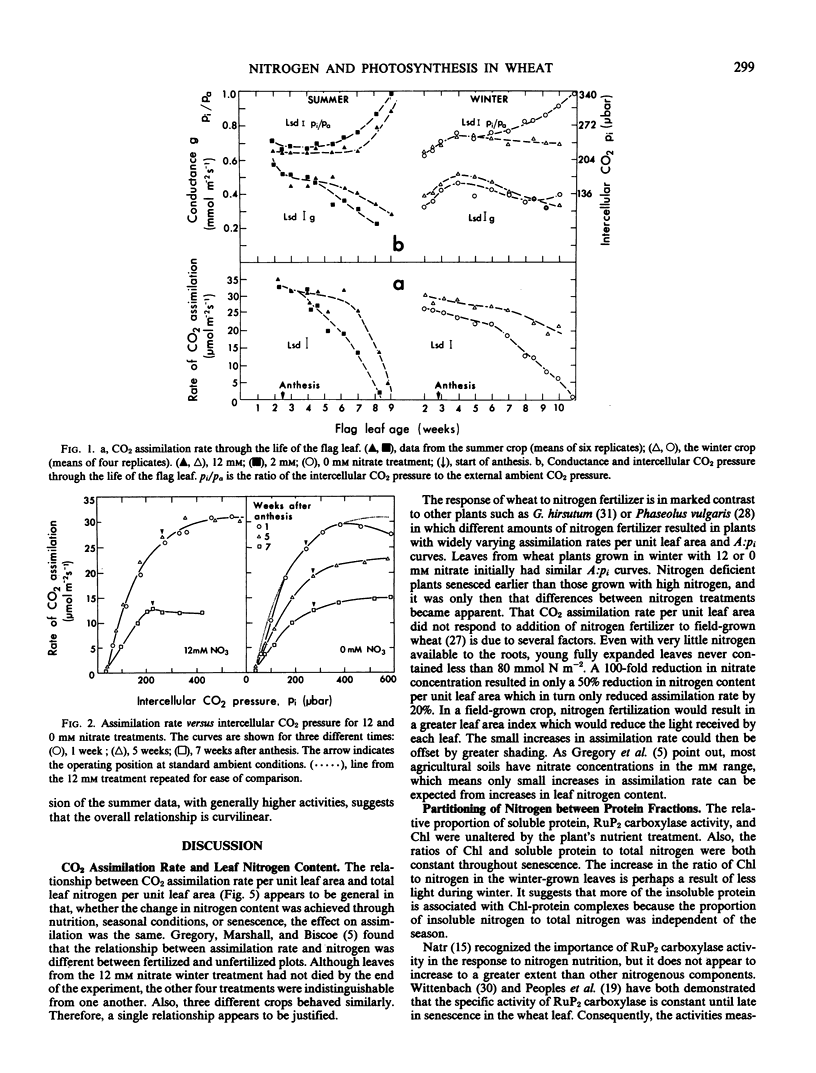
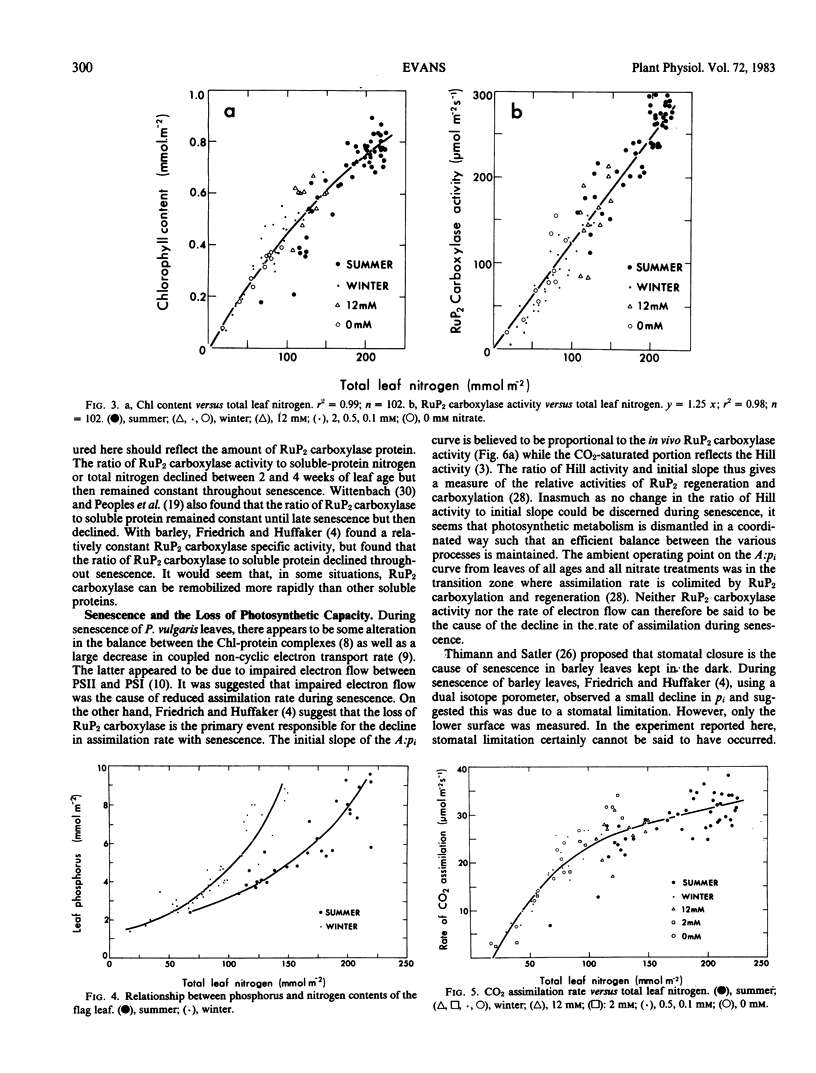
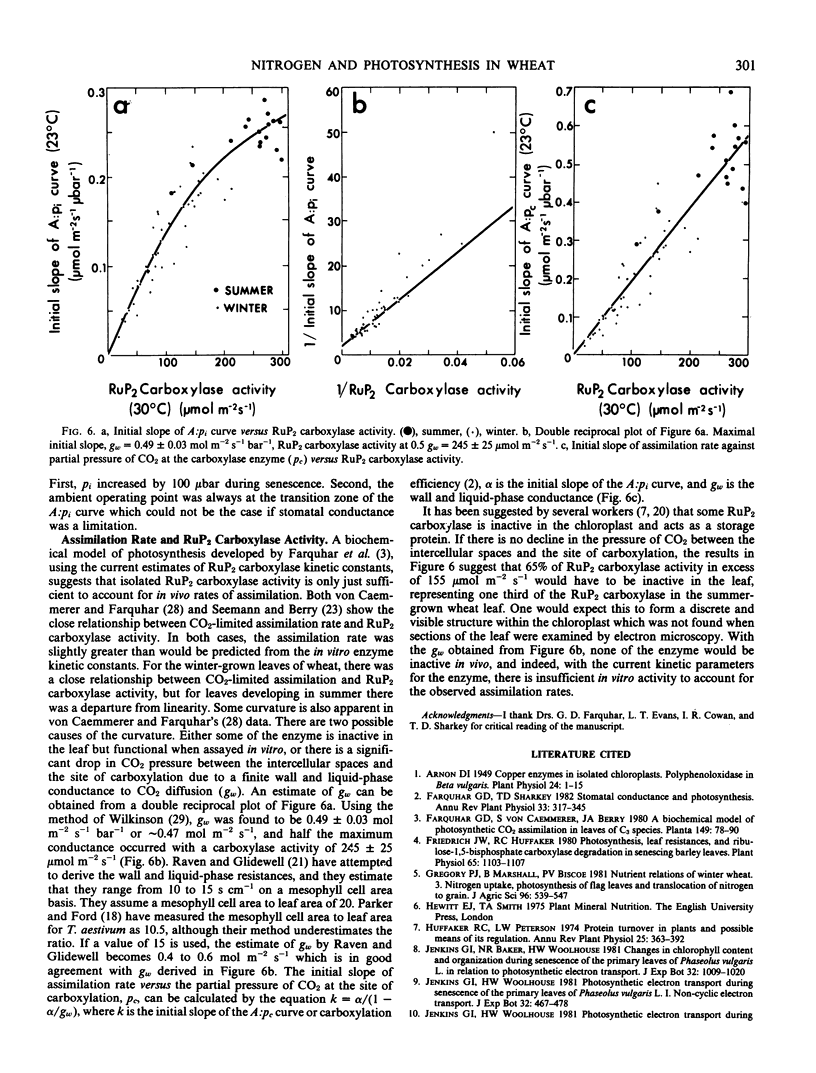
Abstract
Wheat (Triticum aestivum L. cv Yecora 70) plants were grown with various concentrations of nitrate nitrogen available to the roots. Sampling of flag leaves began after they had reached full expansion and continued throughout senescence. Rates of gas exchange, ribulose-1,5-bisphosphate (RuP2) carboxylase activity, and the amounts of chlorophyll, soluble protein, nitrogen, and phosphorus were determined for each flag leaf. Rate of CO2 assimilation was uniquely related to total leaf nitrogen irrespective of nutrient treatment, season, and leaf age. Assimilation rate increased with leaf nitrogen, but the slope of the relationship declined markedly when leaf nitrogen exceeded 125 millimoles nitrogen per square meter. Chlorophyll content and RuP2 carboxylase activity were approximately proportional to leaf nitrogen content. As leaves aged, RuP2 carboxylase activity and calculated Hill activity declined in parallel. With normal ambient partial pressure of CO2, the intercellular partial pressure of CO2 was always such that rate of assimilation appeared colimited by RuP2 carboxylation and RuP2 regeneration capacity.
The initial slope of rate of CO2 assimilation against intercellular partial pressure of CO2 varied nonlinearly with carboxylase activity. It is suggested that this was due to a finite conductance to CO2 diffusion in the wall and liquid phase which causes a drop in CO2 partial pressure between the intercellular spaces and the site of carboxylation. A double reciprocal plot was used to obtain an estimate of the transfer conductance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Imai K., Farquhar G. D., Cowan I. R. A Direct Confirmation of the Standard Method of Estimating Intercellular Partial Pressure of CO(2). Plant Physiol. 1982 Mar;69(3):657–659. doi: 10.1104/pp.69.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. Relation between senescence and stomatal opening: Senescence in darkness. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2770–2773. doi: 10.1073/pnas.76.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Ribulose Bisphosphate Carboxylase and Proteolytic Activity in Wheat Leaves from Anthesis through Senescence. Plant Physiol. 1979 Nov;64(5):884–887. doi: 10.1104/pp.64.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]


