Abstract
Following successful school-based demonstration programs in 2014–2016, the human papillomavirus (HPV) vaccine was introduced nationwide in Senegal for 9-year-old girls in 2018, using a routine service delivery strategy at health facilities, schools, and other outreach sites. We reviewed the HPV vaccine introduction in Senegal to understand the successes, challenges, and lessons learned.
Focusing on three key domains (program decision-making, planning, and implementation), we conducted ten semi-structured interviews during 2019–2020 with purposively selected national-level stakeholders (government, expert advisory committee, key technical and implementation partners) and comprehensive desk reviews of country documents on HPV vaccine introduction.
Due to the global HPV vaccine shortage, the introduction was limited to a single-age cohort; therefore, 9-year-old girls were chosen. This strategy enabled Senegal to potentially reach more girls in primary education because school enrolment rates decline thereafter. Vaccination through routine delivery platforms (i.e., health facility, school-based, and community outreach) was perceived to be more cost-effective than a campaign approach. High-level political commitment and collaborations between immunization and education partners were frequently cited by key informants as reasons for a successful vaccine introduction. All key informants reported that the health care worker (HCW) strike, rumors, and vaccine hesitancy negatively impacted the introduction. Other challenges noted included insufficient information on attitudes towards HPV vaccination among HCWs, teachers, and community members.
Senegal successfully introduced HPV vaccine into the national immunization schedule, using a routine delivery strategy. Strong leadership and a multi-sectoral approach likely contributed to this success. To build sustainability of the HPV vaccination program in the future, it is important to improve the understanding and engagement among all stakeholders, including HCWs and community members, and to strengthen and innovate communication and crisis management strategies. To better understand the efficiency and effectiveness of Senegal’s vaccination strategy, additional assessments of the operational costs and coverage achieved are needed.
Keywords: Human papillomavirus, Vaccination, Vaccine introduction, Senegal
1. Introduction
Cervical cancer is the fourth most common cancer among women worldwide, with an estimated 604,127 new cases and over 341,831 deaths in 2020 [1]. Most (85%) of the burden of morbidity and mortality from cervical cancer occurs in low- and lower-middle-income countries, where routine cervical cancer screening and treatment are not widely available [2]. With 1876 new cases each year and 1367 deaths, cervical cancer is the most common cancer among women and the leading cause of morbidity from all cancers in Senegal [3,4].
The World Health Organization (WHO) recommends human papillomavirus (HPV) vaccination for girls aged 9–14 years to prevent cervical cancer [5]. Senegal’s successful pilot demonstration project using school-based HPV vaccination in two districts (Dakar Ouest, Mékhé), which was supported by Gavi, the Vaccine Alliance (Gavi) in 2014–2016 and a second pilot in a third district (Khombole), using a routine immunization approach, led to the nationwide introduction of the quadrivalent HPV vaccine for 9-year-old girls in October 2018 [6]. The vaccine was administered with a 6-month minimum interval between the two doses, using a routine service delivery strategy at health facilities, schools, and other outreach sites. School-based HPV vaccination was the predominant routine service delivery strategy.
Senegal was the first Gavi-supported country in West Africa to introduce the HPV vaccine into its routine immunization program. Although much has been learned from Gavi-supported pilot demonstration projects about the vaccine delivery to a target population not routinely accessing health services, there are many knowledge gaps in national introduction and scale-up of the HPV vaccine administration, especially regarding successful program decision-making, resources needed, implementation, and critical factors to ensure equity. We reviewed the introduction of the HPV vaccine into the routine immunization program in Senegal to better understand the successes, challenges, and lessons learned in program decision-making, planning, and implementation.
2. Methods
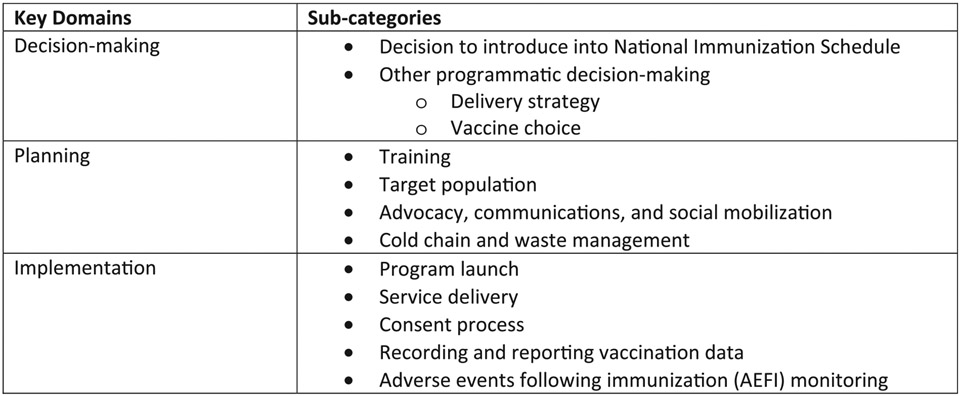
Two interviewers conducted semi-structured interviews in French with purposively selected national-level key informants (N = 10). Participation in these interviews was voluntary. The interview guidance had questions on the descriptive aspects of the program, as well as open-ended questions on successes, challenges, and suggestions for improvement, especially focusing on three key domains as shown in Fig. 1: decision-making (decision to introduce nationwide and other programmatic decision-making), planning (training, target population, advocacy, communications and social mobilization, cold chain, and waste management) and implementation (program launch, service delivery, consent process, recording and reporting vaccination data, and adverse events following immunization).
Fig. 1.
Key domains of data collection tools used in the review of the national introduction of HPV vaccination in Senegal, 2019–2020.
We also conducted a comprehensive review of planning and implementation documents and monitoring data relating to Senegal’s HPV vaccine program, as provided by the Ministry of Health and Social Action (MoHSA) and partners. These primary sources included planning tools, meeting minutes, training and social mobilization materials, program materials (data recording and reporting tools), and reported vaccination data. We observed monitoring and supervision assessments at four purposively selected health facilities and two district health offices. Sites were selected to include both urban and rural populations based on the MoHSA monitoring schedule and their proximity to Dakar. Observations included the review of available microplans, the HPV vaccine materials, and the recorded and reported data.
Data collection took place in March 2019–February 2020.
3. Ethical considerations
In accordance with the human-subjects review procedures, this program review was determined to be non-research by the Human Subjects Office at U.S. Centers for Disease Control and Prevention. Local permissions were obtained by the Senegal MoHSA staff before site visits. All key informants provided verbal informed consent. Data collected were anonymised and stored securely.
4. Results
We completed ten interviews with key informants (Nine in-person and one by telephone). These included representatives from the government (Expanded Program on Immunization, Department of Health Education and Information, Division of Non-Communicable Diseases within the MoHSA, and the Ministry of Education (MoE)), the National Technical Advisory Group for Immunization (NITAG), and key HPV technical and implementation partners (WHO, United Nations International Children’s Emergency Fund–UNICEF, United States Agency for International Development–USAID, and Program for Appropriate Technology in Health –PATH).
4.1. Decision-making
4.1.1. Decision to introduce the HPV vaccine nationwide
As related by key informants, primary drivers for the nationwide introduction of the HPV vaccine were the WHO recommendation [5], funding support for the vaccines and operational costs from Gavi, high demand for the HPV vaccine, and the high burden of cervical cancer in Senegal (Box 1). Given the WHO global recommendation [5] that girls aged 9–14 years are offered HPV vaccination and growing high-level support for an HPV vaccination program in Senegal, there was pre-existing political interest to introduce the HPV vaccine long before its eventual introduction in 2018. However, owing to a lack of domestic funds availability, the introduction was not feasible until Gavi offered financial support for the pilot demonstration projects and the national introduction. All key informants reported that the HPV vaccine introduction was regarded as a political priority in Senegal; civil societies and women’s organizations advocated for the HPV vaccine introduction, citing that it was an important preventive measure for cervical cancer, especially given the lack of cervical cancer screening and treatment. Equity was also a driving factor because the HPV vaccine was already available in the private sector. Key informants noted the scarcity of local data (the HPV burden, vaccine effectiveness, and the perception of key stakeholders) as a challenge to effective decision-making. However, the rates of cervical cancer incidence and mortality were estimated to be high per the Global Cancer Observatory [7] data. This is the preferred cancer data source for Senegal, given the lack of a comprehensive cervical cancer registry and the lack of data on the prevalence of HPV serotypes in the country.
Box 1. Considerations in the decision to introduce the HPV vaccine in the routine immunization program, Senegal 2013–2018.
Primary drivers
WHO recommendation on HPV vaccine introduction
Available GAVI support for introduction of HPV vaccine
High demand for HPV vaccine
High estimated burden of cervical cancer
Political priority
Other factors
Limited cervical cancer screening and treatment
Aim to achieve equitable access to HPV vaccine
Successful demonstration project, in terms of immunization coverage and acceptability of vaccine
Ability of the national immunization program to deliver HPV vaccine
HPV vaccine effectiveness
HPV vaccine safety
Because the NITAG was not established until 2014, an HPV vaccine pilot committee was founded in 2013 to plan for the pilot programs in 2014–2016. Senegal adopted a campaign with a school-based delivery approach for the initial pilot introduction in two districts (Dakar Ouest, Mékhé). Senegal subsequently chose to conduct a pilot in the third district (Khombole), using a routine immunization strategy, in which the HPV vaccine was offered at schools, health facilities, and community posts continuously. This routine delivery strategy proved feasibility at the pilot-scale, without increasing resource burden on the immunization delivery systems. It was noted in the desk review that the pilots successfully demonstrated the acceptability of the HPV vaccine among girls and caregivers [8], and high coverage was achieved in all districts, including the district with the routine immunization approach. Two-dose administrative coverage across the three districts was >90 percent [9]. The key informants reported that the success of these pilots gave the MoHSA and partners the confidence that a nationwide scale-up could be accomplished.
The key informants pointed out that the global evidence on the effectiveness and safety of the HPV vaccine played an important role in the decision for nationwide introduction.
4.1.2. Programmatic decision-making
MoHSA’s key partners in decisions relating to the introduction and implementation were the MoE, WHO (technical advisory role; training), UNICEF (technical advisory role; procurement; logistics), PATH (technical advisory role; global HPV introduction support) and United Nations Population Fund–UNFPA (technical advice specific to target population). Upon the government’s decision for a nationwide introduction, these partners, and other stakeholders (e.g., USAID) held a 5-day residential retreat to make decisions on the implementation strategy. Senegal used the same process for consensual decision-making among partners when planning for other vaccine introductions, and key informants generally regarded it as a strength of the country’s approach. The HPV vaccine pilot committee, established for the demonstration projects, continued the decision-making and planning in the lead-up to national introduction.
MoHSA planned the budget and made the funding decisions, in consultation with the Ministry of Finance, utilizing the WHO Cervical Cancer Prevention and Control Costing tool [10] to estimate the cost. Gavi provided financial support for the vaccine and operational costs for the introduction, and this funding was supplemented by UNICEF while government funds were required to maintain the program. Financial sustainability was addressed during the decision-making stage, and there were no anticipated funding gaps for the HPV vaccine introduction.
MoHSA, its partners, and stakeholders considered a continuous routine delivery platform (i.e., immunization offered at schools, health facilities, and during community outreach) to be the most cost-effective and more sustainable than a school-based campaign approach for the nationwide introduction. However, no local data were available on the cost of this delivery approach. Senegal’s routine immunization system was thought to be robust enough to add another vaccine, and the recent experiences with the other vaccine introductions (the pneumococcal conjugate vaccine in 2013, the rotavirus vaccine in 2014, the measles-containing vaccine second dose in 2014, the inactivated polio vaccine in 2015) informed the planning process for the HPV vaccine introduction.
The country had initially planned a multi-age cohort nationwide introduction and publicly communicated that there would be an HPV vaccination program in 2017 for girls aged 9–14 years. However, the shifting global availability of the HPV vaccine led to delays and changes to the nationwide scale-up. Due to a global HPV vaccine shortage, the introduction was postponed until 2018 and was limited to a single age cohort. Nine-year-old girls (approximately 200,000 per year) were chosen for the single-age cohort introduction so that many girls could be reached during primary school before school enrolment rates decline. This decision was also based on the ease of determining the age of the child and ensuring vaccination before sexual debut in Senegal [11]. However, the change from multi-age to single-age cohort delivery reportedly caused confusion among HCW and community members during the nationwide introduction.
Because the 9-valent vaccine was not available through Gavi support and it was not financially feasible to obtain at a non-Gavi-negotiated price, Senegal MoHSA chose to introduce the quadrivalent vaccine (Gardasil) with Gavi support and for its additional protection against anogenital warts and other anogenital cancers. Although the NITAG was not actively involved in the planning for either the pilot or the scale-up to national level, the NITAG members were consulted on specific technical questions, including the vaccine choice.
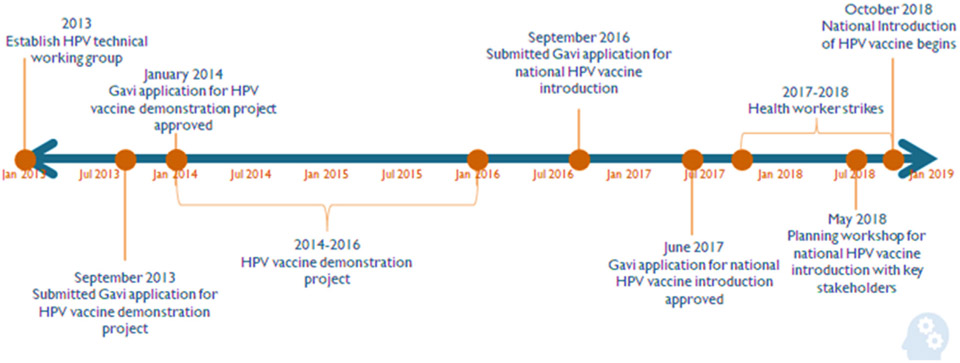
The MoHSA was successful in meeting deadlines during the decision-making and planning timeline (Fig. 2), and the key informants deemed this to be important for ensuring a timely and successful national introduction.
Fig. 2.
Senegal human papillomavirus (HPV) vaccine national introduction decision-making timeline, 2013–2018.
4.2. Planning
4.2.1. Training
Key informants reported that cascaded training was conducted nationwide at the regional and district levels. Trainees included district-level health officers, at least one HCW per facility, and at least one focal person from each school. All HPV training materials were developed in Senegal, including a comprehensive training manual. The one-day training program included seven core modules (Box 2) (HPV infection and cervical cancer, the HPV vaccine, target population and vaccination calendar, vaccine administration, data management, Adverse Events following Immunization (AEFI) management, and communication). In close collaboration with the MoE, other training materials were also created specifically for use in the education sector, including a guide on the HPV vaccine introduction for teachers and a module on primary prevention of cervical cancer for the school curriculum. All training participants were required to complete a pre-test and post-test to evaluate their knowledge. However, key informants reported that trained personnel were not always available at the sites of vaccine delivery, especially at schools, and nor were there reliable contingency plans in case of trained personnel absence. This created challenges in terms of provision of information and consistency of timely service delivery.
Box 2. Core Components of HPV Vaccination Training, Senegal, 2018–2019.
HPV infection and cervical cancer
HPV vaccine
Target population and vaccination calendar
Vaccine administration
Data management
AEFI management
Communication
4.2.2. Target population
The target population was calculated based on population estimates from the National Statistics and Demography Institute (L’Agence Nationale de la Statistique et de la Démographie). Each health facility assumed 1.26% of the catchment area population to be 9-year-old girls—the similar process used for other vaccines included in Senegal’s immunization schedule. Key informants thought that overall, the national level coverage estimates were acceptably accurate when this approach is used, despite over- or under-estimations in some districts. Enumeration of the target population was considered because it was done for the pilot in some areas [11]. However, MoHSA, the partners, and stakeholders deemed the cost and time-burden of enumeration prohibitive for scale-up to the national level.
4.2.3. Advocacy, communications, and social mobilization
An HPV vaccine communication plan was developed that included a gap analysis of the existing infrastructure, as well as an analysis of strengths, weaknesses, opportunities, and threats. The MoHSA led a general public information and advocacy campaign. Decision-makers acknowledged even before the pilots that immunizing girls against cervical cancer and other sexually-transmitted infections would be a delicate issue in certain Senegalese cultures [11]. Reported strengths of the country’s approach included regular early planning meetings and the timely preparation of communication tools. Information on the national introduction of the HPV vaccination was disseminated through the press, radio, and television. The immunization program developed leaflets, banners, posters, and flyers (Fig. 3) for nationwide distribution. Well-known technical experts in Senegal publicly demonstrated their support for the HPV vaccine. The responsible persons at the district and health facility levels coordinated advocacy meetings with the local stakeholders including community and religious leaders.
Fig. 3.
Communication tools used in national introduction of human papillomavirus (HPV) vaccination in Senegal, 2018–2019.
Key informants reported that lessons learned from the pilots helped the decision-makers to anticipate communication challenges, for example in tackling resistance from parents in urban areas, such as Dakar, and in certain educational facilities, such as private schools. However, key informants reported that there was insufficient local data available on attitudes towards the HPV vaccination among key stakeholders (HCWs, teachers, and community members) before the national introduction and the country did not finalize a crisis communication plan. Key informants reported that the necessary funds were not available for the communication efforts required, and social media communication routes were not fully leveraged.
4.2.4. Cold chain and waste management
Senegal had completed a readiness assessment of the cold chain and waste management across the immunization program in 2015 and developed a 2016–2020 cold chain and logistics improvement plan. The plan highlighted the existing gaps in cold chain and waste management at the central, regional, and district levels. Health Systems Strengthening funds from Gavi were used to address the identified gaps (e.g., replacing cold storage) as needed during 2017. The national introduction of the HPV vaccine did not require any additional changes in this program area.
4.3. Implementation
4.3.1. Program launch
The national launch ceremony was held on October 31, 2018, in Diamniado with the President of Senegal, 15 African First Ladies, and 20 African Ministers of Health in attendance. This high-level initiative was regarded by key informants as a strength of the program launch.
However, there were significant challenges with unanticipated issues of vaccine hesitancy and vaccine refusals across Senegal after the launch. A HCW strike because of compensation and working conditions had been going on since March 2018, and it was intensified from October to December 2018, resulting in a HCW boycott of providing immunizations, including the HPV vaccination. HCWs reportedly participated in the propagation of misinformation regarding the HPV vaccination (e.g., false information regarding the adverse events following HPV vaccination and rumors regarding vaccine-related infertility) through interpersonal communications and across social media platforms, such as WhatsApp. All key informants reported that the HCW strike and rumors contributed to the early challenges with HPV vaccine hesitancy, which negatively impacted the introduction and program performance. Although the HCW strike had started before the launch, the country program decided to go ahead with the introduction, given the insecurities around the vaccine supply.
Key informants reported a strong response to remedy vaccine hesitancy at the national level, including implementing a revised communication plan, involvement of community and religious leaders, revival meetings in poorly performing regions, and increased communication efforts to the target population and caregivers through various channels (social media, written press, radio, and television). Yet, there were ongoing difficulties with vaccine hesitancy in Senegal for many months post-introduction, and observations from monitoring and supervision visits at six months post-launch highlighted that many health facilities did not initiate the HPV vaccination activities owing to community hesitancy and, in some cases, resistance. Nevertheless, by July 2019, according to the reported data, more than 100,000 HPV vaccine doses had been administered and almost three quarters of districts were reported to have achieved 80% coverage of the target population with the first dose of HPV vaccine. However, uncertainties were acknowledged regarding the estimated target population with many districts reporting coverage greater than 100%.
4.3.2. Service delivery
Most vaccinations are provided in schools; this is considered the most effective and efficient strategy to reach the highest number of girls, using a continuous routine delivery approach. The focal person at the school listed the 9-year-old girls and liaised with the local health center to arrange a vaccination session. Frequency of these school-based vaccination sessions depended on the health center and school, but many schools were reported to host the vaccination sessions on a quarterly basis. Key informants noted to reflect that the HPV vaccine delivery platform is an opportunity to integrate other adolescent health interventions, but is not being used as such deworming, iron supplementation [11], or other cervical cancer prevention and treatment initiatives for mothers.
Key informants reported several challenges in school-based vaccination, such as absentee girls, vaccination session delays during school vacations, inaccuracies between the date of birth and age listed in school lists. Absentee girls were advised to visit the local health post or health center for vaccination; however, this has not been consistently recorded and monitored. Community health workers worked to sensitize the community and identify out-of-school girls for vaccination during the outreach activities.
4.3.3. Consent process
School principals and teachers were responsible for informing the parents and guardians of female students about the HPV vaccination alongside the public information campaign conducted by MoHSA. Hence, an “opt-out” approach to consent was adopted at most schools, whereby parents and guardians or the girls themselves, had the option to refuse the vaccination. These refusals were documented; however, none of the key informants were aware of any efforts to use refusal data to improve coverage. Some private schools rejected the “opt-out” approach and created their own written consent forms.
4.3.4. Recording and reporting vaccination data
Senegal developed its own HPV recording tools based on tools used during the pilot program. All vaccination doses were recorded in the health facility register, which includes the name of the child and place of vaccination. An HPV tally sheet had to be completed for every vaccination session. Following the first dose, the HCW kept the home-based HPV vaccination card at health facilities. The vaccination card was subsequently given to the girl recipient following the second dose of the HPV vaccine. The reporting system for HPV vaccination utilized the existing health information system with no special tracking for the HPV vaccine. Each health facility completed a monthly report of routine vaccinations administered, including the HPV vaccine, by dose, strategy (e.g., fixed, mobile), and age category of the child. This monthly report was submitted on paper to the district level or entered directly into the electronic health information system (District Health Information Software 2–DHIS2). Key informants noted that achieving a high 2-dose coverage for all girls could be a challenge without having a clear strategy to ensure the receipt of the second dose.
4.3.5. AEFI monitoring
No changes were made to the existing AEFI monitoring system for the HPV vaccine introduction. Standard forms were available for HCWs to report AEFIs. At the time of this data collection, no AEFIs had been reported.
5. Discussion
High-level political commitment and multi-sectoral collaboration between partners, especially between the health and education sectors, were frequently cited as reasons for successful HPV vaccine introduction in Senegal—a finding consistent with other countries’ experiences [12-16]. Senegal’s experience and lessons learned during the pilot demonstration projects and other new vaccine introductions helped the country to accomplish the nationwide scale-up of the HPV vaccination, both from the point of the technical considerations and a well-executed project management. This is critical for a successful new vaccine introduction to ensure that it is high quality, timely, and within budget [13]. Furthermore, though Senegal required support for the introduction and HPV vaccine, the country was able to rely upon its existing processes and infrastructure (e.g., vaccination recording and reporting, AEFI recording and reporting, cold chain systems); hence, it did not need additional investments. However, how this translates to sustainability and coverage is yet to be understood.
All key informants reported that the HCW strike negatively impacted the introduction. Previous studies have shown that adolescents who receive a provider’s recommendation have substantially higher odds of initiating the HPV vaccination compared to those who do not [17]. In Senegal, the providers were on strike at the time of the introduction and for several subsequent months. Additionally, the providers reportedly propagated negative information about the vaccine through interpersonal communication channels and social media tools, such as WhatsApp. Furthermore, reportedly, the parents and teachers were resisting the HPV vaccine introduction in their communities. Other countries introducing HPV vaccine have experienced similar challenges [18,19], however these vaccine hesitancy challenges were unprecedented in Senegal and largely unanticipated, thus highlighting the need for more engagement with key stakeholders (HCWs, teachers, and community members) before scaling up to the national level to gain a better understanding of the attitudes towards HPV and the HPV vaccination. Adequate information should be provided to the HCW to enable them to support appropriate messaging and answer caregiver questions; efforts should be increased to build population resilience against vaccine rumors through ongoing vaccination advocacy activities [20]. A properly funded communication strategy, including a social media plan, as well as a crisis management plan, are essential prerequisites for any new vaccine introduction. It is also notable that the country chose to move forward with the vaccine introduction despite the presence of the HCW strike due to the global vaccine supply insecurity.
Vaccination through routine delivery platforms, primarily school-based delivery, was perceived to be more cost-effective than a campaign approach in Senegal. Other countries in the African Region, such as Tanzania, have similarly had success with introducing HPV into the routine immunization schedule [15], though the longer term 2-dose coverage and cost implications are as yet unknown. Factors favoring a high coverage with this approach in Senegal include the strength of the immunization program and the high rate of primary school enrollment. More data are required to understand how successful and sustainable Senegal’s strategy will be. The expertise of the NITAG can be better leveraged in the planning process and to support ongoing HPV program monitoring, including coverage and safety, and to provide evidence-based recommendations for program strengthening. A separate piece of this evaluation included an analysis of cost. However, the lack of burden data before the introduction makes impact monitoring a challenge; therefore, a future coverage survey will be important to assess whether the country is achieving the global cervical cancer elimination goal to fully vaccinate 90% of girls by age 15 years [21] given the anticipated challenges with reaching girls for the second dose and the uncertainties regarding the estimated target population. Initiating or strengthening the existing adolescent health interventions via ongoing collaboration with partners (e.g., MoE) is vital.
Our evaluation has potential limitations. We selected ten key national-level stakeholders fundamental to the program decision-making, planning, and implementation processes. However, our findings are limited by the select purposive sampling and possible social desirability bias from informant interviews.
6. Conclusions
Key informants indicated the strong leadership and a multi-sectoral approach contributed to the success of the HPV vaccine introduction in Senegal. To build sustainability of the HPV vaccination in the future, it will be important to better understand and engage all stakeholders, including HCWs, teachers, and caregivers. New vaccine introduction requires a robust communication and crisis management plan with a multi-pronged approach. Additional assessments of the operational costs and coverage would be helpful to better understand the efficiency and effectiveness of Senegal’s vaccination strategy.
Key points.
This manuscript describes the successes, challenges, and lessons learned from the national introduction of human papillomavirus (HPV) vaccination in Senegal focusing on three key domains (program decision-making, planning, and implementation).
Acknowledgements
The authors wish to acknowledge the contributions to the design and implementation of this evaluation by the Government of Senegal including the Ministry of Health and Social Action, and the Ministry of Education; partner organizations including PATH, UNICEF, and WHO; and all the evaluation’s key informants.This work was supported by Gavi, the Vaccine Alliance [“Evaluation of Human Papilloma Virus (HPV) Vaccine National Introduction in Low-and-Lower-Middle Income Countries” - Contract No. ME 9422 12 20].
Footnotes
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position, policies, or views of the U.S. Centers for Disease Control and Prevention or the World Health Organization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data statement
I am not linking or uploading my research data. Key informants could be identifiable by their comments and observations during interviews, however all recorded data were anonymized and stored securely accessible only to the evaluation team.
References
- [1].International Agency for Research on Cancer. Cervix uteri cancer fact sheet; 2020. https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf..
- [2].Randall TC, Ghebre R. Challenges in prevention and care delivery for women with cervical cancer in sub-Saharan Africa. Front Oncol 2016;6:160. 10.3389/fonc.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX de SSI, Centre). IC on H and C (HPV I. Human papillomavirus and related diseases in the world. Summary Report; 2019. [Google Scholar]
- [4].International Agency for Research on Cancer. Senegal cancer fact sheet; 2018.
- [5].World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec 2017;19:241–68. [Google Scholar]
- [6].Government of Senegal. Application form for Gavi new vaccine support; 2016.
- [7].Global Cancer Observatory; n.d. https://gco.iarc.fr/ [accessed September 20, 2020].
- [8].Ministere de la Sante et de l’Action Sociale. Rapport de l’Evaluation Post-Introduction du Vaccin contre le Papillomavirus Humain R (VPH) dans les districts de Mekhe et de Dakar-Ouest au Senegal; 2015.
- [9].Ministere de la Sante et de l’Action Sociale. Introduction du vaccine contre le cancer du col de l’uterus dans le programme elargi de vaccination; 2018.
- [10].World Health Organization. WHO cervical cancer prevention and control costing (C4P) tool. WHO; 2020. [Google Scholar]
- [11].Gavi. Proposal for HPV Demo support: Senegal; n.d. https://www.gavi.org/news/document-library/proposal-hpv-demo-support-senegal-0.
- [12].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Griffiths UK, Feletto M, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middleincome countries. PLoS ONE 2017;12. 10.1371/journal.pone.0177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scotney S, Snidal S, Saidu Y, Ojumu A, Ngatia A, Bagana M, et al. Succeeding in new vaccine introduction: lessons learned from the introduction of inactivated poliovirus vaccine in Cameroon, Kenya, and Nigeria. J Infect Dis 2017;216:S130–6. 10.1093/infdis/jiw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buang SN, Ja’afar S, Pathmanathan I, Saint V. Human papillomavirus immunisation of adolescent girls: Improving coverage through multisectoral collaboration in Malaysia. BMJ 2018;363. 10.1136/bmj.k4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tanzania: National introduction of human papillomavirus (HPV) vaccine in Tanzania: programmatic decision-making and implementation; n.d. https://www.sciencedirect.com/science/article/pii/S0264410X21004722?via%3Dihub. [DOI] [PMC free article] [PubMed]
- [16].Nationwide Introduction of HPV Vaccine in Zimbabwe 2018–2019: Experiences with multiple cohort vaccination delivery; n.d. https://www.sciencedirect.com/science/article/pii/S0264410X21006691#!. [DOI] [PMC free article] [PubMed]
- [17].Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systematic review. Hum Vaccines Immunother 2016;12:1454–68. 10.1080/21645515.2015.1129090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: the global response to Japan’s suspension of its HPV vaccine recommendation. Hum Vac Immunother 2014;10:2543–50. 10.4161/21645515.2014.969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larson HJ, Brocard P, Garnett G. The India HPV-vaccine suspension. Lancet 2010;376:572–3. 10.1016/S0140-6736(10)60881-1. [DOI] [PubMed] [Google Scholar]
- [20].World Health Organization. Vaccination and trust. 2017; n.d. https://www.euro.who.int/__data/assets/pdf_file/0004/329647/Vaccines-and-trust.PDF [accessed December 13, 2020].
- [21].World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem; n.d. https://www.who.int/publications/i/item/9789240014107 [accessed July 21, 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I am not linking or uploading my research data. Key informants could be identifiable by their comments and observations during interviews, however all recorded data were anonymized and stored securely accessible only to the evaluation team.