Abstract
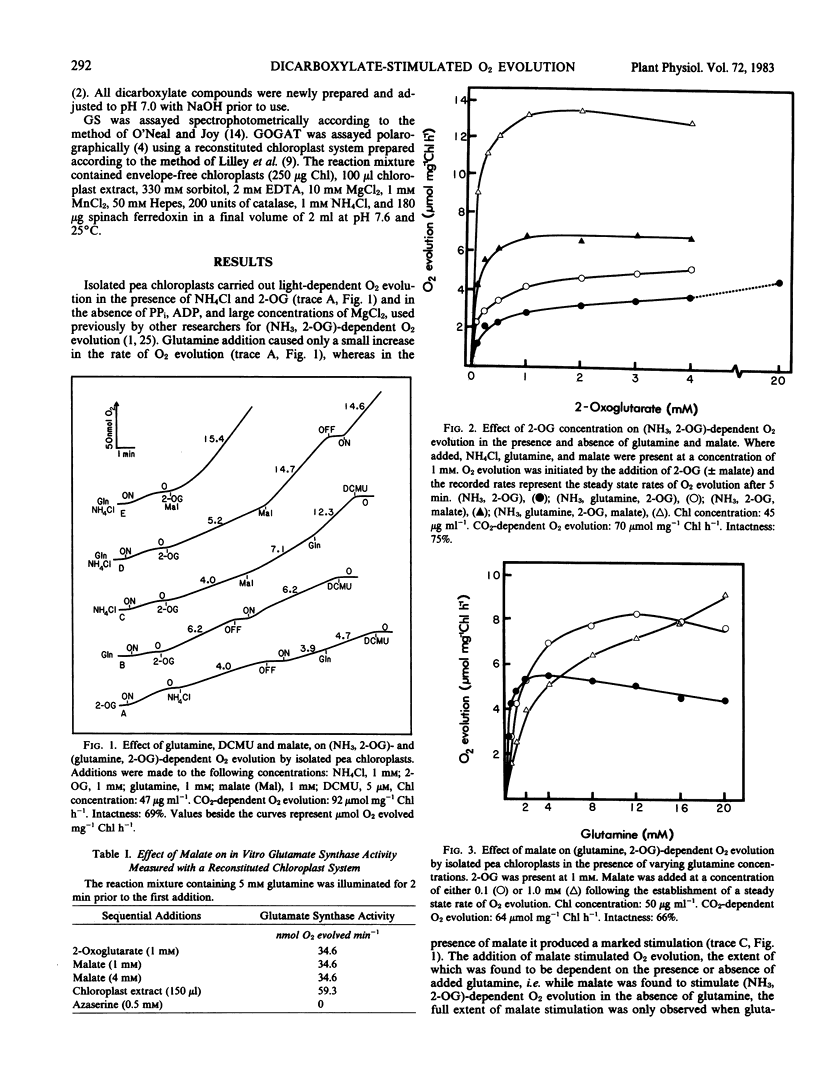
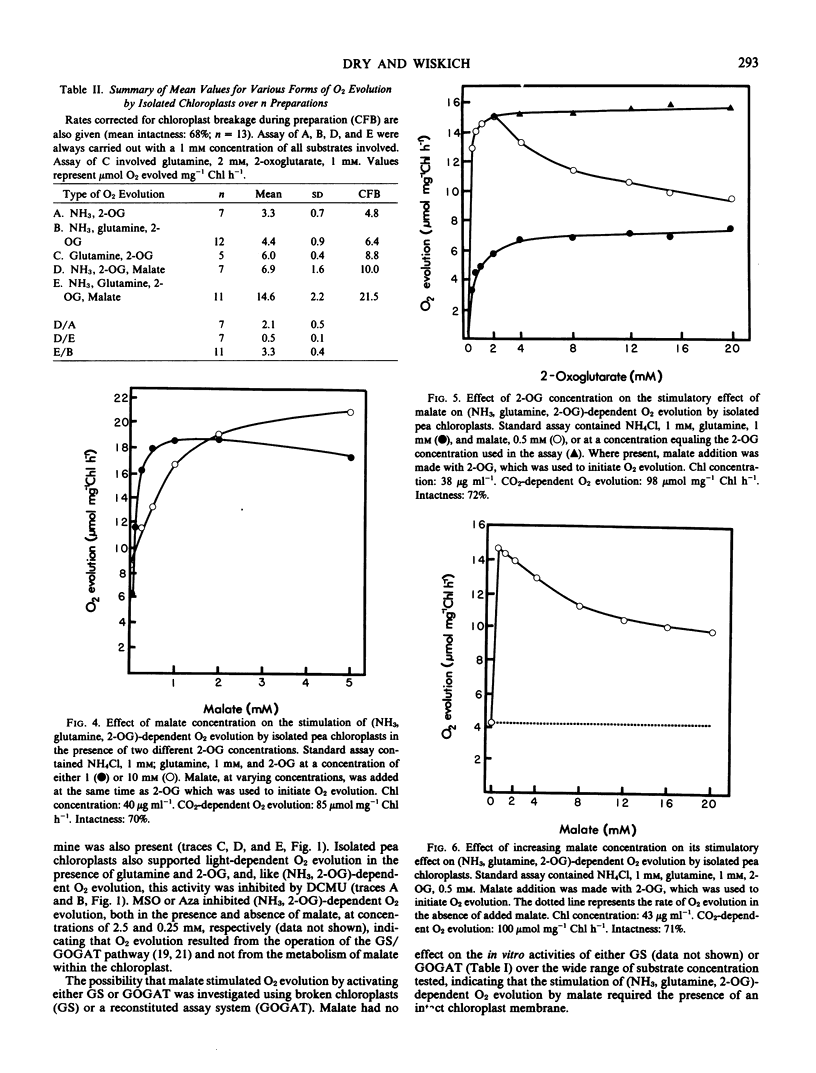
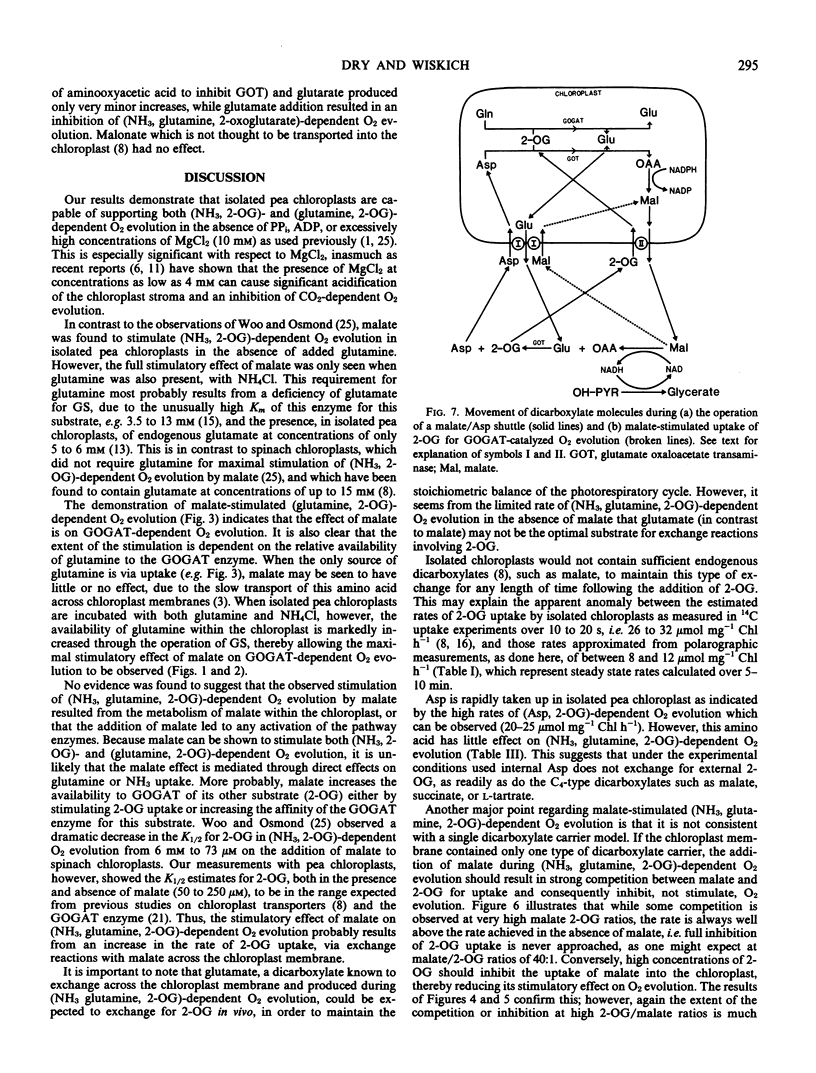
Intact isolated chloroplasts from pea (Pisum sativum) leaves carried out light-dependent (NH3, 2-oxoglutarate) and (glutamine, 2-oxoglutarate)-dependent O2 evolution at rates of 3.3 ± 0.7 (n = 7) and 6.0 ± 0.4 (n = 5) micromoles per milligram chlorophyll per hour, respectively. Malate stimulated the rate of (NH3, 2-oxoglutarate)-dependent O2 evolution 2.1 ± 0.5 (n = 7)-fold in the absence of glutamine, and 3.3 ± 0.4 (n = 11)-fold in the presence of glutamine. Malate also stimulated (glutamine, 2-oxoglutarate)-dependent O2 evolution in the presence of high concentrations of glutamine. The affinity (K1/2) of (NH3, glutamine, 2-oxoglutarate)-dependent O2 evolution for 2-oxoglutarate was estimated at 200 to 250 micromolar in the absence of malate and 50 to 80 micromolar when malate (0.5 millimolar) was present. In contrast to malate and various other dicarboxylates, aspartate, glutarate, and glutamate did not stimulate (NH3, glutamine, 2-oxoglutarate)-dependent O2 evolution in isolated pea chloroplasts. Using both in vitro assays and reconstituted chloroplast systems, malate was shown to have no effect on the activities of either glutamine synthetase or glutamate synthase.
The concentration of malate required for maximal stimulation of O2 evolution was dependent on the concentration of 2-oxoglutarate present. However, the small extent of the competition between malate and 2-oxoglutarate for uptake was not consistent with that predicted by the current `single carrier' model proposed for the uptake of dicarboxylates into chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Neuburger M., Douce R. Role of Glutamate-oxaloacetate Transaminase and Malate Dehydrogenase in the Regeneration of NAD for Glycine Oxidation by Spinach leaf Mitochondria. Plant Physiol. 1981 Mar;67(3):467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Osmond C. B. Stimulation of ammonia and 2-oxoglutarate-dependent o(2) evolution in isolated chloroplasts by dicarboxylates and the role of the chloroplast in photorespiratory nitrogen recycling. Plant Physiol. 1982 Mar;69(3):591–596. doi: 10.1104/pp.69.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]


