Abstract
Photobiomodulation therapy (PBMt) combined or not with oral hypoglycemic medication has not been investigated in type 2 diabetes (T2DM) patients. All ten T2DM patients were assessed randomly at six different occasions (3 with and 3 without regular oral hypoglycemic medication). Capillary glycemia was assessed after overnight fast (pre-prandial), 1h postprandially (standardized meal, 300 kcal), and 30 min, 3h, 6h, 12h post PBMt (830 nm; 25 arrays of LEDs, 80 mW/array). Three doses (0 J – sham, 100 J, 240 J per site) were applied bilaterally on quadriceps femoris muscles, hamstrings, triceps surae, ventral upper arm and forearm; and randomly combined or not with oral hypoglicemic medication, totaling six different therapies applied for all ten TDM2 patients (PBMt sham, PBMt 100 J, PBMt 240 J, PBMt sham + medication, PBMt 100J + medication, PBMt 240J + medication). Cardiac autonomic control was assessed by heart rate variability (HRV) indices. Without medication, there was reduction in glycemia after all PBMt doses, with 100 J as the best dose that persisted until 12 hours and presented lower area under the curve (AUC). With medication, glycemia decreased similarly among doses. No differences between 100 J and sham + medication, but AUC was significantly lower after 100 J, suggesting better glycemic control. Low frequency component of HRV increased after sham + medication and 100 J, suggesting higher sympathetic activation. PBMt showed time- and dose-response effect to reduce glycemia in T2DM patients. Effects on HRV were consistent with glycemic control.
Keywords: dose-response, glycemic control, heart rate variability, hypoglycemic drugs, insulin resistance, photobiomodulation therapy
Graphical Abstract
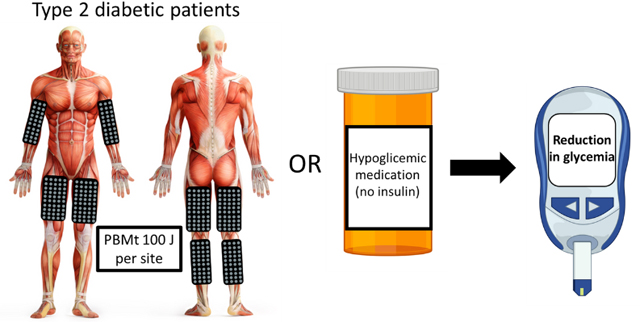
Photobiomodulation therapy can reduce the hyperglycemia and affect the autonomic cardiac autonomic control in Type 2 Diabetes Mellitus patients with a dose and time-response.
1. INTRODUCTION
Type 2 Diabetes Mellitus (T2DM) is the most common type of DM and accounts for approximately 90–95% of cases [1]. T2DM is characterized by insulin resistance, especially in the liver, skeletal muscle and adipose tissue, although many other tissues are also affected by this condition [2]. Pancreatic beta cell dysfunction is also a hallmark of T2DM, where insulin production becomes insufficient to allow the metabolism of glucose in the face of increased insulin resistance [2]. The result is chronic hyperglycemia that (if not controlled) causes damage to blood vessels. The development of macro and microvascular complications can lead to diabetic retinopathy, neuropathy, nephropathy, coronary heart disease, and cerebrovascular disease [3]. In 2019 there were 463 million cases of DM worldwide, which were predicted to rise to 548 million by 2045 [4]. Thus, new approaches to hypoglycemic therapy for T2DM are urgently needed.
In addition to the complications already mentioned, it has been shown that patients with DM suffer from cardiac autonomic dysfunction, which is associated with an increased risk of death in patients with DM [5–8]. Although the mechanisms responsible for cardiac autonomic dysfunction in DM need to be fully elucidated, poor glycemic control, hyperglycemic activation of the polyol pathway, protein kinase C activation, and increased levels of oxidative and nitrosative stress are some factors that may contribute to the development and progression of autonomic neuropathy [9, 10].
Although injections of insulin might eventually be needed, oral hypoglycemic medications are usually the first line of pharmacological treatment in T2DM, along with lifestyle changes [11]. There are many different classes of oral hypoglycemic medications, and their mechanisms of action differ considerably. For instance, metformin is the most frequently prescribed oral medication to reduce hyperglycemia worldwide, and is the first line treatment for pre-diabetic individuals as well as T2DM patients [11]. Even though the mechanisms of action are not fully understood [12], there is evidence that metformin reduces postprandial hepatic glucose production, as well as increasing peripheral insulin sensitivity. Sulfonylureas are the second line of hypoglycemic medications prescribed for T2DM patients [13]. The mechanism of action involves increasing insulin release by depolarizing the pancreatic beta cells through blockage of potassium inflow by ATP-dependent ion channels, which in turn allows the diffusion of calcium into the cytosol, and contraction of the actomyosin filaments responsible for the secretion of insulin [14, 15].
Recently some other hypoglycemic drugs have been developed, and are often prescribed to T2DM patients, such as dipeptidyl peptidase 4 (DPP-4) inhibitors [16] that prevent the degradation of incretin hormone, thus increasing postprandial insulin release. Moreover, sodium-glucose cotransporter-2 (SGLT2) inhibitors [17], can inhibit SGLT-2 in the proximal tubules of the renal glomeruli, thus reducing glucose reabsorption by 90% and producing glycosuria. Other hypoglycemic drugs prescribed to T2DM patients include meglitinides, thiazolidinediones, glucagon-like peptide-1 receptor agonists, and α-glucosidase inhibitors [18, 19]. Notwithstanding the hypoglycemic effect of these medications, they can lose effectiveness over time [20] resulting in the need for higher doses, with more side-effects such as hypoglycemia, gastrointestinal disturbance, nausea, and renal impairment [21]. These side effects means that there is often low adherence to these pharmacological regimens [22]. Altogether, this evidence points to the need to discover other hypoglycemic therapies, combined or not with conventional medications.
It is an undisputed fact that physical exercise is highly beneficial for the prevention and treatment of T2DM [23]. Unfortunately, the adherence of patients to the recommended amount of exercise is often a challenge [24–26]. For this reason, it is very important to investigate the effects of other approaches to improve glycemic control in T2DM patients. In this regard, photobiomodulation therapy (PBMt) has shown some promising results. PBMt involves the application of low levels of red and/or near infrared light to various parts of the body. PBMt is commonly used to reduce pain and inflammation, and to stimulate healing, but can also increase muscle metabolism. In some situations, PBMt has been described as acting like an “exercise mimetic”.
Many studies using animal models have shown improved glucose metabolism in response to PBMt [27–35], and one clinical trial [36] has supported the potential clinical use of PBMt for improving glucose metabolism. PBMt is known to have a biphasic dose-response [37, 38] which is responsible for stimulatory, inhibitory, or even null biological effects. Recently, using a randomized, crossover, double-blind, sham-controlled trial our group [36] reported a dose-response effect of PBMt on glycemia in T2DM patients, where we showed that low and moderate-to-high, but not moderate or high, doses of PBMt reduced glycemia. Furthermore, there is evidence that PBMt effects are also time-dependent [39, 40]. However, in our previous study [36], we only assessed glycemia for 15 min after PBMt irradiation, which might have been too short an interval to reveal the full potential of PBMt to reduce glycemia. Furthermore, we did not investigate the combination of PBMt plus conventional medication, as in that study there was not a “no-medication” arm. As there is evidence that the combination of oral hypoglycemic medications with other hypoglycemic approaches, such as physical exercise, oftentimes produces counterintuitive results [41–44], it is of great importance to investigate the combined effects of PBMt and these commonly prescribed drugs on glycemia in T2DM patients. Thus, clinical studies on the combination of PBMt plus oral hypoglycemic medications are lacking and urgently needed [45].
Therefore, the primary aim of the present study was to investigate the dose-response and time-response effects of PBMt on glycemic control in T2DM patients, combined or not with their usual oral hypoglycemic medications. A secondary aim was to assess the acute effect of PBMt on cardiac autonomic control.
2. MATERIALS AND METHODS
2.1. Subjects
A total of 10 patients previously diagnosed with T2DM at least 12 months before enrolment were invited to participate in the study. In order to be eligible, patients had to be 40 to 80 years-old, with a body mass index between 20 and 39.9 kg/m2 and have taken oral hypoglycemic medication for at least 6 months. Patients were excluded if they had a history of cancer or currently had cancer, acute infections, neurological deficits, coagulation disturbances, or were receiving insulin treatment. Finally, patients had to be approved by their personal physician to take part in the study. Before participation, after being made fully aware of the study’s risk and benefits, patients signed a written informed consent form previously approved by the local Institutional Review Board (opinion #1.959.414). The study protocol was prospectively registered in the Brazilian Clinical Trials Registry, under the number RBR-38wn44 (https://ensaiosclinicos.gov.br/rg/RBR-38wn44). All procedures of this study followed current Brazilian laws regarding human studies and were in accordance with the ethical principles outlined in the Declaration of Helsinki. Participant characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics
| Sex (F/M) | 7/3 |
| Skin phototypes (Fitzpatrick scale) | |
| Type II (n) | 6 |
| Type III (n) | 3 |
| Type IV (n) | 1 |
| Age (years) | 63.10 ± 8.34 |
| Body mass (kg) | 78.84 ± 16.30 |
| Height (m) | 1.57 ± 0.11 |
| Body mass index (kg/m2) | 31.81 ± 5.09 |
| Time since diagnosis (years) | 6.1 ± 3.6 |
Data shown as mean ± SD.
2.2. Study design
This was a randomized, crossover, double-blind, sham-controlled clinical trial. Randomization was performed by a researcher blinded to the treatment protocol using sequentially numbered, opaque sealed envelopes.
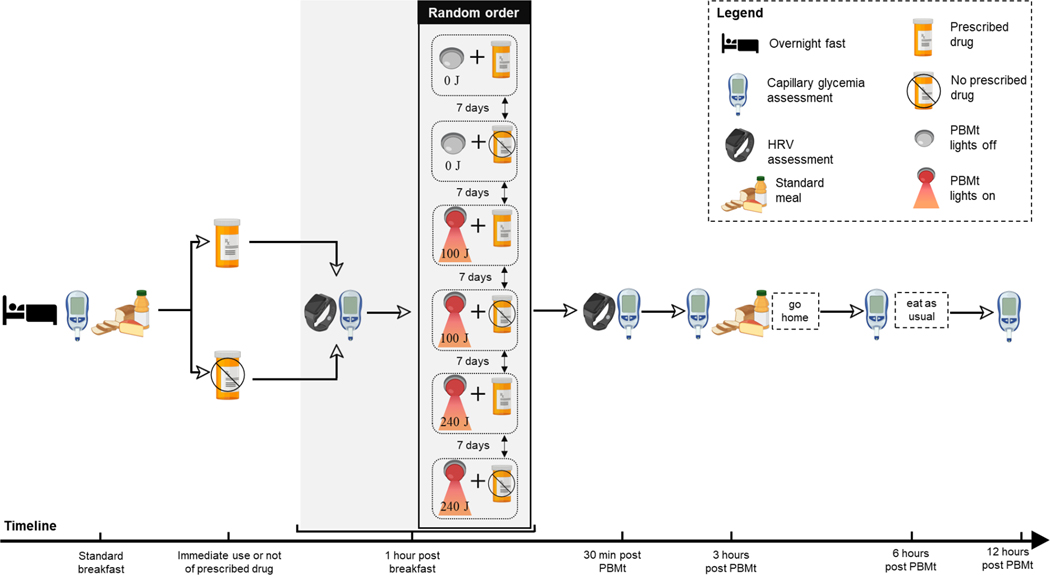
On six different occasions, interspersed by 7 days to allow washout from the previous session, all patients reported to the laboratory in the morning after an overnight fast without having taken their medication. Fasting capillary glycemia (pre-prandial) was first assessed, followed by ingestion of a standardized breakfast (2 slices of whole grain bread, 200 mL of natural fruit juice, 2 slices of cheese, 338 kcal, Carbs: 39 g, Protein: 18.9 g, Fat: 11.8 g). After that, in randomized order, all patients received all the 6 different treatments purposed in the present study: PBMt sham, PBMt 100 J, PBMt 240 J, PBMt sham + medication, PBMt 100 J + medication, PBMt 240 J + medication) sequentially separated by a washout period of 7 consecutive days. Thus, on 3 occasions the patients either took their oral hypoglycemic medication as prescribed by their physician, or on the other 3 occasions the patients refrained from taking their oral hypoglycemic medication for the next 24 hours (Figure 1).
Figure 1.
Study design. PBMt – photobiomodulation therapy; HRV – heart rate variability.
One-hour after breakfast (postprandial), heart rate variability (HRV) and capillary glycemia were assessed, followed by PBMt (100 J or 240 J per site of irradiation) or sham (0 J) to eight anatomical sites described below. Thirty-minutes, 3, 6 and 12 hours after PBMt, the capillary glycemia was reassessed. HRV was reassessed 30-min after PBMt. During the time window of 12 hours of the study, the patients were instructed to rest, i.e., not to engage in any type of exercise.
After the 3-hour glycemia assessment, the same standardized meal described above was provided to patients, then they were dismissed from the laboratory and instructed not to ingest anything more until the 6-hour glycemic assessment, and to rest. Between the 6-hour and the 12-hour assessments patients were allowed to consume their usual diet and instructed also to rest. Thus, the glycemic assessments at 6-hour and the 12-hour timepoints were performed by the patients outside the laboratory and the results were shared with the researchers. Figure 1 depicts the study design.
2.3. Pre-participation assessment
Patients reported to the laboratory, completed a medical history questionnaire, and had their body weight and height measured, and their body mass index calculated. During that visit, they were also familiarized with the study protocol.
2.4. Oral hypoglycemic medications
All 10 patients were receiving metformin treatment, and 5 (50%) were exclusively taking metformin. Three patients were taking a combination of metformin and sulfonylureas (2 on Glibenclamide and 1 on Gliclazide). One patient was taking a combination of metformin, sulfonylurea (Gliclazide), and SGLT2 inhibitor (Sitagliptin), while another one was taking metformin, DPP-4 inhibitor (Sitagliptin) and SGLT2 inhibitor (Empagliflozin).
During the investigation of the isolated effects of PBMt, the use of oral hypoglycemic medications was suspended for 24 hours before and after the application of sham or real photobiomodulation, but with medical consent and supervision. To guarantee the patient’s safety, they were monitored for glycemic levels at the following timepoints: before standardized breakfast; 1 hour after standardized breakfast (pre PBMt); 30 minutes, 3 hours, 6 hours and 12 hours after PBMt; in total there were 6 monitoring timepoints over 24 hours. If blood glucose levels reached ≥ 250 mg/dL at the first measurement after the standardized breakfast, or after lunch or dinner, blood glucose was monitored every 2 hours and, if the blood glucose level was maintained at a high level for two consecutive hours [21], patients were instructed to make immediate use of their hypoglycemic medication(s). In the present study, no participants had blood glucose levels ≥ 250 mg/dL for two consecutive hours, thus there was no need to take additional medication in this situation in any arm of the study. Table 2 lists hypoglycemic medication classes, daily dosage, and the number of patients receiving treatment.
Table 2.
Oral hypoglycemic medication used by patients
| Class | Daily dosage (mg) Mean ± SD (min-max) |
Patients under treatment # (%) |
|---|---|---|
| Biguanides | ||
| Metformin | 1,605 ± 743 (500–2,550) | 10 (100) |
| Sulfonylureas | ||
| Glibenclamide | 33 ± 18 (15–50) | 2 (20) |
| Gliclazide | 60 ± 0 (NA) | 2 (20) |
| DPP-4 inhibitors | ||
| Sitagliptin | 100 ± 0 (NA) | 1 (10) |
| SGLT2 inhibitors | ||
| Empagliflozin | 25 ± 0 (NA) | 2 (20) |
2.5. Capillary glycemia assessment
Handheld glucometers (Accu-Chek Active, Roche®) were used individually by the patients to self-assess capillary glycemia by pricking the fingertip. Immediately after assessment the value was registered by a researcher blinded to the treatment. For the 6-hour and 12-hour assessments, the glycemia value was transferred by the patient via email or text message to the same researcher. All the glucometers of the study were calibrated by the manufacturer and calibrated during the study at each new pack of strips following manufacturer’s recommendations. There was no problem (bad working or loss of calibration) related to any of the glucometers used in the study. All patients enrolled in the study had previous experience in self-assess capillary glycemia to control own diabetes.
2.6. Heart rate variability measurement
This procedure was used to evaluate cardiac autonomic control at rest in the supine position for 15 minutes, and at rest in the orthostatic posture for 15 minutes. Heart rate (HR) records in beats per minute and R-R intervals (iR-R) were collected beat by beat, using a Polar® Frequency Meter model RS800CX (Electro Oi, Finland), for 15-min. Data were captured from a belt with a coded transmitter, placed around the chest region, at the level of the 5th intercostal space and later transferred through an interface to a compatible computer. During the collection period, the volunteers were instructed to maintain normal breathing. We monitored the volunteers to maintain a respiratory rate between 10 and 20 breaths per minute.
For the analysis of HRV, the periods of higher signal stability were selected, which included at least 256 consecutive heart beats [46]. The analysis was made from a linear model in the frequency domain, through spectral analysis: low frequency (LF), high frequency (HF) in normalized units (un), and by the ratio between low and high frequency LF /HF [46]. The HRV was also analyzed by a non-linear model from Shannon entropy and corrected conditional entropy analysis, providing complexity indices of the analyzed iR-R time series [47] such as SDNN (standard deviation of all normal iR-R intervals recorded in milliseconds) and RMSSD (square root of the mean square of the differences between consecutive iR-R expressed in milliseconds), and from the symbolic analysis [48, 49], which makes it possible to quantify the sympathetic and parasympathetic components of autonomic HR modulation. HRV data collection was performed before and 30 min after PBMt, and analysis was conducted by a single researcher blinded to PBMt.
2.7. Photobiomodulation therapy
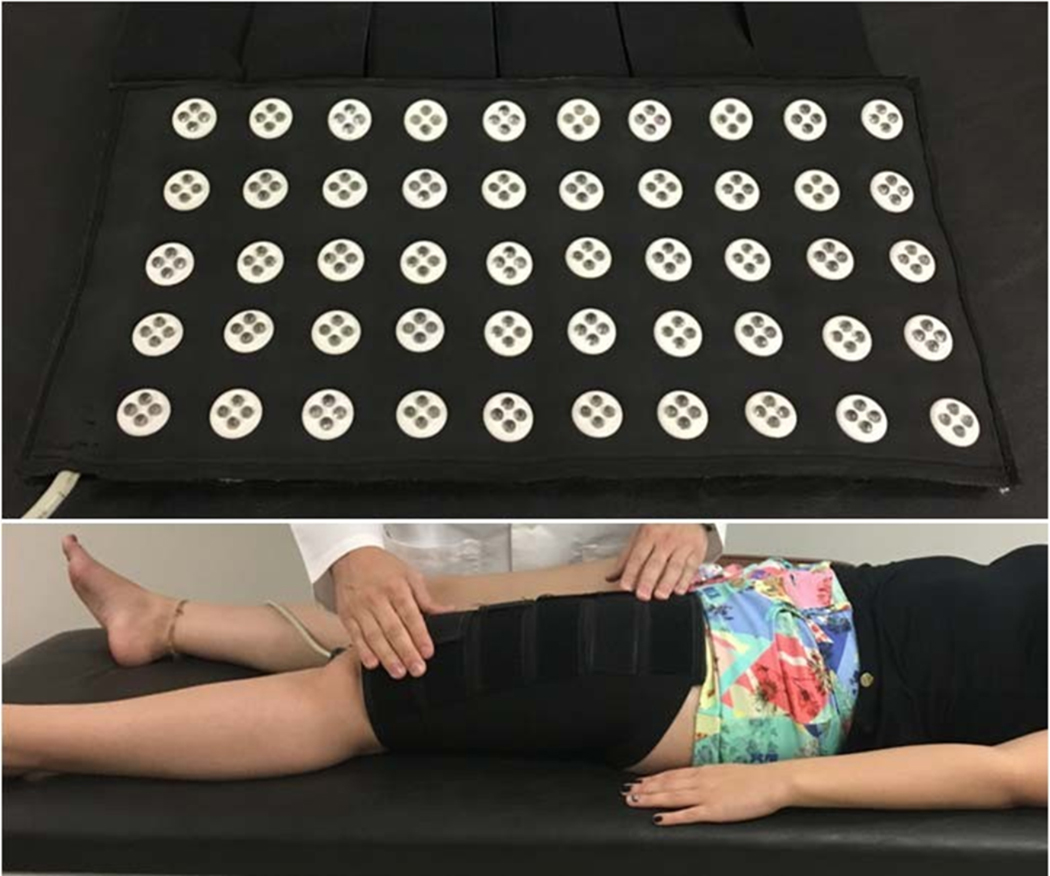
PBMt used low-intensity light emitting diodes (LEDs) contained in a non-commercial array of 200 light emitters (prototype) (Figure 2). This prototype contained 100 LEDs that emit light in the red range (630 ± 10 nm) and 100 LEDs in the infrared range (830 ± 20 nm) arranged in 50 arrays of 4 LEDs each (25 red arrays, 80 mW each; 25 infrared arrays, 80 mW each) distributed on a flexible surface (34×18 cm; 612 cm2) that could be molded to the body surface [50]. Table 3 lists the optical parameters of the light source, as well as the PBMt irradiation parameters, which were calibrated and measured using a power and optical energy meter (PM100D Thorlabs®) equipped with a S130C sensor (area of 0.70 cm2).
Figure 2.
Light-emitting device composed of 200 low-intensity LEDs (light-emitting diodes), containing 25 arrays of 4 LEDs in the red range and 25 arrays of 4 LEDs in the near-infrared range. Only near-infrared light was used in this study. Example of irradiation on the quadriceps femoris muscles.
Table 3 -.
Optical parameters of the light-emitting device and photobiomodulation therapy using LEDs (light-emitting diodes)
| Number of LEDs | 200 (100 infrared and 100 red) |
| Wavelength | 830 ± 20 nm (infrared) and 630 ± 10 nm (red) |
| Frequency | Continuous |
| Optical power (each array of 4 LEDs) | 80 mW (infrared) and 80 mW (red) |
| Total device power | 2 W (only infrared turned on) |
| Area of each LED | 0.2 cm2 |
| Device emission area | 612 cm2 |
| Irradiance (each array of 4 LEDs) | 114.28 mW/cm2 (infrared) and 0 mW/cm2 (red) |
| Irradiation time per each site (sec) | 30, 50, 120 |
| Total energy per site (30 s) | 0 J [infrared 0 J (25 × 0 J); red 0 J (25 × 0 J)] |
| Total energy per site (50 s) | 100 J [infrared 100 J (25 × 4 J); red 0 J (25 × 0 J)] |
| Total energy per site (120 s) | 240 J [infrared 240 J (25 × 9.6 J); red 0 J (25 × 0 J)] |
| Number of sites | 8 (four muscle groups on each side) |
| Total energy per session (30 s) | 0 J |
| Total energy per session (50 s) | 800 J |
| Total energy per session (120 s) | 1,920 J |
| Fluence – 30 s | 0 J/cm2 (infrared) and 0 J/cm2 (red) |
| Fluence – 50 s | 5.71 J/cm2 (infrared) and 0 J/cm2 (red) |
| Fluence – 120 s | 13.71 J/cm2 (infrared) and 0 J/cm2 (red) |
| Method of application | Device in contact with the skin, at 90° and with light pressure |
PBMt using LEDs was applied (wrapping) to eight separate sites on the body, bilaterally on the quadriceps femoris muscles, hamstrings, triceps surae, and ventral upper arm and forearm muscles, similarly to previous studies [50–52]. PBMt was performed according to the irradiation time (30, 50 or 120 seconds) on each site in a random order. The sham treatment did not emit light (0 J and 0 mW), while the light-emitting device recorded the therapy time. It is important to point out that the light in the red range was not used, remaining off during the entire therapy by means of a selector switch on the equipment itself. During irradiation, both subjects and the therapist wore protective eyeglasses and were unaware of real or sham.
PBMt was performed by only a single evaluator throughout the study in a double-blind, randomized, crossover and sham manner. That is, each patient (with hypoglycemic medication or not) received all 3 doses of PBMt randomly, including the sham dose, and without knowing which light dose was delivered. Also, the other study evaluators were blinded to the order of PBMt treatments until the end of the study.
2.7. Statistical analysis
Outcome variables were analyzed for normal distribution using the Shapiro-Wilk test. Then, the data were analyzed using analysis of variance tests (two-way ANOVA) of 6×6 repeated measures (6 different treatments: 3 doses of PBMt without medication, 3 doses of PBMt combined with medication) and 6 levels or repeated measures (pre-prandial, 1 hour postprandial (pre intervention); 30 minutes, 3 hours, 6 hours, and 12 hours after PBMt). Also, the deltas of glycemic variation were compared between the PBMt groups without medication at each evaluation time of the present study also by two-way ANOVA with repeated measures 3×5 (1-hour postprandial minus pre intervention; 30 minutes post PBMt minus pre-intervention; 3 hours post PBMt minus pre-intervention; 6 hours post PBMt minus pre-intervention; and 12 hours post PBMt minus pre-intervention). The same analysis was applied to PBMt groups combined with medication. Area under the curve (AUC) was calculated according to the trapezoidal rule, and analysis was performed by one-way ANOVA. Finally, an independent t-test was used to compare the best results between PBMt alone and PBMt combined with medication. HRV was analyzed using Wilcoxon’s test with Bonferroni correction, as the data were not normally distributed. For all analyses, a significance level of 5% was adopted. The intention-to-treat analysis was used in this study to input values (used the average of the measure) for only 4 missing data points. There were no participants who dropped out or who failed to follow-up.
3. RESULTS AND DISCUSSION
3.1. Pre-prandial and postprandial glycemia
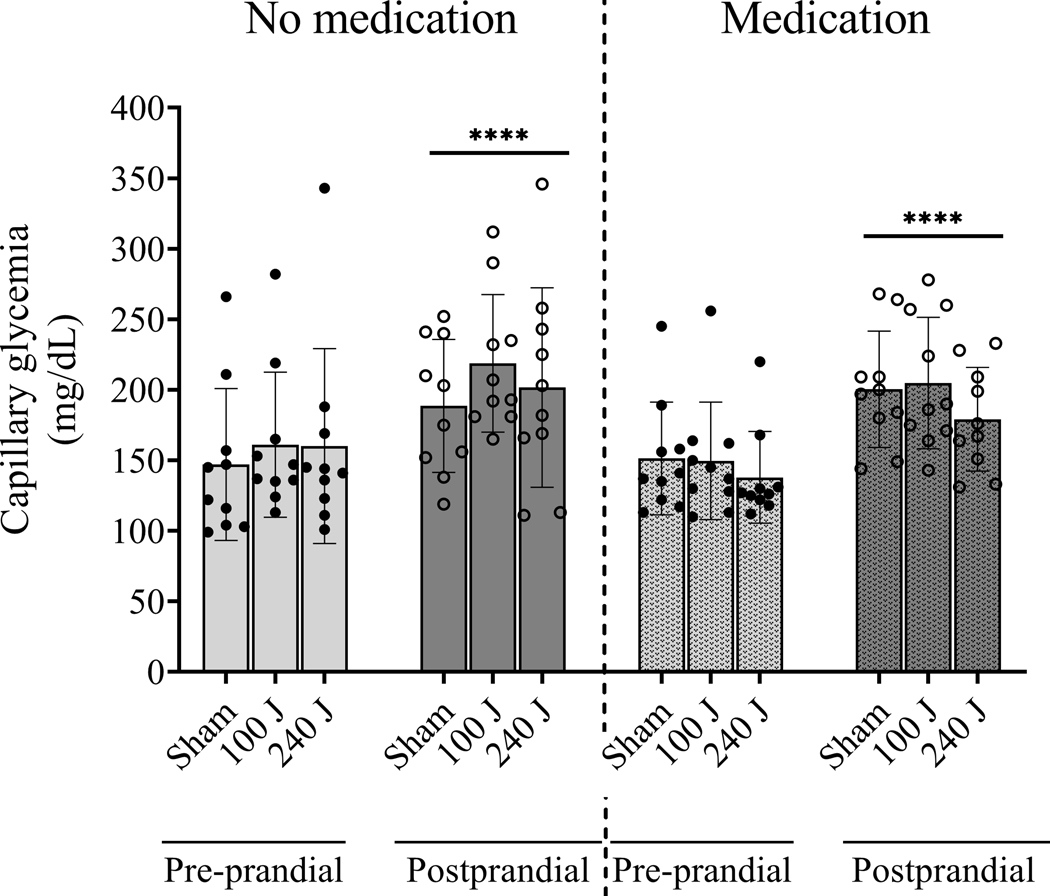
Pre-prandial glycemia was similar among groups (p=0.6111). After consumption of the standardized meal, post-prandial glycemia was similarly increased in all groups or conditions (p<0.0001), and medication intake did not affect this response (p=0.8349). Figure 3 shows pre-prandial and post-prandial glycemia responses.
Figure 3.
Capillary glycemia after an overnight fast (pre-prandial) and after a standardized meal (post-prandial), without oral hypoglycemic medication intake (no medication) or after oral hypoglycemic medication intake (medication) in type 2 diabetic patients (n=10). ****p<0.0001 vs pre-prandial. Values are average and standard error of the mean.
3.2. Glycemic response after PBMt without oral hypoglycemic medication intake
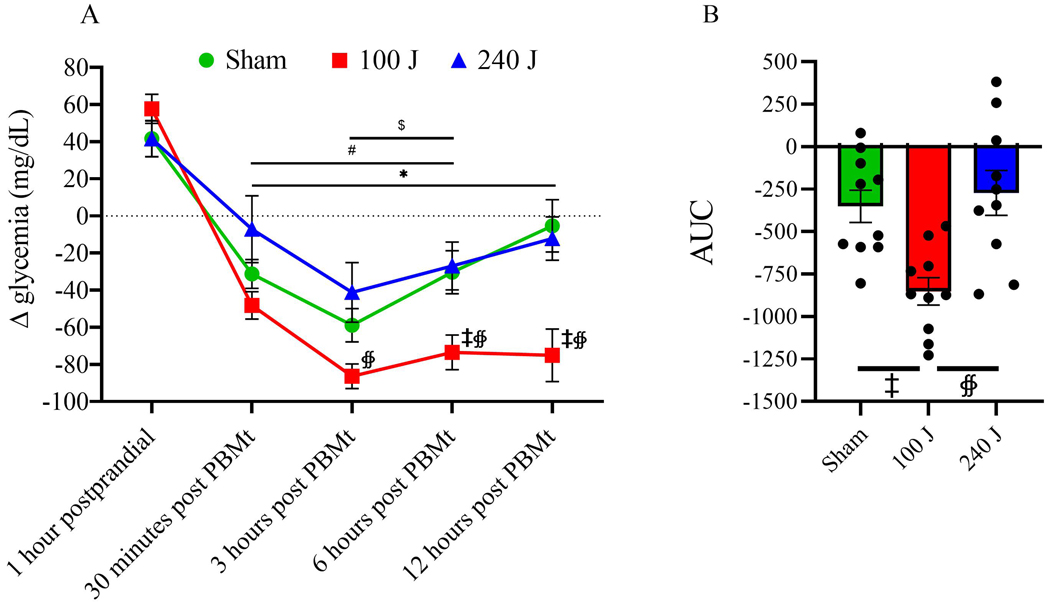
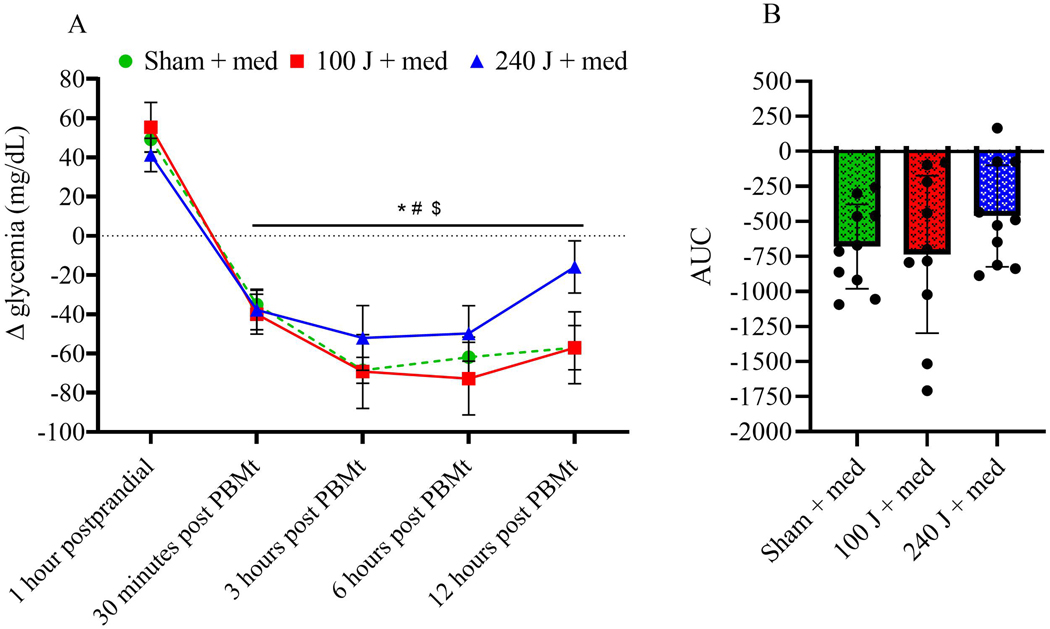
When patients did not take their oral hypoglycemic medication, the increase in glycemia 1 hour post-prandial was not different among groups (p>0.41). Conversely, 30 min after PBMt irradiation, the sham and the 100 J groups showed reduced glycemia compared to 1 hour postprandial (p<0.01 for both). Between 3 and 6 hours after irradiation, all groups showed reduced glycemia compared to postprandial values (p<0.05 for all). However, only the 100 J group still showed reduced glycemia 12 hours after irradiation (p=0.0002). Using a between group comparison, 100 J showed lower glycemia compared to 240 J between 3 and 12 hours; and compared to sham between 6 and 12 hours post irradiation (Figure 4A). The area under the curve (AUC) showed lower glycemia for the 100 J group during the period analyzed after irradiation compared to sham (p=0.0032) and to 240 J (p=0.0152), and no difference between sham and 240 J (p=0.6) (Figure 4B).
Figure 4A –
Change in glycemia response (delta) after the standardized meal (1 hour postprandial), and 30 min, 3, 6, and 12 hours after 0 J (sham), 100 J or 240 J of photobiomodulation therapy (PBMt), in type 2 diabetes patients without oral hypoglycemic medication (n=10). Values are average and standard error of the mean. 4B – Area under the curve (n=10). Values are arbitrary units. Within group comparison vs 1 hour postprandial: #p<0.05 for sham, *p<0.05 for 100 J, $ p<0.05 for 240 J. Between groups comparisons: ‡ p<0.05 vs sham, ∯ p<0.05 vs 240 J.
For the primary outcome (glycemia), the sample size of 10 patients per treatment was appropriate, showing significant difference between PBMt 100 J from the other PBMt doses (sham and 240 J) at 6-hour timepoint regarding the delta of glycemia (mg/dL), for example. The achieved effect size of 0.59 and power of 0.95 for ANOVA (repeated measures, between factors) calculated at GPower 3.1.9, used a standard deviation of 35.72, number of groups equal 3 (PBMt 100 J, PBMt 240 J, PBMt sham), total sample size equal 30 (3 treatments with 10 patients at each treatment), 5 measurements (1 hour post prandial, 30 min post PBMt, 3 hours post PBMt, 6 hours after PBMt, 12 hours after PBMt) and alfa of 5%.
3.3. Glycemic response after PBMt with oral hypoglycemic medication intake
When combined with oral hypoglycemic medication, all groups showed a reduction in glycemia between 30 min and 12 hours after irradiation compared to 1 hour postprandial (p<0.05 for all). No significant differences between conditions were observed (Figure 5A). Analysis of AUC did not reveal a significant main effect (p=0.2614; Figure 5B).
Figure 5A –
Delta glycemia response after a standardized meal (1 hour postprandial), and 30 min, 3, 6, and 12 hours after 0 J (sham), 100 J or 240 J of PBMt, in type 2 diabetes patients associated with oral hypoglycemic medication intake (n=10). Values are average and standard error of the mean. 5B – area under the curve (n=10). Values are arbitrary units. Between groups comparisons vs 1 hour postprandial: #p<0.05 for sham, *p<0.05 for 100 J, $ p<0.05 for 240 J.
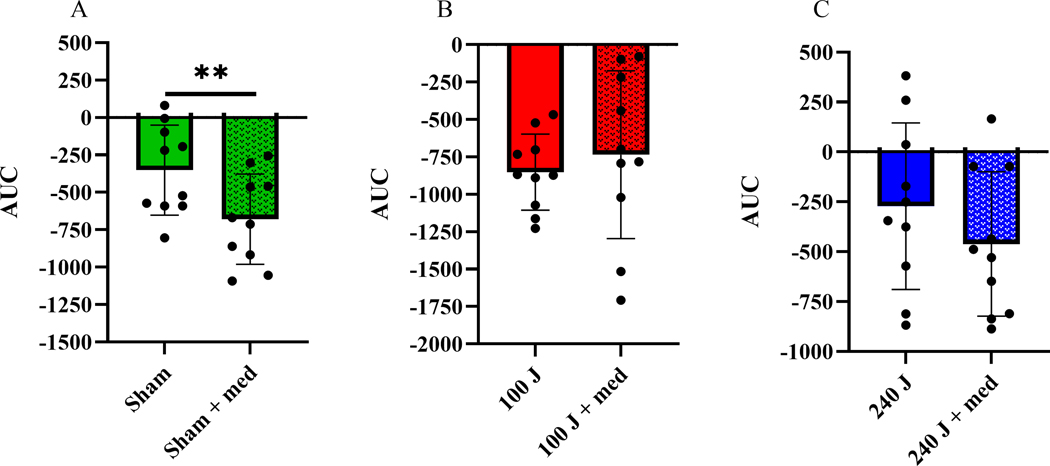
3.4. Comparison between PBMt treatments (combined or not with hypoglycemic medication)
When compared between irradiation treatments, there was a positive effect of the oral hypoglycemic medication in the sham group observed by a reduction in AUC (p<0.01; Figure 6A). Conversely, medication use did not affect the AUC response when combined with PBMt 100 J (p=0.574; Figure 6B) or 240 J (p=0.12; Figure 6C).
Figure 6 –
Glycemia area under the curve without or with oral hypoglycemic medication intake combined with PBMt: sham (0 J) (6A); PBMt 100 J (6B); or PBMt 240 J (6C); (n=10). **p<0.01. Values are arbitrary units.
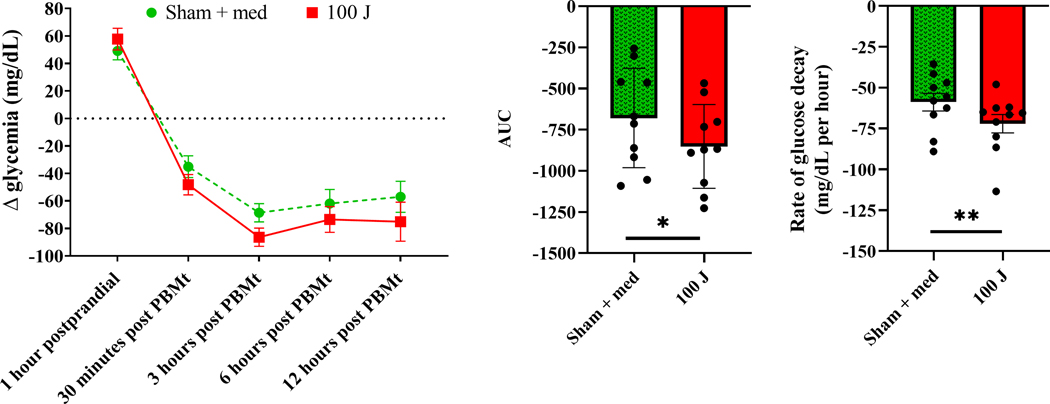
The best result without medication use was observed for the 100 J PBMt (Figures 4A and 4B). Regarding the combination of oral hypoglycemic medication plus PBMt, there was no significant difference between groups (Figures 5A and 5B). However, due to the lower average and standard deviation of the AUC of the sham plus medication group (Figure 6A), we chose this group for comparison with PBMt 100 J without medication. Therefore, we compared these 2 groups and results are shown in Figure 7. Although we did not observe any significant difference during the time window analyzed between sham + medication and 100 J PBMt (p=0.49 for interaction; Figure 7A), the AUC was significantly lower in the 100 J PBMt group (p<0.05) (Figure 7B). Furthermore, the rate of glucose decay calculated from 1 hour postprandial to 3 hours post PBMt showed that the glucose level reduced faster in the 100 J PBMt group (p=0.0064; Figure 7C). Thus, suggesting 100 J PBMt may have induced better glycemic control in T2DM patients.
Figure 7 –
Delta glycemia response after a standard meal (1 hour postprandial), and 30 min, 3, 6, and 12 hours after PBMt sham (0 J) combined with oral hypoglycemic medication intake, and after PBMt 100 J without oral hypoglycemic medication intake in type 2 diabetes patients (n=10) (7A) - values are average and standard error of the mean. Area under the curve (7B) - values are arbitrary units. Rate of glucose decay (7C) - values are average and standard error of the mean. *p<0.05, **p<0.01.
3.5. Effect of PBMt (combined or not) with oral hypoglycemic medication on heart rate variability (HRV)
HRV was analyzed by measuring: a) heart rate (number of beats per minute - bpm); b) the standard deviation of all normal R-R intervals (iR-R) recorded in milliseconds squared (SDNN); c) the square root of the mean square of the differences between consecutive R-R intervals expressed in milliseconds squared (RMSSD), which quantifies abrupt variations in HRV; d) the high frequency (HF) component in normalized units (un), corresponding to respiratory modulation, and an indicator of vagus nerve action on the heart; e) the low frequency (LF) component in normalized units (un), resulting from the joint action of the vagal and sympathetic components on the heart, with a predominance of the sympathetic; f) LF/HF ratio, which reflects the absolute and relative changes between the sympathetic and parasympathetic components of the autonomic nervous system, characterizing the sympathovagal balance affecting the heart. Table 4 presents the results of these measurements. Significant effects (p<0.05) were observed for LF component after 100 J PBMt (without medication) and after sham + medication, suggesting higher sympathetic activation in these groups 30 min after irradiation.
Table 4.
Results of heart rate variability measurements
| HR (bpm) | SDNN (mseg) | RMSSD (mseg) | LF (ms2) | HF (ms2) | LF (un) | LF/HF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | |
| Sham | 70±8 | 65±6 | 19±7 | 19±8 | 20±9 | 22±13 | 156±141 | 142±88 | 185±165 | 249±341 | 46±22 | 45±23 | 1.2±1.2 | 1.2±1.0 |
| 100 J | 72±10 | 69±9 | 18±10 | 21±12 | 21±13 | 22±14 | 104±78 | 218±240 | 278±421 | 250±231 | 41±26 | 53±27* | 1.1±1.2 | 1.1±1.3 |
| 240 J | 69±5 | 66±5 | 20±11 | 23±15 | 22±14 | 26±23 | 184±166 | 237±263 | 232±334 | 408±750 | 45±24 | 48±26 | 1.8±3.1 | 1.5±1.4 |
| Sham+med | 69±6 | 68±7 | 23±14 | 21±13 | 28±25 | 24±23 | 172±126 | 152±68 | 49±26 | 42±23 | 50±23 | 57±23* | 1.9±2.4 | 2.1±2.1 |
| 100 J+med | 71±7 | 67±8 | 29±37 | 25±12 | 32±43 | 29±18 | 370±639 | 225±186 | 119±100 | 323±353 | 56±15 | 49±20 | 1.6±0.9 | 1.2±0.9 |
| 240 J+med | 69±5 | 65±5 | 18±9 | 21±8 | 18±9 | 22±10 | 174±174 | 218±166 | 208±359 | 221±236 | 52±18 | 53±20 | 1.7±2.0 | 1.7±1.8 |
Heart rate (HR); standard deviation of all normal R-R intervals (iR-R) recorded in milliseconds squared (SDNN); square root of the mean square of the differences between consecutive R-R intervals expressed in milliseconds squared (RMSSD); high frequency (HF) component in milliseconds squared (mseg2); low frequency (LF) component in milliseconds squared (mseg2) and in normalized units (un); LF/HF ratio. Statistical significance (p<0.05 vs pre, bold to facilitate visualization) in the Wicoxon test (non-parametric data) with Bonferroni correction; oral hypoglycemic medication (med). pre = 30 min before photobiomodulation therapy; post = 30 min after photobiomodulation therapy.
4. DISCUSSION
The present study aimed to assess the dose-response and time-response of PBMt combined or not with oral hypoglycemic medication on capillary glycemia and cardiac autonomic control in T2DM patients. The main results are as follow: a) when oral hypoglycemic medication was withdrawn, the 100 J PBMt led to the greatest reduction in capillary glycemia compared to sham or 240 J PBMt, especially at 6 and 12 hours post irradiation; b) PBMt, when combined with oral hypoglycemic medication, did not elicit further decrease in capillary glycemia, except for the SHAM group, as expected; c) 100 J PBMt induced better glycemic control than medication intake alone; and d) Cardiac sympathetic modulation increased in the conditions that elicited the best glycemic response, i.e. sham + medication and 100 J PBMt.
To the best of our knowledge this is the first study to provide convincing evidence using a randomized, double-blind, crossover and sham-controlled clinical trial, of the beneficial effects and the dose-response and time-response effects of PBMt, combined or not with the use of oral hypoglycemic medication, on the glycemic control in T2DM patients. Previous studies from our group [33, 34, 53] and from others [30–32, 35, 54, 55] have suggested PBMt could improve glycemic control in insulin resistant T2DM in animal models, however clinical studies on humans are only beginning to emerge.
Recently, we showed that PBMt (LED array composed of 50 diodes, 850 nm, 75 mW/diode) applied over the rectus and oblique abdomen, quadriceps femoris, triceps surae, and hamstring muscle areas in T2DM patients positively affected fasting glycemia in a dose-dependent manner, with reductions observed 15 min after irradiation at 75 and 450 J per site, but not after 150 J, 300 J, or 600 J [36]. The present data are in line with these previous results, as we also observed a positive effect after 100 J, but not after 240 J, which is compatible with the 75 J and the 300 J results from that previous study. However, we should point out that the patients in the previous study took their prescribed medication as usual, which included insulin in 3 out of 13 patients (~23%), and the study protocol did not include an arm without medication, as we had in the present study. We believe these methodological differences preclude more detailed comparisons between the studies. Moreover, the time window investigated in the previous study (15 min post PBMt) might have been too short to capture all the positive effects of PBMt on glycemic control. Nonetheless, despite some limitations, our previous study [36] together with the present results clearly suggest that PBMt exerts a positive effect on glycemic control in T2DM patients.
As stated above, we observed a positive effect of PBMt with 100 J, but not 240 J. It is known that PBMt shows a biphasic dose-response effect [37, 38, 56], which depending on the dosage applied, we can observe stimulatory, inhibitory, or no biological effects. Although we tested only 2 doses, the present results are in agreement with the biphasic PBMt dose-response, and agreed well with previous results from our group [36]. Interestingly, in that previous study we observed a possible triphasic dose-response, where low (75 J) and moderate-to-high (450 J) doses exerted positive effects on glycemia, and moderate (150–300 J) and high (600 J) doses did not. We had already observed a triphasic PBMt effect for muscle glycogen synthesis in rats [53]. Altogether, based on these results, we suggest that for positive effects on glycemic control in T2DM patients predominantly with type II and III skin phototypes, PBMt dose delivery per site of irradiation over multiple sites on the body should be between 75–100 J or around 450 J, but not between 150–300 J or around 600 J.
Our study highlights the importance of using a longer time window for the assessment of PBMt effects on glycemia. For example, if we had stopped analysis at the 3-hour post PBMt time point, we would have missed the positive effects of the 100 J PBMt compared to the sham (Figure 4A). Although we observed positive effects on glycemia after 100 J PBMt when hypoglycemic medication was withdrawn, and when medication was combined with sham irradiation, a combination of these two therapies (100 J + medication) did not produce any improvement in glycemic control. Although this result might seem counterintuitive, one previous study did report that the combination of PBMt and medication did not improve on each therapy used alone [27]. More specifically, Asghari et al [27] reported improved glucose tolerance after PBMt in a rat model of T2DM, but when combined with metformin (the most commonly prescribed oral hypoglycemic medication worldwide [11]) there was no improvement over PBMt alone.
Our results on T2DM patients corroborate these previous animal data. Interestingly, and in line with the present data, metformin did not potentiate the positive effects of an acute bout of resistance [57] or aerobic [58] exercise on insulin resistance in pre-diabetic humans. There have been some reports that metformin could actually be detrimental for glucose metabolism when combined with exercise [44]. For instance, Sharoff et al [43] assessed the combination of metformin with an acute bout of aerobic exercise in insulin resistant (non-diabetic) individuals and observed that metformin significantly attenuated the exercise-induced increase in whole-body insulin sensitivity. It has also been reported that metformin can interfere wirth chronic exercise adaptations [59, 60]. However, this impairment seems to affect naïve metformin users, because in chronic (>6 months) users this response does not seem to occur [57, 58]. All T2DM patients in the present study were chronic metformin users, and our results suggest that, even though no potentiation was observed when 100 J was combined with medication intake, there was no inhibition or attenuation with this combination. These findings suggest that PBMt improves glycemic control in chronic metformin users, but as in the case for exercise, this improvement is not observed when metformin is added to the regimen.
It is important to highlight that patients were not exclusively taking metformin, as we did not interfere with prescription of oral hypoglycemic medication. In fact, only half of the patients were receiving metformin monotherapy, while 3 (30%) received dual therapy (metformin + sulfonylureas), and the other 2 (20%) received triple therapy (metformin + sulfonylurea + SGLT2 inhibitor; or metformin + SGLT2 inhibitor + DPP-4 inhibitor). Furthermore, the mechanisms of action of these hypoglycemic medications vary greatly [61, 62], and the drug dosages also differed among our patients (see Table 2). However, our results showed that regardless of oral hypoglycemic medication type and dose, and whether mono, dual, or triple therapy, the glycemic response was consistent in our T2DM patients. We believe that not interfering with medication prescription increased the overall validity of our study, by simulating the real world in clinical practice. Nevertheless, we suggest that more studies to assess the interaction of different types and doses of oral hypoglycemic medication with PBMt are certainly warranted.
The fact that we observed a better result after 100 J (without medication) compared with sham plus medication (Figures 7A, 7B and 7C) was interesting. Of note, other studies have also reported better results after PBMt compared to anti-inflammatory drugs in skeletal muscle trauma [63], and in Achilles tendinopathy [64]. Moreover, Nunes et al [65] reported that PBMt improved pain symptoms in endodontic treatment compared to ibuprofen administration. Thus, there is evidence in the literature that PBMt can actually exert more positive effects than commonly used medications for inflammation and pain. In this regard, the present results suggest that PBMt might be at least as effective for glycemic control as common oral medications. Nevertheless, we should mention that the present study assessed glycemia after an acute PBMt session for 12 hours only, under controlled conditions. Thus, our results do not not provide enough evidence to suggest that oral hypoglycemic medication should be replaced by PBMt to treat T2DM patients. Nevertheless, we believe the present data are very encouraging and should inspire more studies to fully understand the effects of PBMt on glucose metabolism, as well as the mechanisms involved.
To understand the possible mechanisms involved in the improvement in glycemia control in response to PBMt, we assessed the cardiac autonomic control through the HRV indexes. Interestingly, we observed that after the regimens that induced the greatest reduction in glycemia (sham + medication and PBMt100 J) a significant increase was observed in the LF component of spectral analysis [8]. These findings may reflect an increase in the sympathetic autonomic modulation in response to hypoglycemia induced by 100 J PBMt and medication use. It is well established that sympathetic nervous system plays an important role in increasing glucose production and also in decreasing glucose utilization, thus being considered an important hypoglycemic counterregulatory system [66]. In addition, greater sympathetic activation was previously observed when blood glucose clearance was accelerated by sulfonylureas combined with an acute bout of exercise [66]. Therefore, those findings corroborate the effectiveness of PBMt in reducing capillary blood glucose, and faster rate of glucose decay (figure 7C).
Although in the present study we did not investigate the intracellular mechanisms that might have played a role in the positive effects we observed, we believe that this topic deserves more investigation in the future. Animal [33, 34], as well as cell culture [30–32] studies have provided evidence that PBMt can improve insulin signaling by activating the Akt-AS160-GLUT4 pathway, and thus increasing glucose uptake and glycogen synthesis [53]. In a recent review, we summarized some possible mechanisms of PBMt-induced improvement in glucose metabolism [45]. Therefore, we believe it is reasonable to suggest that similar mechanisms would occur in the present study. Moreover, we cannot overlook the possible effect of PBMt on reducing hepatic glucose production, because it has been demonstrated that PBMt increases reactive oxygen species production, leading to activation of AMP-activated protein kinase (AMPK) [30], which could result in reduced expression of gluconeogenic enzymes [67]. Interestingly, metformin reduces hyperglycemia by similar mechanisms [67], which could help explain the lack of any additive effects of PBMt and metformin in the present study, because these therapies could act on the same pathway and possibly lead to a ceiling effect. However, as discussed previously, our T2DM patients were not exclusively on metformin, making direct inferences on the possible mechanisms responsible for the observed results difficult. There is no doubt that future studies should focus on the mechanisms responsible for the effects of PBMt on blood glycemia in human subjects, and how they interact with oral hypoglycemic medication intake.
Even though we did not observe an additive effect of PBMt and oral hypoglycemic medication, other combinations of hypoglycemic therapies have shown promising results. For instance, some studies have investigated the combination of exercise and PBMt in obese, non-diabetic subjects [68–70] and the results suggested improved glucose metabolism compared to exercise alone. More recently, we [71] reported that PBMt applied before a short-duration acute session of aerobic exercise in T2DM patients reduced glucose levels, which was not observed in sham-treated patients. Thus, there is limited evidence suggesting that a combination of PBMt and exercise is better than exercise alone in improving glucose metabolism. Because physical exercise [23] and oral hypoglycemic medication [11] are the current cornerstones of T2DM prevention and treatment, therefore the present study together with recent literature [36] all suggest that PBMt can improve glycemic control in T2DM patients. A study of the triple combination of medication plus exercise plus PBMt should be undertaken to assess the potential clinically relevant, and additive (or inhibitory) effects on diabetes control and treatment.
5. CONCLUSION
In the present study we demonstrated for the first time in T2DM patients that PBMt applied alone with a dose of 100 Joules per site on the muscles of the lower and upper limbs was able to induce a significant reduction in capillary glycemia from 30 minutes to 12 hours post irradiation. Considering the combined use of PBMt and oral hypoglycemic medication, we observed that there were no inhibitory or additive effects of these therapies. When we compared the best regimens (100 J without medication and sham + medication), we observed there was better glycemic control after 100 J. This observation has clinical relevance, since PBMt is a non-invasive, painless, low-cost, non-pharmaceutical treatment, with no side-effects, and could lead to improved glycemic control compared to hypoglycemic drugs in T2DM patients. We also showed that the HRV was modulated by PBMt and could potentially be used as a tool to identify patients who are responsive to PBMt for glycemic control. However, the mechanisms of action of PBMt on glycemic control and HRV should be further investigated. Finally, we suggest carrying out multicenter trials using the same methodology as the present study to verify the effects of PBMt in different populations.
ACKNOWLEDGMENTS
We would like to thank the Universidade Sagrado Coração; Federal University of São Carlos; National Council for Scientific and Technological Development (CNPQ); the Minas Gerais State Agency for Research and Development (FAPEMIG).
FINANCIAL DISCLOSURE
Flavio de Castro Magalhaes was supported by National Council for Scientific and Technological Development (CNPQ: Grant#407975/2018-7 and # 402091/2021-3) and by the Minas Gerais State Agency for Research and Development (FAPEMIG: Grant# APQ-00008-22). Michael R Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
Abbreviations:
- T2DM
type 2 diabetes mellitus
- HRV
heart rate variability
- PBMt
photobiomodulation therapy
Footnotes
CONFLICT OF INTEREST
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; Hologenix Inc. Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc, Minneapolis-St. Paul MN; Sunlighten, Kansas City, MO; Consulting; USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; Klox Asia, Guangzhou, China. Stockholding: Niraxx Light Therapeutics, Inc, Irvine CA; JelikaLite Corp, New York NY. The other authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- [1].Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ Diabetes Res Clin Pract. 2022, 183, 109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Nature reviews. Disease primers. 2015, 1, 15019. [DOI] [PubMed] [Google Scholar]
- [3].Fowler MJ Clinical Diabetes. 2008, 26, 77–82. [Google Scholar]
- [4].Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Diabetes Research and Clinical Practice. 2019, 157, 107843. [DOI] [PubMed] [Google Scholar]
- [5].Karayannis G, Giamouzis G, Cokkinos DV, Skoularigis J, Triposkiadis F. Expert review of cardiovascular therapy. 2012, 10, 747–765. [DOI] [PubMed] [Google Scholar]
- [6].França da Silva AK, Penachini M. da Costa de Rezende Barbosa F. Marques Vanderlei DG Destro Christofaro LC Marques Vanderlei Ann Noninvasive Electrocardiol. 2016, 21, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD Diabetes care. 2001, 24, 1793–1798. [DOI] [PubMed] [Google Scholar]
- [8].Benichou T, Pereira B, Mermillod M, Tauveron I, Pfabigan D, Maqdasy S, Dutheil F PLoS One. 2018, 13, e0195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Veves A, King GL J Clin Invest. 2001, 107, 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sharma JK, Rohatgi A, Sharma D. The journal of the Royal College of Physicians of Edinburgh. 2020, 50, 269–273. [DOI] [PubMed] [Google Scholar]
- [11].Aschner P. Diabetes Res Clin Pract. 2017, 132, 169–170. [DOI] [PubMed] [Google Scholar]
- [12].Foretz M, Guigas B, Viollet Nature reviews B. Endocrinology. 2019, 15, 569–589. [DOI] [PubMed] [Google Scholar]
- [13].Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W. Front Endocrinol (Lausanne). 2017, 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ashcroft FM Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1996, 28, 456–463. [DOI] [PubMed] [Google Scholar]
- [15].Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, Corlianò F, Fra GP, Bartoli E, Derosa G. Archives of medical science: AMS. 2015, 11, 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gallwitz B Front Endocrinol (Lausanne). 2019, 10, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsia DS, Grove O, Cefalu WT Current opinion in endocrinology, diabetes, and obesity. 2017, 24, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lorenzati B, Zucco C, Miglietta S, Lamberti F, Bruno G. Pharmaceuticals (Basel, Switzerland). 2010, 3, 3005–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ganesan K, Rana MBM, Sultan S. in Oral Hypoglycemic Medications, Vol., StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL), 2022. [PubMed] [Google Scholar]
- [20].Munshi MN, Maguchi M, Segal AR Current diabetes reports. 2012, 12, 239–245. [DOI] [PubMed] [Google Scholar]
- [21].Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman Diabetes care B. 2009, 32, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saraiva EMS, Coelho JLG, Dos Santos Figueiredo FW, do RP Souto Journal of diabetes and metabolic disorders. 2020, 19, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, Kirwan JP, Zierath JR Med Sci Sports Exerc. 2022, 54, 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arzu D, Tuzun EH, Eker L. J Sports Sci Med. 2006, 5, 615–620. [PMC free article] [PubMed] [Google Scholar]
- [25].Carballo-Fazanes A, Rico-Díaz J, Barcala-Furelos R, Rey E, Rodríguez-Fernández JE, Varela-Casal C, Abelairas-Gómez C. Int J Environ Res Public Health. 2020, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eves ND, Plotnikoff Diabetes care RC. 2006, 29, 1933–1941. [DOI] [PubMed] [Google Scholar]
- [27].Asghari M, Kanonisabet A, Safakhah M, Azimzadeh Z, Mostafavinia A, Taheri S, Amini A, Ghorishi SK, JalaliFiroozkohi R, Bayat S, Bayat M. J Photochem Photobiol B. 2017, 169, 63–69. [DOI] [PubMed] [Google Scholar]
- [28].Peplow PV, Baxter GD Photomed Laser Surg. 2014, 32, 500–504. [DOI] [PubMed] [Google Scholar]
- [29].Fukuoka CY, Torres Schröter G, Nicolau J, Simões A. 2016, 9, 1246–1254. [DOI] [PubMed] [Google Scholar]
- [30].Guo S, Gong L, Shen Q, Xing D. J Photochem Photobiol B. 2020, 213, 112075. [DOI] [PubMed] [Google Scholar]
- [31].Gong L, Zou Z, Liu L, Guo S, Xing Aging D. 2021, 13, 10015–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gong L, Zou Z, Huang L, Guo S, Xing D Cell Signal. 2020, 67, 109491. [DOI] [PubMed] [Google Scholar]
- [33].Silva G, Ferraresi C, de Almeida RT, Motta ML, Paixão T, Ottone VO, Fonseca IA, Oliveira MX, Rocha-Vieira E, Dias-Peixoto MF, Esteves EA, Coimbra CC, Amorim FT, Magalhães FC J Biophotonics. 2020, 13, e201960140. [DOI] [PubMed] [Google Scholar]
- [34].Silva G, Ferraresi C, de Almeida RT, Motta ML, Paixão T, Ottone VO, Fonseca IA, Oliveira MX, Rocha-Vieira E, Dias-Peixoto MF, Esteves EA, Coimbra CC, Amorim FT, de F. Castro Magalhães Lasers Med Sci. 2018, 33, 559–571. [DOI] [PubMed] [Google Scholar]
- [35].Yoshimura TM, Sabino CP, Ribeiro MS J Biophotonics. 2016, 9, 1255–1262. [DOI] [PubMed] [Google Scholar]
- [36].Linares SN, Beltrame T, Galdino GAM, Frade MCM, Milan-Mattos JC, Gois MO, Borghi-Silva A, de Biase PF, Manchado-Gobatto FB, Bagnato VS, Parizotto NA, Ferraresi C, Catai AM. 2022, 9, 481. [Google Scholar]
- [37].Huang YY, Sharma SK, Carroll J, Hamblin MR Dose Response. 2011, 9, 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang YY, Chen AC, Carroll JD, Hamblin MR Dose Response. 2009, 7, 358–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR Lasers Med Sci. 2015, 30, 1259–1267. [DOI] [PubMed] [Google Scholar]
- [40].Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, Parizotto NA, Hamblin MR Photochem Photobiol. 2015, 91, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Newman AA, Grimm NC, Wilburn JR, Schoenberg HM, Trikha SRJ, Luckasen GJ, Biela LM, Melby CL, Bell C. J Clin Endocrinol Metab. 2019, 104, 1953–1966. [DOI] [PubMed] [Google Scholar]
- [42].Brinkmann C Front Endocrinol (Lausanne). 2021, 12, 694099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B. Am J Physiol Endocrinol Metab. 2010, 298, E815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boulé NG, Robert C, Bell GJ, Johnson ST, Bell RC, Lewanczuk RZ, Gabr RQ, Brocks DR Diabetes care. 2011, 34, 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Magalhães FC, Ferraresi C Photobiomodulation, photomedicine, and laser surgery. 2022, 40, 597–603. [DOI] [PubMed] [Google Scholar]
- [46].Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ European heart journal. 1996, 17, 354–381.8737210 [Google Scholar]
- [47].Porta A, Guzzetti S, Montano N, Furlan R, Pagani M, Malliani A, Cerutti S. IEEE Trans Biomed Eng. 2001, 48, 1282–1291. [DOI] [PubMed] [Google Scholar]
- [48].Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, Cogliati C, Somers VK, Malliani A, Porta A. Circulation. 2005, 112, 465–470. [DOI] [PubMed] [Google Scholar]
- [49].Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R, Montano N. J Appl Physiol (1985). 2007, 103, 1143–1149. [DOI] [PubMed] [Google Scholar]
- [50].Beltrame T, Ferraresi C, Parizotto NA, Bagnato VS, Hughson RL Lasers Med Sci. 2018, 33, 1065–1071. [DOI] [PubMed] [Google Scholar]
- [51].Ferraresi C, Dos Santos RV, Marques G, Zangrande M, Leonaldo R, Hamblin MR, Bagnato VS, Parizotto NA Lasers Med Sci. 2015, 30, 1281–1287. [DOI] [PubMed] [Google Scholar]
- [52].Ferraresi C, Beltrame T, Fabrizzi F, do Nascimento ES, Karsten M, Francisco Cde O, Borghi-Silva A, Catai AM, Cardoso DR, Ferreira AG, Hamblin MR, Bagnato VS, Parizotto NA Physiother Theory Pract. 2015, 31, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Castro KMR, de Paiva Carvalho RL, Junior GMR, Tavares BA, Simionato LH, Bortoluci CHF, Soto CAT, Ferraresi C. J Photochem Photobiol B. 2020, 207, 111877. [DOI] [PubMed] [Google Scholar]
- [54].Bonifacio M, Benfato ID, de Almeida Cruz M, de Sales DC, Pandolfo IL, Quintana HT, Carvalho CPF, de Oliveira CAM, Renno ACM Lasers Med Sci. 2022, 37, 1799–1809. [DOI] [PubMed] [Google Scholar]
- [55].Paolillo FR, Campos T, Alvarez C, Sene-Fiorese M, Bagnato VS, de Oliveira Duarte ACG, Parizotto NA J Biophotonics. 2021, 14, e202100109. [DOI] [PubMed] [Google Scholar]
- [56].Hamblin MR AIMS biophysics. 2017, 4, 337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moreno-Cabañas A, Ortega JF, Morales-Palomo F, Ramirez-Jimenez M, Alvarez-Jimenez L, Mora-Rodriguez R. Med Sci Sports Exerc. 2022, 54, 1043–1050. [DOI] [PubMed] [Google Scholar]
- [58].Ortega JF, Hamouti N, Fernández-Elías VE, de Prada MV, Martínez-Vizcaíno V, Mora-Rodríguez R. Acta diabetologica. 2014, 51, 749–755. [DOI] [PubMed] [Google Scholar]
- [59].Moreno-Cabañas A, Morales-Palomo F, Alvarez-Jimenez L, Ortega JF, Mora-Rodriguez R. Obesity (Silver Spring, Md.). 2022, 30, 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA Aging Cell. 2019, 18, e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shahar J, Hamdy O. Diabetes spectrum : a publication of the American Diabetes Association. 2015, 28, 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Eckstein ML, Williams DM, O’Neil LK, Hayes J, Stephens JW, Bracken RM Diabet Med. 2019, 36, 349–358. [DOI] [PubMed] [Google Scholar]
- [63].de Almeida P, Lopes-Martins R, Tomazoni SS, Albuquerque-Pontes GM, Santos LA, Vanin AA, Frigo L, Vieira RP, Albertini R, de Carvalho Pde T, Leal-Junior EC Photochem Photobiol. 2013, 89, 501–507. [DOI] [PubMed] [Google Scholar]
- [64].Naterstad IF, Rossi RP, Marcos RL, Parizzoto NA, Frigo L, Joensen J, Lopes Martins PSL, Bjordal JM, Lopes-Martins RAB Photomed Laser Surg. 2018, 36, 137–145. [DOI] [PubMed] [Google Scholar]
- [65].Nunes EC, Herkrath FJ, Suzuki EH, Gualberto Júnior EC, Marques AAF, Sponchiado EC Júnior Lasers Med Sci. 2020, 35, 971–978. [DOI] [PubMed] [Google Scholar]
- [66].Hoffman RP Current diabetes reviews. 2007, 3, 185–193. [DOI] [PubMed] [Google Scholar]
- [67].Rena G, Hardie DG, Pearson ER Diabetologia. 2017, 60, 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].da Silveira Campos RM, Dâmaso AR, Masquio DC, Aquino AE Jr., Sene-Fiorese M, Duarte FO, Tock L, Parizotto NA, Bagnato VS Lasers Med Sci. 2015, 30, 1553–1563. [DOI] [PubMed] [Google Scholar]
- [69].Duarte FO, Sene-Fiorese M, de Aquino Junior AE, da Silveira Campos RM, Masquio DC, Tock L, Garcia AC de Oliveira Duarte, A. R. Dâmaso, V. S. Bagnato, Parizotto NA J Photochem Photobiol B. 2015, 153, 103–110. [DOI] [PubMed] [Google Scholar]
- [70].Sene-Fiorese M, Duarte FO, de Aquino Junior AE, Campos RM, Masquio DC, Tock L, de Oliveira Duarte AC, Dâmaso AR, Parizotto NA, Bagnato VS Lasers Surg Med. 2015, 47, 634–642. [DOI] [PubMed] [Google Scholar]
- [71].Francisco CO, Beltrame T, Hughson RL, Milan-Mattos JC, Ferroli-Fabricio AM, Galvão Benze B, Ferraresi C, Parizotto NA, Bagnato VS, Borghi-Silva A, Porta A, Catai AM Complement Ther Med. 2019, 42, 178–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.