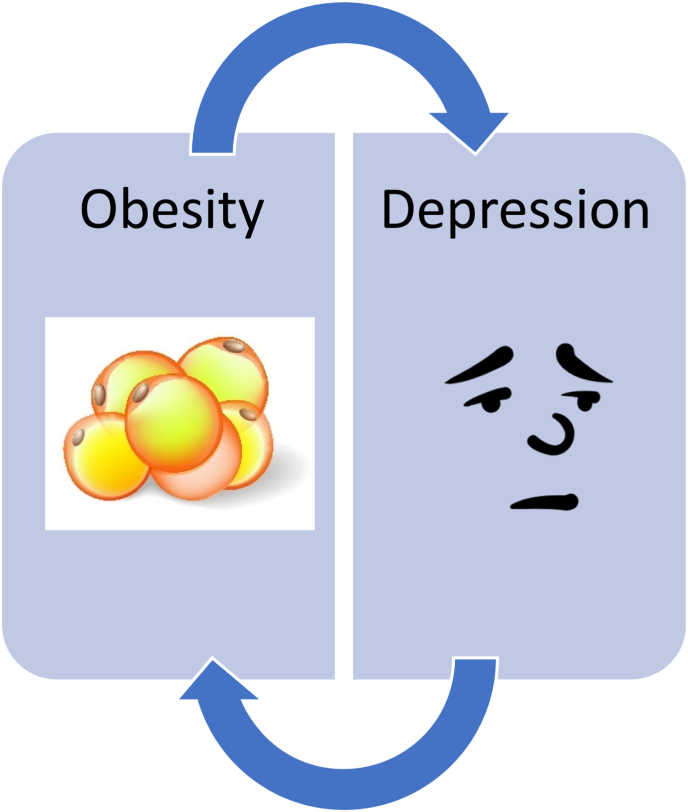
Obesity and mood disorders frequently occur together and can have a bidirectional relationship. Obesity is a risk factor for mood disorders, and mood disorders are a risk factor for obesity [97]. (See Fig. 13). The bidirectional relationship between obesity and depression may be stronger among women than among men [53,92,93,98]. Obesity and psychiatric diseases share pathogenic pathways involving the endocrine and immune system, hypothalamic and pituitary axis, and nervous system [99]. While several antiobesity medications list potential suicidal behavior and ideations within their prescribing information [109], even among patients with mental illness, antiobesity medications may not only improve body weight, improve adiposopathic related metabolic diseases, and improve eating behavior, but may also improve depression, anxiety, and stress levels [112]. Nonetheless, it is prudent to monitor patients treated with antiobesity medications for changes in mood (See Table 1 and Table 2).
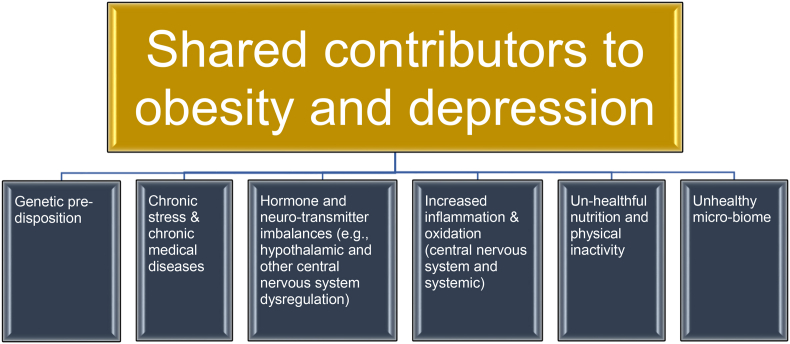
Fig. 14.
Shared contributors to obesity and depression. Obesity and depression share common factors that may lead to altered eating patterns, altered mood, and social stigma, which may in turn worsen obesity and depression. Healthful nutrition, routine physical activity, and attaining a healthy body weight may improve depression [[94], [95], [96]].
Fig. 13.
Obesity and Depression are bidirectional. Obesity is a risk factor for mood disorders; mood disorders are a risk factor for obesity [53,92,93].
Table 1.
Ten takeaway messages: obesity and mental stress. Shown are takeaway messages regarding obesity and mental stress.
| 1. | Shorter-term “fight or flight” stress response increases sympathomimetic activity [4]. |
| 2. | Shorter-term adrenergic stress responses may improve cognition and physiologic function, as well as alter tolerance to pain and immune function [4]. |
| 3. | Longer-term “submit and stay” stress may increase hypothalamic corticotropic activity and worsen sleep patterns [4,51]. |
| 4. | Longer-term hypothalamic stress responses may increase food cravings, promote pain intolerance, increase blood pressure, worsen glucose metabolism, and dysregulate immune responses [4,[51], [52], [53]]. |
| 5. | Chronic stress-induced adiposopathic responses may adversely affect the limbic system [4,51], which is responsible for behavior and emotional responses. |
| 6. | Dysregulation of the limbic system with chronic stress may affect hunger, food choice, and emotional modulation of food intake [4]. |
| 7. | Dysregulation of the limbic system with chronic stress may affect reward-seeking behavior [4]. |
| 8. | Mental stress may adversely affect the cerebrum, which may contribute to prioritization of personal, work, or other behaviors and activities, with less prioritization of healthful behaviors and activities (i.e., healthful nutrition and routine physical activity) [2,4,54]. |
| 9. | Mental stress may impair self-regulation and promote choosing unhealthful (immediately rewarding ultra-processed) foods over more healthful (delayed-gratification unprocessed) foods [2,4,54]. |
| 10. | Obesity and its adverse health complications may increase mental stress, which may contribute to unhealthful behavior, endocrinopathies and immunopathies, which, in turn, may further worsen obesity and its complications, resulting in an adiposopathic stress cycle [4,52,55] (See Fig. 10). |
Table 2.
Ten takeaway messages: obesity and psychiatric disease. Obesity and mood disorders frequently occur together and can have a bidirectional relationship.
| 1. | Obesity and mood disorders frequently occur together [97]. |
| 2. | The relationship between obesity and depression is bidirectional. Obesity is a risk factor for mood disorders; mood disorders are a risk factor for obesity [53,92,93]. |
| 3. | The association between depression and obesity may be stronger among women [53,98]. |
| 4. | Obesity and psychiatric diseases may share pathogenic pathways involving the endocrine and immune systems, hypothalamic and pituitary axis, and nervous system (e.g., autonomic nervous system, monoamines, synapses, neurogenesis, and neuroinflammation) [99]. |
| 5. | Psychiatric diseases may independently contribute to overnutrition and/or consumption of foods rich in carbohydrates and fats [97]. |
| 6. | Individuals have unique body weight responses to medications used to treat psychiatric disease [100,101]. |
| 7. | Study populations support that some psychiatric medications may generally increase body weight, while other psychiatric medications may promote body weight neutrality or weight reduction [3]. |
| 8. | Weight reduction in patients with obesity may improve mood in patients without clinical depression [95,96]. |
| 9. | Non-surgical, intentional weight reduction in patients with obesity may reduce symptoms of depression [92]. |
| 10. | Bariatric surgery often improves mental health conditions (e.g., depression and binge eating disorders); however, bariatric surgery is sometimes associated with recurring or new psychiatric disorders, alcohol or substance abuse, eating disorders, and suicidality [[102], [103], [104], [105]]. |
Regarding bariatric surgery, weight reduction in patients with obesity may improve mood and improve mental health conditions [102]. That said, bariatric surgery is also associated with recurring or new psychiatric disorders, higher risk of new alcohol or substance abuse, eating disorders, and suicidality [[103], [104], [105],113]. Additionally, malabsorptive bariatric surgery may impair the absorption of some, but not all commonly used psychiatric medications, reducing their bioavailability, and thus potentially worsening or exacerbating psychiatric disease previously well-controlled by more therapeutic blood levels of psychiatric medications [114]. Table 2 lists takeaways regarding obesity and psychiatric disease.
3.1. Obesity and depression
In addition to sharing a bidirectional relationship (see Fig. 13), obesity and depression share potential contributors such as genetic predisposition, chronic stress, chronic diseases, hormone imbalances, neurotransmitter imbalances, increased inflammation, increased oxidation, unhealthful nutrition, low physical activity, lower socioeconomic status, adverse life experiences (e.g., abuse) and an unhealthy microbiome [53,94,[115], [116], [117], [118]]. These may lead to altered eating patterns, altered mood, and social stigma, as well as potential worsening of both obesity and depression. Depression is a predictor of the development of obesity [106]. Conversely, healthful nutrition, routine physical activity, and attaining a healthy body weight may improve depression [95,96].
3.1.1. Obesity and depression: treatment and anti-depressant medications
Obesity and depression are linked in part through the effects of nutrition and physical activity [97]. Management of both obesity and depression optimally involve a comprehensive, patient-centered approach including medical history, physical exam, laboratory evaluation if beneficial, appropriate diagnosis, and psychological support void of bias [106]. Healthful nutrition and routine physical activity may likewise improve obesity and depressive symptoms. Like obesity, medications are often indicated to treat depression for patients who do not respond to non-pharmacologic therapy. However, clinicians and patients should be aware of the potential weight effects of anti-depression therapy, especially in patients with obesity or at risk for obesity [100,101,106] (See Table 3, Table 4).
Table 3.
Effect of antidepressants on body weight [3]. Wide variance exists in individual weight responses to psychiatric medications. Relatively few studies have directly compared individual drugs within the context of a prospective, randomized, controlled, dedicated body weight trial. Given the lack of available data and variance of data, it is challenging to objectively rank medications within a class, regarding their effects on body weight. However, certain antidepressant medications are described to be especially associated with weight gain, such as amitriptyline, paroxetine, and mirtazapine. Antidepressants with variable effects on body weight include: (a) some tricyclic antidepressants (secondary amines) such as desipramine, nortriptyline, and protriptyline; (b) some selective serotonin reuptake inhibitors, such as escitalopram and sertraline; (c) some serotonin and norepinephrine re-uptake inhibitors, such as desvenlafaxine and duloxetine; (d) irreversible monoamine oxidase inhibitors such as tranylcypromine; and (e) serotonergic agents such as vortioxetine.
| Antidepressants | Effect on body weight |
|---|---|
| Tricyclic antidepressants (tertiary amines) such as amitriptyline, doxepin, imipramine, and dosulepin | ↑ |
| Selective serotonin reuptake inhibitors such as paroxetine and citalopram | ↑ |
| Selective serotonin reuptake inhibitors such as fluoxetine | ↓ (variable) [106] |
| Selective serotonin and norepinephrine reuptake inhibitors such as venlafaxine | ↑ |
| Irreversible monoamine oxidase inhibitors such as isocarboxazid and phenelzine | ↑ |
| Trazodone, mirtazapine, and brexpiprazole | ↑ |
| Bupropion | ↓ |
Table 4.
Effect of mood stabilizing medications on body weight [3]. Wide variance exists in individual weight responses to psychiatric medications. Relatively few studies have directly compared individual drugs within the context of a prospective, randomized, controlled, dedicated body weight trial. Given the lack of available data and variance of data, it is challenging to objectively rank medications within a class, regarding their effects on body weight. Listed are mood stabilizing medications described to be especially associated with weight gain. Mood stabilizing medications with variable effects on body weight include lamotrigine and oxcarbazepine.
| Mood stabilizing medications | Effect on body weight |
|---|---|
| Gabapentin | ↑ |
| Divalproex | ↑ |
| Lithium | ↑ |
| Valproate | ↑ |
| Vigabatrin | ↑ |
| Cariprazine | ↑ |
| Carbamazepine | ↑ |
An important principle for virtually all psychiatric medications, including anti-depressant medications, is that substantial variance exists in individual weight effects [100,101]. The reported weight effects of medications have limitations. Reported weight effects of psychiatric medications are often derived from observations of independent studies, rather than a direct head-to-head comparison within the same controlled clinical trial [3]. Additionally, psychiatric medications are sometimes used for multiple psychiatric conditions, and studies often report variable weight effects depending on the condition being treated [3]. Finally, antidepressants and other medications may be useful for treating eating disorders in addition to depression [[119], [120], [121]]:
-
•
Fluoxetine is an approved treatment for bulimia nervosa
-
•
Lisdexamfetamine is an approved treatment for binge eating disorder [109].
-
•
Selective serotonin reuptake inhibitors may be effective for binge eating disorder (e.g., sertraline and especially fluoxetine) [122].
-
•
Selective serotonin re-uptake inhibitors (e.g., sertraline), or the anti-migraine/seizure medication topiramate may help night eating syndrome
-
•
Topiramate is reported to be potentially useful for binge eating disorders, depression, and other psychiatric diseases [123,124].
Anti-obesity medications have the potential for drug interactions with anti-depressant drugs. Phentermine and topiramate/phentermine are contraindicated during or within 14 days following monoamine oxidase inhibitors due to risk of hypertensive crisis [125,126]. According to the prescribing information, the safety and efficacy of phentermine with serotonergic agents have not been established [125,126]. Orlistat may cause malabsorption and thus potentially decrease drug absorption and blood levels; however, the prescribing information for orlistat does not specifically list drug interactions with anti-depressive agents [127]. Concomitant use of naltrexone/bupropion with MAO inhibitors may increase risk of hypertensive reactions [128]. Bupropion inhibits CYP2D6 and can increase concentrations of antidepressants, (e.g., selective serotonin reuptake inhibitors and many tricyclics) [128]. Concomitant use of CYP2B6 inducers (e.g., carbamazepine) may reduce bupropion exposure [128]. While glucagon-like protein-1 (GLP-1) receptor agonists may decrease gastric emptying, the prescribing information does not specifically list potential drugs interactions with anti-depressant drugs [129,130].
3.2. Obesity and psychosis
As with depression, other neuropsychiatric disorders with shared pathogenic pathways include mood disorders, dementia, and schizophrenia and major neurocognitive disorders. Both obesity and neuropsychiatric disorders can involve low-grade systemic inflammation, neuroinflammation, neuroendocrine changes (e.g., hypothalamic-pituitary-adrenal (HPA) axis), brain stress systems, and gut microbiota [131]. Another common clinical interrelationship between obesity and psychosis is that anti-psychotic medications often contribute to weight gain (See Table 5). However, as with anti-depressant therapy, not all patients have the same weight response to the same anti-psychotic medications [100,132,133]. If a patient is stable while being treated with an anti-psychotic medication, then changes in medications for the purposes of improvement in body weight should occur with caution and with involvement of a clinician qualified in the treatment of psychosis (i.e., a psychiatrist or psychiatric nurse practitioner).
Table 5.
Effect of antipsychotic medications on body weight [3]. Wide variance exists in individual weight responses to psychiatric medications. Relatively few studies exist that have directly compared individual drugs within the context of a prospective, randomized, controlled, dedicated body weight trial. Given the lack of available data and variance of data, it is challenging to objectively rank medications within a class, regarding their effects on body weight. Certain antipsychotic medications are described to be especially associated with weight gain, such as clozapine, olanzapine, and zotepine. Antipsychotic medications with variable effects on body weight include amisulpride, aripiprazole, asenapine, cariprazine, haloperidol, loxapine, lurasidone, ziprasidone, paliperidone, and perphenazine.
| Antipsychotic medications | Effect on body weight |
|---|---|
| Clozapine | ↑ |
| Olanzapine | ↑ |
| Zotepine (not available in U.S.) | ↑ |
| Chlorpromazine | ↑ |
| Brexpiprazole | ↑ |
| Iloperidone | ↑ |
| Lithium | ↑ |
| Quetiapine | ↑ |
| Risperidone | ↑ |
| Thioridazine | ↑ |
3.2.1. Obesity and psychosis: antipsychotic medications
Table 5 lists antipsychotic medications often associated with body weight gain [133,134]. Metformin and GLP-1 receptor agonists may help mitigate weight gain due to antipsychotic medications [132,135]. Topiramate may also help mitigate antipsychotic medication weight gain and may have a therapeutic effect in patients with psychosis [135]. Phentermine is contraindicated in patients with agitated states, cardiac dysrhythmias, and uncontrolled hypertension [125,126]. Orlistat may cause malabsorption; however, the prescribing information for orlistat does not specifically list drug interactions with anti-psychotic agents [127].
The bupropion component of naltrexone/bupropion may inhibit CYP2D6 and therefore potentially affect antipsychotics metabolized by CYP2D6 [128]. CYP2D6 substrates include the atypical antipsychotics aripiprazole, clozapine, olanzapine, quetiapine, and risperidone, and the typical antipsychotics thioridazine and zuclopenthixol [136,137]. CYP2D6 inhibitors include the atypical antipsychotic asenapine and typical antipsychotics of chlorpromazine, fluphenazine, haloperidol, levomepromazine, perphenazine, and pimozide [136,137]. While liraglutide and semaglutide may decrease gastric emptying, neither of the respective prescribing information specifically lists potential drugs interactions with anti-psychotic drugs [129,130].
4. Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on “Stress, Psychiatric Disease, and Obesity” is one of a series of OMA CPSs designed to assist clinicians in better understanding the physiology and treatment of obesity, and particularly the relationship between obesity and mental health. The information presented in this CPS may aid clinicians in improving the health and wellbeing of their patients with obesity and who are experiencing stress or psychiatric disease, especially those with metabolic, physiological, and psychological complications.
Transparency [138]
This manuscript was derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees. This was followed by a similar Pediatric “Obesity Algorithm,” with updates approximately every two years by OMA authors. Authors of prior years’ version of the Obesity Algorithm are included in Supplement #1.
Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. (Supplement #1) The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
After transcription of the applicable sections of the prior OMA Obesity Algorithm to an initial draft, HEB substantially reviewed, updated, and reformatted the manuscript. SMC, CV, VG, and LW reviewed, edited, and approved the document.
Managing disclosures and dualities of interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Diclosures
SMC reports no relevant disclosures.
CV reports no relevant disclosures.
VG reports being speaker for Novo Nordisk.
LW reports no relevant disclosures.
HEB's research site institution has received research grants from 89Bio, Allergan, Alon Medtech/Epitomee, Altimmune, Amgen, Boehringer Ingelheim, Eli Lilly, NovoNordisk, Pfizer, and Vivus. HEB has served as a consultant/advisor for 89Bio, Altimmune, Amgen, and Boehringer Ingelheim.
Acknowledgements and Funding
Medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who helped implement author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.obpill.2022.100041.
Contributor Information
Sandra M. Christensen, Email: sam.chris@im-wm.com.
Catherine Varney, Email: cwv9v@uvahealth.org.
Vivek Gupta, Email: drgupta@mindfulweightloss.com.
Lori Wenz, Email: lori.wenz@att.net.
Harold Edward Bays, Email: hbaysmd@outlook.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Bays H.E., McCarthy W., Burridge K., Tondt J., Karjoo S., Christensen S., Ng J., Golden A., Davisson L., Richardson L. Obesity Algorithm eBook. 2021. www.obesityalgorithm.orghttps://obesitymedicine.org/obesity-algorithm/ presented by the Obesity Medicine Association, Accessed = September 18, 2021)
- 2.Freshwater M., Christensen S., Oshman L., Bays H.E. Behavior, motivational interviewing, eating disorders, and obesity management technologies: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tondt J., Bays H.E. Concomitant medications, functional foods, and supplements: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau Y.H., Potenza M.N. Stress and eating behaviors. Minerva Endocrinol. 2013;38:255–267. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharif K., Watad A., Coplan L., Lichtbroun B., Krosser A., Lichtbroun M., et al. The role of stress in the mosaic of autoimmunity: an overlooked association. Autoimmun Rev. 2018;17:967–983. doi: 10.1016/j.autrev.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Kivimäki M., Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruk J., Aboul-Enein B.H., Bernstein J., Gronostaj M. Psychological stress and cellular aging in cancer: a meta-analysis. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/1270397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stojanovich L., Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7:209–213. doi: 10.1016/j.autrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Song H., Fang F., Tomasson G., Arnberg F.K., Mataix-Cols D., Fernández de la Cruz L., et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA, J Am Med Assoc. 2018;319:2388–2400. doi: 10.1001/jama.2018.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus E., Bays H.E. Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarwer D.B., Polonsky H.M. The psychosocial burden of obesity. Endocrinol Metab Clin N Am. 2016;45:677–688. doi: 10.1016/j.ecl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Valk E.S., Savas M., van Rossum E.F.C. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7:193–203. doi: 10.1007/s13679-018-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morry J., Ngamcherdtrakul W., Yantasee W. Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017;11:240–253. doi: 10.1016/j.redox.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sher L.D., Geddie H., Olivier L., Cairns M., Truter N., Beselaar L., et al. Chronic stress and endothelial dysfunction: mechanisms, experimental challenges, and the way ahead. Am J Physiol Heart Circ Physiol. 2020;319:H488–H506. doi: 10.1152/ajpheart.00244.2020. [DOI] [PubMed] [Google Scholar]
- 16.Umriukhin P.E., Ershova E.S., Filev A.D., Agafonova O.N., Martynov A.V., Zakharova N.V., et al. Human leukocytes. Genes (basel) vol. 13. 2022. The psychoemotional stress-induced changes in the abundance of SatIII (1q12) and telomere repeats, but not ribosomal DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma W., Liu P., Zheng J., Lü J., Zhao Q., Li D., et al. Immune and nonimmune mechanisms mediate the mental stress-induced tumor growth in a xenograft model of breast cancer. Cell Death Dis. 2021;12:987. doi: 10.1038/s41419-021-04280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth L., Rombouts M., Schrijvers D.M., Lemmens K., De Keulenaer G.W., Martinet W., et al. Chronic intermittent mental stress promotes atherosclerotic plaque vulnerability, myocardial infarction and sudden death in mice. Atherosclerosis. 2015;242:288–294. doi: 10.1016/j.atherosclerosis.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Polsky L.R., Rentscher K.E., Carroll J.E. Stress-induced biological aging: a review and guide for research priorities. Brain Behav Immun. 2022;104:97–109. doi: 10.1016/j.bbi.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dar T., Radfar A., Abohashem S., Pitman R.K., Tawakol A., Osborne M.T. Psychosocial stress and cardiovascular disease. Curr Treat Options Cardiovasc Med. 2019;21:23. doi: 10.1007/s11936-019-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mifsud S., Schembri E.L., Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med. 2018;79:634–639. doi: 10.12968/hmed.2018.79.11.634. [DOI] [PubMed] [Google Scholar]
- 22.Widjaja D., Orini M., Vlemincx E., Van Huffel S. Cardiorespiratory dynamic response to mental stress: a multivariate time-frequency analysis. Comput Math Methods Med. 2013;2013 doi: 10.1155/2013/451857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuenyongchaiwat K. Cardiovascular response to mental stress tests and the prediction of blood pressure. Indian J Psychol Med. 2017;39:413–417. doi: 10.4103/0253-7176.211744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomiyama A.J., Epel E.S., McClatchey T.M., Poelke G., Kemeny M.E., McCoy S.K., et al. Associations of weight stigma with cortisol and oxidative stress independent of adiposity. Health Psychol. 2014;33:862–867. doi: 10.1037/hea0000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertolino G., Quaglia F., Scudeller L., Ceresa I., Balduini C.L. Transient leukocytosis in Emergency Room: an overlooked issue. Ital J Med. 2017;11:41–47. [Google Scholar]
- 26.Hinterdobler J., Schunkert H., Kessler T., Sager H.B. Impact of acute and chronic psychosocial stress on vascular inflammation. Antioxidants Redox Signal. 2021;35:1531–1550. doi: 10.1089/ars.2021.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ince L.M., Weber J., Scheiermann C. Control of leukocyte trafficking by stress-associated hormones. Front Immunol. 2019;9 doi: 10.3389/fimmu.2018.03143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhabhar F.S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhabhar F.S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin Immunol. 2008;4:2. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGregor B.A., Murphy K.M., Albano D.L., Ceballos R.M. Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress. 2016;19:185–191. doi: 10.3109/10253890.2015.1127913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maydych V., Claus M., Dychus N., Ebel M., Damaschke J., Diestel S., et al. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albashir A.A.D. The potential impacts of obesity on COVID-19. Clin Med. 2020;20:e109–e113. doi: 10.7861/clinmed.2020-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghili S.M.M., Ebrahimpur M., Arjmand B., Shadman Z., Pejman Sani M., Qorbani M., et al. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes. 2021;45:998–1016. doi: 10.1038/s41366-021-00776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bays H.E., Golden A., Tondt J. Thirty obesity myths, misunderstandings, and/or oversimplifications: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hebebrand J., Albayrak Ö., Adan R., Antel J., Dieguez C., de Jong J., et al. Eating addiction", rather than "food addiction", better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Ruddock H.K., Christiansen P., Halford J.C.G., Hardman C.A. The development and validation of the addiction-like eating behaviour scale. Int J Obes. 2017;41:1710–1717. doi: 10.1038/ijo.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meule A., Gearhardt A.N. Food addiction in the light of DSM-5. Nutrients. 2014;6:3653–3671. doi: 10.3390/nu6093653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauck C., Cook B., Ellrott T. Food addiction, eating addiction and eating disorders. Proc Nutr Soc. 2020;79:103–112. doi: 10.1017/S0029665119001162. [DOI] [PubMed] [Google Scholar]
- 39.Farr O.M., Li C.R., Mantzoros C.S. Central nervous system regulation of eating: insights from human brain imaging. Metab Clin Exp. 2016;65:699–713. doi: 10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heatherton T.F., Wagner D.D. Cognitive neuroscience of self-regulation failure. Trends Cognit Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes Cortes M., Andrade Louzado J., Galvão Oliveira M., Moraes Bezerra V., Mistro S., Souto Medeiros D., et al. Unhealthy food and psychological stress: the association between ultra-processed food consumption and perceived stress in working-class young adults. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/ijerph18083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenny Paul J. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueiras R., Romero-Picó A., Vazquez M.J., Novelle M.G., López M., Diéguez C. The opioid system and food intake: homeostatic and hedonic mechanisms. Obesity Facts. 2012;5:196–207. doi: 10.1159/000338163. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher P.C., Kenny P.J. Food addiction: a valid concept? Neuropsychopharmacology. 2018;43:2506–2513. doi: 10.1038/s41386-018-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlsson H.K., Tuominen L., Helin S., Salminen P., Nuutila P., Nummenmaa L. Mesolimbic opioid-dopamine interaction is disrupted in obesity but recovered by weight loss following bariatric surgery. Transl Psychiatry. 2021;11:259. doi: 10.1038/s41398-021-01370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyldelund N.B., Byrne D.V., Andersen B.V. Food pleasure profiles-an exploratory case study of the relation between drivers of food pleasure and lifestyle and personality traits in a Danish consumer segment. Foods. 2022;11 doi: 10.3390/foods11050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham C.A., Egeth H.E. The capture of attention by entirely irrelevant pictures of calorie-dense foods. Psychon Bull Rev. 2018;25:586–595. doi: 10.3758/s13423-017-1375-8. [DOI] [PubMed] [Google Scholar]
- 48.Moynihan A.B., Wapv Tilburg, Igou E.R., Wisman A., Donnelly A.E., Mulcaire J.B. Eaten up by boredom: consuming food to escape awareness of the bored self. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blum K., Gardner E., Oscar-Berman M., Gold M. Liking" and "wanting" linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharmaceut Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow N.D., Wang G.J., Baler R.D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cognit Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrell C.S., Gillespie C.F., Neigh G.N. Energetic stress: the reciprocal relationship between energy availability and the stress response. Physiol Behav. 2016;166:43–55. doi: 10.1016/j.physbeh.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thaler J.P., Guyenet S.J., Dorfman M.D., Wisse B.E., Schwartz M.W. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouakinin S.R.S., Barreira D.P., Gois C.J. Depression and obesity: integrating the role of stress, neuroendocrine dysfunction and inflammatory pathways. Front Endocrinol. 2018;9:431. doi: 10.3389/fendo.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adam T.C., Epel E.S. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Moore C.J., Cunningham S.A. Social position, psychological stress, and obesity: a systematic review. J Acad Nutr Diet. 2012;112:518–526. doi: 10.1016/j.jand.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Bali A., Jaggi A.S. Clinical experimental stress studies: methods and assessment. Rev Neurosci. 2015;26:555–579. doi: 10.1515/revneuro-2015-0004. [DOI] [PubMed] [Google Scholar]
- 57.Crosswell A.D., Lockwood K.G. Best practices for stress measurement: how to measure psychological stress in health research. Health Psychol Open. 2020;7 doi: 10.1177/2055102920933072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimsdale J.E. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanai M., Khan M.A.B. StatPearls Publishing LLC.; 2022. Acute stress disorder. StatPearls. Treasure island (FL): StatPearls publishing copyright © 2022. [PubMed] [Google Scholar]
- 60.Juster R.P., Sindi S., Marin M.F., Perna A., Hashemi A., Pruessner J.C., et al. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 2011;36:797–805. doi: 10.1016/j.psyneuen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Bays H.E. Evaluation and practical management of increased visceral fat. J Am Coll Cardiol. 2022;79:1266–1269. doi: 10.1016/j.jacc.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 62.Geva N., Defrin R. Opposite effects of stress on pain modulation depend on the magnitude of individual stress response. J Pain. 2018;19:360–371. doi: 10.1016/j.jpain.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Hannibal K.E., Bishop M.D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rezitis J., Herzog H., Ip C.K. Neuropeptide Y interaction with dopaminergic and serotonergic pathways: interlinked neurocircuits modulating hedonic eating behaviours. Prog Neuro-Psychopharmacol Biol Psychiatry. 2022;113 doi: 10.1016/j.pnpbp.2021.110449. [DOI] [PubMed] [Google Scholar]
- 65.Wiss D.A., Avena N., Rada P. Sugar addiction: from evolution to revolution. Front Psychiatr. 2018;9:545. doi: 10.3389/fpsyt.2018.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motiejunaite J., Amar L., Vidal-Petiot E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann Endocrinol. 2021;82:193–197. doi: 10.1016/j.ando.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Iob E., Steptoe A. Cardiovascular disease and hair cortisol: a novel biomarker of chronic stress. Curr Cardiol Rep. 2019;21:116. doi: 10.1007/s11886-019-1208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jackson S.E., Kirschbaum C., Steptoe A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity. 2017;25:539–544. doi: 10.1002/oby.21733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geer E.B., Lalazar Y., Couto L.M., Cohen V., Lipton L.R., Shi W., et al. A prospective study of appetite and food craving in 30 patients with Cushing's disease. Pituitary. 2016;19:117–126. doi: 10.1007/s11102-015-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mason A.E., Schleicher S., Coccia M., Epel E.S., Aschbacher K. Chronic stress and impulsive risk-taking predict increases in visceral fat over 18 months. Obesity. 2018;26:869–876. doi: 10.1002/oby.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Safi Z.A., Polotsky A., Chosich J., Roth L., Allshouse A.A., Bradford A.P., et al. Evidence for disruption of normal circadian cortisol rhythm in women with obesity. Gynecol Endocrinol. 2018;34:336–340. doi: 10.1080/09513590.2017.1393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomiyama A.J. Stress and obesity. Annu Rev Psychol. 2019;70:703–718. doi: 10.1146/annurev-psych-010418-102936. [DOI] [PubMed] [Google Scholar]
- 73.Brown A., Flint S.W., Batterham R.L. Pervasiveness, impact and implications of weight stigma. EClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomiyama A.J. Weight stigma is stressful. A review of evidence for the Cyclic Obesity/Weight-Based Stigma model. Appetite. 2014;82:8–15. doi: 10.1016/j.appet.2014.06.108. [DOI] [PubMed] [Google Scholar]
- 75.Dunkelberger J.R., Song W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 76.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Capuron L., Lasselin J., Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology. 2017;42:115–128. doi: 10.1038/npp.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishitani N., Sakakibara H. Association of psychological stress response of fatigue with white blood cell count in male daytime workers. Ind Health. 2014;52:531–534. doi: 10.2486/indhealth.2013-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Incollingo Rodriguez A.C., Epel E.S., White M.L., Standen E.C., Seckl J.R., Tomiyama A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology. 2015;62:301–318. doi: 10.1016/j.psyneuen.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Morin J.P., Rodríguez-Durán L.F., Guzmán-Ramos K., Perez-Cruz C., Ferreira G., Diaz-Cintra S., et al. Palatable hyper-caloric foods impact on neuronal plasticity. Front Behav Neurosci. 2017;11:19. doi: 10.3389/fnbeh.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baumeister R.F., Bratslavsky E., Muraven M., Tice D.M. Ego depletion: is the active self a limited resource? J Pers Soc Psychol. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- 82.Regard M., Landis T. Gourmand syndrome": eating passion associated with right anterior lesions. Neurology. 1997;48:1185–1190. doi: 10.1212/wnl.48.5.1185. [DOI] [PubMed] [Google Scholar]
- 83.Kullmann S., Abbas Z., Machann J., Shah N.J., Scheffler K., Birkenfeld A.L., et al. Investigating obesity-associated brain inflammation using quantitative water content mapping. J Neuroendocrinol. 2020;32 doi: 10.1111/jne.12907. [DOI] [PubMed] [Google Scholar]
- 84.Seong J., Kang J.Y., Sun J.S., Kim K.W. Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res (Seoul) 2019;42:383–392. doi: 10.1007/s12272-019-01138-9. [DOI] [PubMed] [Google Scholar]
- 85.Dfarhud D., Malmir M., Khanahmadi M. Happiness & health: the biological factors- systematic review article. Iran J Public Health. 2014;43:1468–1477. [PMC free article] [PubMed] [Google Scholar]
- 86.Blum K., Werner T., Carnes S., Carnes P., Bowirrat A., Giordano J., et al. Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. J Psychoact Drugs. 2012;44:38–55. doi: 10.1080/02791072.2012.662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mizoguchi A., Banno R., Sun R., Yaginuma H., Taki K., Kobayashi T., et al. Glucocorticoid receptor signaling in ventral tegmental area neurons increases the rewarding value of a high-fat diet in mice. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L., Lu Y.P., Chen H.Y., Huang S.N., Guo Y.R., Zhang J.Y., et al. Ventral tegmental area GABAergic neurons induce anxiety-like behaviors and promote palatable food intake. Neuropharmacology. 2020;173 doi: 10.1016/j.neuropharm.2020.108114. [DOI] [PubMed] [Google Scholar]
- 89.Raglan G.B., Schmidt L.A., Schulkin J. The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocr Connect. 2017;6:R1–r7. doi: 10.1530/EC-16-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sander J., Moessner M., Bauer S. Depression, anxiety and eating disorder-related impairment: moderators in female adolescents and young adults. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/ijerph18052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Macedo I.C., de Freitas J.S., da Silva Torres I.L. The influence of palatable diets in reward system Activation: a mini review. Adv Pharmacol Sci. 2016;2016 doi: 10.1155/2016/7238679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabricatore A.N., Wadden T.A., Higginbotham A.J., Faulconbridge L.F., Nguyen A.M., Heymsfield S.B., et al. Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int J Obes. 2011;35:1363–1376. doi: 10.1038/ijo.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jantaratnotai N., Mosikanon K., Lee Y., McIntyre R.S. The interface of depression and obesity. Obes Res Clin Pract. 2017;11:1–10. doi: 10.1016/j.orcp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Kurhe Y., Mahesh R. Mechanisms linking depression co-morbid with obesity: an approach for serotonergic type 3 receptor antagonist as novel therapeutic intervention. Asian J Psychiatr. 2015;17:3–9. doi: 10.1016/j.ajp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Kvam S., Kleppe C.L., Nordhus I.H., Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 96.Krogh J., Hjorthoj C., Speyer H., Gluud C., Nordentoft M. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wurtman J., Wurtman R. The trajectory from mood to obesity. Curr Obes Rep. 2018;7:1–5. doi: 10.1007/s13679-017-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohn J.N., Cabrera Y., Dimitrov S., Guay-Ross N., Pruitt C., Shaikh F.D., Hong S. Sex-specific roles of cellular inflammation and cardiometabolism in obesity-associated depressive symptomatology. Int J Obes (Lond). 2019 Oct;43(10):2045–2056. doi: 10.1038/s41366-019-0375-3. Epub 2019 May 14. PMID: 31089263; PMCID: PMC6774832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajan T.M., Menon V. Psychiatric disorders and obesity: a review of association studies. J Postgrad Med. 2017;63:182–190. doi: 10.4103/jpgm.JPGM_712_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hasnain M., Vieweg W.V., Hollett B. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: a review for primary care physicians. PGM (Postgrad Med) 2012;124:154–167. doi: 10.3810/pgm.2012.07.2577. [DOI] [PubMed] [Google Scholar]
- 101.Uguz F., Sahingoz M., Gungor B., Aksoy F., Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatr. 2015;37:46–48. doi: 10.1016/j.genhosppsych.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 102.Kubik J.F., Gill R.S., Laffin M., Karmali S. The impact of bariatric surgery on psychological health. J. Obesity. 2013;2013 doi: 10.1155/2013/837989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dawes A.J., Maggard-Gibbons M., Maher A.R., Booth M.J., Miake-Lye I., Beroes J.M., et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA, J Am Med Assoc. 2016;315:150–163. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- 104.Green D.D., Engel S.G., Mitchell J.E. Psychological aspects of bariatric surgery. Curr Opin Psychiatr. 2014;27:448–452. doi: 10.1097/YCO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 105.Peterhansel C., Petroff D., Klinitzke G., Kersting A., Wagner B. Risk of completed suicide after bariatric surgery: a systematic review. Obes Rev : an official journal of the International Association for the Study of Obesity. 2013;14:369–382. doi: 10.1111/obr.12014. [DOI] [PubMed] [Google Scholar]
- 106.Ghusn W., Bouchard C., Frye M.A., Acosta A. Weight-centric treatment of depression and chronic pain. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiss D.A., Brewerton T.D. Adverse childhood experiences and Adult obesity: a systematic review of plausible mechanisms and meta-analysis of cross-sectional studies. Physiol Behav. 2020;223 doi: 10.1016/j.physbeh.2020.112964. [DOI] [PubMed] [Google Scholar]
- 108.Blokhin I.O., Khorkova O., Saveanu R.V., Wahlestedt C. Molecular mechanisms of psychiatric diseases. Neurobiol Dis. 2020;146 doi: 10.1016/j.nbd.2020.105136. [DOI] [PubMed] [Google Scholar]
- 109.Bays H.E., Fitch A., Christensen S., Burridge K., Tondt J. Anti-obesity medications and investigational agents: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022 doi: 10.1016/j.obpill.2022.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siskind D., Hahn M., Correll C.U., Fink-Jensen A., Russell A.W., Bak N., et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metabol. 2019;21:293–302. doi: 10.1111/dom.13522. [DOI] [PubMed] [Google Scholar]
- 111.Durell N., Franks R., Coon S., Cowart K., Carris N.W. Effect of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38:283–288. doi: 10.1177/87551225221110850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tham M., Chong T.W.H., Jenkins Z.M., Castle D.J. The use of anti-obesity medications in people with mental illness as an adjunct to lifestyle interventions - effectiveness, tolerability and impact on eating behaviours: a 52-week observational study. Obes Res Clin Pract. 2021;15:49–57. doi: 10.1016/j.orcp.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 113.King W.C., Chen J.Y., Courcoulas A.P., Dakin G.F., Engel S.G., Flum D.R., et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis : Off. J. Am. Soc. Bariatric. Surg. 2017;13:1392–1402. doi: 10.1016/j.soard.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braun A., Bruxner G. Little more than a gut feeling?"-considerations when prescribing psychotropic medications to patients undergoing bariatric surgery. Australas Psychiatr : Bull. Royal. Aus. Zealand. Coll. Psychiatr. 2021;29:272–274. doi: 10.1177/1039856220956468. [DOI] [PubMed] [Google Scholar]
- 115.Newton S., Braithwaite D., Akinyemiju T.F. Socio-economic status over the life course and obesity: systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ettman C.K., Adam G.P., Clark M.A., Wilson I.B., Vivier P.M., Galea S. Wealth and depression: a scoping review. Brain Behav. 2022;12:e2486. doi: 10.1002/brb3.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palmisano G.L., Innamorati M., Vanderlinden J. Life adverse experiences in relation with obesity and binge eating disorder: a systematic review. J Behav Addict. 2016;5:11–31. doi: 10.1556/2006.5.2016.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radell M.L., Abo Hamza E.G., Daghustani W.H., Perveen A., Moustafa A.A. The impact of different types of abuse on depression. Depress Res Treat. 2021;2021 doi: 10.1155/2021/6654503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crow S.J. vol. 42. The Psychiatric clinics of North America; 2019. pp. 253–262. (Pharmacologic treatment of eating disorders). [DOI] [PubMed] [Google Scholar]
- 120.Bello N.T., Yeomans B.L. Safety of pharmacotherapy options for bulimia nervosa and binge eating disorder. Expet Opin Drug Saf. 2018;17:17–23. doi: 10.1080/14740338.2018.1395854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Milano W., De Rosa M., Milano L., Capasso A. Night eating syndrome: an overview. J Pharm Pharmacol. 2012;64:2–10. doi: 10.1111/j.2042-7158.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 122.Duan H., Zhu L., Li M., Zhang X., Zhang B., Fang S. Comparative efficacy and acceptability of selective serotonin reuptake inhibitor antidepressants for binge eating disorder: a network meta-analysis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.949823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arnone D. Review of the use of Topiramate for treatment of psychiatric disorders. Ann Gen Psychiatr. 2005;4:5. doi: 10.1186/1744-859X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brownley K.A., Berkman N.D., Peat C.M., Lohr K.N., Cullen K.E., Bann C.M., et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 2016;165:409–420. doi: 10.7326/M15-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phentermine HCL/topiramate extended release prescribing information (QSYMIA) http://www.vivus.com/docs/QsymiaPI.pdf Accessed.
- 126.LOMAIRA™ (phentermine hydrochloride USP) tablets, CIV) https://www.lomaira.com/Prescribing_Information.pdf Accessed.
- 127.XENICAL® (orlistat https://www.xenical.com/pdf/PI_Xenical-brand_FINAL.PDF Capsules. Accessed.
- 128.Naltrexone HCL/bupropion HCL extended release prescribing information (CONTRAVE) http://general.takedapharm.com/content/file.aspx?filetypecode=CONTRAVEPI&cacheRandomizer=c5f9d506-7c0a-4c03-b357-2a926ba14990 Accessed.
- 129.Liraglutide prescribing information for treatment of obesity (SAXENDA) https://www.novo-pi.com/saxenda.pdf Accessed.
- 130.Semaglutide injection 2.4 mg (WEGOVY) prescribing information. https://www.novo-pi.com/wegovy.pdf Accessed.
- 131.Martins L.B., Monteze N.M., Calarge C., Ferreira A.V.M., Teixeira A.L. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. doi: 10.1016/j.nut.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 132.Dayabandara M., Hanwella R., Ratnatunga S., Seneviratne S., Suraweera C., de Silva V.A. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatric Dis Treat. 2017;13:2231–2241. doi: 10.2147/NDT.S113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pillinger T., McCutcheon R.A., Vano L., Mizuno Y., Arumuham A., Hindley G., et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatr. 2020;7:64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Solmi M., Murru A., Pacchiarotti I., Undurraga J., Veronese N., Fornaro M., et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Therapeut Clin Risk Manag. 2017;13:757–777. doi: 10.2147/TCRM.S117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhuo C., Xu Y., Liu S., Li J., Zheng Q., Gao X., et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393. doi: 10.3389/fphar.2018.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Urichuk L., Prior T.I., Dursun S., Baker G. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metabol. 2008;9:410–418. doi: 10.2174/138920008784746373. [DOI] [PubMed] [Google Scholar]
- 137.Mora F., Molina J.D., Zubillaga E., López-Muñoz F., Álamo C. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol Physiol. 2015;5:1–10. doi: 10.4172/2161-1459.1000176. https://www.longdom.org/open-access/cyp450-and-its-implications-in-the-clinical-use-of-antipsychotic-drugs-2161-1459-1000176.pdf [DOI] [Google Scholar]
- 138.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.