Abstract
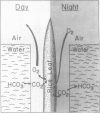
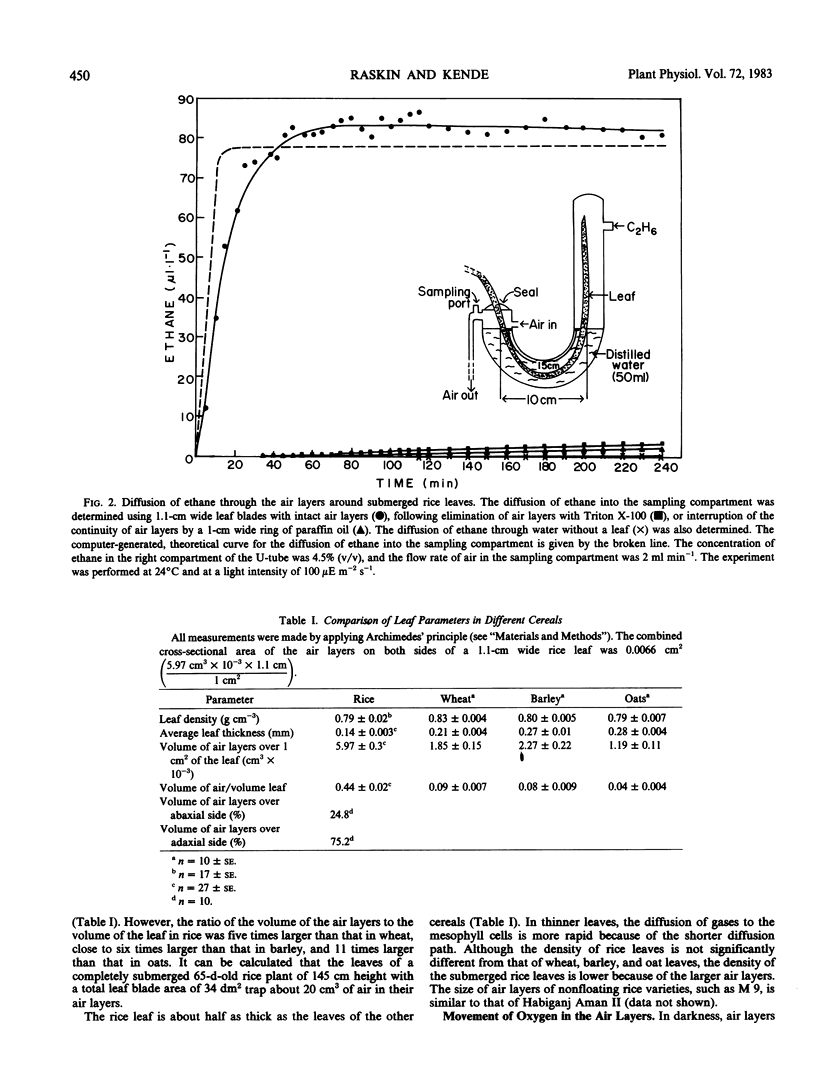
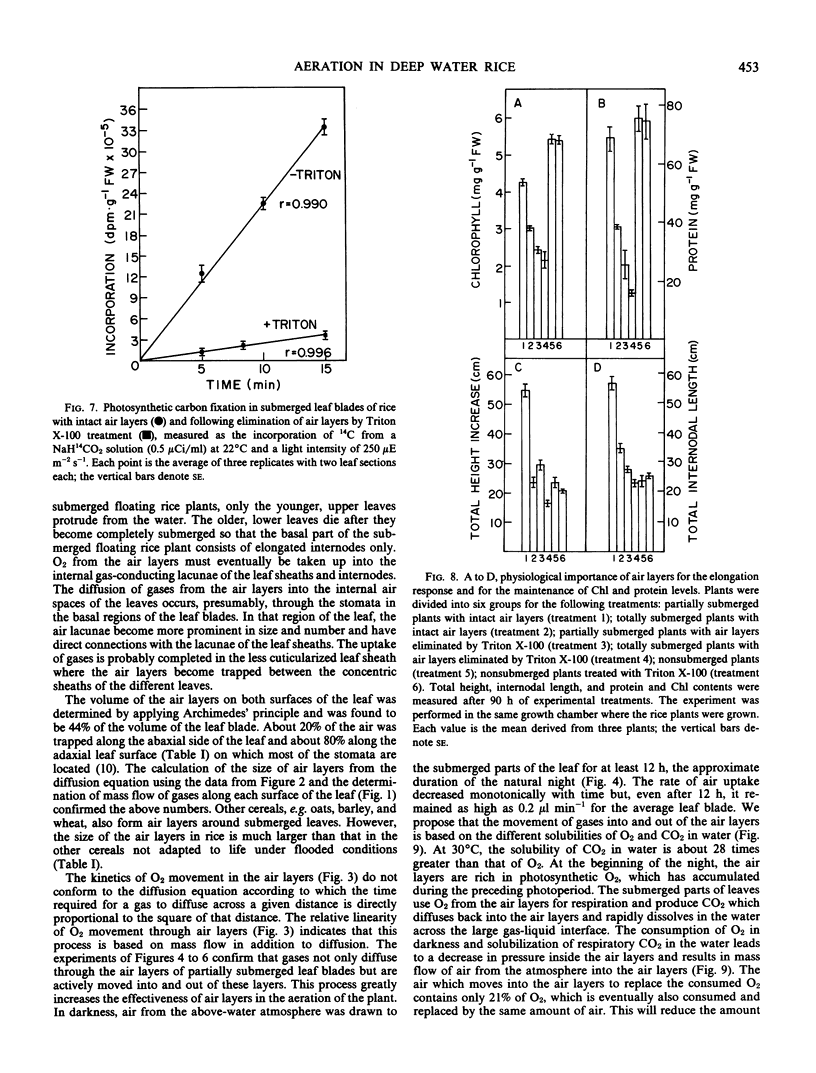
In partially flooded deep water rice (Oryza sativa L. cv Habiganj Aman II), continuous air layers trapped between the hydrophobic, corrugated surface of the leaf blades and the surrounding water constitute the major path of aeration. The conduction of gases through the internal air spaces of the leaf is negligible compared to the conduction of gases through the external air layers. The total volume of the air layers on both sides of a leaf blade is about 45% of the volume of the leaf blade itself. The size of the air layers around submerged leaf blades of cereals not adapted to conditions of partial flooding, e.g. of oats, barley, and wheat, is considerably smaller than that of rice. Gases move through the air layers not only by diffusion but also by mass flow. In darkness, air is drawn down from the atmosphere through the air layers along a pressure gradient created by solubilization of respiratory CO2 in the surrounding water. In light, photosynthetic O2 is expelled through the air layers to the atmosphere because the solubility of O2 in water is much lower than that of CO2. Air layers greatly increase the rate of photosynthetic carbon fixation by enlarging the surface of the gas-liquid interface available for CO2 uptake from the water. Air layers are vital for the survival of the partially submerged rice plant. When leaves are washed with a dilute solution of a surfactant (Triton X-100), no air layers are formed under water. Plants without air layers do not grow in response to submergence, and the submerged parts of the plant deteriorate as evident by rapid loss of chlorophyll and protein. Air layers provide a significant survival advantage even to completely submerged rice plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983 Jun;72(2):441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]