Abstract
Background
The scientific literature examining effective treatments for opioid dependent adults clearly indicates that pharmacotherapy is a necessary and acceptable component of effective treatments for opioid dependence. Nevertheless, no studies have been published that systematically assess the effectiveness of the pharmacological detoxification among adolescents.
Objectives
To assess the effectiveness of any detoxification treatment alone or in combination with psychosocial intervention compared with no intervention, other pharmacological intervention or psychosocial interventions on completion of treatment, reducing the use of substances and improving health and social status.
Search methods
We searched the Cochrane Central Register of Controlled Trials (2014, Issue 1), PubMed (January 1966 to January 2014), EMBASE (January 1980 to January 2014), CINHAL (January 1982 to January 2014), Web of Science (1991‐January 2014) and reference lists of articles.
Selection criteria
Randomised controlled clinical trials comparing any pharmacological interventions alone or associated with psychosocial intervention aimed at detoxification with no intervention, placebo, other pharmacological intervention or psychosocial intervention in adolescents (13 to 18 years).
Data collection and analysis
We used standard methodological procedures recommended by The Cochrane Collaboration
Main results
Two trials involving 190 participants were included. One trial compared buprenorphine with clonidine for detoxification. No difference was found for drop out: risk ratio (RR) 0.45 (95% confidence interval (CI): 0.20 to 1.04) and acceptability of treatment: withdrawal score mean difference (MD): 3.97 (95% CI ‐1.38 to 9.32). More participants in the buprenorphine group initiated naltrexone treatment: RR 11.00 (95% CI 1.58 to 76.55), quality of evidence moderate.
The other trial compared maintenance treatment versus detoxification treatment: buprenorphine‐naloxone maintenance versus buprenorphine detoxification. For drop out the results were in favour of maintenance treatment: RR 2.67 (95% CI 1.85, 3.86), as well as for results at follow‐up RR 1.36 [95% CI 1.05to 1.76); no differences for use of opiate, quality of evidence low.
Authors' conclusions
It is difficult to draw conclusions on the basis of two trials with few participants. Furthermore, the two studies included did not consider the efficacy of methadone that is still the most frequent drug utilised for the treatment of opioid withdrawal. One possible reason for the lack of evidence could be the difficulty in conducting trials with young people due to practical and ethical reasons.
Plain language summary
Detoxification treatments for opiate dependent adolescents
Detoxification treatment for heroin dependents adolescents
Review question
We reviewed the evidence on the effect of detoxification treatment compared with pharmacological maintenance treatment or psychosocial intervention in achieving abstinence on adolescents heroin dependents.
Background
Substance abuse among adolescents (13 to 18 years old) is a serious and growing problem. It is important to identify effective treatments for those who are opioid dependent. For adults, pharmacotherapy is a necessary and acceptable part of effective treatment. Detoxification agents are used to reduce withdrawal symptoms during managed withdrawal but the rate of completion of detoxification tends to be low, and rates of relapse are high. Withdrawal symptoms, particularly drug craving, may continue for weeks and even months after detoxification. The period of recovery from dependence is typically influenced by a range of psychological, social and treatment‐ related factors. Detoxification treatments include methadone, buprenorphine, and alpha2‐adrenergic agonists.
Study characteristics
The review authors searched the literature for randomised controlled trials investigating pharmacological interventions with or without psychosocial intervention aimed at detoxification in adolescents. They found only two trials, both conducted in the USA; one compared 28‐day treatment with buprenorphine, using tablets placed under the tongue, to wearing a clonidine patch in 36 opiate dependent adolescents who were treated as outpatients. The other trial compared maintenance treatment versus detoxification treatment: buprenorphine‐naloxone maintenance versus buprenorphine detoxification.
Key results
The trial comparing buprenorphine with clonidine reported a trend in favour of buprenorphine in reducing the drop‐out rate but no difference between treatments in the duration and severity of withdrawal symptoms. More participants in the buprenorphine group went on to long‐term naltrexone treatment. Side effects were not reported. In the second trial comparing buprenorphine maintenance versus buprenorphine for detoxification, for drop out the results were in favour of maintenance treatment, At one‐year follow‐ up, self‐reported opioid use was clearly less in the maintenance group and more adolescents were enrolled in other addiction programs. Conducting trials with young people may be difficult for both practical and ethical reasons.
Quality of the evidence
This review was limited by the very few number of trials retrieved and the quality of the evidence was moderate for the comparison between buprenorphine and clonidine and low for the comparison between buprenorphine detoxification and buprenorphine maintenance. The evidence is current to January 2014.
Summary of findings
Summary of findings for the main comparison. Buprenorphine versus clonidine for opiate dependent adolescents.
| Buprenorphine versus clonidine for opiate dependent adolescents | ||||||
| Patient or population: patients with opiate dependent adolescents Settings: Intervention: buprenorphine versus clonidine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Buprenorphine versus clonidine | |||||
| Drop out Number of participants who did not complete the detoxification treatment Follow‐up: 28 days | Study population | RR 0.45 (0.2 to 1.04) | 36 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| 611 per 1000 | 275 per 1000 (122 to 636) | |||||
| Moderate | ||||||
| 611 per 1000 | 275 per 1000 (122 to 635) | |||||
| Duration and severity of signs and symptoms of withdrawal Adjective rating scale Follow‐up: 28 days | The mean duration and severity of signs and symptoms of withdrawal in the control groups was ‐18.8 score | The mean duration and severity of signs and symptoms of withdrawal in the intervention groups was 3.97 higher (1.38 lower to 9.32 higher) | 32 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| Initiation of naltrexone treatment Number of participants initiating naltrexone Follow‐up: 28 days | Study population | RR 11 (1.58 to 76.55) | 36 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| 56 per 1000 | 611 per 1000 (88 to 1000) | |||||
| Moderate | ||||||
| 56 per 1000 | 616 per 1000 (88 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 only one study included 2 only one study with 36 participants included
Summary of findings 2. Buprenorphine detox compared with buprenorphine maintenance for opiate dependent adolescents.
| Buprenorphine detox compared with buprenorphine maintenance for opiate dependent adolescents | ||||||
| Patient or population: patients with opiate dependent adolescents Settings: Intervention: buprenorphine detox Comparison: buprenorphine maintenance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Buprenorphine maintenance | Buprenorphine detox | |||||
| Drop out Number of participants who dropped out from the study Follow‐up: 12 weeks | Study population | RR 2.67 (1.85 to 3.86) | 152 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 297 per 1000 | 794 per 1000 (550 to 1000) | |||||
| Moderate | ||||||
| 297 per 1000 | 793 per 1000 (549 to 1000) | |||||
| Patients with positive urine at the end of treatment Number of participants with urine positive for opiates Follow‐up: 12 weeks | Study population | RR 1.03 (0.82 to 1.28) | 152 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 662 per 1000 | 682 per 1000 (543 to 848) | |||||
| Moderate | ||||||
| 662 per 1000 | 682 per 1000 (543 to 847) | |||||
| Self‐reported use at 12 months follow‐up Number of participants who reported heroin used at follow‐up Follow‐up: 12 months | Study population | RR 1.36 (1.05 to 1.76) | 152 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 527 per 1000 | 717 per 1000 (553 to 928) | |||||
| Moderate | ||||||
| 527 per 1000 | 717 per 1000 (553 to 928) | |||||
| Enrolment in addiction treatment at 12 month follow‐up Number of participants enrolled in addiction treatment at follow‐up Follow‐up: 12 months | Study population | RR 0.75 (0.53 to 1.07) | 152 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 527 per 1000 | 395 per 1000 (279 to 564) | |||||
| Moderate | ||||||
| 527 per 1000 | 395 per 1000 (279 to 564) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 no allocation concealment 2 only one study with 154 participants 3 participants, providers and outcome assessor not blinded 4
Background
Description of the condition
Several studies have demonstrated that adolescent (less than 18 years old) substance abuse is a serious and growing problem (Altobelli 2005).
In Europe, the estimate of lifetime prevalence of use for young adults 15 to 34 years old is of 32.5% for cannabis, 6.3 % for cocaine, ranging from 0.7 % to 13.6 % in different countries, 5.5 % for amphetamines, ranging from under 0.6 % to 12.4 %; most countries reported estimates in the range of 2.1 to 5.8 % for ectasy and from 0.1 % to 5.4 % for LSD. National estimates vary widely between countries in all measures of prevalence. Opioids, mainly heroin, were cited as the primary drug by more than 200,000 clients reported entering specialist drug treatment in 29 European countries in 2010, or 48 % of all reported treatment entrants (EMCDDA 2012).
In Europe in 2011, the European School Survey Project on Alcohol and Other Drugs (ESPAD) collected data on substance use of more than 100,000 15 to 16‐year‐old European students from 36 countries. Nearly one in three (29%) students in the ESPAD countries perceived cannabis to be (fairly or very) easily available. On average, 18% of students have tried illicit drugs at least once during their lifetime. Most of them (17%) have used cannabis while 6% reported experience with drugs other than cannabis. After cannabis, amphetamines and ecstasy are in second position, each being mentioned by 3% of the students. Lifetime use of cocaine, crack and LSD or other hallucinogens was reported by fewer students (2%) and the rates for heroin and GHB were even lower (1%). Use of cannabis in the past 12 months was 13%, while use in the past 30 days was claimed to be 7% (ESPAD 2012).
In the USA, recent household survey data indicate 9.5 % of youths aged 12 to 17 were current illicit drug users. This rate was similar to the rates of current illicit drug use in 2005 to 2011, but it was lower than the rates from 2002 to 2004. In addition, 7.2 % of youths aged 12 to 17 were current users of marijuana, 2.8 % were current non medical users of psychotherapeutic drugs, 0.8 % were current users of inhalants, 0.6% were current users of hallucinogens, and 0.1 % were current users of cocaine (SAMHSA 2013).
In the USA after 1992, the proportion of young Americans with lifetime use of any drugs rose considerably to a recent high point of 55% in 1999; it then declined gradually to 47% in 2007 through 2009, and stands at 49% in 2012. The annual prevalence of heroin use among 12th graders fell by half between 1975 and 1979, from 1.0% to 0.5%. The rate then held amazingly steady until 1994. Use rose in the mid and late 1990s, along with the use of most drugs; it reached peak levels in 1996 among 8th graders (1.6%), in 1997 among 10th graders (1.4%), and in 2000 among 12th graders (1.5%), suggesting a cohort effect. Since those peak levels, use has declined, with annual prevalence in all three grades fluctuating between 0.7% and 0.9% from 2005 through 2011. Use has declined some in the past two years; in the three grades combined, the 2011 to 2012 decline from 0.7% to 0.6% was significant (Monitoring the Future 2013).
In 2010, most Australians aged 14 years and over (60%) had never used an illicit drug. However, around 15% had used one or more illicit drugs in the past 12 months. Cannabis was the most common illicit drug used recently (10.3%), followed by ecstasy (3.0%) and amphetamines and cocaine (each used by 2.1% of people). Many people who used an illicit drug in 2010 also used other drugs, illicit or licit ( AIHW 2011).
Patterns of drug use have changed over time. An analysis of treatment entry data between 2000 and 2009 showed a decrease in drug injection among primary heroin clients in all European countries (from 58 % to 36 %), particularly in western Europe (EMCDDA 2012). In addition, among opioid users entering treatment in outpatient settings since 2009, those smoking the drug outnumbered those injecting it (EMCDDA 2012).
Description of the intervention
Numerous medications have been successfully used in the treatment of adolescents with a broad array of psychiatric disorders (Hunt 1990; Kaminer 1995). In contrast, medications have been infrequently used in treating substance abuse disorders among adolescents, nevertheless they have generally been shown to be a promising component of such interventions (Kaminer 1995). The scientific literature examining effective treatments for opioid dependent adults clearly indicates that pharmacotherapy is a necessary and acceptable component of effective treatments for opioid dependence. Nevertheless, when young people must be treated, it probably is necessary to monitor the interventions in order to adapt them to this specific population. Different pharmacological agents have been used as detoxification agents to ameliorate withdrawal symptoms, however, the rate of completion of detoxification tends to be low, and rates of relapse to opioid use following detoxification are high (Gossop 1989; Vaillant 1988). Methadone may still be the medication that is most widely used but buprenorphine is seen as having some advantages for adolescents because of its excellent safety profile and the absence of long‐term complications (Levy 2007; Smith 2012). Younger patients who present for treatment of drug dependence often have a shorter history of drug use than treatment‐seeking adults. Treatment early in the course of the disorder presents the opportunity to prevent co‐morbidities associated with drug use, including acute and chronic medical conditions, and psychiatric and social complications (Levy 2007).
How the intervention might work
Managed withdrawal, or detoxification, is not in itself a treatment for dependence (Lipton 1983; Mattick 1996) but detoxification remains a required first step for many forms of longer‐term treatment (Kleber 1982). Withdrawal symptoms, particularly drug craving, may continue to be experienced for weeks and even for months after detoxification, and the period of recovery from dependence is typically influenced by a range of psychological, social and treatment‐ related factors.
Why it is important to do this review
We did not find any reviews in the published literature that assessed the effectiveness of detoxification treatment for adolescents. Many other Cochrane systematic reviews have been published on the effectiveness of various detoxification treatments: methadone (Amato 2013), buprenorphine (Gowing 2009), alpha2‐adrenergic agonists (Gowing 2014), opioid antagonists with minimal sedation (Gowing 2009b) and under heavy sedation (Gowing 2010), psychosocial combined with detoxification treatment (Amato 2011) and one review comparing inpatient versus outpatient settings for opioid detoxification (Day 2005), but none address the question of the effectiveness of treatments for adolescents.
Objectives
To assess the effectiveness of any detoxification treatment alone or in combination with psychosocial intervention compared with no intervention, other pharmacological intervention or psychosocial interventions on completion of treatment, reducing the use of substances and improving health and social status.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs).
Types of participants
Opiate dependent adolescents (13 to 18 years of age). There was no restriction for participants with physical or psychological illness.
Types of interventions
Experimental intervention
Any pharmacological interventions (methadone, buprenorphine, adrenergic agonists, symptomatics) alone or associated with psychosocial intervention aimed at detoxification
Control intervention
No intervention
Other pharmacological interventions
Psychosocial interventions alone
Types of comparisons foreseen
Any detoxification treatment versus no treatment
Any detoxification treatment versus other pharmacological treatment (e.g. methadone versus buprenorphine)
Any pharmacological treatment plus psychosocial treatment versus any pharmacological treatment alone
Any detoxification treatment versus any psychosocial treatment
Types of outcome measures
Primary outcomes
Drop outs measured as number of participants who did not complete the detoxification treatment.
Use of primary substance measured as number of participants with opiate positive urine analysis during and at the end of treatment or self‐reported data.
Acceptability of the treatment as A) duration and severity of signs and symptoms of withdrawal, including patient self‐rating B) side effects.
Results at follow‐up measured as number of participants who relapsed at the end of follow‐up.
Secondary outcomes
Engagement in further treatment measured as number of participants who enrolled in any psychosocial or pharmacological treatment.
Use of other substances of abuse.
Overdose, fatal or nonfatal.
Criminal activity.
Social functioning (integration at school or at work, family relationship).
Search methods for identification of studies
Electronic searches
For this update, we revised the search strategy and re‐ran searches in the following databases.
Cochrane Drugs and Alcohol Group's trials register (Jannuary 2014).
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 1).
MEDLINE (PubMed) (from 1966 ‐ to Jannuary 2014).
EMBASE (embase.com) (from 1980 ‐ to Jannuary 2014).
CINAHL (EBSCO) (1982 ‐ to Jannuary 2014).
Web of Science (1991 ‐ to Jannuary 2014).
Databases were searched using a strategy developed by incorporating the filter for identification of RCTs (Lefebvre 2011) combined with selected MeSH terms and free‐text terms related to alcohol dependence. For details on searchesseeAppendix 1; Appendix 3; Appendix 4; Appendix 5; Appendix 6.
We also searched some of the main electronic sources of ongoing trials.
Current Controlled Trials (www.controlled‐trials.com/).
Clinical Trials.gov (www.clinicaltrials.gov/).
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en).
Searching other resources
We also searched the following.
References of the articles obtained by any means.
Conference proceedings likely to contain trials relevant to the review (Annual Scientific Meeting of the College on Problems of Drug Dependence, European College of Neuropsychopharmacology, American Psychiatric Association).
By contacting investigators, and relevant trial authors seeking information about unpublished or incomplete trials.
All searches included non‐English language literature and studies with English abstracts were assessed for inclusion. When considered likely to meet inclusion criteria, studies were translated.
Data collection and analysis
Selection of studies
Two review authors (SM, CB) independently inspected the search 'hits' by reading titles and abstracts. Each potentially relevant study located in the search was obtained in full text and assessed for inclusion independently by two review authors (SM, LA). Any disagreements were resolved by discussion between the authors.
Data extraction and management
Three review authors (SM, LA, CB) independently extracted data. Any disagreements were discussed and resolved by consensus.
Assessment of risk of bias in included studies
We changed the criteria to assess methodological quality of included studies from that outlined in the protocol to conform the review to the recommended methods outlined in the Cochrane Reviewers Handbook version 5.0.0 and to the requirements of RevMan5 (Cochrane Handboook 2008).
The recommended approach for assessing risk of bias in studies included in Cochrane Review is a two‐part tool, addressing six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of "Yes" indicates low risk of bias, "No" indicates high risk of bias, and "Unclear" indicates unclear or unknown risk of bias. To make these judgments we used the criteria indicated by the handbook and their applicability in the addiction field. For a detailed description of the criteria used see Cochrane Handboook 2008.
The domains of sequence generation, allocation concealment (avoidance of selection bias) and selective outcome reporting (avoidance of reporting bias) have been addressed in the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessors (avoidance of performance bias and detection bias) was considered separately for objective outcomes (drop out, use of substance of abuse measured by urine‐analysis, subjects relapsed at the end of follow up, subjects engaged in further treatments) and subjective outcomes (duration and severity of signs and symptoms of withdrawal, including patient self‐rating, side effects, social functioning as integration at school or at work, family relationships).
Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except for drop out from the treatment, which is very often the primary outcome measure in trials on addiction. It have been assessed separately for results at the end of the study period and for results at follow up.
For drop out from treatment we judged that only sequence generation and allocation concealment could be relevant because lack of blinding is unlikely to influence data collection and incomplete outcome data could not be used for this outcome. For use of substances assessed by urine analysis we judged that sequence generation, allocation concealment and incomplete outcome data could influence results. For subjective outcomes we judged that also lack of blinding of outcome assessor could influence data.
Measures of treatment effect
Dichotomous outcomes were analysed calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed by their confidence intervals (CIs). Continuous outcomes were analysed calculating the mean difference (MD), again with 95% CIs.
The drop out from treatment was reported as the number of participants who did not complete the detoxification treatment. The use of primary substance was reported as the number of participants with opiate positive urine analysis during and at the end of treatment, or self‐report data.The results at follow‐up were measured as number of participants who had relapsed at the end of follow‐up. We did not use data presented as number of positive urine tests over total number of tests in the experimental and control group as a measure of substance abuse. This is because using the number of tests instead of the number of participants as the unit of analysis violates the hypothesis of independence among observations. In fact, the results of tests done in each participant are not independent. For outcomes assessed by scales, we compared and pooled the mean score differences from the end of treatment to baseline (post minus pre) in the experimental and control group. In case of missing data about the standard deviation of the change, we imputed this measure using the standard deviation at the end of treatment for each group.
Assessment of heterogeneity
Heterogeneity was analysed by means of the I2 statistic and Chi2 test for heterogeneity.The cut‐off points were I2 > 50% and P of the Chi2 test < 0.1.
Assessment of reporting biases
We planned to use funnel plots (plots of the effect estimate from each study against the sample size or effect standard error) to assess the potential for bias related to the size of the trials, which could indicate possible publication bias, but did not as we included only two trials.
Data synthesis
We planned to combine the relative risk (RR) or the weighted mean difference (WMD) from the individual trials through meta‐analysis where possible (comparability of intervention and outcomes between trials) using a random‐effects model as some variability was expected in the studies included. We included only two trials with different comparisons, which prevented the possibility of performing meta‐analysis. Accordingly, we used a fixed‐effect method with risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data..
Sensitivity analysis
To incorporate assessment of risk of bias in the review process, we planned to first plot intervention effects estimates stratified for risk of bias for each relevant domain. If differences in results were present among studies at different risk of bias, we planned to perform sensitivity analysis excluding from the analysis studies with a high risk of bias. This was not done because only one study was included in each comparison of the review.
Results
Description of studies
Results of the search
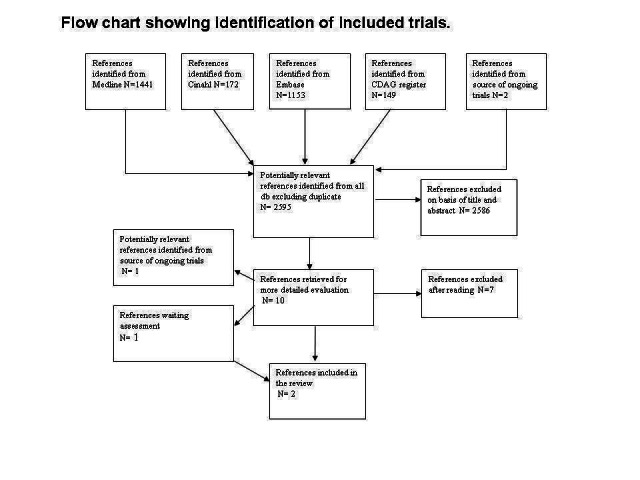
This is an update of a Cochrane review first published in 2009. In the first version of our review we identified 2917 references. After excluding duplicate articles, we identified 2595 potentially relevant references. We excluded 2586 on the basis of title and abstracts leaving 10 studies which were acquired in full text for more evaluation. Out of these, seven studies were excluded, one was included, one is an ongoing trial and one study was classified as study awaiting assessment because it is finished but not yet published and the authors could not give us the data. Immediately before the publication of the review (November 2008) the ongoing study was published, so we decided to include it. See Figure 1.
1.
Flow chart of studies of the review published in 2009
In the 2014 update, we retrieved 1004 further references after excluding duplicates. We further excluded 986 articles on the basis of title and abstract and 15 were acquired in full text for more detailed evaluation. Out of the 15 studies, two (Woody 2009 and Woody 2013) were errata corrige of Woody 2008. One study (with two conference proceedings) was classified as awaiting classification because although the study is finished, it is not yet published and the authors could not give us the data (Marsh 2009).The other 11 retrieved studies were excluded. See Figure 2.
2.

Study flow diagram. 2014 update
For substantive descriptions of studies seeCharacteristics of included studies and Characteristics of excluded studies tables.
Included studies
Two studies met the inclusion criteria (Marsch 2005; Woody 2008). No further studies were retrieved for inclusion in the 2014 update.
Type of comparison: buprenorphine sublingual tablets versus clonidine patch (Marsch 2005; buprenorphine‐naloxone maintenance versus buprenorphine detox (Woody 2008)
Participants: 190 opiate dependent adolescents (13 to 21 years old)
Duration of the trial: 28 days (Marsch 2005); 12 weeks (Woody 2008)
Setting: outpatients
Country: USA
Excluded studies
Overall, 18 studies did not meet the criteria for inclusion in this review. The grounds for exclusion were: study design not in the inclusion criteria: not RCT or CCT: five studies (Ebner 2007; Fiellin 2008; Godley 2004; Lloyd 1974; Moore 2014); experimental intervention not in the inclusion criteria: only psychosocial intervention without pharmacological detoxification: two studies (Baer 2007; Kemp 2007); maintenance treatment: one study (Lehmann 1973); different psychosocial intervention given to two groups receiving the same pharmacological intervention: one study (Forcehimes 2008); outcome not in the inclusion criteria: six studies (Chakrabarti 2010, Hill 2013, Polsky 2010; Subramaniam 2011; Warden 2012; Wilcox 2013); participant not in the inclusion criteria: one study (Mannelli 2011); study design and participants not in the inclusion criteria: one study (Mullen 2010); secondary analysis of the Marsch 2005 study without distinction between experimental and control condition (Moore 2011).
Risk of bias in included studies
3.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation and allocation concealment: we judged one study as at unclear risk of bias (Marsch 2005). The other study (Woody 2008) was judged to be at low risk of bias for sequence generation and at high risk of bias for allocation concealment.
Blinding
Both studies were judged to be at a low risk of bias for objective outcomes. For subjective outcomes, one study (Marsch 2005) was judged at low risk of bias, whereas the other (Woody 2008), was judged to be at a high risk of bias.
Incomplete outcome data
Both studies were judged as at low risk of bias.
Selective reporting
Both studies were judged as at low risk of bias.
Effects of interventions
No meta‐analysis was performed because the two studies assessed different comparisons.
Comparison 1: any detoxification treatment versus other pharmacological treatment: buprenorphine versus clonidine
See Table 1
Primary outcomes
Drop out from treatment: risk ratio (RR): 0.45 (95% confidence interval (CI): 0.20 to 1.04); the result is not statistically significant but there is a trend in favour of buprenorphine. See Analysis 1.1.
1.1. Analysis.
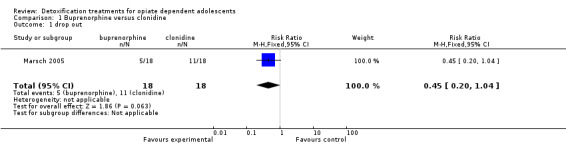
Comparison 1 Buprenorphine versus clonidine, Outcome 1 drop out.
Acceptability of the treatment:
duration and severity of signs and symptoms of withdrawal: Adjective rating scale: mean difference (MD): 3.97 (95% CI ‐1.38 to 9.32); the result is not statistically significant. See Analysis 1.2.
side effects: side effects were not reported in the study.
1.2. Analysis.
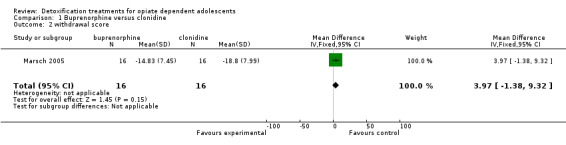
Comparison 1 Buprenorphine versus clonidine, Outcome 2 withdrawal score.
Secondary outcomes
Engagement in further treatment: measured as the number of participants who enrolled in any psychosocial or pharmacological treatment: initiation of naltrexone treatment: RR 11.00 (95% CI 1.58 to 76.55); the result is in favour of buprenorphine. See Analysis 1.3.
1.3. Analysis.
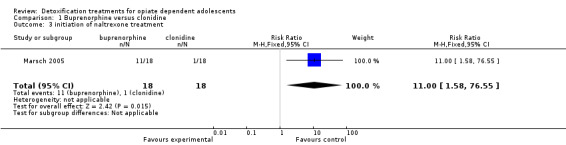
Comparison 1 Buprenorphine versus clonidine, Outcome 3 initiation of naltrexone treatment.
Comparison 2: maintenance treatment versus detoxification treatment: buprenorphine‐naloxone maintenance for nine weeks then tapered to 12 weeks versus buprenorphine detoxification 14 days
See Table 2
Primary outcomes
Drop out from treatment: RR 2.67 (95% CI 1.85 to 3.86); in favour of maintenance treatment. See Analysis 2.1.
Use of substance of abuse: no significant difference. See Analysis 2.2.
Results at follow‐up: self‐reported heroin use at 12 months: RR 1.36 (95% CI 1.05 to 1.76); in favour of maintenance treatment. See Analysis 2.3.
Enrolment in addiction treatment at 12 months: RR: 0.75 (0.53 to 1.07); there is a trend in favour of maintenance treatment. See Analysis 2.4.
2.1. Analysis.
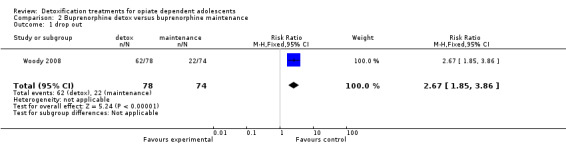
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 1 drop out.
2.2. Analysis.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 2 patients with positive urine at the end of treatment.
2.3. Analysis.
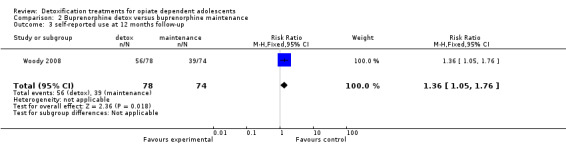
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 3 self‐reported use at 12 months follow‐ up.
2.4. Analysis.
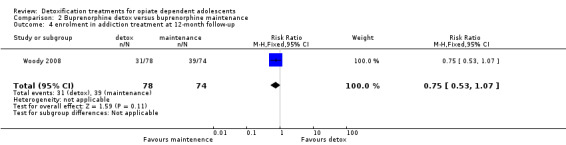
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 4 enrolment in addiction treatment at 12‐month follow‐up.
Secondary outcomes
Use of other substances of abuse: no significant difference for alcohol and marijuana; RR 8.54 (95%CI 1.11 to 65.75); in favour of maintenance treatment. See Analysis 2.5; Analysis 2.6; Analysis 2.7.
Side effects: the authors reported that no serious side effects attributable to buprenorphine‐naloxone were reported and no patients were removed from the study for side effects. The most common side effect was headache, which was reported by 16% to 21% of patients in both groups.
Mortality any cause: one death for methadone overdose occurred in the maintenance group in a patient who dropped out after three doses and was not located until her obituary appeared in a newspaper three months later.
2.5. Analysis.
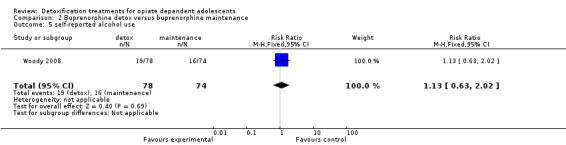
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 5 self‐reported alcohol use.
2.6. Analysis.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 6 self‐reported marijuana use.
2.7. Analysis.
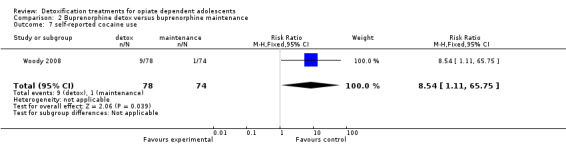
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 7 self‐reported cocaine use.
Discussion
Summary of main results
Despite a comprehensive search of published and unpublished literature only two studies were found. One (Marsch 2005) compared buprenorphine and clonidine for detoxification of adolescents. The study found no difference in drop‐out rate and in withdrawal symptoms even if the difference in drop‐out rate is nearly significant in favour of buprenorphine. More participants in the buprenorphine group initiated naltrexone treatment. The other study (Woody 2008) compared maintenance treatment with buprenorphine‐naloxone for nine weeks then tapered until 12 weeks with 14 days detoxification with buprenorphine. Maintenance treatment seems more efficacious in retaining patients in treatment but not in reducing patients with positive urine at the end of the study. Self‐reported opioid use at one year follow‐up was significantly lower in the maintenance group even if both groups reported high level of opioid use and more patients in the maintenance group were enrolled in other addiction treatment at 12‐month follow‐up.
Overall completeness and applicability of evidence
One study (Marsch 2005) with few participants has too little evidence to draw any conclusions about the superiority of buprenorphine over clonidine. Moreover, there are no studies comparing pharmacological detoxification with psychosocial intervention alone, which is the most used approach to treat opioid dependent adolescents. The study of Woody 2008 compares a short‐term maintenance treatment with a 14‐day detoxification. More than a maintenance treatment, the 12 week buprenorphine‐naloxone could be considered a long‐term detoxification following a two‐month stabilisation period. Only one study with 150 participants has too little evidence to draw any firm conclusions.
Quality of the evidence
The quality of evidence was judged as moderate for the comparison between buprenorphine versus clonidine . The main reason for downgrading was due to the fact that only one study with 36 participants was found for this comparison. The quality of evidence was judged as low for the comparison between buprenorphine detoxification versus buprenorphine maintenance The reasons were that there was no allocation concealment, no blinding of participants, personnel and outcome assessor, and because only one trial with 154 participants has been found for this comparison.
Potential biases in the review process
A particularly important component of a review is the identification of relevant studies. Publication bias has long been recognised as a problem in this regard since it means that the likelihood of finding studies is related to the results of those studies. One way to investigate whether a review is subject to publication bias is to prepare a ‘funnel plot’ and examine this for signs of asymmetry. We could not explore the possibility of publication bias by funnel plot because only two studies was retrieved. We looked for all potentially relevant studies by a comprehensive search, which considered also conference proceedings and registers of ongoing trials. We wrote to the author of the only published trial asking for other trials but she did not answer. We also looked at references of published narrative reviews.
Authors' conclusions
Implications for practice.
It is difficult to draw conclusions on the basis of two trials with few participants. One possible reason for the lack of evidence could be the difficulty in conducting trials with young people due to practical and ethical reasons.
Implications for research.
There is an urgent need of randomised controlled trials comparing pharmacological detoxification versus psychosocial intervention and trials comparing pharmacological intervention plus psychosocial intervention versus psychosocial intervention alone before realising trials which compare different pharmacological approaches. These studies should have a long follow‐up measuring results of relapse after the end of treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2014 | New citation required but conclusions have not changed | No new studies included. |
| 10 April 2014 | New search has been performed | New search. Backround updated, 'Summary of findings' tables created. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 28 July 2009 | Amended | We emended the abstract's errors |
| 10 February 2009 | New search has been performed | to be published as review in the issue 2, 2009 |
| 15 October 2008 | New search has been performed | stage changed in review |
| 20 March 2008 | Amended | Converted to new review format. |
| 27 June 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
We want thank Zuzana Mitrova for her help in all the steps of the review process and Simona Vecchi who performed the bibliographic search.
Appendices
Appendix 1. Cochrane Drugs and Alcohol Group's trials register search strategy
Free text=detox* or withdraw* or abstinen* or abstain* AND Diagnosis = opiate* or opioid*
Appendix 2. CENTRAL search strategy
MeSH descriptor: [Opioid‐Related Disorders] explode all trees
((drug or substance) near (abuse* or addict* or dependen* or disorder*)):ti,ab,kw (Word variations have been searched)
((opioid* or opiate*) near (abuse* or addict* or dependen*)):ti,ab,kw (Word variations have been searched)
(detox* or desintoxi* or disintoxi*):ti,ab,kw
#1 or #2 or #3 or #4
MeSH descriptor: [Heroin] explode all trees
(opioid* or opiate* or opium or heroin):ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Methadone] explode all trees
"methadone":ti,ab,kw (Word variations have been searched)
#6 or #7 or #8 or #9
#5 AND #10
Appendix 3. PubMed search strategy
"Opioid‐Related Disorders"[MeSH]
(detox*[tiab] OR withdraw*[tiab] OR abstinen*[tiab] OR abstain*[tiab])
(opioid*[tiab] AND (abuse*[tiab] OR addict*[tiab] OR dependen*[tiab]))
((drug[tiab] OR substance[tiab]) AND (use*[tiab] OR abuse*[tiab] OR misuse*[tiab] OR addict*[tiab] OR dependen*[tiab] OR disorder*[tiab]))
#1 OR #2 OR #3 OR #4
heroin [MeSH]
heroin [tiab]
opioid*[tiab] OR opiate* [tiab]
methadone [MeSH]
methadone [MeSH]
#6 OR #7 OR #8 OR #9 OR #10
adolescent [MeSH]
adolescen* OR teen* OR young people OR young person* OR young adult* OR youth* OR girl* OR boy* OR juvenile*
#12 OR #13
randomized controlled trial [pt]
controlled clinical trial [pt]
random*[tiab]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trials [tiab]
groups [tiab]
#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22
animals [mh] NOT humans [mh]
#23 NOT #24
#5 AND #11 AND #14 AND #25
Appendix 4. Embase search strategy
drug abuse/exp
addiction/exp
((drug OR substance) NEAR/5 (abuse* OR depend* OR addict*)):ab,ti
detox*:ab,ti OR withdraw*:ab,ti OR abstinen*:ab,ti OR abstain*:ab,ti
#1 or #2 or #3 or #4
'diamorphine'/exp
'methadone'/exp
heroin:ab,ti OR methadone:ab,ti OR opioid*:ab,ti OR opiate*:ab,ti
#6 or #7 or #8 or 9
'adolescent'/exp OR adolescen*:ab,ti OR teen*:ab,ti OR youth*:ab,ti OR girl$*:ab,ti OR boy*:ab,ti OR juvenile*:ab,ti OR (young NEAR/3 people):ab,ti OR (young NEAR/3 person*):ab,ti OR (young NEAR/3 adult*):ab,ti
'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR 'randomized controlled trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
#5 AND #9 AND #10 AND #11AND [embase]/lim
Appendix 5. CINAHL search strategy
(MH "Substance Use Disorders+")
TX(detox* or withdraw* or abstinen* or abstain*)
TX((opioid* or opiate*) and (abuse* or addict* or dependen*))
S1 or S2 or S3
MH heroin or TX heroin
TX (opioid* or opiate*)
TX opium
MH methadone or TX methadone
S5 or S6 or S7 or S8
MH adolescence
TI adolescen* or TI teen* or TI young people or TI young person* or TI young adult* or TI youth* or TI girl* OR TIboy* or TI juvenile*
AB adolescen* or AB teen* or AB young people or AB young person* or AB young adult* or AB youth* or AB girl* OR AB boy* or AB juvenile*
S10 or S11 or S12
MH "Clinical Trials+"
PT Clinical trial
TI clinic* N1 trial* or AB clinic* N1 trial*
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
TI randomi?ed control* trial* or AB randomi?ed control* trial*
MH "Random Assignment"
TI random* allocat* or AB random* allocat*
MH "Placebos"
TI placebo* or AB placebo*
MH "Quantitative Studies"
S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24
S4 and S9 and S13 and S25
Appendix 6. Web of Science search strategy
TS=((( heroin OR opiate* OR opioid* OR methadone) same (abuse* OR depend* OR addict* OR disorder* OR detox* OR withdraw* OR abstinen* OR abstain*))) AND TS=(adolescen* OR teen* OR young people OR young person* OR young adult* OR early adult* OR youth* OR girl* OR boy* OR juvenile*)
TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#2 AND #1
Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=2008‐2013
Data and analyses
Comparison 1. Buprenorphine versus clonidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drop out | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.20, 1.04] |
| 2 withdrawal score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 3.97 [‐1.38, 9.32] |
| 3 initiation of naltrexone treatment | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [1.58, 76.55] |
Comparison 2. Buprenorphine detox versus buprenorphine maintenance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drop out | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.85, 3.86] |
| 2 patients with positive urine at the end of treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.28] |
| 3 self‐reported use at 12 months follow‐ up | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.76] |
| 4 enrolment in addiction treatment at 12‐month follow‐up | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |
| 5 self‐reported alcohol use | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.02] |
| 6 self‐reported marijuana use | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.83, 3.00] |
| 7 self‐reported cocaine use | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.54 [1.11, 65.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Marsch 2005.
| Methods | Randomised controlled trial. Recruitment modality: self‐referred participants. | |
| Participants | 36 adolescents (13‐18 years) who met the DSM‐IV criteria for opioid dependence. Pregnant women and patients with significant psychiatric disorders (e.g. psychosis) or medical illnesses (e.g. cardiovascular disease) were excluded. Mean age. 17.35 years; 39% male; 97% white. Injection route of opiate use: 36%; other drug dependence alcohol: 17.5%, cannabis: 17%, cocaine: 10%, amphetamine: 6%. |
|
| Interventions | (1) Buprenorphine detoxification: sublingual buprenorphine tablets daily with flexible dosing procedure based on weight and self‐reported opiate use at intake (starting dose range: 6 mg‐ 8 mg). Buprenorhine dose that decreased by 2 mg every 7 days. Behavioural therapy 3 one‐hour individual sessions per week. Contingency management approach: participants could earn a voucher on the provision of opioid negative urine samples. At the end of the study, participants were offered naltrexone. (2) Transdermal clonidine patches 0.1 mg on intake day and day 1; a second patch was added on day 2 and worn until day 6. An optional third patch (depending on the severity of withdrawal symptoms) may have been added on day 4 and worn until day 6. All patches were removed on day 7 and replaced with a 0.2 mg doses. On day 14 the patches were removed again and replaced with a 0.1 mg dose patch. On day 21 the patches were removed again and replaced with a 0 mg dose. Behavioural therapy 3 one‐hour individual session per week. Contingency management approach: participants could earn a voucher on the provision of opioid negative urine samples. At the end of the study, participants were offered naltrexone. Durattion of the trials: 28 days. |
|
| Outcomes | Drop out from treatment measured as the percentage of patients who did not complete the entire detoxification treatment. Time retained in treatment. Opiate abstinence as the percentage of scheduled urine samples opiate negative. Other drug use as percentage of urine samples positives. Acceptability of the treatment: withdrawal effect measured by the Adjective rating scale. Initiation of naltrexone treatment as percentage of patients who initiated. | |
| Notes | Country: USA Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to either detoxification with clonidine or with buprenorphine. In this process participants were stratified for sex and past month route of opiate use (injection vs intranasal)" |
| Allocation concealment (selection bias) | Unclear risk | "participants were randomly assigned to either detoxification with clonidine or with buprenorphine. In this process participants were stratified for sex and past month route of opiate use (injection vs intranasal)" |
| Blinding (performance bias and detection bias) objective outcomes (drop out, use of substance measured by urine‐analysis, abstinent at follow‐up, initiation of naltrexone treatment) | Low risk | "The study used a parallel group, double blind, double dummy design". Participants in the clonidine group received placebo buprenorphine tablets and patients in the buprenorphine group received placebo clonidine patches" COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | "The study used a parallel group, double blind, double dummy design". Participants in the clonidine group received placebo buprenorphine tablets and patients in the buprenorphine group received placebo clonidine patches" COMMENT: blinding of participants and personnel. Not specified if research staff members who assessed subjective outcomes were blind but we judge that they probably were. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "the primary analysis (drop out, time retained, use of substance) included all participants randomised independently to drop out/non compliance, consistent with an intention to treat approach. All secondary outcomes (withdrawals symptoms and signs) were confined to the data from treatment intake to the end of the first week when retention was still high in both condition" |
| Selective reporting (reporting bias) | Low risk | |
Woody 2008.
| Methods | Multicentre randomised controlled trial. Recruitment modality described. | |
| Participants | 154 participants who met the DSM IV diagnostic criteria for opioid dependence and who sought outpatient treatment.152 randomised. Mean age: 19 years. Only one participant was 15 years old and no participants were 14 years old. Male: 59%. White: 56%. | |
| Interventions | (1) Maintenance group:12 weeks buprenorphine. Naloxone: up to 24 mg/day buprenorphine and 0.5 mg naloxone for 9 weeks and then tapered to week 12. :74 patients. (2) Detoxification group: 2 weeks buprenorphine. Naloxone: up to 14 mg/day buprenorphine and then tapered to day 14: 78 patients. Both groups were offered 1 weekly individual and 1 group counselling. |
|
| Outcomes | Primary outcome: opioid positive urine test results at weeks 4, 8 and 12. Secondary outcomes: drop out, self‐reported use, enrolment in addiction treatment outside the assigned condition, other drug use, adverse events. Results at 6,9,12 months follow‐up: self‐reported opioid use, self‐reported other drug use, other addiction treatment received. |
|
| Notes | Country: USA Setting: outpatients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred through an automated 24‐hour service at the Veterans Affairs Cooperative Studies Program in Perry Point, Maryland, that was programmed
to randomise patients separately by site. At each site, a biased coin randomisation protected against severe imbalance of sex, ethnicity, route of administration, and age across the treatment groups. Age was dichotomised as 14 to 18 years or 18 to 21 years, ethnicity as the majority ethnic group vs all others within the site, and route of administration as injecting or non injecting. |
| Allocation concealment (selection bias) | High risk | Balance was assessed by comparing the group sum of the binary indicators as each new patient was randomised. If both groups were balanced when a new patient was being randomised, then each group had an allocation probability of 1/2; if there was an imbalance, then the group with the higher score on the sum of indicators received an allocation probability of 1/3 and the other group a probability of 2/3. |
| Blinding (performance bias and detection bias) objective outcomes (drop out, use of substance measured by urine‐analysis, abstinent at follow‐up, initiation of naltrexone treatment) | Low risk | Patients and providers impossible to be blinded for the nature of the intervention (14 days detox vs 12 weeks maintenance). COMMENT: objective outcomes unlikely to be biased by lack of blinding. |
| Blinding (performance bias and detection bias) Subjective outcome | High risk | Patients and providers impossible to be blinded for the nature of the intervention (14 days detox vs 12 weeks maintenance) Outcome assessor not blinded: "Research assistant likely knew groups assignment because the study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants withdrawn from the study reported for each group. Reason for withdrawal given. Analysis on the basis of the Intention‐to‐treat principle: "patients were contacted at all assessment point regardless of whether they remained in treatment". |
| Selective reporting (reporting bias) | Low risk | |
DSM IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baer 2007 | Type of intervention not in the inclusion criteria: only psychosocial intervention without pharmacological detoxification |
| Chakrabarti 2010 | Outcome not in the inclusion criteria: baseline patient characteristics of Woody 2008 trial |
| Ebner 2007 | Study design not in the inclusion criteria: not RCT or CCT |
| Fiellin 2008 | Study design not in the inclusion criteria: not RCT or CCT |
| Forcehimes 2008 | Type of intervention not in the inclusion criteria: psychosocial intervention; the same pharmacological intervention given to both groups |
| Godley 2004 | Study design not in the inclusion criteria: not RCT or CCT |
| Hill 2013 | Outcome not in the inclusion criteria: association between cannabis use during opioid dependence treatment and positive urine drug screens for opioids; no raw data about cannabis use in the two groups provided |
| Kemp 2007 | Type of intervention not in the inclusion criteria: only psychosocial intervention without pharmacological detoxification |
| Lehmann 1973 | Type of intervention not in the inclusion criteria: maintenance treatment |
| Lloyd 1974 | Study design not in the inclusion criteria: not RCT or CCT |
| Mannelli 2011 | Participants not in the inclusion criteria: adults |
| Moore 2011 | Secondary analysis of the all sample of the Marsch 2005 study without distinction between experimental and control condition |
| Moore 2014 | Study design not in the inclusion criteria: qualitative study |
| Mullen 2010 | Study design and participants not in the inclusion criteria: observation cohort study on adult population |
| Polsky 2010 | Outcome not in the inclusion criteria: cost effectiveness analysis of the Woody 2008 trial |
| Subramaniam 2011 | Outcome not in the inclusion criteria: Predictors of Abstinence: secondary analysis of the Woody 2008 trial |
| Warden 2012 | Outcome not in the inclusion criteria:Predictors of attrition: secondary analysis of the Woody 2008 trial |
| Wilcox 2013 | Outcome not in the inclusion criteria: Concordance between self‐report and urine drug screen data: secondary analysis of the Woody 2008 trial |
CCT: controlledclinical trial RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Marsh 2009.
| Methods | Double blind randomised controlled trial |
| Participants | 53 opioid dependents adolescents and young adults (age 13‐24 eligible) |
| Interventions | Experimental: buprenorphine taper of 28 days Control: buprenorphine taper of 63 days |
| Outcomes | Retention in treatment; use of primary substance of abuse measured by urine analysis |
| Notes | Author contacted; study ended but definite results not yet published |
Differences between protocol and review
We changed the criteria to assess methodological quality of included studies from that described in the protocol to conform to the recommended methods outlined in the Cochrane Handboook 2008 and to the requirements of RevMan5.
Contributions of authors
Silvia Minozzi and Cristina Bellisario inspected the search 'hits' by reading titles and abstracts. Silvia Minozzi, Laura Amato and Cristina Bellisario extracted data, Silvia Minozzi wrote the review, Laura Amato commented and drafted conclusions. Marina Davoli commented on the final draft.
Sources of support
Internal sources
Department of Epidemiology, ASL RM E, Italy.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Marsch 2005 {published data only}
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, et al. Comparison of pharmacological treatments for opioid‐dependent adolescents. Archives of General Psychiatry 2005;62(10):1157‐64. [DOI] [PubMed] [Google Scholar]
Woody 2008 {published data only}
- Woody GE. Extended vs short‐term buprenorphine‐naloxone for treatment of opioid‐addicted youth: a randomized trial (Errata corrige). JAMA 2009;301(8):830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE. Extended vs short‐term buprenorphine‐naloxone for treatment of opioid‐addicted youth: a randomized trial (Errata corrige). JAMA 2013;319(14):1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short‐term buprenorphine‐naloxone for treatment of opioid‐addicted youth. JAMA 2008;300(17):2003‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baer 2007 {published data only}
- Baer JS, Garret SB, BeadnellB, Wells EA, Peterson PL. Brief motivational intervention with homeless adolescents: evaluating effects on substance use and service utilization. Psychology of Addictive Behaviors 2007;21(4):582‐6. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2010 {published data only}
- Chakrabarti A, Woody GE, Griffin ML, Subramaniam G, Weiss RD. Predictors of buprenorphine‐naloxone dosing in a 12‐week treatment trial for opioid‐dependent youth: secondary analyses from a NIDA Clinical Trials Network study. Drug and Alcohol Dependence 2010;107(2‐3):253‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebner 2007 {published data only}
- Ebner R, Zierer C, Schreiber W. After detoxification is before detoxification? Long term outcome of drug dependent adolescent [Nach dem Entzug=vor demEntzug? Langzeitvrlauf bei drogenabhangigen Jugendlichen]. Psychiatrische Praxis 2007;34(Suppl 1):s42‐3. [Google Scholar]
Fiellin 2008 {published data only}
- Fiellin DA. Treatment of adolescent opioid dependence: no quick fix. JAMA 2008;300(17):2057‐9. [DOI] [PubMed] [Google Scholar]
Forcehimes 2008 {published data only}
- Forcehimes AA, Bogenschutz MP, Tonigan JS, Woody GE. Impacy of therapeutic alliance on treatment outcome in opioid dependent adolescent and young adult treated with buprenorphine. Proceedings of the70th Annual Scientific Meeting of the College on Problems of Drug Dependence. 2008.
Godley 2004 {published data only}
- Godley SH, Dennis ML, Godely MD, Funk RR. Thirty‐months relapse trajectory cluster groups among adolescents discharged from out‐patients treatment. Addiction 2004;99(Suppl 2):129‐39. [DOI] [PubMed] [Google Scholar]
Hill 2013 {published data only}
- Hill KP, Bennett HE, Griffin ML, Connery HS, Fitzmaurice GM, Subramaniam G, et al. Association of cannabis use with opioid outcomes among opioid‐dependent youth. Drug and Alcohol Dependence 2013;132(1‐2):342‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 2007 {published data only}
- Kemp R, Harris A, Vurel E, Siharthan T. Stop using stuff: trial of a drug and alcohol intervention for young people with comorbid mental illness and drug and alcohol problems. Australasian Psychiatry 2007;15(6):490‐3. [DOI] [PubMed] [Google Scholar]
Lehmann 1973 {published data only}
- Lehmann WX. The use of 1‐alpha‐acetyl‐methadol (LAAM) as compared to methadone in the maintenance and detoxification of young heroin addicts. NIDA monograph 1973;8:82‐3. [PubMed] [Google Scholar]
Lloyd 1974 {published data only}
- Lloyd RA, Katon RN, DuPont RL. Evolution of a treatment approach for young heroin addicts. Comparison of three treatment modalities. International Journal of Addiction 1974;9(2):229‐39. [DOI] [PubMed] [Google Scholar]
Mannelli 2011 {published data only}
- Mannelli P, Peindl K, Patkar AA, Wu LT, Tharwani HM, Gorelick D A. Problem drinking and low‐dose naltrexone‐assisted opioid detoxification.. Journal of Studies on Alcohol and Drugs 2011;72(3):507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011 {published data only}
- Moore SK, Marsch LA, Badger GJ, Solhkhah R, Hofstein Y. Improvement in psychopathology among opioid‐dependent adolescents during behavioral‐pharmacological treatment. Journal of Addiction Medicine 2011;5(4):264‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014 {published data only}
- Moore SK, GuarinoH, Marsch. L. A. This is not who I want to be:" experiences of opioid‐dependent youth before, and during, combined buprenorphine and behavioral treatment. Substance Use & Misuse 2014;49(3):303‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullen 2010 {published data only}
- Mullen L, Keenan E, Barry J, Long J, Mulholland D, Grogan L, et al. Factors predicting completion in a cohort of opiate users entering a detoxification programme. Irish Journal of Medical Science 2010;179(4):569‐73. [DOI] [PubMed] [Google Scholar]
Polsky 2010 {published data only}
- Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE. Cost‐effectiveness of extended buprenorphine‐naloxone treatment for opioid‐dependent youth: data from a randomized trial. Addiction 2010;105(9):1616‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2011 {published data only}
- Subramaniam GA, Warden D, Minhajuddin A, Fishman MJ, Stitzer ML, Adinoff B, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid‐dependent youth. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(11):1120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warden 2012 {published data only}
- Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Adaptive Behavior 2012;37(9):1046‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcox 2013 {published data only}
- Wilcox CE, Bogenschutz MP, Nakazawa M, Woody G. Concordance between self‐report and urine drug screen data in adolescent opioid. Adaptive Behavior 2013;38(10):2568‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Marsh 2009 {published data only}
- March L, Moore SK, Solhkhah R, Badger GJ. Predictors of outcome in Buprenorphine treatment for opioid‐dependent youth. Proceedings of the 71th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2009 June 20‐25; Reno/Sparks, Nevada, USA. 2009.
- Marsch LA, MooreS, Solhkhah R. A randomized, controlled trial of buprenorphine dosing regimens for opioid dependent youth. Proceedings of the 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence; 2011 June 18‐23, Hollywood, Florida. 2011.
Additional references
AIHW 2011
- Australian Institute of Health and Welfare (AIHW). Drugs in Australia 2010: tobacco, alcohol and other drugs. Drug statistics series no. 27. Cat. no. PHE 154. Canberra 2011.
Altobelli 2005
- Altobelli E, Rapacchietta L, Tiberti S, Petrocelli R, Cicioni L, Orio F, et al. Association between drug, alcohol and tobacco use in adolescents and socio‐familiar factors [Associazione tra l'uso di sostanze stupefacenti, alcol e tabacco negli adolescenti e contesto socio‐familiare]. Annali d'Igiene 2005;17:57‐65. [PubMed] [Google Scholar]
Amato 2011
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD005031] [DOI] [PubMed] [Google Scholar]
Amato 2013
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003409.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handboook 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration.. Available from www.cochrane‐handbook.org 2008.
Day 2005
- Day E, Ison J, Strang J. Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004580] [DOI] [PubMed] [Google Scholar]
EMCDDA 2012
- European Monitoring Centre for Drugd & Drug Addiction (EMCDDA). Annual Report: The state of the drugs problem in Europe. European Union and Norway. Office for publications of the European Communities, Luxembourg 2012.
ESPAD 2012
- Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, et al. The 2011 ESPAD report: substance use among students in 36 European countries. Swedish Council for Information on Alcohol and Other Drugs, Stockholm, Sweden 2012.
Gossop 1989
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. British Journal of Psychiatry 1989;154:348‐53. [DOI] [PubMed] [Google Scholar]
Gowing 2009
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002025.pub4] [DOI] [Google Scholar]
Gowing 2009b
- Gowing L, Ali R, White J. Opioid antagonists with minimal sedation for opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002021.pub3] [DOI] [PubMed] [Google Scholar]
Gowing 2010
- Gowing L, Ali R, White J. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002022.pub3] [DOI] [Google Scholar]
Gowing 2014
- Gowing L, Farrell M, Ali R, White J. Alpha2‐adrenergic agonists for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD002024.pub4] [DOI] [Google Scholar]
Hunt 1990
- Hunt RD, Capper L, O'Connel P. Clonidine in child and adolescent psychiatry. Journal of Child Adolescent Psychofarmacology 1990;1:87‐102. [DOI] [PubMed] [Google Scholar]
Kaminer 1995
- Kaminer Y. Pharmacotherapy for adolescents with psychoactive substance use disorders. NIDA Research Monograph 1995;156:291‐324. [PubMed] [Google Scholar]
Kleber 1982
- Kleber HD, Riordan CE. The treatment of narcotic withdrawal: a historical review. Journal of Clinical Psichiatry 1982;43(6):30‐4. [PubMed] [Google Scholar]
Levy 2007
- Levy S, Vaughan BL, Angulo M, Knight JR. Buprenorphine replacement therapy for adolescents with opioid dependence: early experience from a children's hospital‐based outpatient treatment program. Journal of Adolescent Health 2007;40(5):477‐82. [DOI] [PubMed] [Google Scholar]
Lipton 1983
- Lipton DS, Maranda MJ. Detoxification from heroin dependency: an overview of method and effectiveness. Advances in Alcohol and Substance Abuse 1983;2(1):31‐55. [Google Scholar]
Mattick 1996
- Mattick RP, Hall W. Are detoxification programmes effective?. Lancet 1996;347:97‐100. [DOI] [PubMed] [Google Scholar]
Monitoring the Future 2013
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 2012 Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan. 2013 2013.
SAMHSA 2013
- Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H‐46, HHS 2013;Publication No. (SMA) 13‐4795. Rockville 2013.
Smith 2012
- Smith B, Fagan J, Kernan K. Outcomes of heroin dependent adolescents presenting for opiate substitution treatment. Journal of Substance Abuse Treatment 2012;42(1):35‐44. [DOI] [PubMed] [Google Scholar]
Vaillant 1988
- Vaillant GE. What can long‐term follow‐up teach us about relapse and prevention of relapse in addiction?. British Journal of Addiction 1988;83(10):1147‐57. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Minozzi 2009
- Minozzi S, Amato L, Davoli M. Detoxification treatments for opiate dependent adolescents. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006749.pub2] [DOI] [PubMed] [Google Scholar]



