Abstract
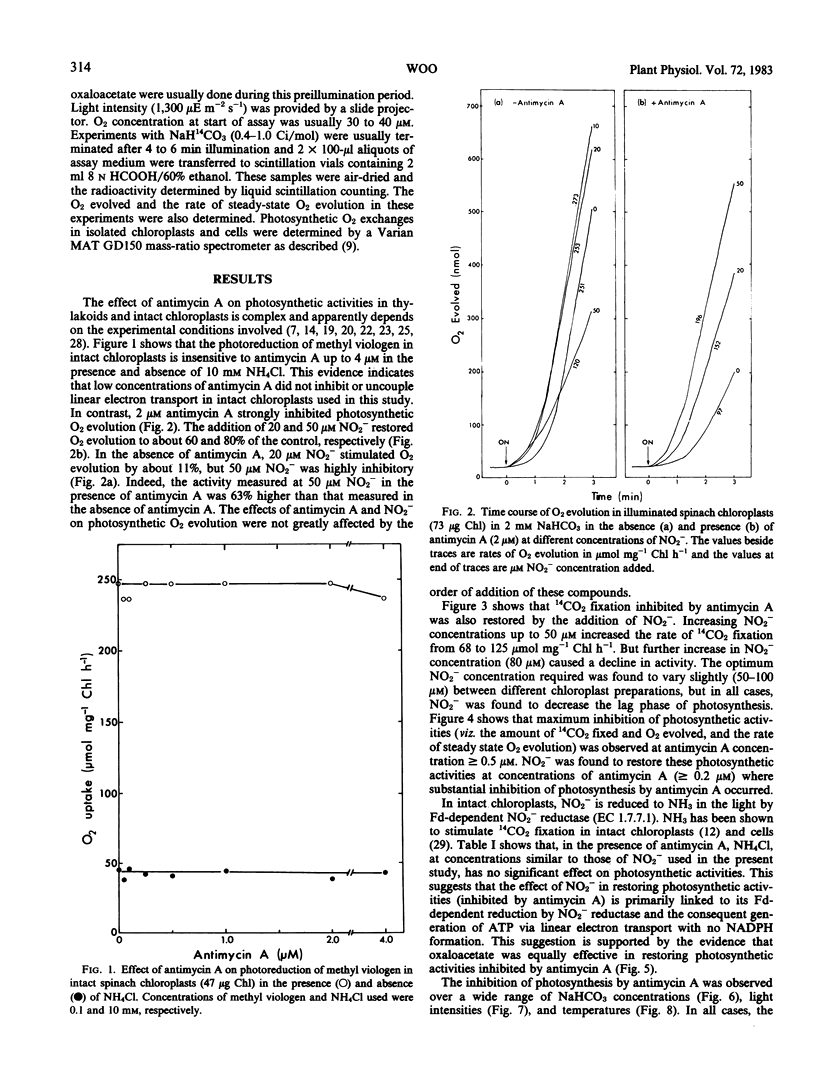
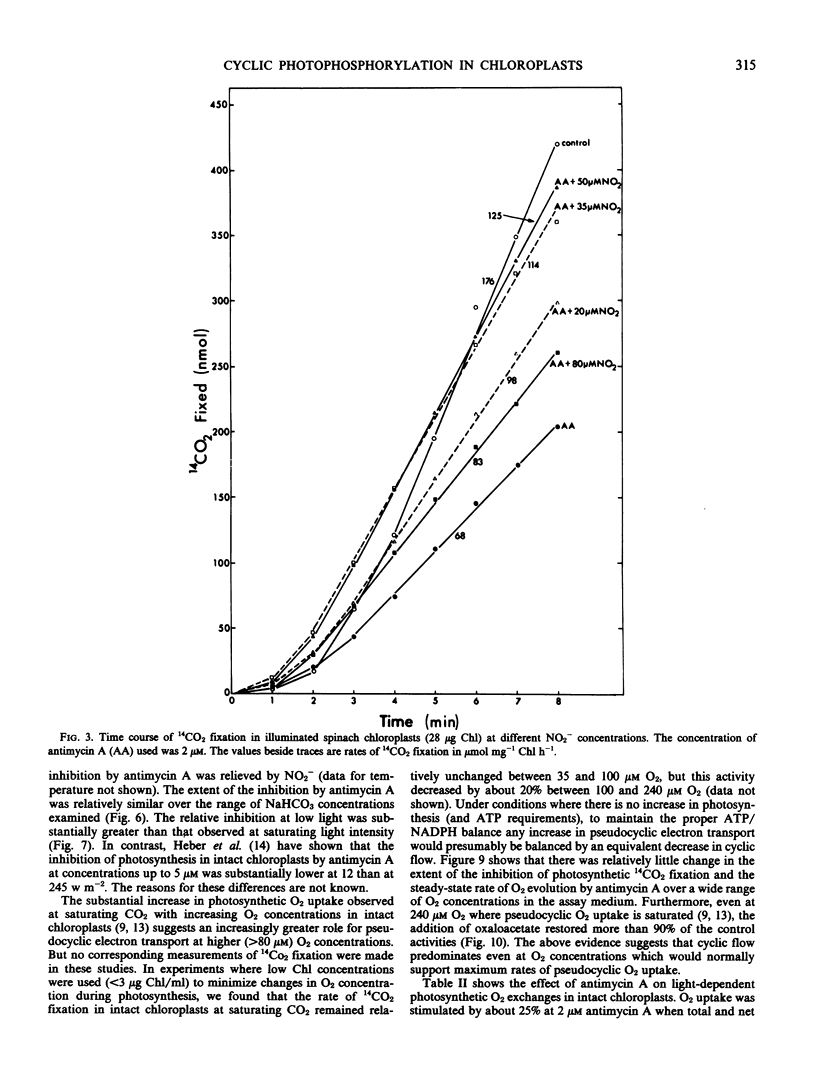
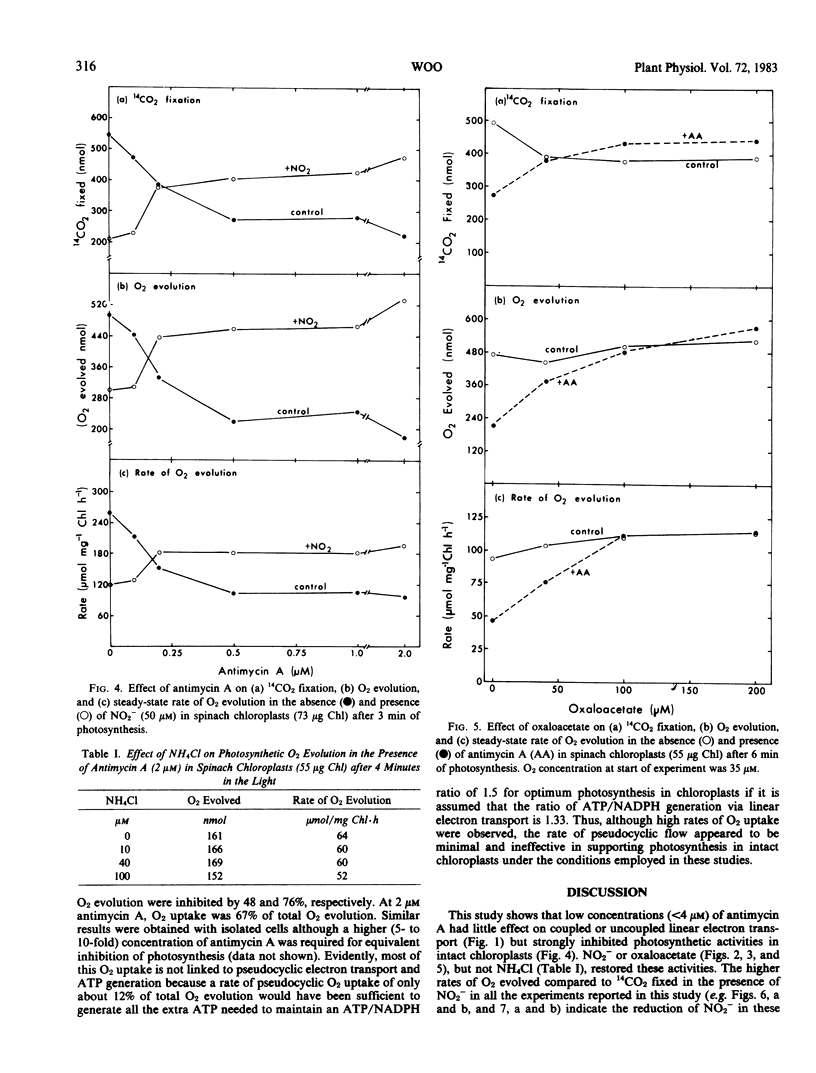
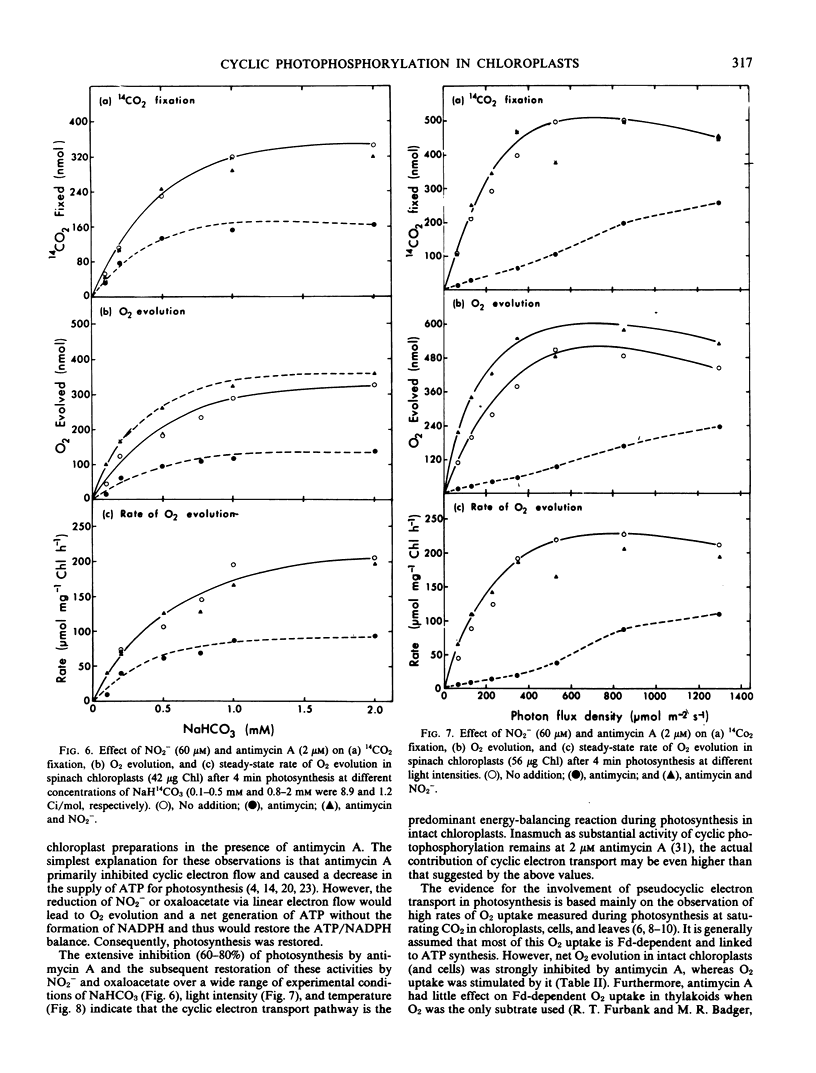
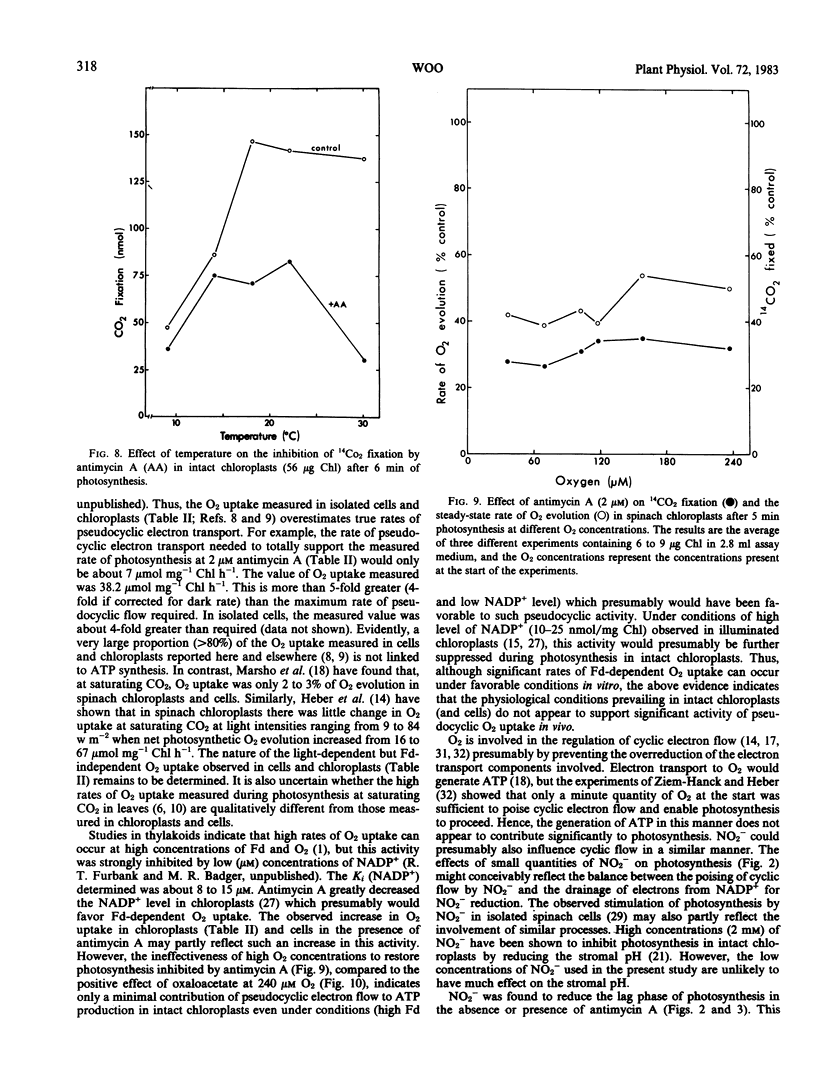
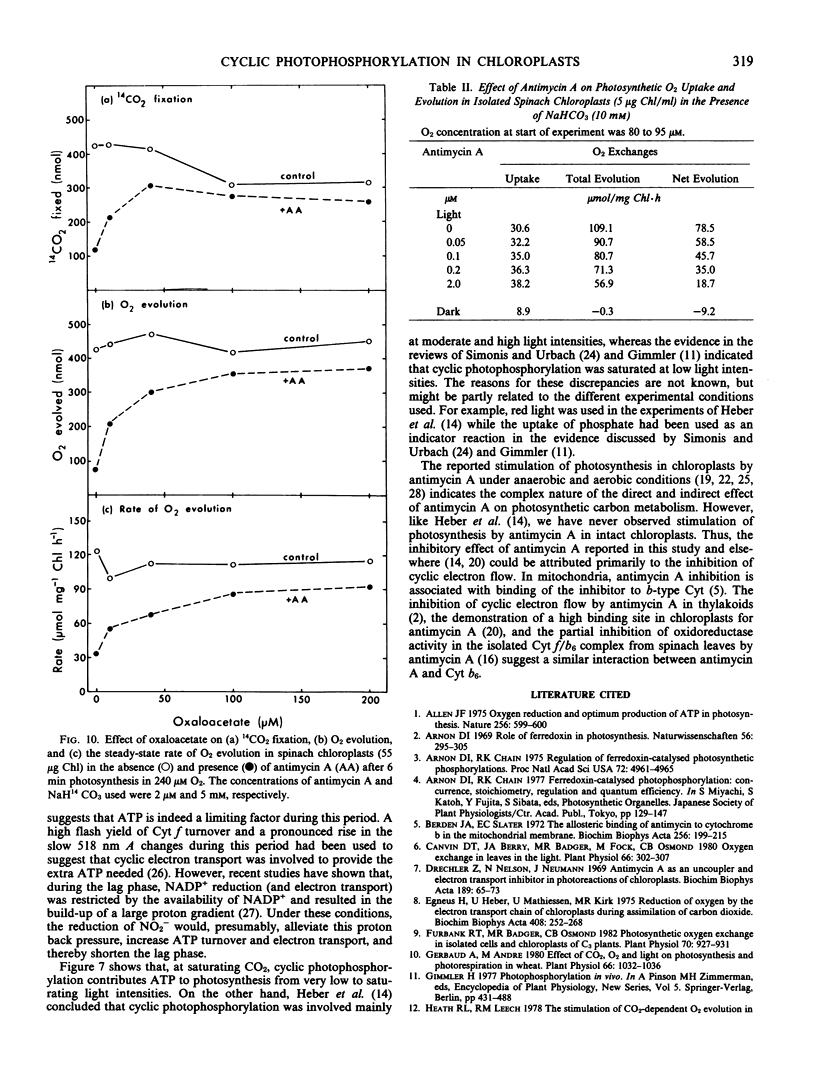
This study examines the effect of antimycin A and nitrite on 14CO2 fixation in intact chloroplasts isolated from spinach (Spinacia oleracea L.) leaves. Antimycin A (2 micromolar) strongly inhibited CO2 fixation but did not appear to inhibit or uncouple linear electron transport in intact chloroplasts. The addition of small quantities (40-100 micromolar) of nitrite or oxaloacetate, but not NH4Cl, in the presence of antimycin A restored photosynthesis. Antimycin A inhibition, and the subsequent restoration of photosynthetic activities by nitrite or oxaloacetate, was observed over a wide range of CO2 concentration, light intensity, and temperature. High O2 concentration (up to 240 micromolar) did not appear to influence the extent of the inhibition by antimycin A, nor the subsequent restoration of photosynthetic activity by nitrite or oxaloacetate. Studies of O2 exchanges during photosynthesis in cells and chloroplasts indicated that 2 micromolar antimycin A stimulated O2 uptake by about 25% while net O2 evolution was inhibited by 76%. O2 uptake in chloroplasts in the presence of 2 micromolar antimycin A was 67% of total O2 evolution. These results suggest that only a small proportion of the O2 uptake measured was directly linked to ATP generation. The above evidence indicates that cyclic photophosphorylation is the predominant energy-balancing reaction during photosynthesis in intact chloroplasts. On the other hand, pseudocyclic O2 uptake appears to play only a minimal role.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Chain R. K. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Berden J. A., Slater E. C. The allosteric binding of antimycin to cytochrome b in the mitochondrial membrane. Biochim Biophys Acta. 1972 Feb 28;256(2):199–215. doi: 10.1016/0005-2728(72)90053-9. [DOI] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler Z., Nelson N., Neumann J. Antimycin A as an uncoupler and electron transport inhibitor in photoreactions of chloroplasts. Biochim Biophys Acta. 1969 Sep 16;189(1):65–73. doi: 10.1016/0005-2728(69)90226-6. [DOI] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Furbank R. T., Badger M. R., Osmond C. B. Photosynthetic oxygen exchange in isolated cells and chloroplasts of c(3) plants. Plant Physiol. 1982 Oct;70(4):927–931. doi: 10.1104/pp.70.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., André M. Effect of CO(2), O(2), and Light on Photosynthesis and Photorespiration in Wheat. Plant Physiol. 1980 Dec;66(6):1032–1036. doi: 10.1104/pp.66.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. Rates and properties of endogenous cyclic photophosphorylation of isolated intact chloroplasts measured by CO2 fixation in the presence of dihydroxyacetone phosphate. Biochim Biophys Acta. 1976 Jan 15;423(1):91–102. doi: 10.1016/0005-2728(76)90103-1. [DOI] [PubMed] [Google Scholar]
- Marsho T. V., Behrens P. W. Photosynthetic oxygen reduction in isolated intact chloroplasts and cells in spinach. Plant Physiol. 1979 Oct;64(4):656–659. doi: 10.1104/pp.64.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miginiac-Maslow M., Champigny M. L. Relationship between the Level of Adenine Nucleotides and the Carboxylation Activity of Illuminated Isolated Spinach Chloroplasts: A Study with Antimycin A. Plant Physiol. 1974 Jun;53(6):856–862. doi: 10.1104/pp.53.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. D., Slovacek R. E., Hind G. Cyclic electron transport in isolated intact chloroplasts. Further studies with antimycin. Biochim Biophys Acta. 1978 Nov 9;504(2):298–309. doi: 10.1016/0005-2728(78)90178-0. [DOI] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Schacter B., Bassham J. A. Antimycin A Stimulation of Rate-limiting Steps of Photosynthesis in Isolated Spinach Chloroplasts. Plant Physiol. 1972 Mar;49(3):411–416. doi: 10.1104/pp.49.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Crowther D., Hind G. Relative activities of linear and cyclic electron flows during chloroplast CO2-fixation. Biochim Biophys Acta. 1980 Oct 3;592(3):495–505. doi: 10.1016/0005-2728(80)90094-8. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Influence of antimycin a and uncouplers on anaerobic photosynthesis in isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):538–542. doi: 10.1104/pp.60.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Gerbaud A., Furbank R. T. Evidence for Endogenous Cyclic Photophosphorylation in Intact Chloroplasts: CO(2) Fixation with Dihydroxyacetone Phosphate. Plant Physiol. 1983 Jun;72(2):321–325. doi: 10.1104/pp.72.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Osmond C. B. Stimulation of ammonia and 2-oxoglutarate-dependent o(2) evolution in isolated chloroplasts by dicarboxylates and the role of the chloroplast in photorespiratory nitrogen recycling. Plant Physiol. 1982 Mar;69(3):591–596. doi: 10.1104/pp.69.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziem-Hanck U., Heber U. Oxygen requirement of photosynthetic CO2 assimilation. Biochim Biophys Acta. 1980 Jul 8;591(2):266–274. doi: 10.1016/0005-2728(80)90158-9. [DOI] [PubMed] [Google Scholar]


