Abstract
In vivo CO2 fixation activity and in vitro phosphoenolpyruvate carboxylase activity were demonstrated in effective and ineffective nodules of alfalfa (Medicago sativa L.) and in the nodules of four other legume species. Phosphoenolpyruvate carboxylase activity was greatly reduced in nodules from both host and bacterially conditioned ineffective alfalfa nodules as compared to effective alfalfa nodules.
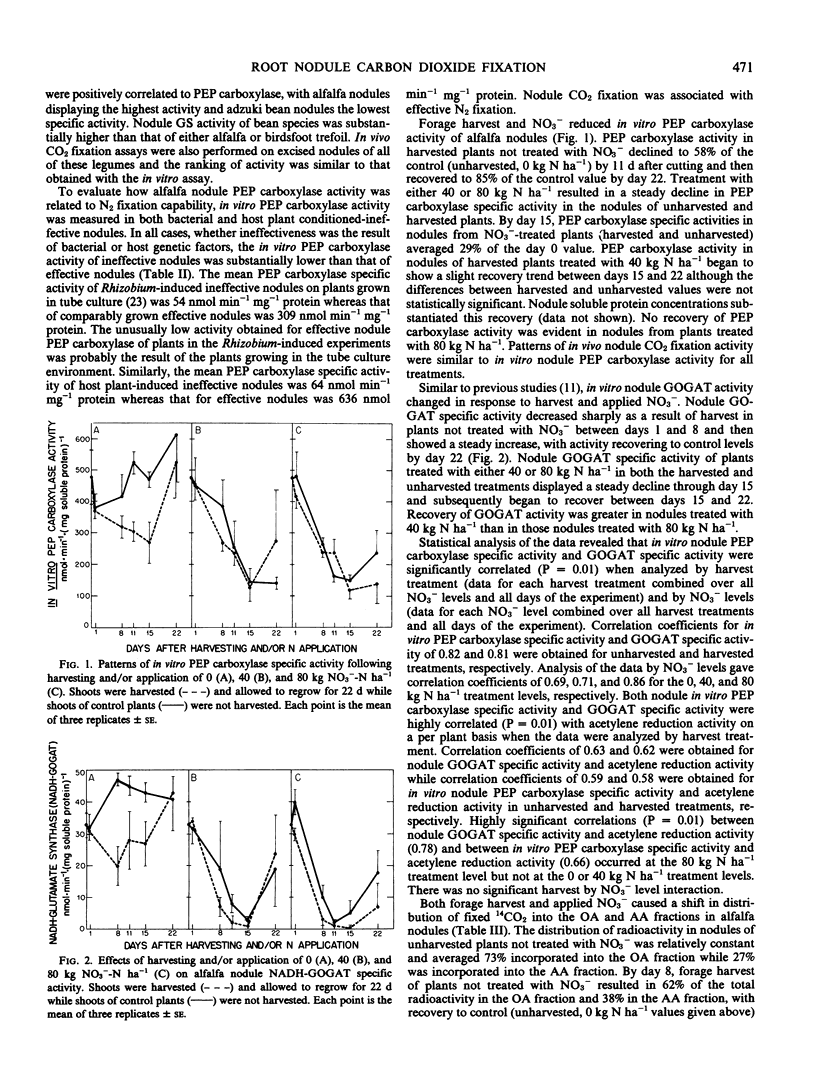
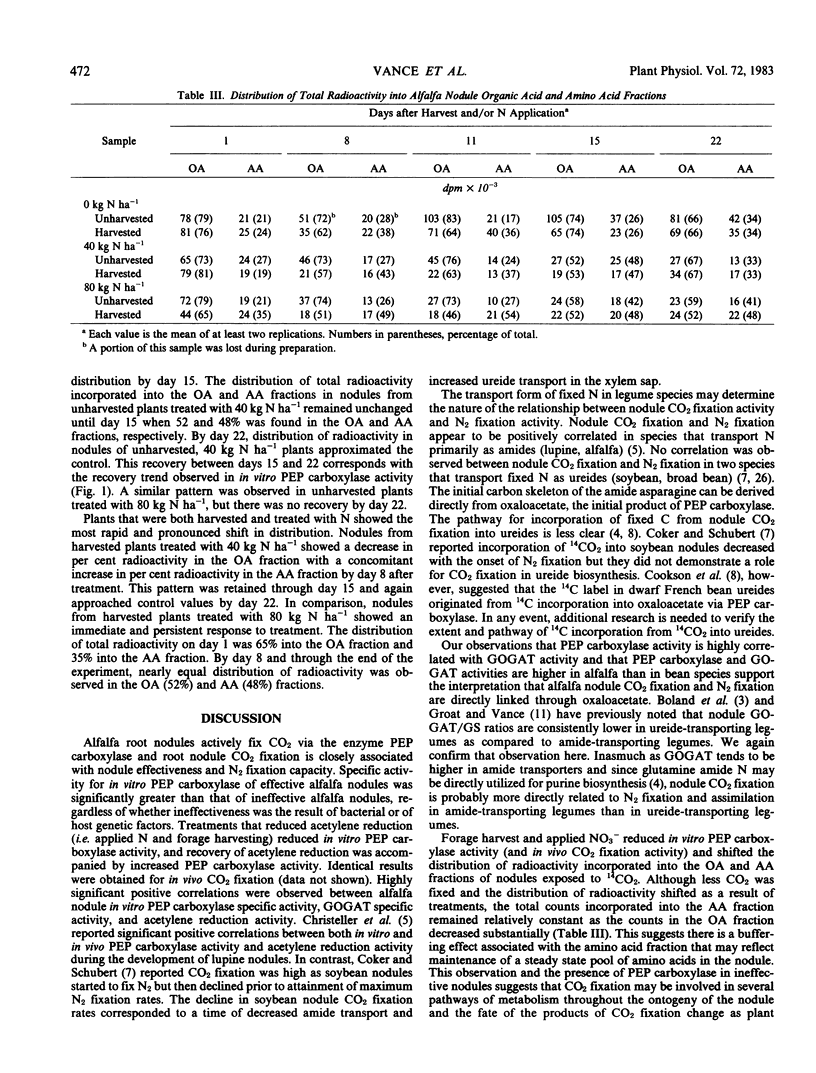
Forage harvest and nitrate application reduced both in vivo and in vitro CO2 fixation activity. By day 11, forage harvest resulted in a 42% decline in in vitro nodule phosphoenolpyruvate carboxylase activity while treatment with either 40 or 80 kilograms nitrogen per hectare reduced activity by 65%. In vitro specific activity of phosphoenolpyruvate carboxylase and glutamate synthase were positively correlated with each other and both were positively correlated with acetylene reduction activity.
The distribution of radioactivity in the nodules of control plants (unharvested, 0 kilograms nitrogen per hectare) averaged 73% into the organic acid and 27% into the amino acid fraction. In nodules from harvested plants treated with nitrate, near equal distribution of radioactivity was observed in the organic acid (52%) and amino acid (48%) fractions by day 8. Recovery to control distribution occurred only in those nodules whose in vitro phosphoenolpyruvate carboxylase activity recovered.
The results demonstrate that CO2 fixation is correlated with nitrogen fixation in alfalfa nodules. The maximum rate of CO2 fixation for attached and detached alfalfa nodules at low CO2 concentrations (0.13-0.38% CO2) were 18.3 and 4.9 nanomoles per hour per milligram dry weight, respectively. Nodule CO2 fixation was estimated to provide 25% of the carbon required for assimilation of symbiotically fixed nitrogen in alfalfa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland M. J., Schubert K. R. Purine biosynthesis and catabolism in soybean root nodules: incorporation of 14C from 14CO2 into xanthine. Arch Biochem Biophys. 1982 Feb;213(2):486–491. doi: 10.1016/0003-9861(82)90574-4. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.) : DEVELOPMENTAL PATTERNS AND RESPONSE TO APPLIED NITROGEN. Plant Physiol. 1981 Jun;67(6):1198–1203. doi: 10.1104/pp.67.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Evans H. J. Phosphoenolpyruvate carboxylase from soybean nodule cytosol. Evidence for isoenzymes and kinetics of the most active component. Biochim Biophys Acta. 1979 Apr 12;567(2):445–452. doi: 10.1016/0005-2744(79)90130-x. [DOI] [PubMed] [Google Scholar]
- Vance C. P., Heichel G. H., Barnes D. K., Bryan J. W., Johnson L. E. Nitrogen Fixation, Nodule Development, and Vegetative Regrowth of Alfalfa (Medicago sativa L.) following Harvest. Plant Physiol. 1979 Jul;64(1):1–8. doi: 10.1104/pp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


