Abstract
A biofilter which eliminated ethylene (C2H4) from the high parts-per-million range to levels near the limit for plant hormonal activity (0.01 to 0.1 ppm) was developed. Isolated ethylene-oxidizing bacteria were immobilized on peat-soil in a biofilter (687 cm3) and subjected to an atmospheric gas flow (73.3 ml min−1) with 2 or 117 ppm of C2H4. Ethylene was eliminated to a minimum level of 0.017 ppm after operation with 2.05 ppm of C2H4 for 16 days. Also, the inlet C2H4 concentration of 117 ppm was reduced to <0.04 ppm. During operation with 2 and 117 ppm of C2H4, an increase in the C2H4 removal rate was observed, which was attributed to proliferation of the immobilized bacteria, notably in the first 0- to 5-cm segment of the biofilter. The maximal C2H4 elimination capacity of the biofilter was 21 g of C2H4 m−3 day−1 during operation with 117 ppm of C2H4 in the inlet gas. However, for the first 0- to 5-cm segment of the biofilter, an elimination capacity of 146 g of C2H4 m−3 day−1 was calculated. Transition of the biofilter temperature from 21 to 10°C caused a 1.6-fold reduction in the C2H4 removal rate, which was reversed during operation for 18 days. Batch experiments with inoculated peat-soil demonstrated that C2H4 removal still occurred after storage at 2, 8, and 20°C for 2, 3, and 4 weeks. However, the C2H4 removal rate decreased with increasing storage time and was reduced by ca. 50% after storage for 2 weeks at all three temperatures. The biofilter could be a suitable tool for C2H4 removal in, e.g., horticultural storage facilities, since it (i) removed C2H4 to 0.017 ppm, (ii) had a good operational stability, and (iii) operated efficiently at 10°C.
Ethylene (C2H4) is a gaseous plant hormone and air pollutant which has a strong effect on plant physiological processes such as ripening, senescence, and aging (2). The threshold limit for C2H4 sensitivity varies among plant species (22), but a general dose response is no effect below 0.01 ppm, half-maximal effect at 0.1 ppm, and maximal effect at 1 to 10 ppm (1). The lower threshold limit of 0.01 ppm is only slightly higher than the atmospheric background concentration (0.003 to 0.005 ppm) and may be exceeded in polluted areas (1, 2, 13, 17, 18). Also, significant accumulation of C2H4 may occur in horticultural storage facilities due to endogenous production by the plant material (2, 12). To reduce the detrimental effects of such C2H4 (14), chemical C2H4 scrubbers are widely used in storage facilities for horticultural produce (16, 19). A drawback of such scrubbers is the cost of operation and the need for replenishment of the C2H4-removing agents (2, 7). Therefore, the use of biological catalysts (3) for C2H4 removal is an interesting alternative in horticulture (11, 19). Likewise, biological catalysts could be used to reduce high ethylene concentrations at point sources, such as petrochemical facilities, where ethylene and polyethylene are produced (13).
Previously, biological waste gas purification has been reported for several air pollutants (see, e.g., references 6 and 11), including C2H4 (4, 10, 20, 21). So far, however, no biological system for C2H4 removal which has a satisfactory operational stability and a sufficient efficiency to reduce the C2H4 concentration to levels near the lower threshold limit for the plant hormonal response (0.01 ppm) has been described.
Here I report the outcome of a study with a biofilter based on isolated ethylene-oxidizing bacteria that were immobilized on peat-soil. During stable operation with inlet levels of 2 and 117 ppm of C2H4, it was possible to reduce the outlet levels to less than 0.04 ppm with a minimum level of 0.017 ppm.
MATERIALS AND METHODS
Preparation of cell suspension.
The ethylene-oxidizing bacterial strain RD-4 was isolated and cultivated as described by Elsgaard and Andersen (8). For the present purpose, the strain was grown in 5-liter vessels containing 2 liters of defined mineral medium without added C sources. The medium in each bottle was inoculated with 20 ml of a pregrown culture, and C2H4 was added to a headspace concentration of ca. 1%. The cultures were incubated with shaking (120 rpm) at 30°C, and the C2H4 concentration was replenished to ca. 1% when it dropped below 0.5% (assayed by gas chromatographic [GC] analysis). After incubation for 5 days, the ethylene-oxidizing bacteria were harvested by centrifugation (16.000 × g for 15 min) and resuspended in 1.5 liters of autoclaved tap water. The density of the cell suspension was estimated to be 2 × 108 cells ml−1 by direct microscopic counts in a Bürker-Türk counting chamber. The cell suspension was stored at 0 to 2°C until needed for biofilter experiments (1 week later) and batch experiments (2 weeks later).
Biofilter construction.
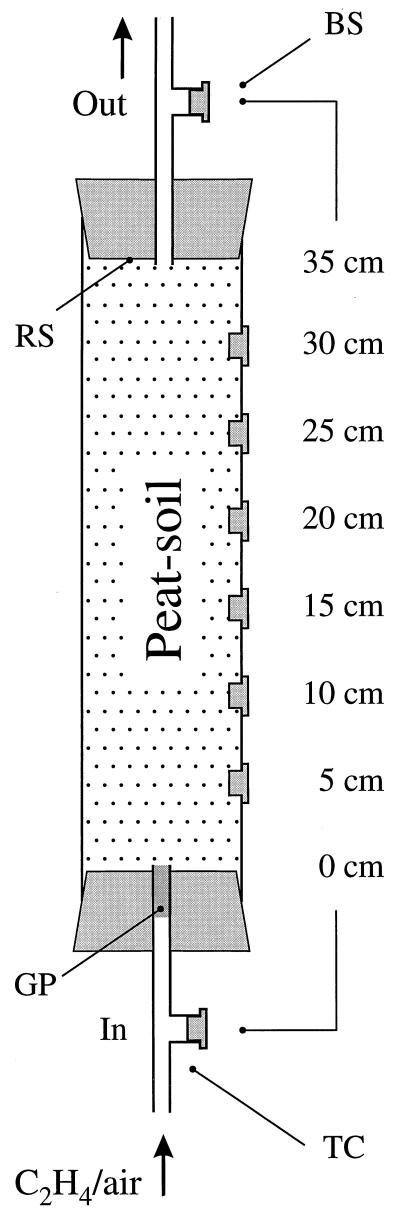
A laboratory-scale biofilter was made from an acrylic core with an inner diameter of 5 cm and a length of 40 cm (Fig. 1). Six butyl rubber stoppers (for gas sampling) were inserted in holes at a vertical distance of 5 cm apart. The core was stoppered with rubber stoppers, each equipped with a T-connector. The T-connectors served as channels for the gas flow and had a butyl rubber stopper inserted for gas sampling (Fig. 1). In operational mode, the biofilter had a volume of 687 cm3 and could be sampled at depths from 0 to 35 cm (inlet and outlet, respectively) at increments of 5 cm.
FIG. 1.
Peat-soil biofilter with butyl stoppers (BS) for gas sampling at the inlet (In), for each 5-cm depth increment, and at the outlet (Out). A flow of humidified air with ethylene (C2H4/air) was applied to the inlet from two mass flow controllers. GP, glass wool plug; RS, rubber stopper; TC, T-connector.
A mixture of atmospheric air and C2H4 in N2 (AGA SpecialGas, Lidingö, Sweden) was supplied to the biofilter by use of two mass flow controllers, with a resulting flow rate (F) of 73 ml min−1. The pseudo-residence time (θp = V/F) of the biofilter was 9.4 min. Before reaching the inlet, the gas was humidified by being bubbled through a flask containing distilled water. To verify the gas flow was constant during the experiments, a digital flowmeter (Jour Research, Onsala, Sweden) was connected to the outlet of the biofilter and the readings of the flowmeter were checked on a daily to weekly basis.
Biofilter experiments.
Immobilized ethylene-oxidizing bacteria were prepared by mixing 300 g of peat-soil (Pindstrup blend 2; Pindstrup Mosebrug) with 200 ml of distilled water and 100 ml of bacterial cell suspension. The soil sample was thoroughly mixed and allowed to equilibrate at room temperature (20 to 22°C) for 1 h. The inoculated peat-soil was loosely packed in the biofilter to a density of 0.44 g cm−3. The final dry-matter content (9) of the inoculated peat-soil was 20.5%.
(i) Operation with 2 ppm of C2H4.
A mixture of 10 ppm of C2H4 and atmospheric air (1:4) was applied to the biofilter. During an operational period of 16 days at 20 to 22°C, gas samples (0.6 ml) were collected from all depths of the biofilter and analyzed for C2H4. Gas samples (0.2 to 0.5 ml) for measurement of O2 and CO2 at the biofilter inlet and outlet were collected after 1 day of operation.
(ii) Operation with different C2H4 concentrations.
After 16 days of operation with 2 ppm of C2H4, the biofilter was operated with atmospheric air for 25 days (i.e., the biofilter was starved for C2H4). Subsequently, the inlet level was adjusted to 2 ppm of C2H4 for 8 days and then to 117 ppm for 80 days. The latter concentration was obtained by mixing 1,000 ppm of C2H4 and atmospheric air (1:8). Gas samples for C2H4 analysis were withdrawn regularly.
(iii) Transition from 21 to 10°C.
During operation with 117 ppm of C2H4, the influence of temperature was tested by placing the biofilter at 10°C in a thermostated incubator. Control experiments showed that temperature equilibrium at 10°C was reached in the center of the biofilter within 5 h. Ethylene concentrations were measured at the biofilter inlet and outlet during operation for 18 days.
Batch experiments.
Batch experiments were done to test the effect of storage on the C2H4 removal rate of the inoculated peat-soil. A sample of immobilized bacteria was prepared as described above, while a control sample was prepared by mixing 100 g of peat-soil with 100 ml of distilled water. Each of the soil samples was divided into three portions that were placed at 2, 8, and 20°C. To test the C2H4 removal rate before storage, triplicate 10-g soil samples were transferred to 120-ml serum bottles that were purged with atmospheric air and closed with butyl rubber stoppers. Ethylene was added to a headspace concentration of ca. 20 ppm, and the time course of C2H4 removal (20 to 22°C) was monitored by GC analysis of withdrawn gas samples (0.4 ml). Similar assays were done with the stored soil samples after 2, 3, and 4 weeks of storage. Before these assays, the 10-g subsamples were allowed to equilibrate at room temperature for 1 h.
Gas analysis.
Gas samples for analysis of C2H4, CO2, and O2 were collected with 1-ml gas-tight syringes (Pressure-Lok). Generally, C2H4 was quantified with a Hewlett-Packard 5840A gas chromatograph equipped with a flame ionization detector (8). For injection of a 0.6-ml gas sample, the C2H4 detection limit was 0.04 ppm for a signal-to-noise ratio of 3.
Occasionally, samples from the biofilter were collected by passing the outlet flow through a stoppered 120-ml serum bottle for 1 to 2 h with two hypodermic needles. The bottle was then removed from the gas stream, and subsamples of 1.0 ml were analyzed for C2H4 with a Photovac 10Splus gas chromatograph equipped with a photoionization detector (8). The ethylene detection limit was 0.002 ppm for a sample volume of 1.0 ml.
Oxygen (0.5-ml samples) was measured on a Varian 3700 gas chromatograph with a thermal conductivity detector (TCD) and a molecular sieve 5A column. The oven temperature was 30°C, and the carrier gas was He (flow rate, 76 ml min−1). Carbon dioxide (0.2-ml samples) was measured on a GC 82 (Mikrolab, Højbjerg, Denmark) with a TCD. The column (Porapak N) was operated at 60°C with He as the carrier gas (flow rate, 43 ml min−1).
Statistics.
Data from replicate samples are presented as mean ± standard error (SE) with the number of samples (n) indicated. The significance of differences between data from the biofilter inlet and outlet was tested by use of a two-tailed Student t test (23).
RESULTS
Biofilter experiments.
During the experiments, the flow rate measured at the outlet of the biofilter ranged from 71.4 to 75.9 ml min−1 with a mean and SE of 73.3 ± 0.3 ml min−1 (n = 23). In a control experiment, it was demonstrated that the flow rates at the inlet and the outlet were identical (76.5 to 76.8 ml min−1) and equaled the sum of the flow rates for the two gaseous components that were mixed (67.9 and 8.7 ml min−1). Furthermore, it was demonstrated that with an empty biofilter, the outlet C2H4 concentration (2.19 ± 0.03 ppm; n = 3) was equal to the inlet concentration (2.18 ± 0.03 ppm; n = 3). This showed that no significant leakage or adsorption occurred.
(i) Operation with 2 ppm of C2H4.
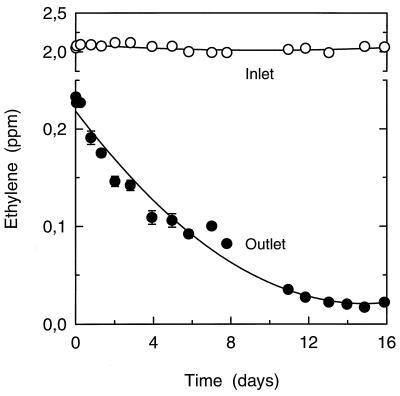
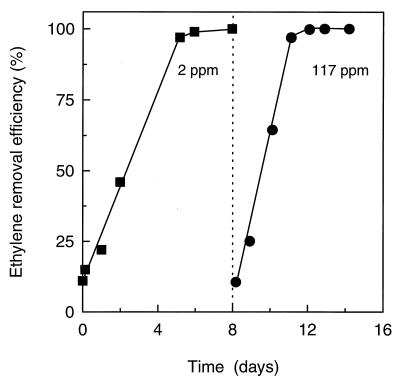
During the first experiment, a stable inlet concentration of 2.05 ± 0.01 ppm (n = 57) was applied to the biofilter (Fig. 2). Measurements of the outlet C2H4 concentration after 1 h of operation (0.23 ± 0.01 ppm; n = 4) demonstrated that 89% of the incoming C2H4 had already been removed by the biofilter at this early stage (Fig. 2). During the experiment, the efficiency of C2H4 removal gradually increased to 99%. Thus, at the end of the experiment the biofilter had a stable performance with an outlet concentration of only 0.017 to 0.020 ppm of C2H4 (Fig. 2).
FIG. 2.
Ethylene concentration in the gas flow at the inlet and at the outlet of the biofilter during operation for 16 days with 2.05 ppm of C2H4 (73.3 ml min−1). Data represent the mean ± SE of three or four samples. Note the scale break on the y axis.
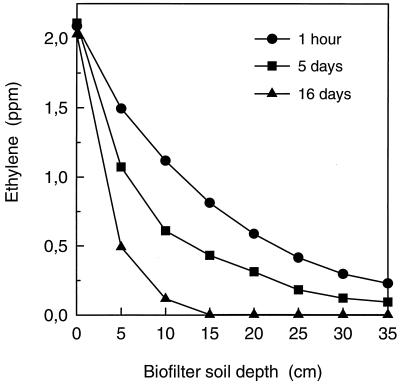
Gas analysis at different biofilter soil depths showed that after 1 h of operation all the soil layers were exposed to C2H4 at concentrations ranging from 2.05 to 0.23 ppm (Fig. 3). However, as the rate of C2H4 removal increased in the soil layers close to the inlet, removal of the incoming C2H4 ultimately occurred within the first 15 cm of the biofilter (Fig. 3). Segment-specific C2H4 removal rates were calculated as follows:
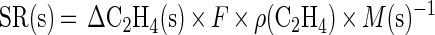 |
1 |
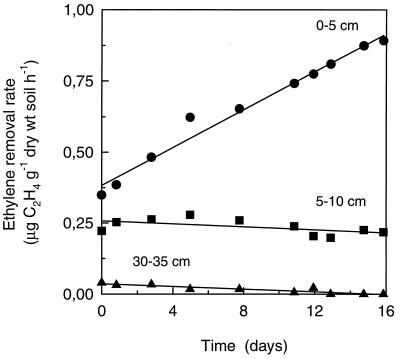
where SR(s) is the specific C2H4 removal rate (micrograms of C2H4 per gram [dry weight] of soil per hour) by a given 5-cm segment (s), ΔC2H4(s) is the difference between the inlet and outlet C2H4 concentration of the segment (microliters per liter), F is the flow rate (4.4 liters h−1), ρ(C2H4) is the density of C2H4 at 21°C (1.16 μg μl−1), and M(s) is the soil dry-matter content in a 5-cm segment (8.8 g). These calculations showed that the initial C2H4 removal rate in each segment (after 1 h of operation) depended linearly on the C2H4 concentration (data not shown). In the 0- to 5-cm segment, the C2H4 removal rate increased from 0.35 to 0.89 μg of C2H4 g (dry weight) of soil−1 h−1 during the experiment (Fig. 4). In the subsequent segments, a relatively stable or decreasing rate was observed (Fig. 4).
FIG. 3.
Ethylene concentration at different soil depths of the biofilter after operation with 2.05 ppm of C2H4 for 1 h, 5 days, and 16 days. Soil depths of 0 and 35 cm represent the biofilter inlet and outlet, respectively.
FIG. 4.
Ethylene removal rates by individual 5-cm segments of the biofilter during operation for 16 days with 2.05 ppm of C2H4. Data are shown for the segments from 0 to 5 cm, 5 to 10 cm, and 30 to 35 cm.
Oxygen measurements after 1 day of operation showed that the O2 content of the inlet gas and the outlet gas was 15.6% ± 0.1% (n = 3) and 15.2% ± 0.1% (n = 3), respectively. This demonstrated that oxic conditions prevailed throughout the whole soil column. At the same time, the CO2 concentrations at the inlet and the outlet were 0.018% ± 0.001% (n = 4) and 0.024% ± 0.001% (n = 3), respectively. Thus, the outlet CO2 concentration was significantly higher than the inlet CO2 concentration (P < 10−4), demonstrating that a net mineralization of organic C occurred in the biofilter.
(ii) Operation with different C2H4 concentrations.
Operation for 25 days without C2H4 in the inlet gas caused a decrease in the biofilter efficiency of C2H4 removal. When 2 ppm C2H4 was reapplied, only 11 to 15% of the incoming C2H4 was removed after 0.5 to 3 h of operation (Fig. 5); however, after operation for 8 days, the efficiency of C2H4 removal was recovered and >98% of the incoming C2H4 was removed by the biofilter (Fig. 5). Transition of the inlet C2H4 level from 2 to 117 ppm similarly caused a transient decrease in the efficiency of the biofilter, which initially (after 0.5 h) removed only 10% of the incoming C2H4 (after the transition, the inlet concentration was 117.2 ± 0.4 ppm [n = 76] during the subsequent experiments). However, after operation at 117 ppm of C2H4 for 4 days, the C2H4 concentration at the outlet of the biofilter was below the detection limit of 0.04 ppm. Thus, more than 99.9% of the incoming C2H4 was removed by the biofilter (Fig. 5).
FIG. 5.
Ethylene removal efficiency of the biofilter after transition of the inlet C2H4 concentration from 0 to 2 ppm and from 2 to 117 ppm. The dotted line indicates the latter transition. Before time zero, the biofilter was starved for C2H4 for 25 days. Data are the mean ± SE of three pairs of analyses of inlet and outlet concentrations.
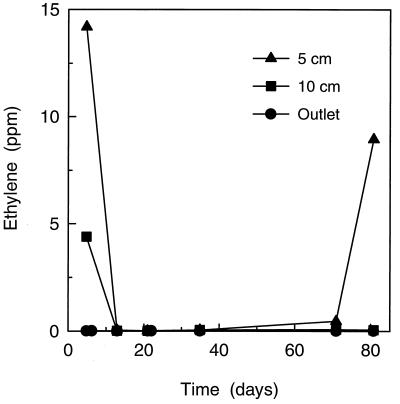
The long-term operational stability of the biofilter was tested with the inlet level of 117 ppm of C2H4 (Fig. 6). It was demonstrated that for more than 75 days of constant operation, the biofilter was able to reduce the outlet C2H4 concentration to less than 0.04 ppm. Most of the C2H4 was removed during passage through the first 0 to 5 cm of the biofilter (Fig. 6). However, after 70 days of operation, the C2H4 removal by the 0- to 5-cm segment started to decrease but the removal was then accomplished within the 5- to 10-cm segment (Fig. 6).
FIG. 6.
Ethylene concentration in the gas flow of the biofilter during operation for 80 days with 117 ppm of C2H4. Data are shown for the 5-cm depth the 10-cm depth, and the outlet. At time zero, the inlet C2H4 concentration was changed from 2 to 117 ppm.
(iii) Transition from 21 to 10°C.
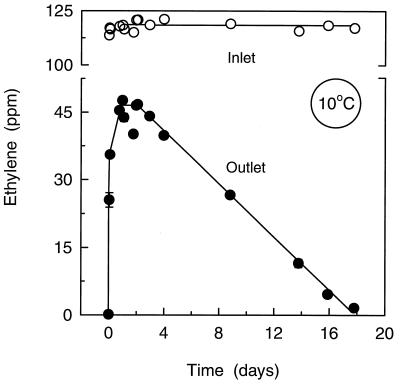
When the biofilter was transferred from 21 to 10°C (Fig. 7), the outlet C2H4 concentration gradually increased from <0.04 to 46.6 ± 0.3 ppm (n = 3). However, after 2 days of operation at 10°C, the outlet concentration started to decrease with a linear time course (Fig. 7). Thus, when the experiment was stopped after 18 days, the outlet concentration was 1.6 ± 0.1 ppm C2H4 (n = 3), which was equivalent to a removal efficiency of 98.6%.
FIG. 7.
Ethylene concentration in the gas flow at the inlet and the outlet of the biofilter after transition from 21 to 10°C. Temperature equilibrium at 10°C was reached after operation for 5 h. Data are mean ± SE (n = 3). Note the scale break on the y axis.
Batch experiments.
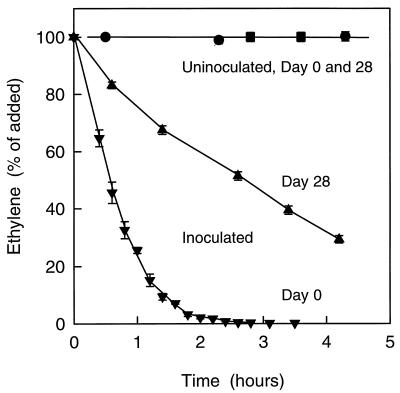
With freshly inoculated peat-soil, rapid depletion of the added ∼20 ppm of C2H4 was observed (Fig. 8). During the first 1 h of the experiment (Fig. 8), C2H4 was removed at rate of 17.1 ppm h−1, which was equal to a specific rate of 1.06 μg of C2H4 g (dry weight) of soil−1 h−1 (8). After storage of the inoculated peat-soil for 28 days at 20°C, a constitutive C2H4 removal still occurred but took place at a lower rate than for the freshly inoculated soil (Fig. 8), so that the data for the first 1.5 h of the experiment showed a removal rate of 3.3 ppm h−1 (0.21 μg of C2H4 g [dry weight] of soil−1 h−1). Within the assay time of 5 h, no C2H4 removal occurred in samples of uninoculated peat-soil either immediately or after 28 days of storage at 20°C (Fig. 8).
FIG. 8.
Time course of C2H4 removal in batch experiments with inoculated or uninoculated peat-soil. Ethylene was added to ca. 20 ppm. Results are shown for freshly inoculated peat-soil (▾), inoculated peat-soil stored at 20°C for 28 days (▴), uninoculated peat-soil prior to storage (•), and uninoculated peat-soil after storage at 20°C for 28 days (■). Data are mean ± SE (n = 3).
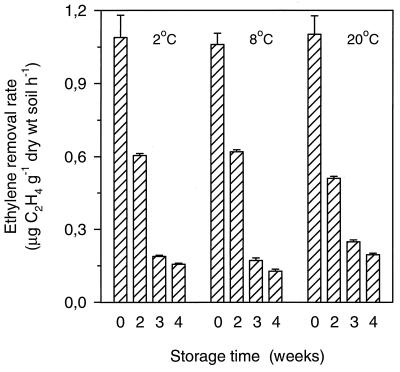
All time courses of C2H4 removal by inoculated peat-soil stored at 2, 8, or 20°C for 0, 2, 3, and 4 weeks fell within the range depicted by the data from day 0 and day 28 in Fig. 8. Thus, for comparison, the initial C2H4 removal rate for each assay was calculated from the first two or three data points obtained during 0 to 1.5 h of incubation. These rates demonstrated that the C2H4 removal rate decreased as a result of longer storage times (Fig. 9). However, the extent of the decrease caused by the storage time was almost unaffected by the storage temperature (Fig. 9).
FIG. 9.
Initial C2H4 removal rates in batch experiments with inoculated peat-soil. Soil samples were assayed before storage and after storage at 2, 8, or 20°C for 2, 3, and 4 weeks. Data are mean ± SE (n = 3).
DISCUSSION
Efficiency and capacity of C2H4 removal.
The biofilter showed an initial removal efficiency of 89%, which increased to 99% during 2 weeks of operation with an inlet concentration of 2.05 ppm C2H4. In a comparable study, van Ginkel et al. (20) reported the removal of C2H4 by a biofilter operating at 30°C with Mycobacterium strain E3 immobilized on compost. With an inlet concentration of 2 ppm of C2H4, an initial removal efficiency of ca. 67% (outlet concentration, ca. 0.66 ppm) was obtained, which increased during the first 2 weeks and stabilized at ca. 87% (outlet concentration, ca. 0.27 ppm) during operation for 8 weeks (calculated from Fig. 2 in reference 20). However, because the study by van Ginkel et al. (20) was performed with different reactor characteristics (V = 15 cm3, F = 8 ml min−1, θp = 1.9 min), the higher efficiency in the present study was at least partly related to a longer pseudo-residence time (9.4 min). When the data from the two biofilters were evaluated in terms of the ethylene elimination capacity, defined as the amount of ethylene degraded per unit of reactor volume and time (15), it was found that the capacity of the biofilter in the present study was 0.4 g of C2H4 m−3 day−1 while the capacity of biofilter of van Ginkel et al. (20) was 1.5 g of C2H4 m−3 day−1. However, as C2H4 was removed from 2.05 to 0.49 ppm within the first 5-cm segment of the biofilter in the present study, the ethylene elimination capacity of this segment was 1.9 g of C2H4 m−3 day−1. This capacity was slightly higher than that calculated for the biofilter of van Ginkel et al. (20).
De heyder et al. (4) studied the removal of C2H4 by a packed granular activated carbon biobed (19 to 23°C) inoculated with Mycobacterium strain E3. During operation with 127 ppm of C2H4 in the inlet gas, they obtained a maximal elimination capacity of 250 g of C2H4 m−3 day−1, which corresponded to a removal efficiency of 84.9% (4). However, this was a maximal rate obtained during cycles of wetting and drying out of the biobed. Under regular operation (i.e., with constant wetting), the typical elimination capacity was 112 g of C2H4 m−3 day−1 (4). In comparison, the elimination capacity obtained with the biofilter in the present study was 21 g of C2H4 m−3 day−1 during stable operation (75 days) with 117 ppm C2H4 in the inlet gas. However, since C2H4 was eliminated mainly within the first 5-cm segment of this biofilter (Fig. 6), the maximal elimination capacity found within this segment was 146 g of C2H4 m−3 day−1. Thus, the maximal elimination capacity of the biofilter material was somewhat lower than in the studies of De heyder et al. (4), but on the other hand, the C2H4 outlet concentrations (<0.04 ppm) in the biofilter in the present study were more than 400-fold lower than those reported (ca. 19 ppm) by De heyder et al. (4).
In summary, the biofilter in the present study appeared to be an interesting tool for C2H4 removal because it combined a high removal efficiency, a good elimination capacity, and an extremely low outlet C2H4 concentration.
Depth dependence and stability of C2H4 removal.
Data from the 0- to 5-cm segment demonstrated that with an inlet concentration of 2.05 ppm, there was an increase in the C2H4 removal rate from 0.35 to 0.89 μg of C2H4 g (dry weight) of soil−1 h−1 during operation for 16 days. This could be due to (i) microbial adaptation to the C2H4 concentration, (ii) an increase in the cell number in the segment, or (iii) a combination of these effects. If the rate increase were due to an increase in the number of bacteria (with a constant C2H4 removal rate per cell), it was calculated that the population in the 0- to 5-cm segment should increase from the initial number of 1.6 × 108 to 4.1 × 108 cells g (dry weight) of soil−1. This could be obtained just by slow growth of the RD-4 bacteria, which had a doubling time of ca. 22 h when cultivated in mineral medium with a headspace of 1% C2H4 (data not shown). No increases in the C2H4 removal rate occurred in the soil layers above the 0- to 5-cm segment, indicating that no growth of the added bacteria occurred in these layers.
After C2H4 starvation for 25 days, the biofilter lost 89% of its C2H4 removal efficiency. However, recovery of the efficiency occurred within 4 days after reapplication of C2H4. This demonstrated that the bacteria were able to survive in the peat-soil without an external source of C2H4. Bacterial growth after alleviation of the C2H4 starvation was indicated by the progressive increase in C2H4 removal after transition from 0 to 2 ppm of C2H4 and from 2 to 117 ppm of C2H4.
The operational stability of the biofilter was demonstrated by the performance of the 0- to 5-cm segment, which caused almost complete removal of 117 ppm of C2H4 for more than 75 days of constant operation. In turn, subsequent reduction of the removal efficiency, which may have resulted from depletion of (unknown) bacterial growth factors supplied by the peat-soil, was noted. Correspondingly, an enhancement of biological ethylene removal by soluble microbial products has recently been indicated in studies with Mycobacterium strain E3 (5). In the present study, the decrease in the efficiency of the 0- to 5-cm segment had no influence on the overall performance of the biofilter, because the next soil layers were able to remove the incoming C2H4 to less than 0.04 ppm.
Effect of temperature.
After transition of the biofilter temperature from 21 to 10°C, the difference between the inlet and outlet C2H4 concentrations decreased from 117 to 71 ppm in 1 day. This corresponded to a decrease in the specific C2H4 removal rates from 9.7 to 6.1 μg of C2H4 g (dry weight) of soil−1 h−1, as calculated (equation 1) for the whole biofilter, using ρ(C2H4) = 1.20 μg μl−1 at 10°C. Previously (21), it was observed that the activity of immobilized Mycobacterium strain E3 declined rapidly below 10°C and was almost absent at 4°C. In the present study, the activity below 10°C was not assayed, and so it is not clear whether strain RD-4 would produce a similar response to lower temperatures. However, after 3 days of operation at 10°C, the biofilter outlet concentration started to decrease, indicating that the C2H4-removing bacteria proliferated at 10°C. Such a potential could be important for the application of strain RD-4 in biofilters for use with horticultural produce, which is often kept at temperatures below 10°C. However, a more detailed study of the temperature response of the strain RD-4 should be performed before this suitability can be properly evaluated.
Effect of storage.
During storage of inoculated peat-soil at 2, 8, or 20°C, the bacterial cells lost about half (47 to 58%) of their C2H4 removal activity within 2 weeks. In comparison, it was reported that Mycobacterium strain E3 stored as free cell suspensions at 4°C lost about half of its activity within 2 days (21). Before being used in the present biofilter studies, the RD-4 suspension was stored at 0 to 2°C for 1 week. The effect of this storage was not evaluated, but probably an even more efficient C2H4 removal could be obtained in biofilter experiments if a freshly harvested cell suspension was used as the inoculum.
It was found that storage at 2, 8, or 20°C had a similar influence on the initial C2H4 removal rates of the inoculated peat-soil. Thus, after 4 weeks of storage at all three temperatures, the C2H4 removal rate represented 12 to 17% of the rate observed before storage. These data agreed with the reduction in the C2H4 removal efficiency (from 99 to 11%) that was observed for the biofilter after C2H4 starvation for 25 days. The comparable responses to storage at 2, 8, and 20°C indicated that the bacteria did not proliferate at the expense of organic substrates in the peat-soil, because such growth would be expected to be higher at 20 than at 2°C.
Concerning the application of inoculated peat-soil for purposes of C2H4 removal in biofilters, the results demonstrated that cold storage did not improve the keeping quality of the inoculated peat-soil. Rather, the results suggested that even at 20°C, the inoculated peat-soil could be starved for C2H4 for up to 2 weeks and still retain half of the original activity. This would allow a delay between the preparation of a biofilter and its subsequent use for C2H4 removal.
Conclusions.
Ethylene was removed to 0.017 to 0.020 ppm in a peat-soil biofilter with immobilized bacteria. Reduction of C2H4 to such extremely low levels is attractive at industrial point sources and is an important prerequisite for the development of a biofilter for use in horticultural storage facilities. Other characteristics of the biofilter which were favorable for such use included the observations that (i) the operational stability extended for more than 75 days, (ii) the biofilter adapted to C2H4 removal at 10°C, and (iii) storage of the inoculated peat-soil for 2 weeks at 20°C caused only a halving of the C2H4 removal rate.
ACKNOWLEDGMENTS
I thank Gitte Hastrup Andersen for skillful laboratory assistance and Lise Andersen for analysis of gas samples on the Photovac portable GC. Also, I thank Hubert de Jonge, B. T. Christensen, and two anonymous reviewers for helpful comments on the manuscript.
This work was done as a part of the research program “Prydplantepakken” at the Danish Institute of Agricultural Sciences.
REFERENCES
- 1.Abeles F B. Ethylene as an air pollutant. Agric For Bull. 1982;5:4–12. [Google Scholar]
- 2.Abeles F B, Morgan P W, Saltweit M E., Jr . Ethylene in plant biology. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1992. [Google Scholar]
- 3.de Bont J A M. Oxidation of ethylene by soil bacteria. Antonie Leeuwenhoek. 1976;42:59–71. doi: 10.1007/BF00399449. [DOI] [PubMed] [Google Scholar]
- 4.De heyder B, Overmeire A, Van Langenhove H, Verstraete W. Ethene removal from a synthetic waste gas using a dry biobed. Biotechnol Bioeng. 1994;44:642–648. doi: 10.1002/bit.260440511. [DOI] [PubMed] [Google Scholar]
- 5.De heyder B, van Elst T, Van Langenhove H, Verstraete W. Enhancement of ethene removal from waste gas by stimulating nitrification. Biodegradation. 1997;8:21–30. doi: 10.1023/a:1008204803231. [DOI] [PubMed] [Google Scholar]
- 6.Dragt A J, van Ham J, editors. Biotechniques for air pollution abatement and odour control policies. Amsterdam, The Netherlands: Elsevier Biomedical Press; 1992. [Google Scholar]
- 7.El Blindi A, Rigal L, Malmary G, Molinier J, Torres L. Ethylene removal for long term conservation of fruits and vegetables. Food Quality Preference. 1993;4:119–126. [Google Scholar]
- 8.Elsgaard, L., and L. Andersen. Microbial ethylene consumption in peat-soil during ethylene exposure of Begonia elatior. Plant Soil, in press.
- 9.Forster J C. Soil physical analysis. In: Alef K, Nannipiri P, editors. Methods in applied soil microbiology and biochemistry. London, United Kingdom: Academic Press, Ltd.; 1995. pp. 105–115. [Google Scholar]
- 10.Frye R J, Welsh D, Berry T M, Stevenson B A, McCallum T. Removal of contaminant organic gases from air in closed systems by soil. Soil Biol Biochem. 1992;24:607–612. [Google Scholar]
- 11.Hartmans S, de Bont J A M, Harder W. Microbial metabolism of short-chain unsaturated hydrocarbons. FEMS Microbiol Rev. 1989;63:235–264. doi: 10.1016/0168-6445(89)90034-x. [DOI] [PubMed] [Google Scholar]
- 12.Høyer L. Investigations of the ethylene build-up during transport of pot plants in controlled temperature trucks. Postharvest Biol Technol. 1995;5:101–108. [Google Scholar]
- 13.Jack T R, McBrien R K, Dowsley B. Impact assessment for ethylene emissions at a petrochemical site. In: Kanellis A K, et al., editors. Biology and biotechnology of the plant hormone ethylene. Amsterdam, The Netherlands: Kluwer Academic Publishers; 1997. pp. 283–288. [Google Scholar]
- 14.Kadar A A. Ethylene-induced senescence and physiological disorders in harvested horticultural crops. HortScience. 1985;20:54–57. [Google Scholar]
- 15.Ottengraf S P P, Diks R M M. Process technology of biotechniques. In: Dragt A J, van Ham J, editors. Biotechniques for air pollution abatement and odour control policies. Amsterdam, The Netherlands: Elsevier Biomedical Press; 1992. pp. 17–31. [Google Scholar]
- 16.Rudolphij J W, Jr, Boerrigter H A M. Scrubbers voor ethyleen. Bedrijfsontwikkeling. 1981;12:307–312. [Google Scholar]
- 17.Sawada S. Fundamental studies on dynamics of ethylene in an ecosystem. I. Atmospheric ethylene concentrations in a Fagus crenata forest and above paddy fields during burning of agricultural wastes. Jpn J Ecol. 1985;35:215–223. [Google Scholar]
- 18.Sawada S. Fundamental studies on dynamics of ethylene in an ecosystem. II. Atmospheric ethylene levels in a tropical rain forest of the Colombial Amazon region. Jpn J Ecol. 1985;35:291–295. [Google Scholar]
- 19.Sherman M. Control of ethylene in the postharvest environment. HortScience. 1985;20:57–60. [Google Scholar]
- 20.van Ginkel C G, Welten H G J, de Bont J A M. Growth and stability of ethene-utilizing bacteria on compost at very low substrate concentrations. FEMS Microbiol Ecol. 1987;45:65–69. [Google Scholar]
- 21.van Ginkel C G, Welten H G J, de Bont J A M, Boerrigter H A M. Removal of ethene to very low concentrations by immobilized Mycobacterium E3. J Chem Technol Biotechnol. 1986;36:593–598. [Google Scholar]
- 22.Woltering E J. Effects of ethylene on ornamental pot plants: a classification. Sci Hortic. 1987;31:283–294. [Google Scholar]
- 23.Zar J H. Biostatistical analysis. 3rd ed. Upper Saddle River, N.J: Prentice-Hall International, Inc.; 1996. [Google Scholar]